Abstract
Background:
Erectile dysfunction (ED) caused by pelvic neurovascular injury (PNVI) is often refractory to treatment. In many cases erectogenic therapy is administered in a delayed fashion.
Aims:
To evaluate penile hemodynamic effects and histological changes associated with delayed low-intensity extracorporeal shock wave therapy (Li-ESWT) after PNVI ED in a rat model. We visualized images using immunofluorescence and 3-dimensional imaging of solvent cleared organs (3DISCO), a novel imaging technique.
Methods:
32 Sprague-Dawley male rats aged 12 weeks-old were divided equally into four groups: sham surgery as normal controls (NC), PNVI controls (PC), PNVI with very-low-energy Li-ESWT (PVL), and PNVI with low-energy Li-ESWT (PL) group. Bilateral cavernous nerve (CN) crush and internal pudendal bundle (IPB) ligation were performed in the PNVI groups. Li-ESWT was administered twice a week for four weeks in the PL and PVL groups starting 4 weeks after PNVI.
Outcomes:
Intracavernous pressure (ICP) studies (normalized to Mean Arterial Pressure, MAP) were conducted in all subject animals. After testing, tissue was harvested for immunofluorescence staining and 3DISCO.
Results:
Mean ICP/MAP was lower in PC compared to NC animals (0.37 ± 0.03 vs 0.91 ± 0.03, respectively, p=0.001). The ICP/MAP ratio was significantly higher in PVL and PL animals (0.66 ± 0.07 and 0.82 ± 0.05, respectively) compared to PC animals (p=0.002 and 0.001, respectively). Detailed microstructures and trajectories of nerves and vessels were identified with immunofluorescence and 3DISCO. The PC group had lower density of nerves, axons, nNOS positive nerves, and Schwann cells in the dorsal penis. Animals in the PL group had significantly higher expression of all of these markers compared to PC animals.
Clinical Implications:
Li-EWST may have utility in the management of severe ED related to PNVI from severe pelvic injury or radical pelvic surgeries, even when administered in a delayed fashion.
Strengths and Limitations:
This study of a severe ED phenotype involved treatment administered in a delayed fashion, which is more consistent with how therapy would likely be delivered in a real world clinical context. Moreover, since the treatment commenced 4 weeks after injury when nerve and tissue atrophy have already occurred, the results imply that Li-ESWT can be used for regenerative therapy. Additional studies on dose optimization and treatment interval are required to inform the design of human clinical trials
Conclusions:
Li-ESWT ameliorates the negative functional and histological effects of severe pelvic neurovascular injury in a rat model system. 3DISCO provided high-resolution images of neuroanatomy and neural regeneration.
Keywords: pelvic neurovascular injury (PNVI), low-intensity shock wave therapy (Li-ESWT), Erectile dysfunction (ED), 3-dimensional imaging of solvent cleared organs (3DISCO), nerve regeneration
INTRODUCTION
Erectile dysfunction (ED) is prevalent in men of all ages and may strongly influence quality of life1. ED may be the result of psychogenic, hormonal, medication-induced, neuronal, or vascular causes2. ED is common in the context of radical pelvic surgery, in which setting combined neurovascular injury is common3. ED may be managed with phosphodiesterase type 5 (PDE5) inhibitors4, testosterone5, intracavernous injections6, and penile prosthesis implantation7. However, pelvic surgery patients are often refractory to medical therapy3 and many patients do not desire surgical intervention for ED. Furthermore, these modalities do not resolve the underlying medical condition. A novel means to improve penile hemodynamics in post-pelvic surgery patients would markedly improve clinical outcomes and patient quality of life.
Low-intensity extracorporeal shock wave therapy (Li-ESWT) has been applied to musculoskeletal disorders since the 1990s8, 9,10, 11. In 2012, Vardi et al. published the first randomized, double-blind controlled study demonstrating the positive effects of Li-ESWT for men with ED12. Since 2012 a number of studies have indicated a beneficial effect on penile Li-ESWT on erectile function13. Although Li-ESWT shows promise as a management for ED, studies on efficacy, dose optimization, and safety remain limited.
There is evidence that the negative effects of pelvic neurovascular injury are, in part, time dependent. The optimal timing for administration of Li-ESWT have not been determined. Early treatment with Li-ESWT in a rodent model of Pelvic Neurovascular Injury (PNVI) has demonstrated efficacy in amelioration of impaired penile hemodynamics14. However, many pelvic surgery patients do not receive erectogenic or rehabilitative therapy until substantial periods of time have passed. Studies on delayed ESWT after PNVI are warranted.
