Abstract
Background:
Recent conceptual models argue that early life adversity (ELA) accelerates development, which may contribute to poor mental and physical health outcomes. Evidence for accelerated development in youth comes from studies of telomere shortening or advanced pubertal development following circumscribed ELA experiences and neuroimaging studies of circuits involved in emotional processing. It remains unclear whether all ELA is associated with accelerated development across global metrics of biological aging or whether this pattern emerges following specific adversity types.
Methods:
In 247 children and adolescents aged 8–16 years with wide variability in ELA exposure, we evaluated the hypothesis that early environments characterized by threat, but not deprivation, would be associated with accelerated development across two global biological aging metrics: DNA methylation (DNAm) age and pubertal stage relative to chronological age. We also examined whether accelerated development explained associations of ELA with depressive symptoms and externalizing problems.
Results:
Exposure to threat-related ELA (e.g., violence) was associated with accelerated DNAm age and advanced pubertal stage, but exposure to deprivation (e.g., neglect, food insecurity) was not. In models including both ELA types, threat-related ELA was uniquely associated with accelerated DNAm age (ß=0.18) and advanced pubertal stage (ß=0.28), whereas deprivation was uniquely associated with delayed pubertal stage (ß=−0.21). Older DNAm age was related to greater depressive symptoms, and a significant indirect effect of threat exposure on depressive symptoms was observed through DNAm age.
Conclusions:
Early threat-related experiences are particularly associated with accelerated biological aging in youth, which may be a mechanism linking ELA with depressive symptoms.
Keywords: DNA methylation age, pubertal stage, early life adversity, threat, deprivation, youth
Introduction
Environmental experiences in childhood and adolescence play a meaningful role in shaping health across the lifespan. In particular, early life adversity (ELA)—experiences that represent a deviation from the expectable environment and require adaptation, encompassing physical and sexual abuse, neglect, and institutionalization—has been associated with deleterious mental and physical health outcomes(1–6).
Accumulating evidence suggests that ELA is associated with accelerated development and that this stress-induced acceleration may have negative downstream consequences for health(7,8). For example, some forms of ELA are associated with accelerated neural development, characterized by more mature function in fronto-amygdala circuits involved in emotional processing and learning(7,9). Other work has examined whether ELA is related to accelerated aging using more global metrics of development, including cellular and reproductive strategy indicators. Specifically, ELA has been associated with shorter telomere length, suggesting that telomere erosion may be a cellular mechanism by which ELA is biologically embedded and undermines long-term health(8,10–13). Additionally, the onset of puberty is a reliable marker of maturation in youth that exhibits significant variation regarding age at onset and pace of progression(14). ELA has been associated with faster sexual maturation, including earlier pubertal timing and age of menarche(15–17). Although these lines of work have often been conducted independently, a recent model by Belsky and Shalev(8) posits that associations of ELA with shorter telomeres and accelerated pubertal maturation may reflect the same evolutionary-developmental process. This process is consistent with an evolutionary, life history perspective(15,16,18,19) whereby reproductive fitness is prioritized over growth and maintenance in the context of adverse early environments(8). Although accelerated aging may increase reproductive fitness, it can have negative consequences for physical and mental health.
Here, we examine whether ELA is associated with both cellular and reproductive strategy metrics of biological aging in youth, focusing on a promising, but understudied, cellular metric in studies of ELA in youth: accelerated epigenetic age. Recently established epigenetic clocks indicate that it is possible to quantify biological age relative to chronological age based on genome-wide DNA methylation (DNAm) data(20). DNAm age correlates strongly with chronological age(21), and advanced DNAm age relative to chronological age (i.e., accelerated epigenetic aging) indicates disproportionate biological aging(20). Accelerated epigenetic age has been associated with numerous aging-related risk factors and outcomes, including all-cause mortality(22,23), cancer- and cardiovascular-related mortality(22), cognitive decline(24,25), brain aging(25), and obesity(26). Although DNAm age is heritable, it is sensitive to environmental influences(20,27), and advanced epigenetic aging has been associated with ELA in adults(28–31). In the only study examining this link in children, Jovanovic and colleagues found that direct violence exposure was associated with advanced DNAm age, but witnessing violence was not(32). Accelerated epigenetic age was also associated with a more adult-like cardiovascular response to stress. This study provides preliminary evidence that advanced epigenetic aging during childhood may be one mechanism through which violence exposure impacts health outcomes. For our reproductive strategy metric of biological aging, we focused on pubertal stage relative to chronological age. Pubertal stage is one of the clearest markers of development and physical maturation in youth that can be easily reported and assessed. Early pubertal timing—an indicator of accelerated biological aging—has frequently been associated with numerous negative health outcomes over the lifespan(33,34).
We extend prior work by examining whether accelerated development following ELA is global or specific to certain types of ELA. ELA encompasses a wide range of experiences, including physical and sexual abuse, neglect, chronic poverty, and others. A recent conceptual model posits that ELA reflects several underlying dimensions of environmental experience, which have neurodevelopmental consequences that are at least partially distinct(35–37). In particular, experiences of threat that reflect potential harm and those involving deprivation, or an absence of expected environmental inputs, have been shown to have distinct associations with neurodevelopmental outcomes(35–43). We posit that ELA characterized by threat (i.e., violence exposure) should be particularly likely to result in accelerated biological aging. Life history theory argues that exposure to harsh (e.g., violent) environments should favor the development of fast life history strategies and accelerated maturation, including early onset of puberty(15,18). In contrast, exposure to deprived environments in which bioenergetic resources are scarce should favor the development of slower life history traits that conserve resources and delay puberty and reproduction(18). Thus, early exposure to threat-related ELA (e.g., physical abuse, violence exposure) as opposed to deprivation (e.g., neglect, food insecurity) may be especially likely to trigger accelerated biological aging. Consistent with this idea, research suggests that threat- related ELA—including harsh parenting and maltreatment—is related to earlier onset of puberty(17,44–47). In contrast, children who experience deprivation, including food insecurity, neglect, and parental absence, have been found to exhibit delayed onset of puberty(48–51). We are unaware of research examining how different ELA types influence epigenetic aging in youth.
