Abstract
BACKGROUND:
Stressors affect populations exposed to them as well as offspring. Strategies preventing the intergenerational propagation of effects of stress would benefit public health. Olfactory cue-based fear conditioning provides a framework to address this issue.
METHODS:
We 1) exposed adult male mice to an odor, acetophenone (Ace) or Lyral (parental generation [F0]-Exposed), 2) trained mice to associate these odors with mild foot shocks (F0-Trained), and 3) trained mice to associate these odors with mild foot shocks and then extinguished their fear toward these odors with odor-only presentations (F0-Extinguished). We then examined sensitivity of future generation (F1) offspring to these odors, expression of M71 odorant (Ace-responsive) and MOR23 odorant (Lyral-responsive) receptor-expressing cell populations in F1 offspring, and DNA methylation at genes encoding the Ace- (Olfr151, Olfr160) and Lyral- (Olfr16) responsive receptors in F0 sperm.
RESULTS:
Extinguishing fear toward Ace or Lyral of F0 male mice (F0-Extinguished) that had been fear conditioned with Ace or Lyral, respectively, results in F1-Extinguished offspring that do not demonstrate behavioral sensitivity to Ace or Lyral, respectively, and do not have enhanced representation for M71 or MOR23 odorant receptors in the olfactory system, as is observed in F1-Trained-Ace or F1-Trained-Lyral cohorts, respectively. The promoters of genes encoding Olfr151 and Olfr160 receptors are less methylated in F0-Trained-Ace sperm compared with F0-Exposed-Ace sperm. The Olfr16 promoter is less methylated in F0-Trained-Lyral sperm compared with F0-Exposed-Lyral sperm, and F0Extinguished-Lyral sperm have methylation levels comparable to F0-Exposed-Lyral sperm.
CONCLUSIONS:
Our study demonstrates the potential of using extinction-based behavioral strategies to reverse influences of parental stress in offspring and in the parental germline.
Keywords: Behavioral sensitivity, Extinction training, Glomeruli, Olfaction, Olfactory sensory neurons, Sperm
To model intergenerational influences of stress in mammals, studies have manipulated parental environments and shown effects on offspring (1–11). While interventions can be applied to offspring to reverse these effects (12), there is a translational need to prevent the intergenerational passage of stress from the stressed population to future offspring. Exposure-based psychotherapy involves exposure to or recollection of traumatic cues in a safe context and uses the neural process of extinction to diminish emotional responding to trauma cues when reencountered. Extinction training has been used to successfully diminish fears within a generation (13,14). To our knowledge, such approaches have not been applied to a parental (F0) generation to prevent the propagation of stress to offspring, nor have they been demonstrated to reverse behavioral, neuroanatomical, and epigenetic influences of intergenerational stress.
Olfaction in mice provides an ideal framework to ask how parental experiences influence structure and function in the nervous system of offspring and to examine how extinction training might reverse this influence. First, mice can be trained to exhibit fearlike behavior on presentations of a specific odor after olfactory fear conditioning (odor + shock presentations) (15–17). Second, olfactory sensory neurons (OSNs) in the main olfactory epithelium (MOE) detect odors by expressing odorant receptors (ORs) that can be visualized in transgenic mice (18–21). Previous work has shown that training mice to associate presentations of an odorant, acetophenone (Ace), with mild foot shocks results in an increase in the number of Aceresponsive OSNs in the MOE (those expressing the M71 OR) and a larger M71 OR glomerular area in the olfactory bulb (15,17). Third, specific odors activate specific ORs encoded in the genome, thereby highlighting a location within the genome at which to examine influences of salient olfactory experience (19,22).
We recently reported (23) that subjecting an F0 generation of mice to either Ace + shock or propanol + shock pairings resulted in odor-naïve future (F1 and F2) generations showing enhanced behavioral sensitivity to the odor used to condition the F0 generation. Conditioning F0 M71-LacZ transgenic mice with Ace also resulted in an increased number of Ace-responsive M71+ OSNs in the MOE and larger M71 glomeruli in the olfactory bulbs of behaviorally naïve F1 and F2 generations. Alterations in DNA methylation in F0 and F1 sperm around the gene encoding the M71 receptor suggested that paternal olfactory experience interacted with the genome in the germline. Aversive or appetitive odor exposures influence odor-related perception and neuroanatomy in rodents and fruit flies, demonstrating the generality of this phenomenon (24–26). Exposing previously olfactory fear–conditioned adult mice to extinction training (odor presentations without foot shocks) diminishes their fear toward the conditioned odor and also restores olfactory neuroanatomy to baseline (17). These data set the stage to examine whether exposing an F0 generation to extinction training reverses intergenerational influences of olfactory conditioning.
Here, we 1) exposed adult male mice to an odor only (F0-Exposed), 2) trained mice to associate odor presentation with mild foot shocks (F0-Trained), or 3) trained mice to associate odor presentations with mild foot shocks and then extinguished their fear toward that odor via extinction training with odor-only presentations (F0-Extinguished), followed by conducting experiments on their male F1 offspring (F1-Exposed, F1-Trained, and F1-Extinguished) (Supplemental Figures S1 and S2). We used three different mouse lines in our F0 treatments: C57BL/6J wild type, M71-LacZ mice in which Ace-responsive M71+ OSNs can be visualized and LacZ expression can be quantified, and MOR23-GFP (green fluorescent protein) mice in which the GFP expression associated with Lyral-responsive MOR23+ OSNs can be quantified. Finally, we used prior knowledge (19,22,27,28) that Ace activates ORs encoded by the Olfr151 (M71) and Olfr160 (M72) genes, while Lyral activates the OR encoded by the Olfr16 (MOR23) gene, and examined DNA methylation at these genes in the sperm of F0 male mice.