3-dimensional imaging of solvent cleared organs (3DISCO) is a novel imaging technology that provides 3-dimensional, detailed anatomical structures for whole unsectioned tissues15. An understanding of three-dimensional relationships can be gleaned from 3DISCO images much more readily than with conventional 2-dimensional microscopy. To date, 3DISCO has been applied primarily to studies of the central nerve system16. To our knowledge 3-dimensional images of the cavernous nerves (CN) and major pelvic ganglion (MPG) have never been reported. The anatomical changes related to pelvic nerve injury (and treatments such as Li-EWST) may be of great interest to researchers investigating the basic mechanisms of ED related to neurovascular disruption.
The purposes of the current study were to evaluate the therapeutic effects of two intensities of Li-ESWT on the hemodynamic and physiologic changes that could reflect on the therapeutic effects of Li-ESWT on PNVI ED in the rat model and to examine tissues with 3DISCO to assess 3-dimensional structural changes related to PNVI and Li-ESWT. We hypothesized that animals treated with Li-ESWT would have superior penile hemodynamic and histological parameters compared to control animals.
MATERIALS AND METHODS
Animals and Experimental Design
32 Sprague-Dawley male rats aged 12 weeks were delivered from Charles River Laboratories. All experiments were approved by the Institutional Animal Care at our institution and Institutional Review Board approval was obtained for our research protocol. All rats were housed in standard cages with 12-hour light/dark cycles and had ad libitum access to standard rat chow and water. The rats were divided equally into four groups: sham surgery (negative control, NC), PNVI (positive control, PC), PNVI with very-low-energy Li-ESWT (PVL), and PNVI with low-energy Li-ESWT (PL) group.
Pelvic Neurovascular Injury
All animals underwent general anesthesia and midline vertical incisions on the suprapubic region and transverse incisions over the perineum. The MPG and CN were identified bilaterally adjacent to the prostate (Figure 1A). The IPB was identified as the neurovascular tissue between the ischiocavernous and bulbospongiosus muscle (Figure 1B,1C). In the three PNVI groups, CN were crushed bilaterally by clamping them with a curved hemostat 3 mm from the MPG for 3 minutes. IPB ligations were performed bilaterally using 4–0 nylon suture as previously reported. Sham surgeries were performed for the NC group.
Figure 1.
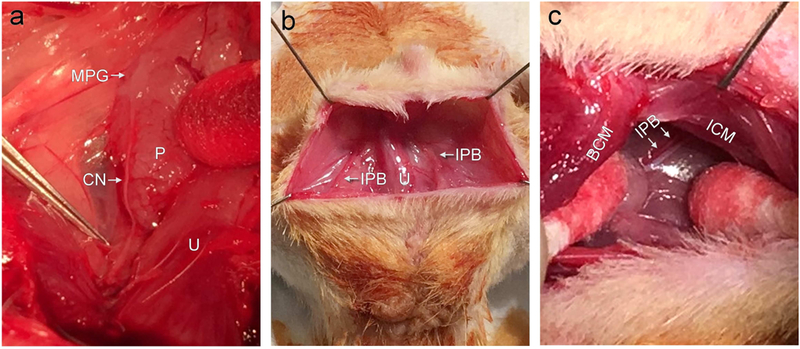
Surgical approach to creating our PNVI model. MPG: major pelvic ganglion, CN: cavernous nerve, IPB: internal pudendal bundle, P: prostate, U: urethra, ICM: ischiocavernous muscle, and BCM: bulbospongiosus muscle.
Low-intensity Extracorporeal Shock Wave Therapy
Li-ESWT was initiated 4 weeks after PNVI surgery. A four-week protocol of twice weekly treatments was administered. All animals received general anesthesia with isoflurane for all treatments. All treatments were administered with the animal in the supine position. A compact electromagnetic unit with defocused shockwave source probe (LiteMed Inc, Taipei, Taiwan) was used. The Li-ESWT probe was placed on the suprapubic region and oriented towards the penis. The PVL group received 0.02 mJ/mm2 energy flux density (EFD) at 3 Hz for 300 pulses per treatment; the PL group received 0.04 mJ/mm2 EFD at 3 Hz for 800 pulses.