In this study, we examined associations of multiple ELA types across the dimensions of threat and deprivation with advanced epigenetic age and pubertal development in a large sample of children and adolescents with wide variability in ELA exposure. We additionally investigated associations of these biological aging metrics with psychiatric symptoms in domains linked to accelerated pubertal development: depression and externalizing behaviors(33,34,52,53). Finally, we tested whether accelerated biological aging accounted for associations of ELA with psychiatric symptoms. We hypothesized that early threat, but not deprivation, exposure would be related to accelerated DNAm age and advanced pubertal stage relative to chronological age and that these biological aging indicators would partially explain associations with depressive symptoms and externalizing problems.
Methods and Materials
Participants and Procedure
Children aged 8–16 years and a parent or guardian were recruited to participate in a study examining ELA, emotion regulation, and psychopathology. Between January 2015 and January 2017, 262 children were enrolled from the community in Seattle, WA (see Supplemental Methods). All procedures were approved by the University of Washington Institutional Review Board. Written informed consent was obtained from legal guardians; children provided written assent.
ELA Exposure
We used a multi-informant, multi-method approach for assessing ELA exposure. Children completed interview and self-report measures assessing child maltreatment, violence exposure, and other ELA; caregivers also completed several questionnaire measures assessing children’s exposure to maltreatment, trauma, and other adversities (see Supplemental Methods). Across these validated measures, multiple ELA experiences reflecting threat and deprivation were assessed for all participants. Threat-related ELA included physical abuse, sexual abuse, emotional abuse, domestic violence, and exposure to other forms of interpersonal violence; deprivation included emotional neglect, physical neglect, food insecurity, and low cognitive stimulation (i.e., cognitive deprivation). Children reported their age of first exposure for threat- related experiences of physical abuse, sexual abuse, and domestic violence; this information was not queried for other forms of ELA.
We created threat and deprivation exposure composites by summing the total number of threat and deprivation experiences, respectively, endorsed by the child and/or caregiver. Child and caregiver reports were combined using an “or” rule; each ELA was coded present if endorsed by the child or caregiver (see Supplemental Methods).
Psychiatric Symptoms
Children completed the Children’s Depression Inventory-2, a widely used self-report measure of depressive symptoms in youth with sound psychometric properties(54,55). To assess externalizing problems, children and caregivers completed the Youth Self-Report and Child Behavior Checklist(56). These widely used scales utilize normative data to generate age- standardized estimates of symptom severity. The externalizing scale has demonstrated validity in discriminating between youths with and without psychopathology(56–58). We used the highest externalizing problems T-score from child or caregiver.
Pubertal Stage
Pubertal stage was determined using self-report Tanner staging(59–61). Using schematic drawings of two secondary sex characteristics (pubic hair and breast/testes development), participants reported their developmental stage on a scale of 1–5. We computed an average score of these ratings. A Tanner stage of 1 signifies no pubertal development has begun; a stage of 5 signifies adult levels of pubertal maturation. Self-report Tanner stage scores correlate with physicians’ physical examinations of pubertal development(62,63).
DNAm Age
Saliva samples were collected using Oragene® kits (DNA Genotek, Ontario, Canada). DNA extraction and methylation profiling were conducted by AKESOgen (Atlanta, GA). The Illumina Infinium MethlyationEPIC BeadChip kit was used to assess methylation levels at >850,000 methylation sites. Horvath DNAm age estimates were calculated based on raw (non- normalized) probe data (see Supplemental Methods). As in prior research(28,32), we regressed DNAm age on chronological age; the unstandardized residuals indicated epigenetic age acceleration and were used as the dependent variables in analyses. Positive residuals indicate that DNAm age is overestimated compared to chronological age, whereas negative residuals indicate DNAm age is underestimated(28).
Covariates
We adjusted for sex, race/ethnicity, and family poverty in all analyses as these represent plausible confounders of associations of threat and deprivation with DNAm age and pubertal stage. Children reported on their sex and race/ethnicity. Caregivers reported total household income, which was used to assess whether the family was living in poverty. The income-to-needs ratio was calculated by dividing total household income by the 2015 U.S. census-defined poverty line for a family of that size, with a value <1 indicating a family was living below the poverty line. We included poverty as a covariate because it is a context that can increase the likelihood of exposure to experiences of threat and deprivation as well as other environmental risks that have unknown effects on biological aging (e.g., exposure to toxins, differences in parenting, crowding)(64). Adjusting for poverty allowed us to examine threat and deprivation after removing the variance associated with these additional environmental factors. Importantly, our pattern of results was similar with and without adjustment for poverty.
Analytic Approach
We examined if threat- and deprivation-related ELA exposure were each associated with our biological aging metrics using linear regression, with DNAm age residuals and Tanner stage residuals (both residualized on chronological age) as dependent variables. Given high co- occurrence of threat and deprivation, we also estimated a model that included both forms of ELA to evaluate unique associations of each ELA type with biological aging.
To investigate whether ELA and our biological aging metrics were related to depressive symptoms and externalizing problems, we used linear regression. These models adjusted for age; thus we used DNAm age and Tanner stage as independent variables, rather than the residuals. We investigated whether there were significant indirect effects of ELA on symptoms through biological aging metrics using bootstrapping with bias-corrected confidence intervals, conducted with the PROCESS macro for SPSS(65).
We conducted two sensitivity analyses. First, because the threat composite had a greater range than the deprivation composite, we ran a sensitivity analysis to ensure this wider variability did not explain findings. We created a threat composite that summed the number of experiences across physical, sexual, and emotional abuse, and domestic violence and then added a standardized score (M=0, SD=1) of number of types of directly experienced interpersonal violence rather than a count variable. This made the ranges of the threat and deprivation composites comparable. We re-ran all analyses using this alternate threat composite. Second, we covaried the estimated proportion of epithelial (buccal) cells in each sample in analyses with DNAm age variables; the proportion of epithelial cells in saliva varies across individuals and can influence DNAm(66). Consistent with prior research(32,66), we estimated the proportion of epithelial (buccal) cells using the method of Houseman and colleagues(67,68) and a reference from buccal cells (GSE46573) from the Gene Expression Omnibus. The proportion of epithelial cells was included along with other relevant covariates. We used a two-sided significance level of .05 for all analyses. The data for this paper are available on the Open Science Framework (https://osf.io/43hfq/).