METHODS AND MATERIALS
Animals
Experiments were conducted with 2-month-old sexually inexperienced and odor-inexperienced animals. For the F0 generation, C57BL/6J animals, M71-LacZ animals maintained in mixed 129/Sv × C57BL/6J background, and MOR23-GFP animals maintained in mixed 129/Sv × C57BL/6J background were bred in our animal facility. For the F1 generation, 10 days after the F0 treatment, F0 male mice were mated with naïve sexually inexperienced C57BL/6J female mice, matings were separated after 12 days, and F1 offspring were weaned after 21 days (Supplemental Figure S1). Our treatments of F0 animals were efficient at eliciting fear toward the odor in F0Trained animals and diminishing fear toward the odor after extinction training in F0-Extinguished animals (Supplemental Figure S2). This study was explicitly aimed at replicating our initial study that used F0 and F1 male mice (23) and at examining the possibility of reversing our effects using extinction training of F0 animals at the level of the F0 germline. Female mice that could be equally, less, or more affected by olfactory experience were not included in our study, and future work will address this omission.
Animals were housed on a 12-hour light/dark cycle in standard group cages (≤ 5/cage) with ad libitum access to food and water, with all experiments conducted during the light half of the cycle. All procedures were approved by the Institutional Animal Care and Use Committee of Emory University and followed National Institutes of Health guidelines.
Behavior
Behavior was performed in a double-blind manner, and data were acquired using automated software (SR-LAB; San Diego Instruments, San Diego, CA).
F0 Olfactory Treatments.
F0-Trained male mice were trained to associate Ace or Lyral presentation with mild foot shocks. The animals were trained on 3 consecutive days, with each training day consisting of five odor presentations for 10 seconds each, coterminating with a 0.25-second 0.4-mA foot shock. F0-Exposed male mice were treated like the F0-Trained group except that odor presentations were not accompanied by foot shocks. F0-Extinguished male mice were first treated like the F0-Trained group. The next day, and for 2 days thereafter, these male mice were placed in a different context and exposed to 30 presentations of the odor with which they had previously been conditioned but without foot shocks (see Supplement for details).
Odor-Potentiated Startle of Adult F1 Offspring.
We measured baseline behavioral sensitivity of the F1 offspring to odors using an odor-potentiated startle (OPS) assay that we had used previously and that measures acoustic startle response to a noise burst (23) (see Supplement for details).
The number of litters examined in the conditions were as follows. For Ace OPS: F1-Exposed, 7 litters; F1-Trained, 10 litters; F1-Extinguished, 12 litters; F1-Home, 7 litters. For Lyral OPS: F1-Exposed, 7 litters; F1-Trained, 8 litters; F1-Extinguished, 5 litters; F1-Home, 7 litters.
Neuroanatomy on F1 Olfactory Bulb and MOE
β-Galactosidase Staining and Measurement of Glomerular Area in the Olfactory Bulb.
The olfactory bulbs of M71-LacZ animals were processed for β-galactosidase staining, and then the glomerular area was quantified using previously published protocols (15). See Supplement for details.
The number of litters examined in the conditions were as follows. F1-Exposed, 4 litters; F1-Trained, 4 litters; F1-Extinguished, 4 litters.
Western Blotting.
Western blotting was carried out using standard procedures. See the Supplement for details. We performed several control experiments to demonstrate that the bands we report do in fact represent detection of LacZ and GFP (Supplemental Figure S3).
For detecting LacZ, the numbers of litters examined in the conditions were as follows. F1-Exposed, 4 litters; F1-Trained, 4 litters; F1-Extinguished, 4 litters.
For detecting GFP, the numbers of litters examined in the conditions were as follows. F1-Exposed, 8 litters; F1-Trained, 7 litters; F1-Extinguished, 7 litters.
Quantitative Polymerase Chain Reaction for Olfr151, Olfr160, and Olfr16 in MOE of F1 Animals
MOE was extracted from animals and RNA was isolated using the Qiagen Total RNeasy Kit (Qiagen, Germantown, MD). Complementary DNA was reverse transcribed using the Qiagen RT2 First Strand cDNA Synthesis Kit. Quantitative polymerase chain reaction was performed on an Applied Biosystems 7500 machine with SYBR Green Master Mix (Applied Biosystems, Foster City, CA) using the primers listed in the Supplement.
Methyl DNA Immunoprecipitation on DNA in F0 Sperm
Moleculargeneticanalyseshaveidentifiedpromotersofthegenes encoding the Ace-responsive M71 (Olfr151) and M72 (Olfr160) and Lyral-responsive MOR23 (Olfr16) ORs: Olfr151 (Chr9: 37,540,846–37,541,182), Olfr160 (Chr9: 37,523,691–37,523,991), and Olfr16 (174,880,337–174,880,639) (Supplemental Figure S4). We measured methylation around these promoters in F0 sperm DNA immunoprecipitated using an anti-5-methyl cytosine antibody (Active Motif, Carlsbad, CA) (see Supplemental Figure S5 for quality control; see Supplement for details).
Statistics
GraphPad Prism (GraphPad Software, San Diego, CA) was used to conduct most statistical analyses. Data were analyzed using either a one-way analysis of variance (ANOVA) or an unpaired t test. All ANOVA main effects were followed by Tukey post hoc tests. To account for litter effects, we ran mixed-effects models including litter as a random effect (29) (random intercept) using the R package nlme. To determine the importance of litter effects, we compared models with and without a random effect of litter using a likelihood ratio test. This method tests whether the variance explained by litter is significantly greater than zero. However, the likelihood ratio test returns a conservative p value when assessing significance of random effects, and this was corrected for by dividing the resulting p value by 2 as suggested by Suur et al. (30). In none of the cases where we tested for litter effects did including litter as a random variable significantly improve the model (all p values > .10). Therefore, all results included in the Results section and figures are from the classical (nonmixed) models described above. All results are presented as mean 6 SEM with *p < .05, **p < .01, ***p < .001, and ****p < .0001 as measures of significance.
