Abstract
The design of cell-based therapies for vocal fold tissue engineering requires an understanding of how cells adapt to the dynamic mechanical forces found in the larynx. Our objective was to compare mechanotransductive processes in therapeutic cell candidates (mesenchymal stromal cells from adipose tissue and bone marrow, AT-MSC and BM-MSC) to native cells (vocal fold fibroblasts-VFF) in the context of vibratory strain. A bioreactor was used to expose VFF, AT-MSC, and BM-MSC to axial tensile strain and vibration at human physiological levels. Microarray, an empirical Bayes statistical approach, and geneset enrichment analysis were used to identify significant mechanotransductive pathways associated with the three cell types and three mechanical conditions. Two databases (Gene Ontology, Kyoto Encyclopedia of Genes and Genomes) were used for enrichment analyses. VFF shared more mechanotransductive pathways with BM-MSC than with AT-MSC. Gene expression that appeared to distinguish the vibratory strain condition from polystyrene condition for these two cells types related to integrin activation, focal adhesions, and lamellipodia activity, suggesting that vibratory strain may be associated with cytoarchitectural rearrangement, cell reorientation, and extracellular matrix remodeling. In response to vibration and tensile stress, BM-MSC better mimicked VFF mechanotransduction than AT-MSC, providing support for the consideration of BM-MSC as a cell therapy for vocal fold tissue engineering. Future research is needed to better understand the sorts of physical adaptations that are afforded to vocal fold tissue as a result of focal adhesions, integrins, and lamellipodia, and how these adaptations could be exploited for tissue engineering.
Keywords: vocal folds, vibration, mechanotransduction, mesenchymal stromal cells, fibroblasts
INTRODUCTION
Mechanical forces contribute to tissue disease (e.g., chronic hypertension can lead to hypertrophic cardiac thickening) and repair (e.g., massage can reduce dermal scarring) (Brilla et al., 1990; Nishiyama et al., 1986; Shin and Bordeaux, 2012). In the larynx, heavy voice use can lead to benign lesions and other voice disorders, partially due to the stress of vibration, tension change, and shear (Altman, 2007; Gunter, 2003; Johns, 2003; Roy et al., 2004; Titze, 1994). It has been proposed that altering phonation stresses with low impact, large amplitude voicing may reduce acute inflammation and potentially minimize tissue injury (Verdolini Abbott et al., 2012), but behavioral intervention alone cannot resolve tissue fibrosis. Cell-based treatment for vocal fold fibrosis may be an alternative. Vocal fold fibroblasts (VFF) maintain and repair vocal fold ECM (Hansen and Thibeault, 2006), but a source of healthy VFF is not clinically available. Bone marrow-derived mesenchymal stromal cells (BM-MSC) and adiposederived mesenchymal stromal cells (AT-MSC) have demonstrated potential as VFF alternatives for vocal fold engineering. BM-MSC and AT-MSC have similar cell surface marker expression, differentiation potential, and immunophenotype as human VFF (Hanson et al., 2010). In other parts of the body, MSC have demonstrated unique immunomodulatory properties that may allow use of clinical grade, allogeneic MSC without a detrimental immune response (Kebriaei et al., 2009; Le Blanc et al., 2008; Uccelli et al., 2007). This is a critical consideration because of proximity between the larynx and airway.
As many laryngeal engineering investigations have been performed in animal models (Johnson et al., 2010; Kanemaru et al., 2003; Lee et al., 2006), there is a paucity of data that considers the unique biomechanical loads of the human vocal fold. Previously, we have reported on cell differentiation markers and wound healing pathways in VFF, AT-MSC, and BM-MSC following stimulation in a bioreactor that mimics vocal fold forces (Bartlett et al., 2015). Design of cell-based therapy also necessitates an understanding of the mechanotransductive molecules and structures (e.g., actin cytoskeleton, stress fibers) underlying the downstream processes (e.g., collagen synthesis) previously reported. The mechanisms underlying how laryngeal fibroblasts adapt their extracellular matrix (ECM) to changes in mechanical load are being explored (Branski et al., 2007; Gaston et al., 2012; Titze et al., 2004; Webb et al., 2006; Wolchok et al., 2009; Wolchok and Tresco, 2013). In vitro, fibroblasts are physically coupled to ECM through cell surface receptors and integrins linked to their cytoskeleton (Ingber, 2006, 1997). Through these connections, a myriad of downstream pathways are propagated that control cell fate processes and tissue remodeling (Chiquet, 1999; MacKenna et al., 2000; Wang et al., 2007).
In the present work, we compared mechanotransduction pathways of vibrated AT-MSC and BM-MSC to VFF to better understand the similarities and differences between these cell types in response to vibratory strain. To do this, we used cDNA microarray and statistical tests of enrichment. Tests for enrichment of common function are used to evaluate the expression of biologically related gene sets, such as signaling pathway genes (Newton et al., 2007; Subramanian et al., 2005). As compared to single gene analyses, enrichment analyses are more reproducible and more capable of identifying biologically meaningful patterns (Subramanian et al., 2005). We hypothesized that vibratory strain would encourage VFF to upregulate expression of cytoskeletal rearrangement and cell adhesion genes as compared to static controls.
MATERIALS AND METHODS
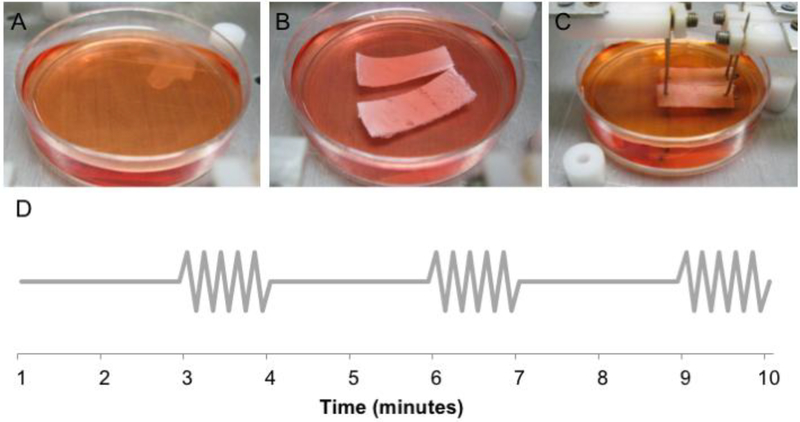
We cultured three donors of each cell type (AT-MSC, BM-MSC, VFF) in three mechanical conditions, resulting in 27 microarrays (3×3×3). Mechanical conditions included cells grown on polystyrene (POLY), cells seeded in scaffolds (SCA), and cells seeded in scaffolds and exposed to vibratory strain in a bioreactor (VIB) (Fig 1).