Results
Participant Characteristics
Our analytic sample comprised 247 participants with data on ELA threat and/or deprivation composites, plus DNAm age (n=205) and/or Tanner stage (n=221). Socio- demographic characteristics of participants and ELA exposures are presented in Table 1. The majority of youth who experienced instances of threat-related ELA (74.6%) were first exposed in early life, before age 8 years. DNAm age ranged from 5.28–28.33 years (M=16.08, SD=4.67) and was positively correlated with chronological age, r=.62, p<.0001. Mean Tanner stage was 3.18 (SD=1.32, range=1–5). Older age was associated with higher Tanner stage, r=.79, p<.0001. DNAm age and Tanner stage were positively correlated, r=.52, p<.0001. Following prior work(28,32), the residual of DNAm age on chronological age was used to measure epigenetic age acceleration [M<0.00001 (SD=3.65), range=−10.55–11.53]. Similarly, the residual of Tanner stage on chronological age was used to measure advanced pubertal development [M<0.00001 (SD=0.81), range=−1.80–2.39]. Positive scores on residual measures indicated accelerated biological aging. Table 2 presents associations of participant socio-demographics with biological aging metrics.
Table 1.
Participant characteristics.
| Values | Valid n | |
|---|---|---|
| Socio-demographics | ||
| Age, years | 12.7 (2.6) [8–16] | 247 |
| Female sex, % | 47.8 (118) | 247 |
| Race/ethnicity, % | 247 | |
| White | 38.9 (96) | |
| Black | 27.9 (69) | |
| Latino | 12.1 (30) | |
| Other | 21.1 (52) | |
| Family below the poverty line, % | 26.8 (62) | 231 |
| Early life adversity exposure | ||
| Threat exposure composite | 5.2 (3.6) [0–14] | 241 |
| Physical abuse, % | 41.7 (103) | 247 |
| Sexual abuse, % | 24.7 (61) | 247 |
| Emotional abuse, % | 32.0 (79) | 247 |
| Domestic violence, % | 45.7 (113) | 247 |
| Number of types of directly | 3.7 (2.6) [0–10] | 241 |
| experienced interpersonal violence | ||
| Deprivation exposure composite | 0.9 (1.1) [0–4] | 241 |
| Physical neglect, % | 27.5 (68) | 247 |
| Emotional neglect, % | 24.7 (61) | 247 |
| Food insecurity, % | 16.3 (40) | 245 |
| Cognitive deprivation, % | 23.2 (56) | 241 |
| Psychiatric Symptoms | ||
| Depressive symptoms | 8.8 (7.4) [0–39] | 247 |
| Externalizing problems | 56.8 (10.9) [34–81] | 247 |
Mean (SD) [range] or % (n).
Table 2.
Associations of participant socio-demographics with biological aging metrics.
| DNAm Age Residual | Tanner Stage Residual | |||||
|---|---|---|---|---|---|---|
| F-statistic | M (SE) | n | F-statistic | M (SE) | n | |
| Sex | F(1, 203)=4.22, p=.041 | F(1, 219)=0.11, p=.745 | ||||
| Male | −0.49 (0.35) | 108 | 0.02 (0.08) | 111 | ||
| Female | 0.55 (0.37) | 97 | −0.02 (0.08) | 110 | ||
| Race/ethnicity | F(3, 201)=1.15, p=.332 | F(3, 217)=5.65, p=.001 | ||||
| White | −0.55 (0.41) | 81 | −0.22 (0.08)b | 90 | ||
| Black | 0.19 (0.50) | 54 | 0.33 (0.10)a | 57 | ||
| Latino | 0.27 (0.71) | 26 | 0.05 (0.15)ab | 29 | ||
| Other | 0.63 (0.55) | 44 | −0.02 (0.12)ab | 45 | ||
| Family poverty status | F(1, 188)=0.99, p=.321 | F(1, 206)=4.50, p=.035 | ||||
| Above poverty line | 0.18 (0.31) | 140 | −0.05 (0.06) | 155 | ||
| Below poverty line | −0.42 (0.51) | 50 | 0.22 (0.11) | 53 | ||
Note. DNAm=DNA methylation. In the Tanner stage residual analysis, means that have no superscript in common for the race/ethnicity categories are significantly different from each other (Bonferroni adjustment for multiple comparisons, p<.05). Epigenetic age acceleration differed by sex, such that females had more positive DNAm age residuals than males. Tanner stage residual score varied by race/ethnicity, with Black youth having accelerated pubertal development compared to White youth. Tanner stage residuals were also greater for those with families below, rather than above, the poverty line.
ELA and Biological Aging
ELA experiences characterized by threat and deprivation were positively correlated, r=0.61, p<.0001. However, only threat, and not deprivation, was associated with accelerated biological aging (Table 3). Higher threat exposure was associated with epigenetic age acceleration (Figure 1A) and advanced pubertal stage (Figure 2A). In contrast, deprivation was not associated with either biological aging metric (Figures 1B and 2B).
Table 3.
Regression parameters and 95% confidence intervals for associations of early life adversity experiences of threat and deprivation with biological aging metrics.
| DNAm Age Residual | Tanner Stage Residual | |||||||
|---|---|---|---|---|---|---|---|---|
| b (95% CI) | β | p | n | b (95% CI) | β | p | n | |
| Threat exposure score | ||||||||
| Model 1a | 0.16 (0.01–0.32) | 0.17 | .042 | 186 | 0.04 (0.01–0.07) | 0.17 | .025 | 204 |
| Model 2b | 0.17 (−0.02–0.36) | 0.18 | .080 | 184 | 0.06 (0.02–0.10) | 0.28 | .002 | 201 |
| Deprivation exposure score | ||||||||
| Model 1a | 0.32 (−0.24–0.87) | 0.10 | .260 | 188 | −0.05 (−0.16–0.07) | −0.06 | .425 | 205 |
| Model 2c | −0.05 (−0.73–0.64) | −0.02 | .890 | 184 | −0.16 (−0.29– −0.02) | −0.21 | .022 | 201 |
Note. DNAm=DNA methylation. CI=confidence interval.
Model adjusted for sex, race/ethnicity, and family poverty status.
Model 1 further adjusted for deprivation exposure score.
Model 1 further adjusted for threat exposure score.
Figure 1.