RESULTS
Extinction Training of Previously OlfactoryConditioned F0 Male Mice Reverses Behavioral Sensitivity to the Conditioning Odor in F1 Offspring
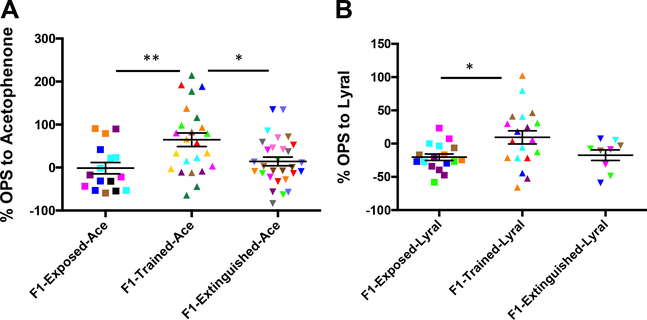
Extinguishing fear responses to Ace of F0 male mice (F0Extinguished-Ace) that had been previously fear conditioned with Ace resulted in F1-Extinguished-Ace offspring no longer demonstrating behavioral sensitivity to Ace as was observed in F1-Trained-Ace offspring (Figure 1A) (F1-Exposed-Ace n = 16, F1-Trained-Ace n = 24, F1-Extinguished-Ace n = 29; ANOVA: F2,66 = 6.497, p = .0027; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace **p .01, F1-Trained-Ace vs. F1-ExtinguishedAce *p .05) (test for litter effect: p = .50). We also replicated our previously reported (23) effect that F1-Trained-Ace animals showed a higher sensitivity to Ace compared with F1-Exposed-Ace animals. F1-Exposed-Ace animals did not show any difference in sensitivity to Ace compared with F1-Home animals (Supplemental Figure S6A) (F1-Home n = 16, F1-ExposedAce n = 16, p > .05) (test for litter effect: p = .21), suggesting that odor exposure alone in the F0 generation is not sufficient to influence F1 offspring.
Figure 1.
Reversal of behavioral sensitivity of salient parental olfactory experience tested using odor-potentiated startle (OPS). (A) Parent generation (F0) male mice treated with acetophenone (Ace) and future generation (F1) mice tested with Ace. Exposing F0 male mice that had been previously fear conditioned with Ace to extinction training (F1-Exposed-Ace), which decreases their fear toward Ace), results in F1-Extinguished-Ace offspring that did not demonstrate behavioral sensitivity to Ace, as is observed in the F1-Trained-Ace cohort. As we have reported previously, F1-Trained-Ace animals sired by F0 male animals that had been conditioned with Ace + mild foot shocks show a higher sensitivity to Ace compared with F1-Exposed-Ace animals sired by F0 male animals that had been exposed only to Ace presentations. (B) F0 male mice treated with Lyral and F1 mice tested with Lyral. F1-Trained-Lyral animals sired by F0 male mice that had been conditioned with Lyral + mild foot shocks showed a higher sensitivity to Lyral compared with F1-Exposed-Lyral animals sired by F0 male mice that had been exposed only to Lyral presentations. Exposing F0 male mice that had been previously fear conditioned with Lyral to extinction training resulted in F1-Extinguished-Lyral offspring that did not demonstrate behavioral sensitivity to Lyral, as is observed in the F1-Trained-Lyral cohort. Data are presented as mean ± SEM. *p < .05, **p < .01. Same colorswithin a group represent individuals from the same litter.
Extinguishing fear responses to Lyral in F0 male mice that had previously received Lyral presentations paired with foot shocks (F0-Extinguished-Lyral) resulted in F1-Extinguished-Lyral animals that no longer demonstrated enhanced behavioral sensitivity to Lyral as was observed in F1-Trained-Lyral offspring (Figure 1B) (F1-Exposed-Lyral n = 17, F1-Trained-Lyral n = 19, F1-Extinguished-Lyral n = 9; ANOVA: F2,42 = 4.260, p = .0207; post hoc: F1-Exposed-Lyral vs. F1-Trained-Lyral *p < .05) (test for litter effect: p = .50). In addition, F1-Trained-Lyral animals showed a higher sensitivity to Lyral compared with F1-ExposedLyral animals. F1-Exposed-Lyral animals did not show any difference in sensitivity to Lyral compared with F1-Home animals (Supplemental Figure S6B) (F1-Home n = 15, F1-Exposed-Lyral n = 17, p > .05) (test for litter effect: p = .22).
To test whether behavioral sensitivity and reversals of behavioral sensitivity of F1 offspring sired by F0 male mice that had been conditioned and extinguished with one odor generalize to other odors, we tested F1 offspring of F0-Exposed-Ace, F0-TrainedAce, and F0-Extinguished-Ace male mice with Lyral. Conversely, we tested F1 offspring of F0-Exposed-Lyral, F0-Trained-Lyral, and F0-Extinguished-Lyral male mice with Ace. F1-Exposed-Ace, F1-Trained-Ace, and F1-Extinguished-Ace animals sired by F0 male mice that had been treated with Ace did not show any differences in sensitivity to another odorant (Lyral) (Supplemental Figure S7A) (F1-Exposed-Ace n = 10, F1-Trained-Ace n = 6, F1-Extinguished-Ace n = 10, p > .05). Similarly, F1-ExposedLyral, F1-Trained-Lyral, and F1-Extinguished-Lyral animals sired by F0 male mice that had been treated with Lyral did not show a higher sensitivity to another odorant (Ace) (Supplemental Figure S7B) (F1-Exposed-Lyral n = 11, F1-Trained-Lyral n = 12, F1-Extinguished-Lyral n = 18, p > .05).