Fig 1. Three experimental conditions.
Cells were cultured on A) polystyrene dishes (POLY), B) scaffolds (SCA), or C) mechanically stimulated scaffolds (VIB) according to the schedule (D). During the first 12 hours of stimulation, the VIB cells were exposed to simultaneous 20% tensile stress and 200 Hz vibration every third minute. During the second 12 hours of stimulation, the cells were exposed to 0% tensile stress and no vibration.
Scaffolds
Polyether polyurethane scaffolds were described previously (5% w/v mass concentration, see Gaston et al., 2015, 2014 for mechanical properties and porosity). Briefly, two grams of Tecoflex SG-80A beads (Thermedics, Wickliffe) were dissolved in DMAC (39.1 mL; Fisher Scientific, Pittsburgh) and added to one side of a 2:1 dual component adhesive cartridge. The other side contained Pluronic 10R5 (18.95 mL; Sigma-Aldrich, St Louis). A dispensing gun pushed the materials through the cartridge and helical static mixer, and into molds. Scaffold sheets were placed in 70% ethanol/dry ice bath (−40°C, 20 minutes), rinsed in de-ionized water (48 hours), trimmed (25 mm x 10 mm x 2 mm), lyophilized, and sterilized.
Cells
Human AT-MSC and BM-MSC (Lonza PT-2501, PT-5006, Walkersville) were cultured according to manufacturer instructions (Table 1). Human primary VFF were obtained from the senior author, and were previously derived with IRB approval from healthy vocal fold tissue (Chen and Thibeault, 2009; Thibeault et al., 2008). Cell-specific media (Bartlett et al., 2015) were refreshed every three days.
Table 1.
Cell Donor Demographics
| Cell Type | Donor | Sex | Age (yrs) |
|---|---|---|---|
| AT-MSC | Donor 1 |
F |
40 |
| Donor 2 |
M |
51 |
|
| Donor 3 |
F |
38 |
|
|
Mean |
43.0 |
||
| BM-MSC | Donor 1 |
M |
22 |
| Donor 2 |
M |
43 |
|
| Donor 3 |
F |
19 |
|
|
Mean |
28.0 |
||
| VFF | Donor 1 |
M |
21 |
| Donor 2 |
F |
59 |
|
| Donor 3 |
F |
77 |
|
|
Mean |
52.3 |
||
| Overall | 41.1 |
On Day 1, scaffolds were soaked in fibronectin (20 μg/ml) to facilitate cell attachment. On Day 2, 1×106 cells in 100 ul of media were added to each scaffold. The seeding procedure was repeated on the other scaffold side on Day 3. During seeding, all VFF and MSC were at passage 4 or 5. On Day 4, VIB scaffolds were attached to the bioreactor and exposed to the stimulation described below for 24 hours (Fig 1D), and SCA scaffolds were placed in new polystyrene dishes. On Day 5, RNA was extracted from VIB, SCA, and POLY samples.
Mechanical Stimulation
VIB cells were stimulated for 24 hours in a bioreactor that was designed to mimic the vocal load of an average day (Day 4, Fig 1D). During the first twelve hours of Day 4, VIB scaffolds were exposed to 200 Hz vibration and 20% axial tensile stress every third minute (~30% of each hour) to mimic voice use during a work day. During the final twelve hours of Day 4, the VIB scaffolds were exposed to no vibratory strain, to mimic rest later in the day. The vibratory frequency (200 Hz) reflects average female fundamental frequency (Linke, 1973). Axial tensile strain (20%) approximates deformation during phonation (Nishizawa, 1989; Nishizawa et al., 1988). The vibration schedule (~30% of every hour) approximates voice use data from a study of teachers who wore ambulatory monitoring devices (Hunter and Titze, 2010).
The bioreactor was designed and built at the UW-Madison and has been described previously (Bartlett et al., 2015). Eight polystyrene dishes containing pairs of cell-seeded scaffolds and media were mounted on the platform. Scaffolds were exposed to axial tensile strain and to compression forces imparted through vibration of paired scaffolds. A servo motor was used to stretch the scaffolds in one dimension, along their primary axis, to simulate ‘lengthening’ of the vocal folds during normal phonation. Simultaneously, a voice coil actuator was used to rapidly bring both scaffolds into direct contact with each other, resulting in compressive forces and vibration at the desired frequency. Notably, all vibration occurred within one plane; a fluid flow driving mucosal wave from the inferior to superior dimension was not present.
RNA Extraction and Microarray
Cells were dissociated from the scaffolds using trypsin and pestles. Total RNA was extracted (Qiagen RNeasy Plus Mini kit, Qiagen, Valencia). RNA quality was evaluated (Agilent 2100 Bioanalyzer). Twenty-seven microarrays (Affymetrix GeneChip Human Gene 2.0 ST, Affymetrix, Santa Clara) were processed at the UW Biotechnology Gene Expression Center, as described previously (Bartlett et al., 2015). Confirmatory reverse transcription - quantitative PCR (RT-qPCR) was previously reported for selected microarray genes (Bartlett et al., 2015).
Microarray Statistical Analysis
Analyses were performed in R using affy, EBarrays, allez, rma software (Gentleman et al., 2004). EBarrays was used to identify genes showing similarities and differences among the cell types and mechanical conditions. It accounts generally for differences among genes in their true underlying expression levels, measurement fluctuations, and distinct expression patterns for a given gene among conditions (Kendziorski et al., 2003). An expression pattern is an arrangement of the true underlying intensities (μ) in each condition. In three conditions, five expression patterns are possible: P1:μ1 = μ2 = μ3; P2:μ1 = μ2 ≠ μ3; P3:μ1 = μ3 = μ2;P4:μ1 ≠ μ2 = μ3 and P5:μ1 ≠ μ2 ≠ μ3. In within cell type analyses, μ1 = POLY, μ2 = SCA, μ3 = VIB. In within mechanical condition analyses, μ1 = ATMSC, μ2 = BMMSC, and μ3 = VFF. The fitted model parameters provide information on the number of genes expected in each pattern and is used to assign probability distributions to every gene. Each gene-specific distribution gives the posterior probability (PP) of that gene’s expression pattern. Thresholds targeted an overall FDR of 5%.