Intracavernous Pressure
After completion of the Li-ESWT protocol and one week wash out period in PVL and PL groups, intracavernous pressure (ICP) was performed for evaluation of penile hemodynamics17. For NC and PC rats, ICP was performed on the 10th week of the experiments. All animals were anesthetized with Ketamine (100mg/kg) and Midazolam (5mg/kg) by intraperitoneal injection. A vertical midline incision was made over the pelvis; the bilateral MPG and CN were identified. The corpus cavernosum was punctured by a 25-gauge needle connected to the pressure transducer (Utah Medical Products®, Midvale, UT). Electrostimulation of the cavernous nerves was performed with the following parameters: 1.5mA, 20Hz, 0.2ms pulse width, 50 seconds duration. Three maximal increases of ICP for each side and average ICP (mean of all stimulations) for each rat were calculated. Mean arterial pressure (MAP) was assessed by puncture of the abdominal aorta after completion of the ICP study. Two ICP-derived parameters were analyzed: the ratio of ICP increase to the MAP (ICP/MAP) and area under ICP curve to the MAP (AUC/MAP) during the 50-second stimulation. The rats were sacrificed by isoflurane overdose and bilateral thoracotomy after ICP for tissue harvest.
Immunofluorescence
All tissue specimens were immersed in 2% formaldehyde and 0.002% picric acid fixation solution followed by 30% sucrose solution for fixation and dehydration. The tissues were subsequently frozen into optimal cutting temperature compound (OCT, Sakura Finetek, Torrance, CA) and sliced into 5 µm-thickness sections. The tissue sections were incubated with primary antibodies: anti-neuronal nitric oxide synthase (nNOS) (Santa Cruz Biotechnologies, Santa Cruz, CA), anti-neurofilament (NF, Abcam, Cambridge, MA) antibody, and anti-s100 antibody (Abcam, Cambridge, MA). Alexa-488 conjugate antibody (Invitrogen, Waltham, MA) was used for secondary antibody staining. Alexa-488 or 594 conjugated phalloidin (Pha, Invitrogen, Waltham, MA) was used for vessel expression. Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, Invitrogen, Waltham, MA).
3-Dimensional Imaging of Solvent Cleared Organs (3DISCO)
MPG and CN were harvested from 3 randomly selected rats of each group for 3DISCO. After fixation and dehydration, the tissues were immersed in Triton X-100 solution for 48 hours. Primary and secondary antibody staining was performed with Triton X-100 solution mixed with 5% fetal bovine serum and 2% bovine serum albumin. The primary antibodies for 3DISCO were as follows: anti-neurofilament and anti-s100 antibody. Alexa-488 conjugate antibody was used for secondary antibody staining. For the tissue clearing steps of 3DISCO, the tissues were immersed, stirred, and rotated in tetrahydrofuran solutions18, and then shifted to dichloromethane and dibenzyl ether solutions. Specimens were placed in a pool of dibenzyl ether on a glass side with dental cement borders. The specimens were then examined with Nikon A1R multi-photon and laser scanning confocal microscope (Nikon Instruments®, Melville, NY).
Image and statistical analysis
3DISCO images were analyzed with Imaris™ (Bitplane, Concord, MA). Immunofluorescence staining was viewed with Nikon Eclipse 80i fluorescent microscope and Retiga-Q image digital camera (Nikon Instruments Inc, Melville, NY). The number of dorsal penile vessels was counted in five 40x power magnification. Quantification of nNOS or S100 in penile dorsal nerve (PDN) was evaluated by Image-Pro Plus® (Media Cybernetics, Rockville, MD) and the ratio of nNOS or S100 to total area of PDN was calculated. A single investigator blinded to group allocation did all the images quantitative assessments. Data are presented as mean ± standard error of the mean. T-test was used for pairwise comparisons and one-way analysis of variance with Turkey-Kramer test for post-hoc comparisons was used for comparison of multiple groups. Significance was set at p<0.05 for all variables. All analyses were conducted using Prism 5® (GraphPad Software Inc, LaJolla, CA).