Extinction Training of Previously OlfactoryConditioned F0 Male Mice Reverses Enhancements in Olfactory Neuroanatomy in F1 Offspring
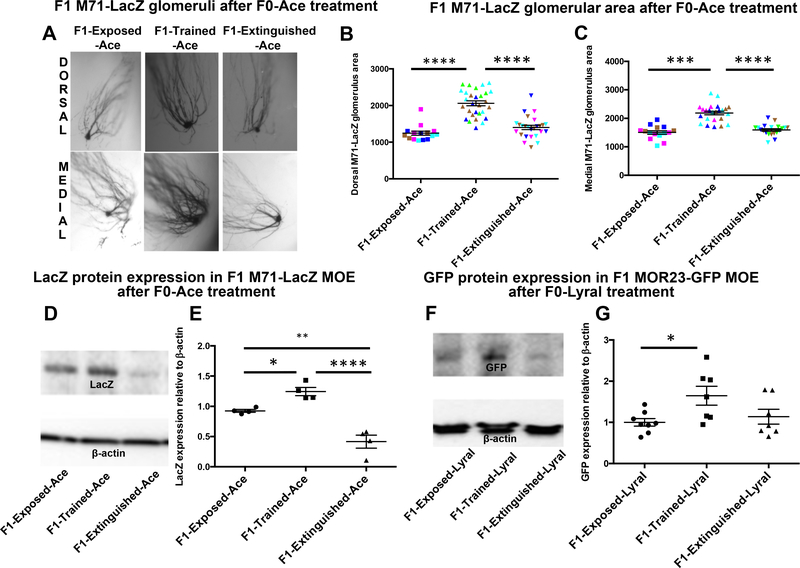
F1-Extinguished-Ace animals have M71-LacZ glomeruli that are no different in size compared with F1-Exposed-Ace animals. Consistent with our previous findings (23), F1-TrainedAce animals had larger M71 glomeruli and therefore an increased representation for Ace compared with these two groups (Figure 2A–C) (dorsal glomeruli: F1-Exposed-Ace n = 15, F1-Trained-Ace n = 29, F1-Extinguished-Ace n = 26; ANOVA: F2,59 = 39.47, p < .0001; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace ****p < .0001, F1-Trained-Ace vs. F1Extinguished-Ace ****p < .0001) (test for litter effect: p = .11) (medial glomeruli: F1-Exposed-Ace n = 15, F1-Trained-Ace n = 24, F1-Extinguished-Ace n = 20; ANOVA: F2,47 = 30.86, p < .0001; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace ***p < .001, F1-Trained-Ace vs. F1-Extinguished-Ace ****p < .0001) (test for litter effect: p = .50). Furthermore, to ensure an objective measure of OR expression in the MOE of F1 offspring, we performed Western blotting of F1-M71-LacZ MOE with a LacZ antibody (Supplemental Figure S3A). This approach revealed that F1-Trained-Ace animals had higher LacZ expression in the MOE than F1-Exposed-Ace animals (Figure 2D, E) (F1-Exposed-Ace n = 4, F1-Trained-Ace n = 4, F1-Extinguished-Ace n = 4; ANOVA: F2,9 = 31.47, p < .0001; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace *p < .05, F1Exposed-Ace vs. F1-Extinguished-Ace **p < .01, F1-TrainedAce vs. F1-Extinguished-Ace ****p < .001). We also detected a significant decrease in LacZ expression in F1-ExtinguishedAce animals compared with F1-Trained-Ace and F1-ExposedAce animals (Figure 2D, E). Finally, we performed quantitative polymerase chain reaction for Olfr151 and Olfr160 (Aceresponsive OR genes) to determine the relative baseline expression of these cell populations in the F1 MOE. We report that F1-Trained-Ace animals have increased messenger RNA (mRNA) for genes encoding the Ace-responsive ORs (Olfr151 and Olfr160) (Supplemental Figure S8A, B) (Olfr151: F1-Exposed-Ace n = 4, F1-Trained-Ace n = 4, F1Extinguished-Ace n = 4; ANOVA: F2,9 = 9.525, p = .0060; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace *p < .05, F1-Trained-Ace vs. F1-Extinguished-Ace **p < .01) (Olfr160: F1-Exposed-Ace n = 6, F1-Trained-Ace n = 5, F1Extinguished-Ace n = 4; ANOVA: F2,12 = 19.83, p = .0002; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace ***p < .001, F1-Trained-Ace vs. F1-Extinguished-Ace *p < .05).
Figure 2.
Reversal of enhanced olfactory neuroanatomy of salient parental olfactory experience. (A–C) β-Galactosidase staining shows that offspring of parent generation (F0) M71-LacZ male mice that had been conditioned to acetophenone (future generation [F1]-Trained-Ace) have larger M71-LacZ glomeruli than offspring of F0 M71-LacZ male mice that had been exposed to Ace (F1-Exposed-Ace). Exposing F0 M71-LacZ male mice that had been previously fear conditioned with Ace to extinction training reversed this enhanced neuroanatomical representation in their F1-Extinguished-Ace offspring. (D, E) Quantitation of LacZ expression in the F1-M71-LacZ-Ace main olfactory epithelium (MOE) using Western blotting. F1-Trained-Ace animals had higher LacZ expression in the MOE compared with F1-Exposed-Ace animals. F1-Extinguished-Ace animals have significantly less LacZ expression in the MOE compared with F1-Exposed-Ace and F1-Trained-Ace animals. (F, G) Quantitation of green fluorescent protein (GFP) expression in the F1-MOR23-GFP-Lyral MOE using Western blotting. F1-Trained-Lyral animals had higher GFP expression in the MOE compared with F1-Exposed-Lyral animals. F1-Extinguished-Lyral animals had comparable amounts of GFP in the MOE as F1-Exposed-Lyral animals. Data are presented as mean ± SEM. *p < .05, **p < .01, ***p < .001, ****p < .001. Same colors within a group represent individuals from the same litter.