KEGG/GO
Tests for enrichment of common biological function among sets of differentially expressed genes was performed with allez (Newton et al., 2007) and two databases. First, Kyoto Encyclopedia of Genes and Genomes (KEGG) provides an electronic representation of the biological system, with pathway maps that model cellular and organism functions. Second, Gene Ontology (GO) terms are categorized into three domains (cellular component, biological process, molecular function) (Gene and Consortium, 2000). The default threshold in allez (normal score |Z|>5) was used to assess significance.
RESULTS
In the EBarrays analysis of each cell type, most of the probes fit pattern one (P1: VIB=SCA=POLY), suggesting that most genes were not sensitive to mechanical stimulation. Specifically, in VFF, 2.6% of probes were differentially expressed across the three mechanical conditions (Analyses 4–6, P2-P5) (PP>0.95). Within the MSC datasets, 3.2% (AT-MSC), and 4.5% (BM-MSC) of probes were differentially expressed across the three mechanical conditions (PP>0.95; data not shown).
Hierarchical Clustering
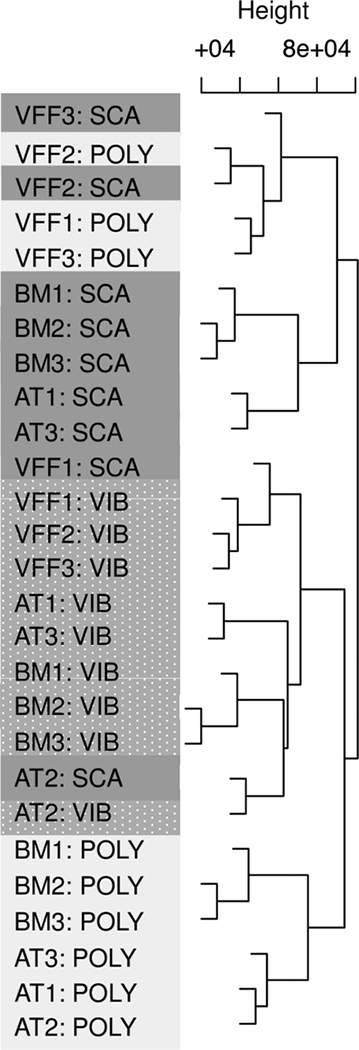
In a hierarchical clustering plot, samples are expected to align according to homogeneity. Typically, clustering is greatest among technical replicates, then biological replicates, and lastly, experimental condition. In dendrogram, the height of each node represents the distance between two child clusters (Fig 2). Microarray samples clustered primarily by mechanical condition, and secondarily by cell type, suggesting that each variable systematically affected gene expression. For example, all but one of the VIB samples were adjacent. Samples did not cluster by donor, experimental date, or microarray batch (data not shown).
Fig 2. Microarray samples clustered primarily by mechanical condition and secondarily by cell type.
Hierarchical clustering plot of the normalized intensity values, with samples denoted by cell type (AT, BM, VFF), donor (1, 2, 3), and mechanical condition (VIB, SCA, and POLY). The top axis (“Height”) denotes the value of the distance metric between clusters, with the axis minimum representing the minimum distance between any two samples.
KEGG
Set means, standard deviations, and enrichment z scores for all significant KEGG pathways in expression patterns 2–4 are provided (Table 2). In VIB analyses, two pathways relevant to the current mechanotransduction investigation were significant in VFF=BM≠AT pattern (ECM-Receptor Interaction-KEGG #4512; Focal Adhesions-KEGG #4510 (|Z|>5). In the POLY analyses, one mechanotransductive pathway (ECM-Receptor Interaction-KEGG #4512) was significant in pattern AT=BM≠VFF (|Z|>5). ECM-Receptor Interaction (KEGG #4512) focuses on the specific linkages between ECM molecules and integrin subunits. Focal adhesions (KEGG #4510) focuses on interaction between key players (cytokines, ECM molecules, and receptors) in signaling pathways (Wnt, P13K, P13K Akt, MAPK, Phosphatidyl inositol). The influence of these interactions on cell fate processes (survival, proliferation, motility) is also included in KEGG annotation. While we have focused on mechanotransduction pathways, we note that other pathways were also enriched (Table 2). These pathways have genes related to ECM and cell signaling (e.g. fibroblast growth factor genes in Melanoma- KEGG #5218, gamma-aminobutyric acid receptor genes in Taste Transduction- KEGG #4742) (Lévi et al., 2002; Sai and Ladher, 2008).
Table 2.
KEGG Pathway Enrichment in Mechanical Condition Analyses