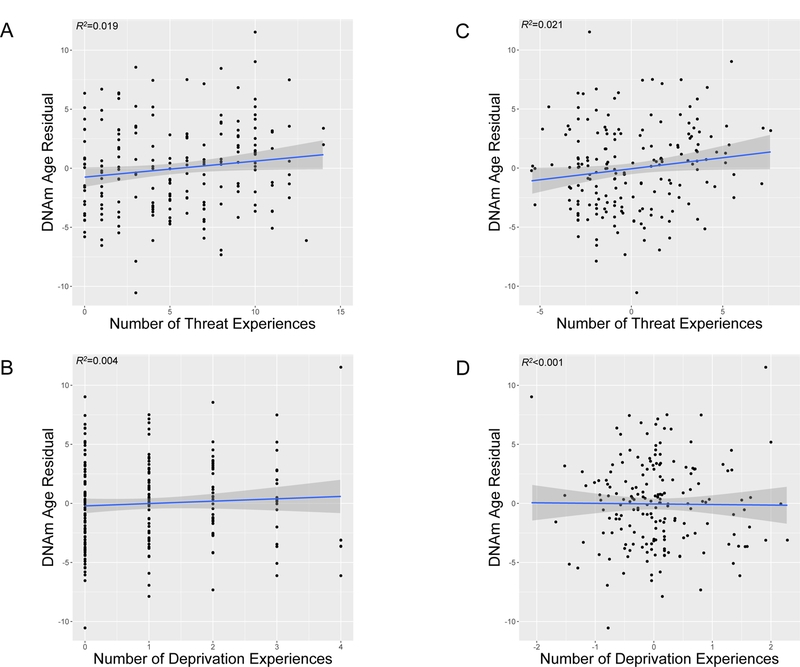
DNA methylation (DNAm) age residuals for youth as a function of the number of types of A) threat experiences and B) deprivation experiences. Scatterplots with regression lines and 95% confidence intervals, along with R-squared (R2) values, are shown. Figures 1A and 1B present unadjusted associations. Figures 1C and 1D present associations with threat and deprivation experiences residualized on covariates in the fully adjusted model (sex, race/ethnicity, family poverty status, and other dimension of early life adversity), respectively. Positive DNAm age residuals indicate accelerated DNAm age relative to chronological age.
Figure 2.
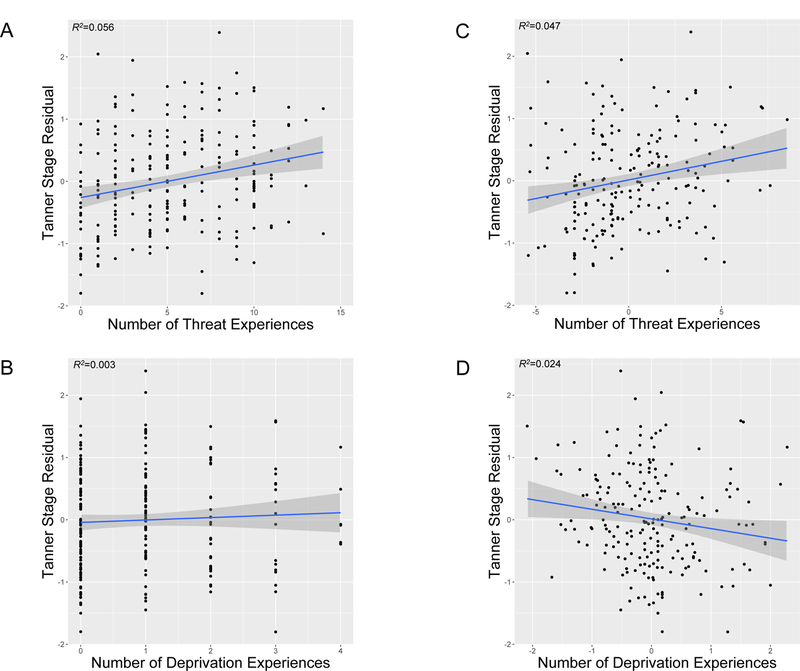
Tanner stage residuals for youth as a function of the number of types of A) threat experiences and B) deprivation experiences. Scatterplots with regression lines and 95% confidence intervals, along with R-squared (R2) values, are shown. Figures 2A and 2B present unadjusted associations. Figures 2C and 2D present associations with threat and deprivation experiences residualized on covariates in the fully adjusted model (sex, race/ethnicity, family poverty status, and other dimension of early life adversity), respectively. Positive Tanner stage residuals indicate advanced pubertal stage relative to chronological age.
Given high co-occurrence of threat and deprivation, we evaluated unique associations of each ELA type with biological aging by including both forms of ELA in the model (Table 3). Effect sizes for threat exposure were unchanged for accelerated epigenetic age (Figure 1C) and larger for advanced pubertal stage (Figure 2C) when adjusting for deprivation. In contrast, deprivation remained unassociated with epigenetic age (Figure 1D) and was associated with delayed pubertal stage (Figure 2D) after adjusting for threat exposure. There were no significant interactions between threat- and deprivation-related ELA in predicting either biological aging metric (ßs≤−0.13, ps>.090). Table S1 presents associations of individual threat and deprivation experiences with the biological aging metrics.
Threat Exposure, Biological Aging, and Psychiatric Symptoms
Threat exposure—the ELA dimension associated with both biological aging metrics—was related to greater depressive symptoms and externalizing problems (Table 4). Deprivation was also associated with higher symptom levels (Table 4). Only DNAm age was related to greater depressive symptoms and, at trend level, externalizing problems; pubertal stage was not associated with either symptom measure (Table 4). DNAm age remained associated with depressive symptoms when threat exposure was included in the model. The indirect effect of threat exposure on depressive symptoms through DNAm age was positive (0.045) and significantly different from zero (95% CI:0.001–0.125)(Figure 3).
Table 4.
Regression parameters and 95% confidence intervals for associations of early life adversity experiences of threat and deprivation and biological aging metrics with depressive symptoms and externalizing problems.
| Depressive Symptoms | Externalizing Problems | |||||||
|---|---|---|---|---|---|---|---|---|
| b (95% CI) | β | p | n | b (95% CI) | β | p | n | |
| Threat | 1.04 (0.80–1.28) | 0.51 | <.0001 | 225 | 1.54 (1.18–1.90) | 0.53 | <.0001 | 225 |
| exposure scorea | ||||||||
| Deprivation | 2.75 (1.82–3.67) | 0.39 | <.0001 | 227 | 4.78 (3.47–6.09) | 0.48 | <.0001 | 227 |
| exposure scorea | ||||||||
| DNAm age | ||||||||
| Model 1a | 0.42 (0.14–0.70) | 0.26 | .004 | 190 | 0.36 (−0.05–0.78) | 0.16 | .084 | 190 |
| Model 2b | 0.26 (0.01–0.52) | 0.16 | .040 | 186 | 0.15 (−0.22–0.52) | 0.06 | .434 | 186 |
| Tanner stage | ||||||||
| Model 1a | 0.34 (−0.92–1.61) | 0.06 | .594 | 208 | 0.49 (−1.27–2.25) | 0.06 | .584 | 208 |
| Model 2b | −0.53 (−1.67–0.61) | −0.09 | .359 | 204 | −0.53 (−2.02–0.97) | −0.07 | .490 | 204 |
Note. CI=confidence interval. DNAm=DNA methylation.