Owing to the position of MOR23-GFP glomeruli on the olfactory bulb, it is challenging to visualize and quantitate their size; instead, we used Western blotting to measure the GFP levels as has been reported (19) (Supplemental Figure S3B). We found a significant increase in GFP expression in the MOE of F1-TrainedLyral and F1-Extinguished-Lyral animals compared with F1-Exposed-Lyral animals (Figure 2F, G) (F1-Exposed-Lyral n = 8, F1-Trained-Lyral n = 7, F1-Extinguished-Lyral n = 7; ANOVA: F2,19 = 3.982, p = .0359; post hoc: F1-Exposed-Lyral vs. F1-Trained-Lyral *p < .05). Quantitative polymerase chain reaction for Olfr16 on the MOE of F1 animals sired by F0 male mice treated with Lyral revealed that F1-Trained-Lyral animals had significantly lower Olfr16 mRNA levels in the MOE compared with F1-Exposed-Lyral animals (Supplemental Figure S8C) (F1-Exposed-Lyral n = 5, F1-Trained-Lyral n = 9, F1Extinguished-Lyral n = 9; ANOVA: F2,20 = 4.9, p = .0185; post hoc: F1-Exposed-Lyral vs. F1-Trained-Lyral *p < .05).
Extinction Training of Olfactory-Conditioned F0 Male Mice Is Accompanied by Restoration of DNA Methylation at the Promoters of Relevant OR Genes in F0 Sperm
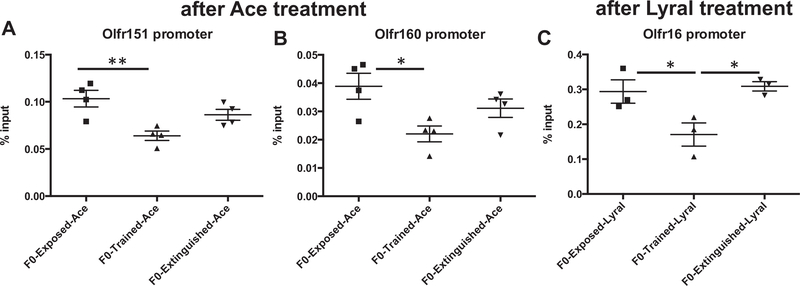
We found that the promoters of genes encoding the Aceresponsive Olfr151 (M71) and Olfr160 (M72) receptors were less methylated in F0-Trained-Ace sperm compared with F0-Exposed-Ace sperm (Figure 3A, B) (Olfr151: F1-Exposed-Ace n = 4, F1-Trained-Ace n = 4, F1-Extinguished-Ace n = 4; ANOVA: F2,9 = 8.61, p = .0081; post hoc: F1-Exposed-Ace vs. F1-TrainedAce **p < .01) (Olfr160: F1-Exposed-Ace n = 4, F1-Trained-Ace n = 4, F1-Extinguished-Ace n = 4; ANOVA: F2,9 = 5.382, p = .029; post hoc: F1-Exposed-Ace vs. F1-Trained-Ace *p < .05). While F0-Extinguished-Ace sperm had methylation levels that appear higher than F0-Trained-Ace sperm levels, we did not find a statistical difference between these groups. Of note, the gene encoding the Lyral-responsive MOR23 OR, Olfr16, was not differentially methylated in F0 sperm across Ace-treated groups (Supplemental Figure S9A) (p < .05). When F0 male mice were exposed, trained, or extinguished to Lyral, we observed differences in methylation levels around the Olfr16 promoter. More specifically, the Olfr16 promoter was less methylated in F0Trained-Lyral sperm compared with F0-Exposed-Lyral sperm, and F0-Extinguished-Lyral sperm had methylation levels that were comparable to F0-Exposed-Lyral levels (Figure 3C) (Olfr16: F1-Exposed-Lyral n = 3, F1-Trained-Lyral n = 3, F1Extinguished-Lyral n = 3; ANOVA: F2,6 = 7.140, p = .0259; post hoc: F1-Exposed-Lyral vs. F1-Trained-Lyral *p < .05, F1-Trained-Lyral vs. F1-Extinguished-Lyral *p < .05). The genes encoding the Ace-responsive M71 (Olfr151) and M72 (Olfr160) ORs were not differentially methylated in F0 sperm across Lyraltreated groups (Supplemental Figure S9B, C) (p > .05).
Figure 3.
Reversal of germline influences of salient parental olfactory experience. (A, B) DNA encoding the acetophenone (Ace)-responsive odorant receptors (Olfr151 and Olfr160) were less methylated at the promoter region in sperm of parent generation (F0) male mice that had been conditioned to Ace (F0-Trained-Ace) compared with F0 male mice exposed to the Ace (F0-Exposed-Ace). The promoters of the Olfr151 and Olfr160 genes in sperm of F0 male mice previously conditioned to Ace and then exposed to extinction training (F0-Extinguished-Ace) were as methylated as the promoters of these genes in sperm of F0-Exposed-Ace male mice. (C) DNA encoding the Lyral-responsive odorant receptor (Olfr16) was less methylated at the promoter region in sperm of F0 male mice that had been conditioned to Lyral (F0-Trained-Lyral) compared with F0 male mice exposed to the Lyral (F0-Exposed-Lyral). The promoter of the Olfr16 gene in sperm of F0 male mice previously conditioned to Lyral and then exposed to extinction training (F0-Extinguished-Lyral) was as methylated as the Olfr16 promoter in sperm of F0-Exposed-Lyral male mice. Six epididymis were used per sample (1 sample = 3 animals). Data are presented as mean ± SEM. *p < .05, **p < .01.