RESULTS
Li-ESWT Ameliorates Impaired Penile Hemodynamics after PNVI
ICP testing results for all groups is presented in figure 2. Mean ICP/MAP was 0.37 ± 0.03 in PC animals compared to 0.93 ± 0.03 in NC animals (p=0.001). Similarly, AUC/MAP was 13.4 ± 1.7 in PC animals versus 35.2 ± 2.1 in NC animals (p=0.001). The PVL and PL groups had significantly higher ICP/MAP (0.66 ± 0.07 and 0.82 ± 0.05, p=0.002 and 0.001, respectively) and AUC/MAP (24.6 ± 2.4 and 29.9 ± 2.2, p=0.005 and 0.001, respectively) compared to the PC group. The difference in ICP/MAP and AUC/MAP between the PVL and PL groups did not attain statistical significance (p>0.05). However, ICP/MAP and AUC/MAP were significantly higher (P=0.007 and 0.009, respectively) in the NC group compared to the PVL group, a relationship not noted when comparing the NC and PL groups.
Figure 2.
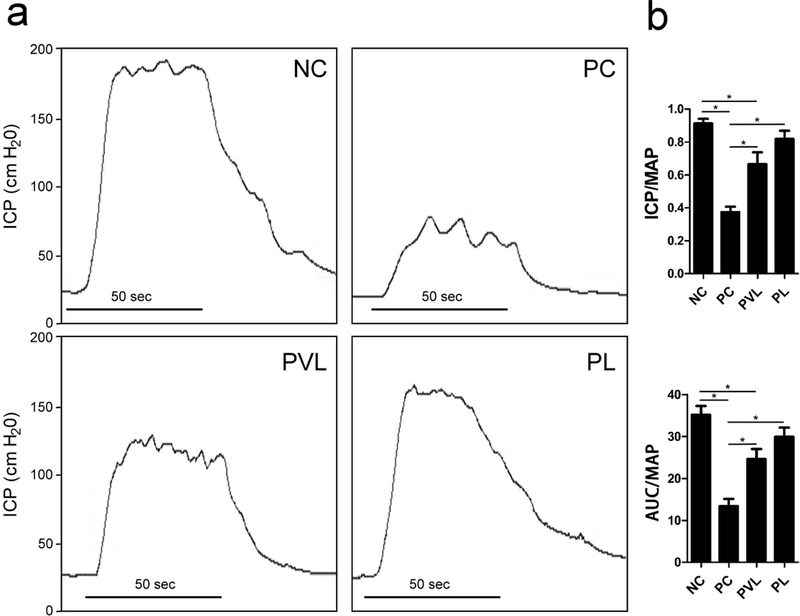
Representative tracing of ICP and mean ICP/MAP and AUC/MAP for each group. The PC group had significantly lower ICP/MAP and AUC/MAP compared to the NC group. Both Li-ESWT groups had significantly higher ICP/MAP and AUC/MAP compared to PC group. *: significant difference at P<0.05. Data are expressed as mean ± standard error of mean. n=7 for each group.
Detailed Microstructure and Nerve Regeneration Assayed with 3DISCO
To explore the 3-dimensional structure changes corresponding to delayed Li-ESWT after PNVI, 3DISCO for CN and MPG was performed. Representative 3DISCO images for each group are presented in Figure 3. In the PC group, atrophied CN and sparse nerve filaments with decreased immunofluorescent signal expression were noted. The PVL and PL groups had thicker nerve bundles with high immunofluorescent signal expression and numerous nerve fibers gathering along CN in the proximal end of nerve crush site. Detailed microstructures of the MPG were noted; marked diversity was noted across different animals.
Figure 3.