Model adjusted for age, sex, race/ethnicity, and family poverty status.
Model 1 further adjusted for threat exposure.
Figure 3.
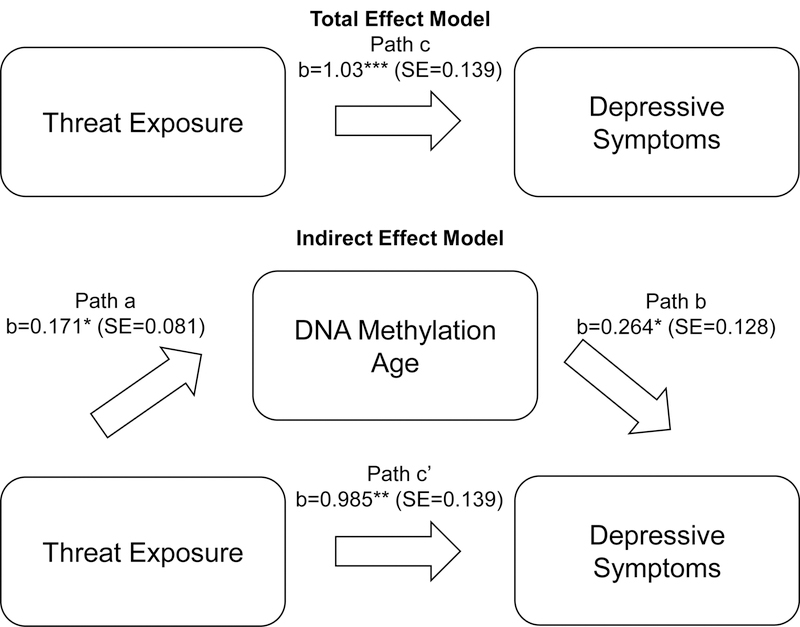
Indirect effect of threat exposure on depressive symptoms through DNA methylation age. Model adjusted for age, sex, race/ethnicity, and family poverty status.
Sensitivity Analyses
To ensure findings were not due to the greater range of our threat, relative to deprivation, composite, we conducted analyses using an alternate threat composite that had a range that was more comparable to the deprivation composite. Threat exposure operationalized in this way remained associated with accelerated epigenetic age and advanced pubertal stage (Table S2); other findings were unchanged (Table S3). The indirect effect of this alternate threat exposure on depressive symptoms through DNAm age remained significant [indirect effect=0.074, (95% CI:0.004–0.222)].
We also covaried estimates of the proportion of epithelial (buccal) cells in each sample in analyses with DNAm age given that the proportion of epithelial cells in saliva can influence DNAm(61). Associations of ELA with DNAm age were largely similar (Table S4), as were associations of DNAm age with depressive symptoms (Table S5). The indirect effect of threat exposure on depressive symptoms through DNAm age [indirect effect=0.043, (95% CI:−0.004– 0.135)] was similar in effect size to the main analyses but not significant.
Discussion
Accelerated aging is a potential mechanism that might contribute to poor health in youth with ELA. Here, we find that ELA is associated with two global metrics of biological aging: advanced DNAm age and pubertal stage compared to chronological age. We extend prior work by demonstrating specificity in the types of ELA that are associated with accelerated biological aging. ELA characterized by threat—specifically interpersonal violence—was associated with both aging metrics. The consistency of this pattern across these biological aging indicators suggests that ELA involving violence is characterized by faster development across multiple levels of analysis. In contrast, ELA characterized by deprivation was not only unrelated to epigenetic age but was associated with delayed pubertal stage relative to chronological age in models adjusting for threat exposure. Findings also suggested that accelerated DNAm age was related to greater depressive symptoms and accounted, in part, for the association of threat exposure with depressive symptoms.
Our findings add to a growing literature suggesting that exposure to ELA may contribute to accelerated development(7,8). They further demonstrate that some ELA experiences are associated with a relatively global pattern of accelerated development, whereas others may have effects on certain neural circuits or systems that are not observable in global aging metrics. Our results are consistent with research on pubertal timing among children with maltreatment(17,44–47) and extend one prior study documenting accelerated epigenetic age in children who experienced violence(32). A similar pattern of accelerated epigenetic aging has been observed in adults with childhood trauma(28). Violence exposure in childhood has also been associated with other cellular measures of accelerated aging, such as telomere shortening(10–13). Together, these results suggest that exposure to threatening early environments is associated with a global pattern of accelerated aging across numerous biological metrics.
In contrast, early exposure to deprivation—including neglect, food insecurity, and an absence of cognitive stimulation—was not associated with accelerated aging. In our model adjusting for threat exposure, deprivation was related to lower pubertal stage relative to chronological age, suggesting that the unique aspects of deprivation that are non-overlapping with threat may be associated with delayed pubertal development. This pattern has been observed in several studies examining neglect and food insecurity and pubertal timing(49,50). Previous research has documented accelerated development in neural circuits underlying emotional processing and shorter telomeres in children exposed to institutionalization(69–72). Future research needs to determine whether the particularly extreme deprivation involved in institutional rearing is associated with other biological aging metrics like accelerated epigenetic age and evaluate whether the deprivation examined here in a community-based sample is associated with neural measures of accelerated development. Determining whether accelerated development is global or specific to certain neural circuits or systems following various forms of ELA is a critical unanswered question.