DISCUSSION
Trauma like the Holocaust and domestic abuse in humans (31,32), and stress protocols in rodents (3,6,33), are broad in their intergenerational effects. This breadth poses a challenge to following intergenerational influences of stress. Olfactory fear conditioning in F0 mice provides an experimental framework that allows us to focus on end points in both the F0 and F1 generations at the level of structure (visualizing OSNs expressing specific ORs), function (behavioral sensitivity toward specific odors), and epigenetics (regulation of expression of OR genes) (15,18,19,21,22,27,28,34–37).
Capitalizing on the aforementioned properties of the olfactory system, we had previously reported (23) that exposing F0 male mice to odor + shock stress (conditioning) resulted in F1 offspring showing 1) behavioral sensitivity to the odor used to condition the F0 generation, 2) increased representation for olfactory neuroanatomy that detects the odor used in the F0 generation, and 3) alterations in DNA methylation in F0 sperm around the gene encoding the OR. These data suggested that olfactory-related stress could be used to study intergenerational influences of stress and that environmental experiences can modify epigenetic marks in the F0 germline. Other studies have also reported intergenerational influences of olfactoryrelated stressors in mice, rats, and fruit flies (24–26).
The ultimate challenge associated with intergenerational influences of stress is to be able to protect offspring that may be vulnerable to parental trauma and stress. Cognitive behavioral therapy that often involves exposure to or recollection of traumatic cues in a safe context is used as a form of extinction training to diminish the detrimental effects of such recollection. Recently, studies using rodents have shown that subjecting animals to extinction training (tone-only presentations) after they had been conditioned to the tone (tone + shock) not only diminished their fear for the tone but also resulted in significant alterations of the microRNA milieu and the DNA methylation status of genes in the prefrontal cortex, a region involved in extinction learning (38–41). We recently reported that exposing previously olfactory fear–conditioned mice to extinction training (odor presentations without foot shocks) diminished their fear responses to the conditioned odor and restored enhancements of olfactory neuroanatomy to baseline (17).
Building on these findings, we hypothesized that extinction training an F0 generation of mice that had been previously subjected to olfactory fear conditioning would reverse the transmission of behavioral and neuroanatomical effects observed in the F1 offspring of conditioned male mice and the influence of such conditioning on epigenetic marks in the sperm of the F0 generation. We found this reversal to occur at the level of behavioral sensitivity (Figure 1), enhanced neuroanatomy (Figure 2), and DNA methylation at OR loci in the germline (Figure 3). To our knowledge, these are the first data to indicate that interventional strategies can be applied to parental generations to prevent or reverse transmission of intergenerational influences of stress. Also important is that our findings present biological correlates of reversal of intergenerational influences of stress and highlight the possibility of using readouts of multiple biological end points to determine the efficacy of interventional strategies. With our use of odorrelated stress in the F0 generation, we focused on the reversal of intergenerational influences of stress at the level of olfactory-related behavior and neuroanatomy in the F1 offspring and epigenetic marks around the genes encoding ORs in the F0 sperm. Future work will need to examine whether such reversals can be observed for other reported intergenerational influences such as those of more generalized stressors, dietary perturbations, and chemical exposures (1–3,5–8,10,42–46).
Odorant exposure and deprivation are salient environmental experiences that affect OR gene expression, olfactory physiology, and behavior (19,27,28,47). While we have previously demonstrated that F1-trained animals demonstrate a behavioral sensitivity that is specific to the odor used in the F0 generation (23), our extinction training protocol that involves 30 odor presentations each day for 3 days could affect sensitivity to other odors in general. Our findings indicate that this is not the case (our effects do not generalize) and that OPS of F1 offspring to an odorant that is independent of the one used during extinction training of the F0 generation is not affected (Supplemental Figure S7). Future work will need to examine whether subjecting F0 animals to conditioning with odor A and then to extinction training with odor B allows for the influences on F1 behavior and neuroanatomy and the F0 germline to persist. Gaining appreciation for such generalizability and specificity of extinction training approaches will suggest optimal strategies to break cycles of intergenerational stress.
Rodent studies have reported changes in gene expression in the central nervous system of offspring sired by male animals subjected to stress. Less is known about whether gene expression in the peripheral nervous system of offspring can be affected by parental experience. We found that the enhancements in LacZ protein expression in the F1-Trained-Ace M71-LacZ olfactory epithelium coincide with increased mRNA levels for Olfr151 and Olfr160 in the F1 olfactory epithelium (Supplemental Figure S8A, B). In contrast, while we observe increased GFP expression in the F1-Trained-Lyral MOR23-GFP olfactory epithelium, Olfr16 mRNA levels are decreased (Supplemental Figure S8C). Cellular plasticity in OSNs in response to odor exposure has been demonstrated to be specific to the olfactory receptorligand pairing (19). Therefore, the observed variability in baseline mRNA levels of the different olfactory receptors might be a consequence of differential gene expression that is dependent on the olfactory receptor being profiled or could be independent of protein expression and stability. Our data do suggest that baseline gene and protein expression in the peripheral nervous system can be influenced by the legacy of paternal olfactory experience.
Many studies have demonstrated that altered RNA, histone modifications, and DNA methylation in sperm of animals exposed to salient environmental events may contribute to intergenerational inheritance (5–8,23,33,43,48). How these changes sculpt the development of specific tissue systems as the single-cell zygote develops into the multicellular organism is an open question that will be answered with sequencing approaches targeted at the developing embryo and the adult brain. Specifically related to our data, we do not know whether and how altered methylation at the OR loci in sperm of F0 olfactory-conditioned animals is relevant to the enhanced representation for these receptors in the nose of adult F1 offspring. These alterations might be coincidental to, and not causal of, the neuroanatomical changes observed in the F1 nose. For this reason, we currently view our methylation data more as a readout that epigenetic marks can be altered in the germline and less as a mechanism of the reported phenomenon. To demonstrate causality, future experiments will need to examine epigenetic signatures around these ORs across embryonic development, manipulate the methylation status at specific OR loci in the germline, and examine the effects of this manipulation on the adult F1 nose.