| Analysis | Pattern | Pathway | Set mean | SetS.D. | # of Genes | Zscore |
|---|---|---|---|---|---|---|
| VIB | AT=BM≠VFF |
None |
- |
- |
- |
- |
| VFF=AT≠BM |
None |
- |
- |
- |
- |
|
| VFF=BM≠AT | ECM-Receptor Interaction | 0.07 | 0.26 | 86 | 6.07 | |
| Focal Adhesions | 0.05 | 0.21 | 206 | 5.49 | ||
| Melanoma |
0.07 |
0.26 |
71 |
5.45 |
||
| SCA | AT=BM≠VFF |
None |
- |
- |
- |
- |
| VFF=AT≠BM |
Collecting Duct Acid Secretion |
0.2 |
0.41 |
27 |
6.41 |
|
| VFF=BM≠AT |
None |
- |
- |
- |
- |
|
| POLY | AT=BM≠VFF |
ECM-Receptor Interaction |
0.17 |
0.38 |
86 |
5.88 |
| VFF=AT≠BM |
Taste Transduction |
0.10 |
0.31 |
53 |
5.14 |
|
| VFF=BM≠AT | None | - | - | - | - |
VIB= vibratory strain, SCA= scaffold only, POLY= polystyrene, AT= adipose derived mesenchymal stromal cells, BM= bone-marrow derived mesenchymal stromal cells, VFF= vocal fold fibroblasts
GO
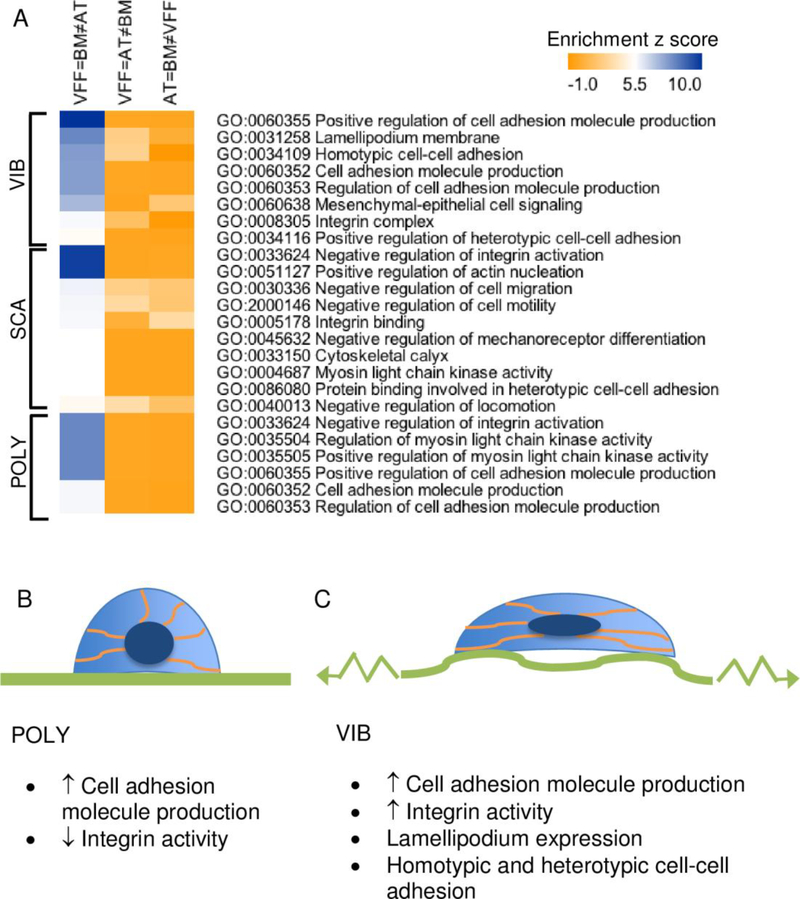
As the KEGG analyses highlighted the significance of Focal Adhesions and ECM-Receptor Interaction in the VFF=BM≠AT pattern, we explored enrichment of GO terms in this dataset to learn more about mechanotransduction. A heat map of significant GO terms related to cell adhesion, mechanotransduction, and cytoskeletal rearrangement manually identified from VFF=BM≠AT pattern is provided (Fig 3, |Z|>5). Z-scores from P2-P3 are included for comparison. Using relevant GO terms from this heat map, we generated schematics of vibratory strain in VFF and BM-MSC (Fig 3B-C). Differences between static and vibratory strain conditions based on the GO analysis involved integrin activity, lamellipodium expression, and homotypic/heterotypic cell-cell adhesion regulation.
Fig 3. Vibrated VFF shared more mechanotransductive similarity with BM-MSC than AT-MSC.
Heat map of the significant GO terms related to mechanotransduction from pattern 2 (AT=BM≠VFF), 3 (VFF=AT≠BM), and 4 (VFF=BM-MSC≠AT-MSC) is provided for VIB, SCA, and POLY data (A). This expression pattern was selected based on the significant KEGG findings. Expected changes to the cell shape (lighter blue), orientation of the actin cytoskeleton (orange), and nucleus (dark blue) for VFF and BMMSC grown on B) polystyrene (POLY), or C) a cell scaffold and exposed to vibratory strain (VIB) are depicted based on GO analyses. Significant GO terms are listed below each schematic from expression pattern 4 (VFF=BM≠AT) for the VIB and POLY data.
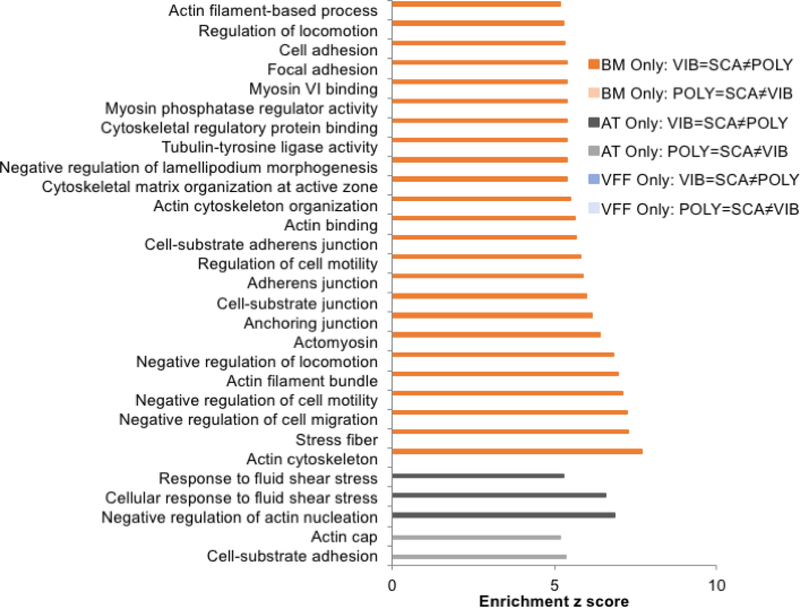
We were also interested in understanding mechanotransduction within each cell type. We searched for significant mechanotransduction GO terms in the VFF Only, ATMSC Only, and BM-MSC Only datasets for P2 (POLY=SCA≠VIB) and P4 (VIB=SCA≠POLY) (Fig 4, |Z|>5). Nearly all significant mechanotransduction GO terms were found in BM MSC (VIB=SCA≠POLY), and related to cell adhesion and cytoskeletal rearrangement. Significant molecules and structures included lamellipodium, stress fibers, actin filament, actin cytoskeleton, actomyosin, myosin VI, anchoring junctions, and adherens junctions. There were only two relevant GO terms for AT-MSC in P2, and three relevant GO terms for AT-MSC in P4. There were no significant mechanotransduction GO terms in VFF in P2 or 4.
Fig 4. Significant mechanotransductive GO terms were primarily associated with BM-MSC in the vibrated and scaffold conditions.
For each cell type analysis, all significant GO terms related to cell adhesion and cytoskeletal rearrangement are provided in the bar chart. Enrichment z scores > 5 were considered significant.