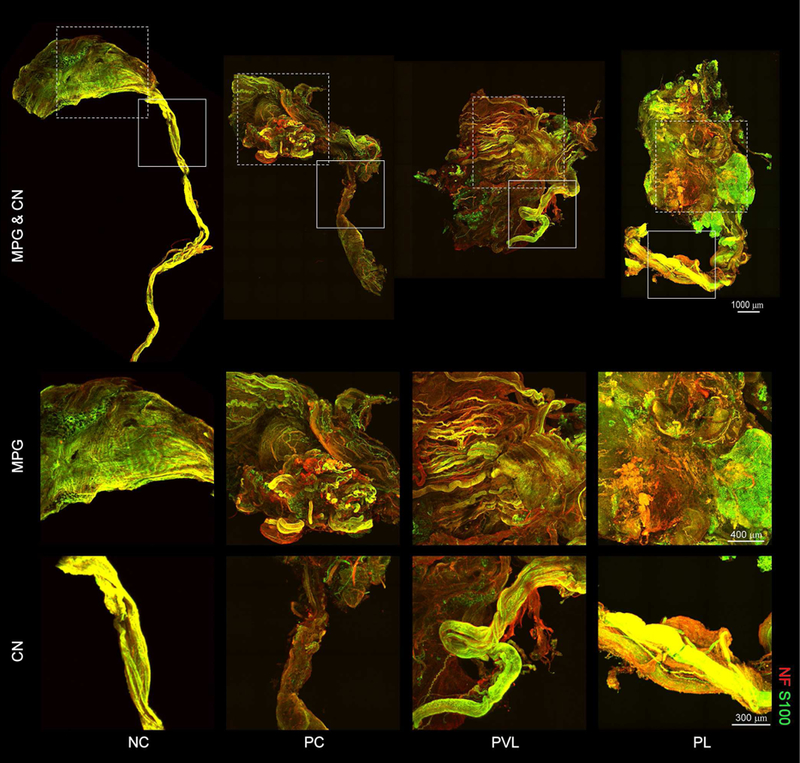
Representative sections of CN, MPG, and CN visualized with 3DISCO for each group. Detailed microstructures and trajectories of the nerve filaments(NF, red) and Schwann cells (S-100, green) were identified clearly. The PC group demonstrated atrophied CN and sparse nerve filaments with decreased immunofluorescence signal expression. Animals treated with Li-ESWT had thick nerve bundles with high immunofluorescence signal expression and numerous nerve fibers within the CN indicative of nerve regeneration. Rectangle regions for magnified images for MPG and CN are shown in the lower 2 rows. n=3 for each group.
Li-ESWT is associated with preservation of vascular integrity after PNVI
Dorsal penile vessel count for all groups is presented in figure 4. The PC group had lower dorsal penile vessel count compared to the NC group but the difference did not attain statistical significance (6.7 ± 1.0 vs 8.8 ± 1.1, respectively, P>0.05). The PVL and PL groups had higher dorsal penile vessel counts (9.6 ± 1.5 and 11.8 ± 1.1, respectively) compared to both the PC and NC groups. The difference was statistically significant only when comparing the PL and PC groups (p=0.024).
Figure 4.
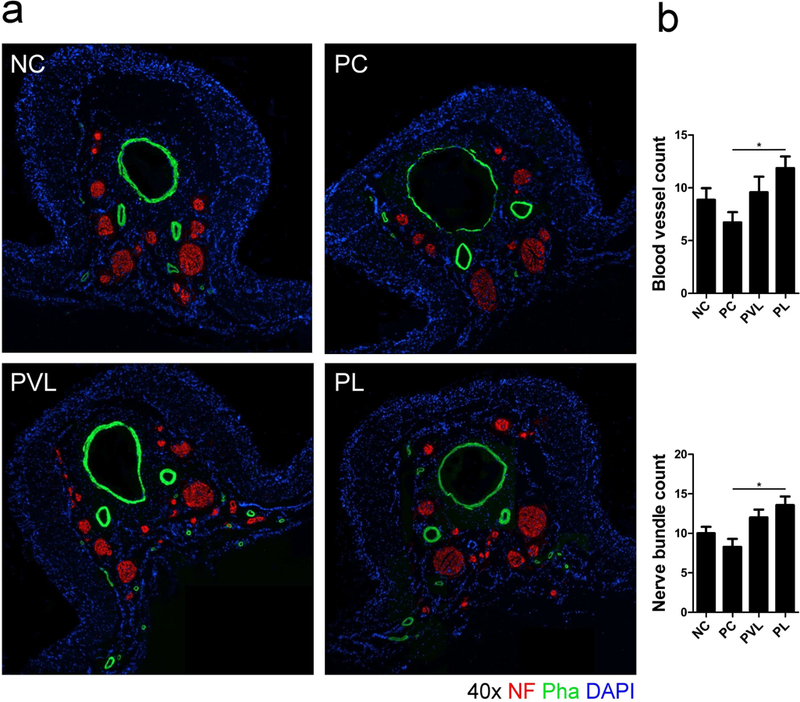
Representative images of nerves (red, NF) and vessels (green, Pha) in the dorsal penis of each group. The PC group had lower dorsal penile vessel counts. Animals treated with Li-ESWT had higher counts of blood vessels. Similarly, the PC group had lower nerve bundle count compared to the NC group. Animals treated with Li-ESWT had higher nerve bundle count compared to PC animals; this difference was statistically significant only for the PL group. *: significant difference at P<0.05. Data are expressed as mean ± standard error of mean. n=7 for each group.