Together, these findings are consistent with life history theory(8,15,16,18,19). This evolutionary-developmental perspective suggests that exposure to harsh environments should favor the development of life history traits consistent with faster maturation, but exposure to deprived environments should favor the development of life history traits that conserve resources and delay reproduction(15,18). Although nutritional deprivation is argued to be a primary driver of slower life history strategies, our findings suggest that cognitive and emotional deprivation may also produce a slower, rather than accelerated, progression of biological aging. Additional studies are needed to replicate this finding. Furthermore, some conceptual models—specifically external prediction models(15,73)—posit that accelerated development after exposure to threat- related ELA reflects an adaptation to an expected future external environment, whereas internal prediction models(74,75) suggest this accelerated development is, at least in part, an adaptation to a compromised internal state resulting from physiological damage from harsh environments. Although external and internal prediction models are not mutually exclusive(74), further research is required to better understand the degree to which accelerated development after threat-related ELA reflects adaptation to internal vs. external cues.
More broadly, these findings add to a growing literature suggesting that different ELA types may have distinct developmental consequences(35–37,76). These specific effects can only be observed in studies that assess multiple dimensions of ELA and examine their unique associations with developmental outcomes(37,77). Given high co-occurrence of ELA types(1,2,78), studies examining single exposure types without considering a range of co- occurring adversities may obscure such specificity. Our findings suggest that considering distinct dimensions of ELA, rather than treating these experiences as a single exposure, holds promise for elucidating the consequences of ELA(35).
Theoretical models suggest that stress-induced acceleration due to ELA may have negative downstream consequences for mental health, but prior research in youth and young adults has been mixed regarding whether accelerated epigenetic age is associated with psychiatric symptoms. For example, accelerated DNAm age was associated with elevated depressive symptoms in one sample(30) but not with internalizing or externalizing symptoms in another sample(32). In the current study, accelerated DNAm age was related to elevated depressive symptoms and marginally with externalizing problems. Additionally, accelerated epigenetic age accounted, in part, for the association of threat exposure with depressive symptoms. Interestingly, we did not observe associations between pubertal stage and psychiatric symptoms. Evidence from population-based samples suggests that accelerated pubertal development is related to depression and externalizing psychopathology in adolescents(34,79,80). It is possible that we were underpowered to detect these associations in the current sample.
Given that our study is cross-sectional, longitudinal research is needed to better understand the mechanisms and consequences of ELA. For example, it is of interest to examine how these biological aging metrics relate to changes in psychopathology and physical health over time and vice versa. The pace of biological aging in healthy adults is associated with aging- related outcomes such as poor physical functioning and cognitive performance even before midlife(81). Investigating whether changes in these biological aging metrics over time are associated with increases or decreases in psychopathology and physical health indicators is an important direction for future research. Moreover, evaluating the degree to which accelerated epigenetic aging is stable or can be ameliorated by positive social experience or intervention is a critical goal for future studies. Further studies are also needed to identify the specific neurobiological processes that are reflected in various aging metrics. DNAm age and Tanner stage were positively correlated in our sample, but a recent study comparing different biological aging measures found relatively low agreement between them(82). Additional research is needed to elucidate what aspects of the aging process these metrics represent and which are most relevant for understanding consequences for physical and mental health.
Our study has several limitations. First, the cross-sectional design limits our ability to draw conclusions about temporality. For example, it is possible that some youth may have reached adrenarche or puberty before experiencing some forms of ELA. Even though the vast majority of youth reported that threat-related experiences first occurred in early childhood (i.e., before the typical age of onset of adrenarche or puberty), the lack of prospective, fine-grained timing information on all ELA forms is a limitation. Further, it is possible that internalizing or externalizing psychopathology could have predated some ELA exposures. Thus, our findings should be considered with these caveats in mind. We plan to explore further some of these results as we continue longitudinal assessments of our sample. Second, we used retrospective reporting (both self- and caregiver-report) of ELA, which has established limitations(83). Additionally, our ELA composites assessed exposure to different types of threat- and deprivation-related experiences but not other aspects of ELA that could influence biological aging, such as severity or duration of exposure. We also considered self-reported pubertal stage and psychiatric symptoms, although externalizing problems were based on child- and caregiver- report. Research has shown high correlations between self-report and physical exam measures of pubertal development, but there is nonetheless variation in accuracy of self-reporting(61–63,84). Future studies should replicate our findings using interview-based measures of psychopathology and physical exam or hormonal metrics of pubertal development. Third, although the racial and ethnic diversity of our sample enhances the generalizability of our findings, such ancestral heterogeneity has been linked to differences in epigenetic age(85) and pubertal timing(86,87). However, race/ethnicity was not significantly associated with epigenetic age acceleration in our sample, and we adjusted for race/ethnicity. Fourth, we used the MethlyationEPIC BeadChip to assess DNAm levels, but the Horvath epigenetic clock was developed on the HumanMethylation450 BeadChip. Although probes are highly overlapping across chips, 16 of the 353 sites for the Horvath epigenetic clock were not on the MethlyationEPIC chip. However, initial work suggests congruence between DNAm levels from both chips(88,89).
Even with these limitations, our study has several unique strengths, including: 1) considering cellular and reproductive strategy metrics of biological aging, 2) using a multi- measure and multi-informant approach to assessing ELA, and 3) examining how ELA dimensions of threat and deprivation relate to biological aging metrics and psychiatric symptoms.