DISCUSSION
BM-MSC and AT-MSC have been shown to attenuate scarring in the myocardium (Makkar et al., 2005), nervous system (Li et al., 2005), skin (McFarlin et al., 2006), and vocal fold (Hertegård et al., 2006; Johnson et al., 2010; Svensson et al., 2010). While pre-clinical trials of cell-based therapies for vocal scar have been promising, it remains unclear if BM-MSC or AT-MSC might be better suited for the unique biomechanical challenges found in the human larynx. Healthy VFF maintain ECM in the lamina propria. Fibroblasts adapt their ECM to changes in mechanical stress through mechanotransduction (Wang et al., 2007). As such, we assume that a desirable cell type for vocal fold engineering would mimic VFF mechanotransduction in response to vibratory strain. KEGG analysis revealed that vibrated VFF shared Focal Adhesions and ECM-Receptor Interaction pathways with vibrated BM-MSC, but not with AT-MSC (Table 2). Further analysis of this expression pattern (VFF=BM≠AT) using the GO database revealed several mechanotransductive responses were shared by these cell types, allowing for conceptualization of vibratory strain schematics (Fig 3). Integrin, focal adhesion, and lamellipodium activity were the primary features that distinguished vibrated from non vibrated VFF and BM-MSC, which may have implications for vocal health and repair.
Cell Adhesion via Integrins
Cell adhesion (cell-cell, cell-ECM) is associated with wound healing and varies with mechanical stress exposure (Juhasz et al., 1993; Shyy and Chien, 1997). VFF and BM-MSC in the vibrated and polystyrene conditions shared significant enrichment for GO terms related to the production of cell adhesion molecules (CAMs) (Fig 3, GO:0060355: Positive Regulation of Cell Adhesion Molecule Production, GO:0060353: Regulation of Cell Adhesion Molecule Production). Enrichment of GO terms related to integrins, a specific family of CAMs, distinguished vibrated VFF and BM-MSC (GO:000835: Integrin Complex) from static controls (GO:0033624: Negative Regulation of Integrin Activation).
Integrins are force-bearing adhesion proteins (Puklin-Faucher and Sheetz, 2009), and as such, our vibratory strain data are not surprising. Integrins sense mechanical loads applied directly to them and to the ECM that they bind (Choquet et al., 1997; Wang et al., 1993). The cytoskeleton rearranges actin microfilaments and cell nuclei proportionate to integrin-transduced mechanical loads, which can strengthen the cell against mechanical distortion (Maniotis et al., 1997; Ralphs et al., 2002; Wang et al., 1993) (Fig 3). The concomitant redistribution of stress in the intracellular machinery may represent microscale shifts that underlie vocal health and disease. Integrin expression has been linked to specific voice disorders. For example, integrin beta 2 is upregulated in vocal polyp as compared to Reinke’s edema, and integrin beta 1 and 3 are upregulated in vocal fold carcinoma (Bartlett et al., 2012; Duflo et al., 2006). Research is needed to understand the relationship between specific integrins and mechanical contributors to fibrosis and treatment. For example, specific integrin subunits may be associated with resilient microenvironments wherein a cell is physically fortified against mechanical damage, and vulnerable microenvironments wherein excessive mechanical stress leads to the production of fibrotic ECM.
Focal Adhesions
Enrichment of Focal Adhesions pathway (KEGG #4510) was shared by vibrated VFF and BM-MSC (Table 2). The role of focal adhesions in laryngeal tissue is unknown. Focal adhesion sites are dynamic and can assemble in response to mechanical stimuli (Balaban et al., 2001; Petroll et al., 2004). Focal adhesion size increases proportionally to traction forces, on a timescale of a few seconds (Balaban et al., 2001). Perhaps focal adhesion sites in the vocal fold cover are routinely adjusted in response to the vibratory and stiffness loads of phonation. Re-anchoring of cells could be protective against mucosal wave propagation. Future research is needed to better understand the physical adaptations that are afforded to vocal tissue from focal adhesions, such as tensional homeostasis and buffering (Brown et al., 1998; Chiquet, 1999; Petroll et al., 2004; Webster et al., 2014).
Lamellipodia
Lamellipodia was another feature of the cell mechanoenvironment that distinguished vibrated VFF and BM-MSC from static controls (Fig 3). Lamellipodia are cytoskeletal projections found at the leading edge of migrating cells. Cell migration is associated with wound healing processes, such epithelial renewal (Ridley et al., 2003). Ingber and others report that the direction of lamellipodia extension can be manipulated by altering scaffold shape, stiffness, and mechanical stress (Dembo and Wang, 1999; Parker et al., 2002; Shemesh et al., 2009; Wang and Ingber, 1994). These findings, in combination with our data, suggest that lamellipodia-mediated cell migration may be exploited in exercises that apply tensile stress to the vocal fold during behavioral voice therapy. For example, upward pitch glide, a presbyphonia exercise (Ziegler et al., 2013), applies tensile stress to the vocal fold cover, which may adjust lamellipodia orientation and position cells for more favorable ECM deposition. It was recently reported that fibroblasts exposed to cyclic strain produced ECM fibers perpendicular to stress direction, but no identifiable ECM orientation was found with vibratory strain (Wolchok and Tresco, 2013). Further inquiry into how VFF and BM-MSC use vibratory strain to direct lamellipodia extension into a stiff, fibrotic bed may provide insights into therapeutic design.
Cell Type Analysis
In addition to analyzing similarities among the cell types, we investigated mechanobiology within each cell type. Contrary to our hypothesis that vibratory strain would encourage VFF to significantly express cytoskeletal rearrangement and cell adhesion-related GO terms, there were no significant mechanotransduction terms in the VFF dataset in the tested patterns (Fig 4). The majority of significant mechanotranduction GO terms were found in BM-MSC, VIB=SCA≠POLY. BM-MSC are keenly mechanosensitive to three-dimensional matrices and mechanical stress (MacQueen et al., 2013). Our data corroborates this, with significant GO terms relating to focal adhesion sites (intracellular proteins linking to actin cytoskeleton, and transmembrane integrins linking to extracellular ligands) and to CAMs. Given the mechanotransductive similarity between VFF and BM-MSC in KEGG and earlier GO data, perhaps these data demonstrate that there was a greater magnitude of difference between the mechanical conditions (VIB=SCA≠POLY) in the BM-MSC dataset than in the VFF dataset.
It was surprising that there were no significant mechanotransduction terms in the VFF dataset in the tested expression patterns (Fig 4). A few possible explanations deserve mention. First, the average age of VFF donors was greater than BM-MSC donors (Table 1). There is some evidence that dermal fibroblasts stiffen with donor age, which can have downstream effects (Kessler et al., 2001; Schulze et al., 2012). Second, perhaps being cultured on polystyrene for four passages prior to the experiment caused the VFF to lose mechanosensitivity. This possibility is less compelling when considering that nuclear stiffness in response to uniform biaxial stress did not differ between earlier and later passages of dermal fibroblasts of healthy controls (Verstraeten et al., 2008). Lastly, in the event that VFF are indeed less mechanosensitive to phonation-like forces than BM-MSC, it may suggest that biomechanical screening of cell sources for vocal fold tissue engineering efforts is less important than the other myriad factors that could be considered (Yang et al., 2008).