Li-ESWT is associated with preservation of neuronal staining in PNVI
Serial immunofluorescence for NF, S100, and nNOS revealed the impact of delayed-stage PNVI and Li-ESWT on the nerve. Nerve bundle count (red) in the dorsal penile region between groups is presented in Figure 4. The PC group had lower mean dorsal penile nerve count compared to the NC group but the difference did not attain statistical significance (8.2 ± 1.0 vs 10.0 ± 0.8, respectively, P>0.05). Mean nerve bundle count was 12.0 ± 1.0 for the PVL group and 13.5 ± 1.1 for the PL group; the difference was statistically significant when comparing the PL and PC group. (P=0.005 ). The proportion of nNOS-positive nerves (green) in the dorsal penile nerve is presented in figure 5. The PC group had significantly lower mean nNOS-positive nerves compared to the NC group (2.6% ± 0.4% vs 4.5% ± 0.5%, respectively, P=0.009). Mean proportion of nNOS fibers was 3.4 ± 0.4% for the PVL group and 4.2 ± 0.4% for the PL group; the difference was statistically significant when comparing the PL and PC groups (P=0.043). The proportion of axonal (NF, red) and Schwann cells (S100, green) in the dorsal penile nerve for each group is presented in Figure 6. Compared to the NC group, the PC group had significantly lower mean axonal (8.6 ± 1.0 % vs 14.9 ± 1.5% for PC and NC, respectively, p=0.008) and Schwann cell content (6.2 ± 1.2 % vs 11.1 ± 1.0% for PC and NC, respectively, p=0.013). Animals treated with Li-ESWT had higher proportion of axonal (12.2 ± 0.9% and 14.2 ± 1.5% for the PVL and PL group, respectively) and Schwann cell (8.9 ± 1.1% and 10.3 ± 0.8% for the PVL and PL group, respectively) content in the dorsal nerve after Li-ESWT compared to the PC group; this difference attained statistical significance only for the PL group (p=0.022 and 0.049 for axonal and Schwann cell content, respectively).
Figure 5.
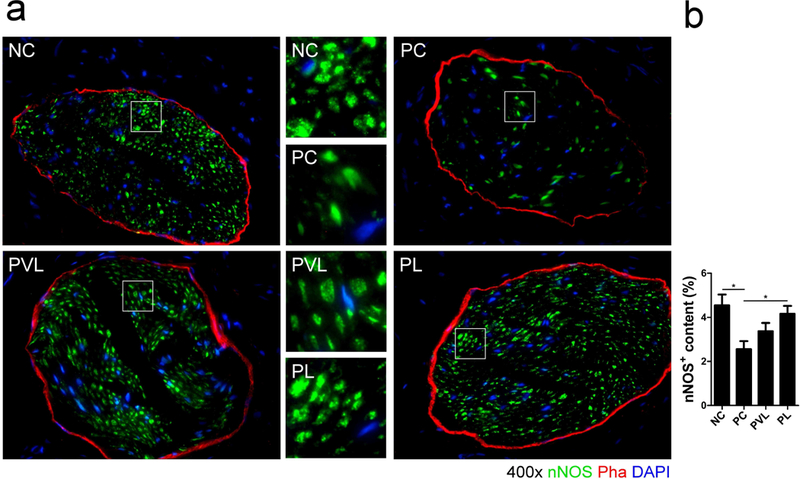
Representative images of nNOS-positive nerves (green) in the dorsal penile nerve for each group. The PC group had significantly lower nNOS-positive nerve content in the dorsal penile nerve compared with the NC group. Animals treated with Li-ESWT had higher expression of nNOS-positive nerves compared to PC animals; this difference was statistically significant only for the PL group. significant difference at P<0.05. Data are expressed as mean ± standard error of mean. n=7 for each group.
Figure 6.