Our findings suggest that salient environmental events with negative (conditioning) and positive (extinction training) valence can influence the germline. How long these influences persist, which sperm precursor cells are affected, and how many sperm bear these influences are important questions to answer in the future. These answers probably hold clues to why not all individuals born to parents with paternal trauma are influenced (not all sperm are influenced by the environmental experience) and whether allowing time to elapse between traumatic events and conception can mitigate intergenerational influences of stress. Finally, the time at which any salient environmental event is registered by the germline in some manner, epigenetic or otherwise, is an important point that requires future analysis. In our study, the time at which the experience of the initial olfactory fear conditioning is registered by the sperm, and whether extinction training prevents this influence or erases it, is food for thought. Given the immediate juxtaposition between the conditioning and extinction training in our protocol, it is highly unlikely, but potentially possible, that erasure of this influence is responsible for the effects of our extinction training. A more likely scenario is that the extinction training that occurs immediately following the conditioning protocol prevents “epigenetic consolidation” of conditioning in the gametes. Building in a significant amount of time between the conditioning and extinction training would be one way to answer this question. In addition, perhaps lessons are to be learned from the general concept of extinction training vis-à-vis behavior—that diminishment of behavior after extinction training is not merely erasure of the initial associative memory but rather a consequence of new associations being made independent of the original learning event (49,50).
In conclusion, our data demonstrate that interventions applied to a parental generation have the potential to reverse behavioral and neuroanatomical influences of parental stress in future offspring and epigenetic signatures in the parental germline. This evidence offers promise for breaking societal cycles of intergenerational stress by intervening in exposed parental populations before conception.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Institute of Health (NIH) Grant Nos. R01-MH108665, R01-HD071982, and R21-HD088931 (to KJR) and by Ruth L. Kirschtein National Research Service Award Predoctoral Fellowship No. F31 MH105237–01 (to FGM). Funding for this study was provided to BGD by the Emory University Department of Psychiatry and Behavioral Sciences, the Emory Brain Health Institute, the Yerkes National Primate Research Center (YNPRC), a Canadian Institute for Advanced Research Azrieli Global Scholar Award, and the Catherine Shopshire Hardman Fund. Additional funding was provided to the YNPRC by the NIH Director’s Office of Research Infrastructure Programs (Grant No. P51OD11132).
HSA, SS, FGM, ND, HL, JB, SH, and HW performed experiments and/or analyzed data. BY and PJ provided input on the methyl DNA immunoprecipitation study. KJR helped with manuscript preparation. BGD designed the study, performed experiments, analyzed data, interpreted data, and wrote the manuscript.
We thank the Veterinary and Animal Care staff in the Yerkes Neuroscience Vivarium for animal husbandry and three anonymous reviewers for constructive feedback on this manuscript.
Footnotes
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://doi.org/10.1016/j.biopsych.2018.07.028.
Contributor Information
Hadj S. Aoued, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia
Soma Sannigrahi, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia.
Nandini Doshi, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia.
Filomene G. Morrison, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia, McLean Hospital, Harvard Medical School, Belmont, Massachusetts.
Hannah Linsenbaum, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia.
Sarah C. Hunter, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia
Hasse Walum, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia.
Justin Baman, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia.
Bing Yao, Yerkes National Primate Research Center, Department of Human Genetics, Emory University School of Medicine, Atlanta, Georgia.
Peng Jin, Yerkes National Primate Research Center, Department of Human Genetics, Emory University School of Medicine, Atlanta, Georgia.
Kerry J. Ressler, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia, McLean Hospital, Harvard Medical School, Belmont, Massachusetts.
Brian G. Dias, Division of Behavioral Neuroscience and Psychiatric Disorders, Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, Georgia,Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia
REFERENCES
- 1.Anway MD (2005): Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308:1466–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babb JA, Carini LM, Spears SL, Nephew BC (2014): Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav 65:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz DM, Laplant Q, Watts EL, Hodes GE, Russo SJ, Feng J, et al. (2011): Paternal transmission of stress-induced pathologies. Biol Psychiatry 70:408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCreary JK, Truica LS, Friesen B, Yao Y, Olson DM, Kovalchuk I, et al. (2016): Altered brain morphology and functional connectivity reflect a vulnerable affective state after cumulative multigenerational stress in rats. Neuroscience 330:79–89. [DOI] [PubMed] [Google Scholar]
- 5.Radford EJ, Ito M, Shi H, Corish JA, Yamazawa K, Isganaitis E, et al. (2014): In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 345:1255903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodgers AB, Morgan CP, Leu NA, Bale TL (2015): Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc Natl Acad Sci U S A 112:13699–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, et al. (2016): Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351:391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, et al. (2014): Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci 17:667–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell E, Klein SL, Argyropoulos KV, Sharma A, Chan RB, Toth JG, et al. (2016): Behavioural traits propagate across generations via segregated iterative-somatic and gametic epigenetic mechanisms. Nat Commun 7:11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saavedra-Rodriguez L, Feig LA (2013): Chronic social instability induces anxiety and defective social interactions across generations. Biol Psychiatry 73:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Short AK, Fennell KA, Perreau VM, Fox A, Bryan MKOr, Kim JH, et al. (2016): Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl Psychiatry 6:e837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCreary JK, Metz GAS (2016): Environmental enrichment as an intervention for adverse health outcomes of prenatal stress. Environ Epigenet 2:dvw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maren S (2011): Seeking a spotless mind: Extinction, deconsolidation, and erasure of fear memory. Neuron 70:830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. (2004): Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 61:1136–1144. [DOI] [PubMed] [Google Scholar]