Limitations
First, given the dearth of vibration data in the mechanotransduction literature, including a condition comprised of cells that were vibrated without tensile stress may have been of interest to the field. Second, only a single time point (24 hours) and stimulation schedule (30%/hour) were included. Recent investigations have yielded contradictory findings, from cells quickly adapting to vibration and other mechanical stimuli, to cells showing greater magnitude of change with exposure time (Robling et al., 2002; Sen et al., 2011; Srinivasan et al., 2007; Thompson et al., 2012). Third, mechanical stimulation in the bioreactor included axial tensile strain and compression forces (imparted through vibration of scaffolds), but not all of the forces present during phonation were included. For example, Mongeau’s group has designed a bioreactor that features airflow-induced self-oscillations (Latifi et al., 2016, 2014). Fourth, there is a possibility that some of the mRNA generated during vibratory strain exposure could have degraded during the twelve-hour rest period (Enholm et al., 1997; Overall et al., 1991). Future work is needed to measure the stability of relevant mRNA transcripts in our experimental paradigm. Fifth, as donors from both sexes across the lifespan were included, findings may not generalize to a specific population. Sixth, gene expression findings were not evaluated with downstream analysis, such as staining actin projections or quantifying integrin isoforms from protein lysates. Finally, the experiment did not include VFF/MSC co-culture, which may be more clinically applicable.
CONCLUSION
KEGG and GO enrichment data revealed that VFF had greater mechanotransductive similarity to BM-MSC than to AT-MSC, which may support the consideration of BM-MSC as a cell therapy for vocal fold regenerative medicine. Our data also highlighted some of the effects of vibratory strain on cells. Integrin, focal adhesion, and lamellipodium activity distinguished vibrated VFF and BM-MSC from those that were not vibrated. This suggests that vibratory strain was associated with cytoarchitectural and ECM remodeling, and cell re-orientation. Future investigations may be able to discern if the presence of specific integrins and other mechanotransducers could be used to better understand the scar microenvironment, to stratify patients with vocal fibrosis into prognostic categories, and to design cell-based therapies.
ACKNOWLEDGMENTS
We thank Thomas Yen for technical assistance and NIH (F31 DC012973, R01 DC4336).
Footnotes
CONFLICT OF INTERST STATEMENT
Rebecca S. Bartlett, PhD – none
Joel D. Gaston, PhD - none
Shuyun Ye, PhD - none
Christina Kendziorski, PhD - none
Susan L. Thibeault, PhD - none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altman K, 2007. Vocal Fold Masses. Otolaryngologic Clinics of North America 40, 1091–1108. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B, 2001. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology 3, 466–472. [DOI] [PubMed] [Google Scholar]
- Bartlett R, Heckman W, Isenberg J, Thibeault S, Dailey SH, 2012. Genetic characterization of vocal fold lesions: leukoplakia and carcinoma. The Laryngoscope 122, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett RS, Gaston JD, Yen TY, Ye S, Kendziorski C, Thibeault SL, 2015. Biomechanical screening of cell therapies for vocal fold scar. Tissue Engineering Part A 21, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branski RC, Perera P, Verdolini K, Rosen CA, Hebda PA, Agarwal S, 2007. Dynamic biomechanical strain inhibits IL-1β-induced inflammation in vocal fold fibroblasts. Journal of Voice 21, 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilla CG, Pick R, Tan LB, Janicki JS, Weber KT, 1990. Remodeling of the rat right and left ventricles in experimental hypertension. Circulation Research 67, 1355–1364. [DOI] [PubMed] [Google Scholar]
- Brown RA, Prajapati R, McGrouther DA, Yannas IV, Eastwood M, 1998. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. Journal of Cellular Physiology 175, 323–332. [DOI] [PubMed] [Google Scholar]
- Chen X, Thibeault S, 2009. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Engineering. Part C, Methods 15, 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, 1999. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biology 18, 417–426. [DOI] [PubMed] [Google Scholar]
- Choquet D, Felsenfeld DP, Sheetz MP, 1997. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, 39–48. [DOI] [PubMed] [Google Scholar]
- Dembo M, Wang Y, 1999. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal 76, 2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflo S, Thibeault S, Li W, Smith M, Schade G, Hess M, 2006. Differential gene expression profiling of vocal fold polyps and Reinke’s edema by complementary DNA microarray. The Annals of Otology, Rhinology, and Laryngology 115, 703–714. [DOI] [PubMed] [Google Scholar]
- Enholm B, Paavonen K, Ristimäki A, Kumar V, Gunji Y, Klefstrom J, Kivinen L, Laiho M, Olofsson B, Joukov V, Eriksson U, Alitalo K, 1997. Comparison of VEGF, VEGF-B, VEGF-C and Ang-1 mRNA regulation by serum, growth factors, oncoproteins and hypoxia. Oncogene 14, 2475–2483. [DOI] [PubMed] [Google Scholar]
- Gaston J, Bartlett R, Klemuk S, Thibeault S, 2015. Formulation and characterization of a porous, elastomeric biomaterial for vocal fold tissue engineering research (Corrigendum). Annals of Otology, Rhinology and Laryngology 124, 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J, Bartlett R, Klemuk SA, Thibeault S, 2014. Formulation and characterization of a porous, elastomeric biomaterial for vocal vold tissue engineering research. Annals Otolology, Rhinology, and Laryngology 123, 866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston J, Rios BQ, Bartlett R, Berchtold C, Thibeault S, 2012. The response of vocal fold fibroblasts and mesenchymal stromal cells to vibration. PLoS ONE 7, e30965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene T, Consortium O, 2000. Gene ontology : tool for the unification of biology. Nature Genetics 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, 2004. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology 5, R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter HE, 2003. A mechanical model of vocal-fold collision with high spatial and temporal resolution. The Journal of the Acoustical Society of America 113, 994–1000. [DOI] [PubMed] [Google Scholar]
- Hansen JK, Thibeault S, 2006. Current understanding and review of the literature: vocal fold scarring. Journal of Voice 20, 110–120. [DOI] [PubMed] [Google Scholar]
- Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, Thibeault S, 2010. Characterization of mesenchymal stem cells from human vocal fold fibroblasts. The Laryngoscope 120, 546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertegård S, Cedervall J, Svensson B, Forsberg K, Maurer FHJ, Vidovska D, Olivius P, Ährlund Richter L, Le Blanc K, 2006. Viscoelastic and histologic properties in scarred rabbit vocal folds after mesenchymal stem cell injection. The Laryngoscope 116, 1248–1254. [DOI] [PubMed] [Google Scholar]
- Hunter EJ, Titze I, 2010. Variations in intensity, fundamental frequency, and voicing for teachers in occupational versus nonoccupational settings. Journal of Speech, Language, and Hearing Research 53, 862–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D, 2006. Cellular mechanotransduction: putting all the pieces together again. The FASEB journal 20, 811–827. [DOI] [PubMed] [Google Scholar]
- Ingber D, 1997. Tensegrity: the architectural basis of cellular mechanotransduction. Annual Review of Physiology 59, 575–599. [DOI] [PubMed] [Google Scholar]
- Johns MM, 2003. Update on the etiology, diagnosis, and treatment of vocal fold nodules, polyps, and cysts. Current Opinion in Otolaryngology & Head and Neck Surgery 11, 456–461. [DOI] [PubMed] [Google Scholar]
- Johnson BQ, Fox R, Chen X, Thibeault S, 2010. Tissue regeneration of the vocal fold using bone marrow mesenchymal stem cells and synthetic extracellular matrix injections in rats. The Laryngoscope 120, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz I, Murphy GF, Yan HC, Herlyn M, Albelda SM, 1993. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. The American Journal of Pathology 143, 1458–1469. [PMC free article] [PubMed] [Google Scholar]
- Kanemaru S, Nakamura T, Omori K, Kojima H, Magrufov A, Hiratsuka Y, Hirano S, Ito J, Shimizu Y, 2003. Regeneration of the vocal fold using autologous mesenchymal stem cells. The Annals of Otology, Rhinology, and Laryngology 112, 915–920. [DOI] [PubMed] [Google Scholar]
- Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, Devetten M, Jansen J, Herzig R, Schuster M, 2009. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biology of Blood and Marrow Transplantation 15, 804–811. [DOI] [PubMed] [Google Scholar]
- Kendziorski CM, Newton MA, Lan H, Gould MN, 2003. On parametric empirical Bayes methods for comparing multiple groups using replicated gene expression profiles. Statistics in Medicine 22, 3899–3914. [DOI] [PubMed] [Google Scholar]
- Kessler D, Dethlefsen S, Haase I, Plomann M, Hirche F, Krieg T, Eckes B, 2001. Fibroblasts in Mechanically Stressed Collagen Lattices Assume a “Synthetic” Phenotype. Journal of Biological Chemistry 276, 36575–36585. [DOI] [PubMed] [Google Scholar]
- Latifi N, Heris HK, Kazemirad S, Mongeau L, 2014. Development of a SelfOscillating Mechanical Model to Investigate the Biological Response of Human Vocal Fold Fibroblasts to Phono-Mimetic Stimulation, in: ASME International Mechanical Engineering Congress and Exposition Montreal, Quebec, pp. 1–7. [Google Scholar]
- Latifi N, Heris HK, Thomson SL, Taher R, Kazemirad S, Sheibani S, Li-Jessen NYK, Vali H, Mongeau L, 2016. A Flow Perfusion Bioreactor System for Vocal Fold Tissue Engineering Applications. Tissue Engineering Part C: Methods 22, 823–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, 2008. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. The Lancet 371, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O, 2004. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet 363, 1439–1441. [DOI] [PubMed] [Google Scholar]
- Lee BJ, Wang S, Lee JC, Jung JS, Bae YC, Jeong HJ, Kim HW, Lorenz RR, 2006. The prevention of vocal fold scarring using autologous adipose tissuederived stromal cells. Cells Tissues Organs 184, 198–204. [DOI] [PubMed] [Google Scholar]
- Lévi S, Grady RM, Henry MD, Campbell KP, Sanes JR, Craig AM, 2002. Dystroglycan is selectively associated with inhibitory GABAergic synapses but is dispensable for their differentiation. The Journal of Neuroscience 22, 4274–4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YI, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, 2005. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia 49, 407–417. [DOI] [PubMed] [Google Scholar]
- Linke CE, 1973. A study of pitch characteristics of female voices and their relationship to vocal effectiveness. Folia Phoniatrica 25, 173–185. [DOI] [PubMed] [Google Scholar]
- MacKenna D, Summerour SR, Villarreal FJ, 2000. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovascular Research 46, 257–263. [DOI] [PubMed] [Google Scholar]
- MacQueen L, Sun Y, Simmons C.a, 2013. Mesenchymal stem cell mechanobiology and emerging experimental platforms. Journal of the Royal Society, Interface 10, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkar RR, Price MJ, Lill M, Frantzen M, Takizawa K, Kleisli T, Zheng J, Kar S, McClelan R, Miyamota T, 2005. Intramyocardial injection of allogenic bone marrow-derived mesenchymal stem cells without immunosuppression preserves cardiac function in a porcine model of myocardial infarction. Journal of Cardiovascular Pharmacology and Therapeutics 10, 225–233. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE, 1997. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America 94, 849–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo M, Araña M, Pelacho B, Prosper F, 2012. Mesenchymal stem cells and cardiovascular disease: a bench to bedside roadmap. Stem Cells International 2012, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS, Bansal M, Li Y, Chopp M, Dulchavsky SA, 2006. Bone marrow derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair and Regeneration 14, 471–478. [DOI] [PubMed] [Google Scholar]
- Newton MA, Quintana FA, den Boon JA, Sengupta S, Ahlquist P, 2007. Random-set methods identify distinct aspects of the enrichment signal in gene-set analysis. The Annals of Applied Statistics 1, 85–106. [Google Scholar]
- Nishiyama S, Kuwahara T, Matsuda I, 1986. Decreased bone density in severely handicapped children and adults, with reference to the influence of limited mobility and anticonvulsant medication. European Journal of Pediatrics 144, 457–463. [DOI] [PubMed] [Google Scholar]
- Nishizawa N, 1989. Stereoendoscopic observation of the larynx--vocal fold length in respiration and in phonation with vocal pitch change. Nihon Jibiinkoka Gakkai Kaiho 92, 1239–1252. [DOI] [PubMed] [Google Scholar]
- Nishizawa N, Sawashima M, Yonemoto K, 1988. Vocal fold length in vocal pitch change Vocal physiology: voice production, mechanisms and functions.Raven Press, New York. [Google Scholar]