Conclusions
Early experiences of threat and violence were associated with accelerated development with respect to epigenetic age and pubertal stage, whereas early experiences of deprivation were not. Advanced epigenetic aging may be one mechanism linking early threat exposure with depressive symptoms. Our findings shed light on how ELA, particularly threat-related experiences, may get under the skin to contribute to negative health outcomes.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (R01MH103291; R01MH103291-S2; K01HL130650; F32MH114317).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC (2010): Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry 67:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC (2012): Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Arch Gen Psychiatry 69:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH (2009): Adverse childhood experiences and the risk of premature mortality. Am J Prev Med 37:389–396. [DOI] [PubMed] [Google Scholar]
- 4.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. (1998): Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 14:245–258. [DOI] [PubMed] [Google Scholar]
- 5.Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, et al. (2012): Physical and sexual abuse in childhood as predictors of early onset cardiovascular events in women. Circulation 126:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suglia SF, Koenen KC, Boynton-Jarrett R, Chan PS, Clark CJ, Danese A, et al. (2018): Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation 137:e15–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaghan BL, Tottenham N (2016): The stress acceleration hypothesis: effects of early- life adversity on emotion circuits and behavior. Curr Opin Behav Sci 7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belsky J, Shalev I (2016): Contextual adversity, telomere erosion, pubertal development and health: two models of accelerated aging—or one? Dev Psychopathol 28:1367–1383. [DOI] [PubMed] [Google Scholar]
- 9.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013): Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, et al. (2018): Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 23:858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, Theall KP (2014): The association of telomere length with family violence and disruption. Pediatrics 134:e128– e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. (2013): Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 18:576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI (2017): Stress-related telomere length in children: a systematic review. J Psychiatr Res 92:47–54. [DOI] [PubMed] [Google Scholar]
- 14.Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ (2011): Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol 47:1389–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belsky J, Steinberg L, Draper P (1991): Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev 62:647–670. [DOI] [PubMed] [Google Scholar]
- 16.Chisolm JS, Quinlivan JA, Petersen RW, Coall DA (2005): Early stress predicts age at menarche and first birth, adult attachment, and expected lifespan. Hum Nat 16:233–265. [DOI] [PubMed] [Google Scholar]
- 17.Mendle J, Ryan RM, McKone KM (2016): Early childhood maltreatment and pubertal development: replication in a population‐based sample. J Res Adolesc 26:595–602. [DOI] [PubMed] [Google Scholar]
- 18.Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL (2009): Fundamental dimensions of environmental risk. Hum Nat 20:204–268. [DOI] [PubMed] [Google Scholar]
- 19.Ellis BJ, Del Giudice M (2014): Beyond allostatic load: rethinking the role of stress in regulating human development. Dev Psychopathol 26:1–20. [DOI] [PubMed] [Google Scholar]
- 20.Horvath S (2013): DNA methylation age of human tissues and cell types. Genome Biol 14:R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, Tsai PC, et al. (2016): DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8:1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perna L, Zhang Y, Mons U, Holleczek B, Saum KU, Brenner H (2016): Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics 8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. (2015): DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol 16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marioni RE, Shah S, McRae AF, Ritchie SJ, Muniz-Terrera G, Harris SE, et al. (2015): The epigenetic clock is correlated with physical and cognitive fitness in the Lothian Birth Cohort 1936. Int J Epidemiol 44:1388–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine ME, Lu AT, Bennett DA, Horvath S (2015): Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging 7:1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath S, Erhart W, Brosch M, Ammerpohl O, von Schönfels W, Ahrens M, et al. (2014): Obesity accelerates epigenetic aging of human liver. Proc Natl Acad Sci USA 111:15538–15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gassen NC, Chrousos GP, Binder EB, Zannas AS (2017): Life stress, glucocorticoid signaling, and the aging epigenome: implications for aging-related diseases. Neurosci Biobehav Rev 74:356–365. [DOI] [PubMed] [Google Scholar]
- 28.Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, et al. (2018): Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology 92:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, et al. (2015): Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol 16:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brody GH, Yu T, Chen E, Beach SR, Miller GE (2016): Family‐centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. J Child Psychol Psychiatry 57:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody GH, Miller GE, Yu T, Beach SR, Chen E (2016): Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: a replication across two longitudinal cohorts. Psychol Sci 27:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, et al. (2017): Exposure to violence accelerates epigenetic aging in children. Sci Rep 7:8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Negriff S, Susman EJ (2011): Pubertal timing, depression, and externalizing problems: a framework, review, and examination of gender differences. J Res Adolesc 21:717–746. [Google Scholar]
- 34.Copeland W, Shanahan L, Miller S, Costello EJ, Angold A, Maughan B (2010): Outcomes of early pubertal timing in young women: a prospective population-based study. Am J Psychiatry 167:1218–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLaughlin KA, Sheridan MA, Lambert HK (2014): Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev 47:578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheridan MA, McLaughlin KA (2014): Dimensions of early experience and neural development: deprivation and threat. Trends Cogn Sci 18:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin KA, Sheridan MA (2016): Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci 25:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheridan MA, Peverill M, Finn AS, McLaughlin KA (2017): Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Dev Psychopathol 29:1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert HK, King KM, Monahan KC, McLaughlin KA (2017): Differential associations of threat and deprivation with emotion regulation and cognitive control in adolescence. Dev Psychopathol 29:929–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Everaerd D, Klumpers F, Zwiers M, Guadalupe T, Franke B, van Oostrom I, et al. (2016): Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology. Neuropsychopharmacology 41:1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dennison MJ, Rosen ML, Sheridan MA, Sambrook KA, Jenness JL, McLaughlin KA (2017): Differential associations of distinct forms of childhood adversity with neurobehavioral measures of reward processing: a developmental pathway to depression [Epub ahead of print, December 21, 2017] Child Dev [DOI] [PMC free article] [PubMed]
- 42.Rosen ML, Sheridan MA, Sambrook KA, Meltzoff AN, McLaughlin KA (2018): Socioeconomic disparities in academic achievement: A multi-modal investigation of neural mechanisms in children and adolescents. Neuroimage 173:298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawson GM, Camins JS, Wisse L, Wu J, Duda JT, Cook PA, et al. (2017): Childhood socioeconomic status and childhood maltreatment: distinct associations with brain structure. PLoS One 12: e0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belsky J, Steinberg LD, Houts RM, Friedman SL, DeHart G, Cauffman E, et al. (2007): Family rearing antecedents of pubertal timing. Child Dev 78:1302–1321. [DOI] [PubMed] [Google Scholar]