- 15.Jones SV, Choi DC, Davis M, Ressler KJ (2008): Learning-dependent structural plasticity in the adult olfactory pathway. J Neurosci 28:13106–13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kass MD, Rosenthal MC, Pottackal J, McGann JP (2013): Fear learning enhances neural responses to threat-predictive sensory stimuli. Science 342:1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison FG, Dias BG, Ressler KJ (2015): Extinction reverses olfactory fear-conditioned increases in neuron number and glomerular size. Proc Natl Acad Sci U S A 112:12846–12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buck L, Axel R (1991): A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65:175–187. [DOI] [PubMed] [Google Scholar]
- 19.Cadiou H, Aoudé I, Tazir B, Molinas A, Fenech C, Meunier N, et al. (2014): Postnatal odorant exposure induces peripheral olfactory plasticity at the cellular level. J Neurosci 34:4857–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman A, Feinstein P, Hirota J, Mombaerts P (2005): The promoter of the mouse odorant receptor gene M71. Mol Cell Neurosci 28:535–546. [DOI] [PubMed] [Google Scholar]
- 21.Vassalli A, Rothman A, Feinstein P, Zapotocky M, Mombaerts P (2002): Minigenes impart odorant receptor-specific axon guidance in the olfactory bulb. Neuron 35:681–696. [DOI] [PubMed] [Google Scholar]
- 22.Bozza T, Feinstein P, Zheng C, Mombaerts P (2002): Odorant receptor expression defines functional units in the mouse olfactory system. J Neurosci 22:3033–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dias BG, Ressler KJ (2013): Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 17:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Debiec J, Sullivan RM (2014): Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A 111:12222–12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Todrank J, Heth G, Restrepo D (2011): Effects of in utero odorant exposure on neuroanatomical development of the olfactory bulb and odour preferences. Proc R Soc B Biol Sci 278:1949–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams ZM (2016): Transgenerational influence of sensorimotor training on offspring behavior and its neural basis in Drosophila. Neurobiol Learn Mem 131:166–175. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Y, Gong NN, Hu XS, Ni MJ, Pasi R, Matsunami H (2015): Molecular profiling of activated olfactory neurons identifies odorant receptors for odors in vivo. Nat Neurosci 18:1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von der Weid B, Rossier D, Lindup M, Tuberosa J, Widmer A, Col JD, et al. (2015): Large-scale transcriptional profiling of chemosensory neurons identifies receptor–ligand pairs in vivo. Nat Neurosci 18:1455–1463. [DOI] [PubMed] [Google Scholar]
- 29.Lazic SE, Essioux L (2013): Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neurosci 14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009): Mixed Effects Models and Extensions in Ecology with R. New York: Springer- Verlag. [Google Scholar]
- 31.Jovanovic T, Smith A, Kamkwalala A, Poole J, Samples T, Norrholm SD, et al. (2011): Physiological markers of anxiety are increased in children of abused mothers. J Child Psychol Psychiatry 52:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. (2016): Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry 80:372–380. [DOI] [PubMed] [Google Scholar]
- 33.Rodgers AB, Morgan CP, Bronson SL, Revello S, Bale TL (2013): Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33:9003–9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colquitt BM, Markenscoff-Papadimitriou E, Duffié R, Lomvardas S (2014): Dnmt3a regulates global gene expression in olfactory sensory neurons and enables odorant-induced transcription. Neuron 83:823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feinstein P, Mombaerts P (2004): A contextual model for axonal sorting into glomeruli in the mouse olfactory system. Cell 117:817–831. [DOI] [PubMed] [Google Scholar]
- 36.Magklara A, Yen A, Colquitt BM, Clowney EJ, Allen W, Markenscoff-Papadimitriou E, et al. (2011): An epigenetic signature for monoallelic olfactory receptor expression. Cell 145:555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vassalli A, Feinstein P, Mombaerts P (2011): Homeodomain binding motifs modulate the probability of odorant receptor gene choice in transgenic mice. Mol Cell Neurosci 46:381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS (2011): MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem 96:89–94. [DOI] [PubMed] [Google Scholar]
- 39.Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming GL, et al. (2013): TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 79:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Wei W, Zhao QY, Widagdo J, Baker-Andresen D, Flavell CR, et al. (2014): Neocortical Tet3-mediated accumulation of 5-hydroxymethylcytosine promotes rapid behavioral adaptation. Proc Natl Acad Sci U S A 111:7120–7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin Q, Wei W, Coelho CM, Li X, Baker-Andresen D, Dudley K, et al. (2011): The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat Neurosci 14:1115–1117. [DOI] [PubMed] [Google Scholar]
- 42.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. (2010): Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi J, et al. (2016): Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351:397–400. [DOI] [PubMed] [Google Scholar]
- 44.Franklin TB, Russig H, Weiss IC, Gräff J, Linder N, Michalon A, et al. (2010): Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry 68:408–415. [DOI] [PubMed] [Google Scholar]
- 45.Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D (2014): Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology 155:3853–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vassoler FM, White SL, Schmidt HD, Sadri-Vakili G, Pierce RC (2013): Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao S, Tian H, Ma L, Yuan Y, Yu CR, Ma M (2013): Activity-dependent modulation of odorant receptor gene expression in the mouse olfactory epithelium. PLoS One 8:e69862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. (2015): Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 350:aab2006. [DOI] [PubMed] [Google Scholar]
- 49.Giustino TF, Maren S (2018): Noradrenergic modulation of fear conditioning and extinction. Front Behav Neurosci 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quirk GJ, Mueller D (2008): Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.