- Overall CM, Wrana JL, Sodek J, 1991. Transcriptional and post-transcriptional regulation of 72-kDa gelatinase/type IV collagenase by transforming growth factorβ1 in human fibroblasts: Comparisons with collagenase and tissue inhibitor of matrix metalloproteinase gene expression. Journal of Biological Chemistry 266, 14064–14071. [PubMed] [Google Scholar]
- Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE, 2002. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. The FASEB Journal 16, 1195–1204. [DOI] [PubMed] [Google Scholar]
- Petroll W, Vishwanath M, Ma L, 2004. Corneal fibroblasts respond rapidly to changes in local mechanical stress. Investigative Opthamology & Visual Science 45, 3466–3474. [DOI] [PubMed] [Google Scholar]
- Puklin-Faucher E, Sheetz MP, 2009. The mechanical integrin cycle. Journal of Cell Science 122, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralphs JR, Waggett AD, Benjamin M, 2002. Actin stress fibres and cell-cell adhesion molecules in tendons: Organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biology 21, 67–74. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR, 2003. Cell migration: integrating signals from front to back. Science (New York, N.Y.) 302, 1704–1709. [DOI] [PubMed] [Google Scholar]
- Robling AG, Hinant FM, Burr DB, Turner CH, 2002. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. Journal of Bone and Mineral Research 17, 1545–1554. [DOI] [PubMed] [Google Scholar]
- Roy N, Merrill RM, Thibeault S, Parsa RA, Gray SD, Smith EM, 2004. Prevalence of voice disorders in teachers and the general population. Journal of Speech, Language, and Hearing Research 47, 281–293. [DOI] [PubMed] [Google Scholar]
- Sai X, Ladher RK, 2008. FGF Signaling Regulates Cytoskeletal Remodeling during Epithelial Morphogenesis. Current Biology 18, 976–981. [DOI] [PubMed] [Google Scholar]
- Schulze C, Wetzel F, Kueper T, Malsen A, Muhr G, Jaspers S, Blatt T, Wittern KP, Wenck H, Käs JA, 2012. Stiffening of human skin fibroblasts with age. Clinics in Plastic Surgery. [DOI] [PubMed] [Google Scholar]
- Sen B, Xie Z, Case N, Styner M, Rubin C, Rubin J, 2011. Mechanical signal influence on mesenchymal stem cell fate is enhanced by incorporation of refractory periods into the loading regimen. Journal of Biomechanics 44, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh T, Verkhovsky AB, Svitkina TM, Bershadsky AD, Kozlov MM, 2009. Role of focal adhesions and mechanical stresses in the formation and progression of the lamellum interface. Biophysical Journal 97, 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin TM, Bordeaux JS, 2012. The role of massage in scar management: A literature review. Dermatologic Surgery 38, 414–423. [DOI] [PubMed] [Google Scholar]
- Shyy JYJ, Chien S, 1997. Role of integrins in cellular responses to mechanical stress and adhesion. Current Opinion in Cell Biology 9, 707–713. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ausk BJ, Poliachik SL, Warner SE, Richardson TS, Gross TS, 2007. Rest-inserted loading rapidly amplifies the response of bone to small increases in strain and load cycles. Journal of Applied Physiology 102, 1945–1952. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette M.a, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP, 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genomewide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 102, 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B, Nagubothu RS, Cedervall J, Le Blanc K, Ährlund Richter L, Tolf A, Hertegård S, 2010. Injection of human mesenchymal stem cells improves healing of scarred vocal folds: Analysis using a xenograft model. The Laryngoscope 120, 1370–1375. [DOI] [PubMed] [Google Scholar]
- Thibeault S, Li W, Bartley S, 2008. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngology--Head and Neck Surgery 139, 816–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WR, Rubin C, Rubin J, 2012. Mechanical regulation of signaling pathways in bone. Gene 503, 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titze I, 1994. Mechanical stress in phonation. Journal of Voice 8, 99–105. [DOI] [PubMed] [Google Scholar]
- Titze I, Hitchcock RW, Broadhead K, Webb K, Li W, Gray SD, Tresco PA, 2004. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. Journal of Biomechanics 37, 1521–1529. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Pistoia V, Moretta L, 2007. Mesenchymal stem cells: a new strategy for immunosuppression? Trends in immunology 28, 219–226. [DOI] [PubMed] [Google Scholar]
- Verdolini Abbott K, Li NYK, Branski RC, Rosen CA, Grillo E, Steinhauer K, Hebda PA, 2012. Vocal exercise may attenuate acute vocal fold inflammation. Journal of Voice 26, 814.e1–814.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraeten VLRM, Ji JY, Cummings KS, Lee RT, Lammerding J, 2008. Increased mechanosensitivity and nuclear stiffness in Hutchinson-Gilford progeria cells: Effects of farnesyltransferase inhibitors. Aging Cell 7, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Thampatty BP, Lin JS, Im HJ, 2007. Mechanoregulation of gene expression in fibroblasts. Gene 391, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Butler JP, Ingber DE, 1993. Mechanotransduction across the cell surface and through the cytoskeleton. Science (New York, N.Y.) 260, 1124–1127. [DOI] [PubMed] [Google Scholar]
- Wang N, Ingber DE, 1994. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophysical journal 66, 2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb K, Hitchcock RW, Smeal RM, Li W, Gray SD, Tresco PA, 2006. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. Journal of Biomechanics 39, 1136–1144. [DOI] [PubMed] [Google Scholar]
- Webster KD, Ng WP, Fletcher DA, 2014. Tensional homeostasis in single fibroblasts. Biophysical Journal 107, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchok J, Brokopp C, Underwood CJ, Tresco P, 2009. The effect of bioreactor induced vibrational stimulation on extracellular matrix production from human derived fibroblasts. Biomaterials 30, 327–335. [DOI] [PubMed] [Google Scholar]
- Wolchok J, Tresco P, 2013. Using vocally inspired mechanical conditioning to enhance the synthesis of a cell-derived biomaterial. Annals of Biomedical Engineering 41, 2358–66. [DOI] [PubMed] [Google Scholar]
- Yang F, Neeley WL, Moore MJ, Karp JM, Shukla A, Langer R, 2008. Tissue Engineering: The Therapeutic Strategy of the Twenty-First Century, in: Nanotechnology and Tissue Engineering: The Scaffold. pp. 3–32. [Google Scholar]
- Ziegler A, Verdolini Abbott K, Johns M, Klein A, Hapner ER, 2013. Preliminary data on two voice therapy interventions in the treatment of presbyphonia. The Laryngoscope 124, 1869–1876. [DOI] [PubMed] [Google Scholar]