- 45.Mendle J, Leve LD, Van Ryzin M, Natsuaki MN, Ge X (2011): Associations between early life stress, child maltreatment, and pubertal development among girls in foster care. J Res Adolesc 21:871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noll JG, Trickett PK, Long JD, Negriff S, Susman EJ, Shalev I, et al. (2017): Childhood sexual abuse and early timing of puberty. J Adolesc Health 60:65–71. [DOI] [PubMed] [Google Scholar]
- 47.Boynton-Jarrett R, Wright RJ, Putnam FW, Lividoti Hibert E, Michels KB, Forman MR, Rich-Edwards J (2013): Childhood abuse and age at menarche. J Adolesc Health 52:241– 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis BJ (2004): Timing of pubertal maturation in girls: an integrated life history approach. Psychol Bull 130:920–958. [DOI] [PubMed] [Google Scholar]
- 49.Belachew T, Hadley C, Lindstrom D, Getachew Y, Duchateau L, Kolsteren P (2011): Food insecurity and age at menarche among adolescent girls in Jimma Zone Southwest Ethiopia: a longitudinal study. Reprod Biol Endocrinol 9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boynton-Jarrett R, Harville EW (2012): A prospective study of childhood social hardships and age at menarche. Ann Epidemiol 22:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson DE, Almas AN, Degnan KA, McLaughlin KA, Nelson CA, Fox NA, Zeanah CH (In press): Pubertal development and the caregiving environment in early adolescence in socially deprived Romanian children: a randomized clinical trial. J Pediatr [DOI] [PMC free article] [PubMed]
- 52.Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J (1997): Is psychopathology associated with the timing of pubertal development? J Am Acad Child Adolesc Psychiatry 36:1768–1776. [DOI] [PubMed] [Google Scholar]
- 53.Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM (2004): Is pubertal timing associated with psychopathology in young adulthood? J Am Acad Child Adolesc Psychiatry 43:718–726. [DOI] [PubMed] [Google Scholar]
- 54.Kovacs M (1992): Children’s Depression Inventory Manual North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- 55.Reynolds WM (1994): Assessment of depression in children and adolescents by self- report measures In: Reynolds WM, Johnston HF, editors. Handbook of Depression in Children and Ddolescents New York, NY: Plenum Press, pp 209–234. [Google Scholar]
- 56.Achenbach TM (1991): Integrative Guide for the 1991 CBCL/4–18, YSR and TRF Profiles Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- 57.Chen WJ, Faraone SV, Biederman J, Tsuang MT (1994): Diagnostic accuracy of the Child Behavior Checklist scales for attention-deficit hyperactivity disorder: a receiver- operating characteristic analysis. J Consult Clin Psychol 62:1017–1025. [DOI] [PubMed] [Google Scholar]
- 58.Seligman LD, Ollendick TH, Langley AK, Baldacci HB (2004): The utility of measures of child and adolescent anxiety: a meta-analytic review of the Revised Children’s Manifest Anxiety Scale, the State-Trait Anxiety Inventory for Children, and the Child Behavior Checklist. J Clin Child Adolesc Psychol 33:557–565. [DOI] [PubMed] [Google Scholar]
- 59.Marshall WA, Tanner JM (1969): Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshall WA, Tanner JM (1970): Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morris NM, Udry JR (1980): Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9:271–280. [DOI] [PubMed] [Google Scholar]
- 62.Coleman L, Coleman J (2002): The measurement of puberty: a review. J Adolesc 25:535– 550. [DOI] [PubMed] [Google Scholar]
- 63.Shirtcliff EA, Dahl RE, Pollak SD (2009): Pubertal development: correspondence between hormonal and physical development. Child Dev 80:327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johnson SB, Riis JL, Noble KG (2016): State of the art review: poverty and the developing brain. Pediatrics 137:e20153075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayes AF (2017): Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach, 2nd ed. New York, NY: Guilford Press. [Google Scholar]
- 66.Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, et al. (2015): DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet 168B:36– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. (2012): DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koestler DC, Christensen B, Karagas MR, Marsit CJ, Langevin SM, Kelsey KT, et al. (2013): Blood-based profiles of DNA methylation predict the underlying distribution of cell types: a validation analysis. Epigenetics 8:816–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, et al. (2012): Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry 17:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Humphreys KL, Esteves K, Zeanah CH, Fox NA, Nelson CA, Drury SS (2016): Accelerated telomere shortening: Tracking the lasting impact of early institutional care at the cellular level. Psychiatry Res 246:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013): Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110:15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Silvers JA, Lumian DS, Gabard-Durnam L, Gee DG, Goff B, Fareri DS, et al. (2016): Previous institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J Neurosci 36:6420–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boyce WT, Ellis BJ (2005): Biological sensitivity to context: I. an evolutionary– developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 17:271–301. [DOI] [PubMed] [Google Scholar]
- 74.Rickard IJ, Frankenhuis WE, Nettle D (2014): Why are childhood family factors associated with timing of maturation? a role for internal prediction. Perspect Psychol Sci 9:3–15. [DOI] [PubMed] [Google Scholar]
- 75.Nettle D, Frankenhuis WE, Rickard IJ (2013): The evolution of predictive adaptive responses in human life history. Proc Biol Sci 280:20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Humphreys KL, Zeanah CH (2015): Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology 40:154–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLaughlin KA (2016): Future directions in childhood adversity and youth psychopathology. J Clin Child Adolesc Psychol 45:361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. (2010): Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry 197:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Platt JM, Colich NL, McLaughlin KA, Gary D, Keyes KM (2017): Transdiagnostic psychiatric disorder risk associated with early age of menarche: a latent modeling approach. Compr Psychiatry 79:70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Natsuaki MN, Biehl MC, Ge X (2009): Trajectories of depressed mood From early adolescence to young adulthood: the effects of pubertal timing and adolescent dating. J Res Adolesc 19:47–74. [Google Scholar]
- 81.Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, et al. (2015): Quantification of biological aging in young adults. Proc Natl Acad Sci USA 112:E4104– E4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, et al. (2018): Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol 187:1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardt J, Rutter M (2004): Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 45:260–273. [DOI] [PubMed] [Google Scholar]
- 84.Dorn LD, Biro FM (2011): Puberty and its measurement: a decade in review. J Res Adolesc 21:180–195. [Google Scholar]
- 85.Horvath S, Gurven M, Levine ME, Trumble BC, Kaplan H, Allayee H, et al. (2016): An epigenetic clock analysis of race/ethnicity, sex, and coronary heart disease. Genome Biol 17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deardorff J, Ekwaru JP, Kushi LH, Ellis BJ, Greenspan LC, Mirabedi A, et al. (2011): Father absence, body mass index, and pubertal timing in girls: differential effects by family income and ethnicity. J Adolesc Health 48:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson SE, Dallal GE, Must A (2003): Relative weight and race influence average age at menarche: results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics 111:844–850. [DOI] [PubMed] [Google Scholar]
- 88.Davis E, Humphreys KL, McEwen LM, Sacchet MD, Camacho MC, MacIsaac JL, et al. (2017): Accelerated DNA methylation age in adolescent girls: associations with elevated diurnal cortisol and reduced hippocampal volume. Transl Psychiatry 7:e1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Logue MW, Smith AK, Wolf EJ, Maniates H, Stone A, Schichman SA, et al. (2017): The correlation of methylation levels measured using Illumina 450K and EPIC BeadChips in blood samples. Epigenomics 9:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