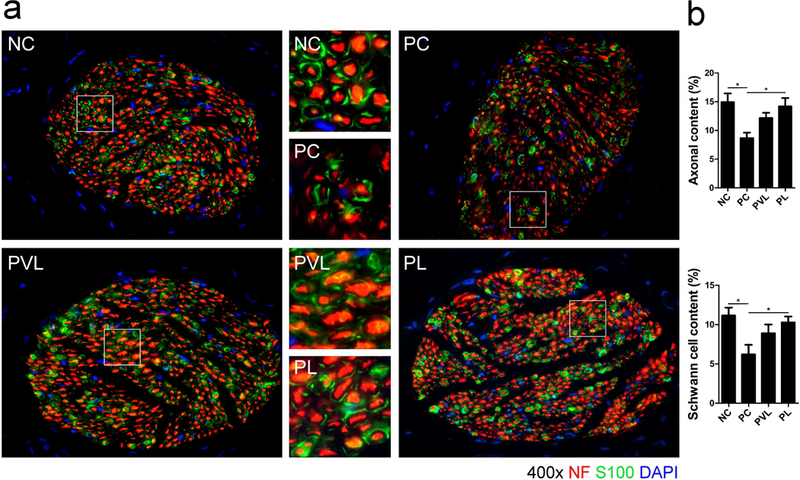
Representative images of Neurofilament (NF, red) and Schwann cell (S100, green) in the dorsal penile nerve for each group. The PC group had significantly decreased neurofilaments and Schwann cell content in the dorsal penile nerve compared with the NC group. Animals treated with Li-ESWT had higher neurofilament and Schwann cell content compared to PC animals; this difference was statistically significant only for the PL group. *: significant difference at P<0.05. Data are expressed as mean ± standard error of mean. n=7 for each group.
DISCUSSION
In the current study, we demonstrated that Li-ESWT ameliorated impairments in penile hemodynamics in an animal model of a severe ED related to pelvic nerve and vessel injury. Immunofluorescence and 3DISCO demonstrated substantially more robust neuronal and vascular tissues in Li-ESWT treated animals, suggesting that preservation or restoration of these structures drives the improvements in penile hemodynamics observed in vivo.
Previous studies have demonstrated the potential mechanisms of Li-ESWT in preserving neuronal and vascular function in various animal models of human conditions. Specifically, Li-ESWT has been shown to induce VEGF-release, suppress apoptosis, and minimize axonal damage after the nerve injury19; promote nerve innervation in both bladder20 and skin21; induce expression of growth associated potein-43 (GAP-43) and activating transcription factor 3(ATF3), both of which promote neurite sprouting from injured ganglia22; activate and induce proliferation of Schwann cells, which are important for guidance of regenerating nerve fibers23. The mechanisms of Li-ESWT mediated Schwann cell activation and proliferation seem to involve p-Erk1/2, p75, brain-derived neurotrophic factor (BDNF), the protein kinase RNA-like endoplasmic reticulum kinase (PERK), and the transcription factor 4 (ATF4) pathway24.
In addition to demonstrating the general efficacy of Li-ESWT therapy, our study investigated timing, treatment focus, and treatment intensity, three issues that are germane to the development of human trials of Li-ESWT for ED. There are basic science data to support the notion of early initiation of penile rehabilitative therapy within a short interval of severe pelvic injury or radical pelvic surgery14. However, many men are more concerned with their injury or cancer prognosis, recovery from surgery, and restoration of urinary continence during the early phases of their recovery; hence some men do not use treatments for erection preservation during this early window. For this reason (among others) many men with ED related to pelvic injury or radical pelvic surgery present later in the course of their disease process. Our study suggests that even delayed intervention with Li-ESWT can have a salubrious effect on erection recovery. In addition, since the treatment commenced 4 weeks after injury when nerve and tissue atrophy have already present14, the results imply that Li-ESWT contributes to the regeneration of the nerve, blood vessel and erectile tissues.
ED related to pelvic injury or surgery may be due to both neuronal and vascular injury with resulting fibrosis. In this study we directed Li-ESWT to both the penis and pelvis. Our positive outcomes, linked with the histological data from the MPG and the penis itself, suggest that a combined approach of treating both neuronal and vascular disease may be most fruitful.
Finally, we investigated two different intensities of Li-ESWT. Mean hemodynamic and immunofluoroscence parameters were superior in both Li-ESWT treated groups compared to controls but the differences were only universally significant for the PL group. The implication is that the PL treatment protocol is likely superior; whether even higher treatment doses would produce additional benefit is unclear. It is likely that beyond a certain point the benefit of additional energy application may be negligible or even harmful.
Prior work has demonstrated that Li-ESWT increases vascular endothelial growth factor (VEGF) and endothelial nitric oxide synthase (eNOS) expression and enhances neovascularization25. It is thought that these mechanism mediate the angiogenic effects of Li-ESWT. Li-EWST has also been applied as a management strategy for ischemic heart disease26, intermittent claudication27, and graft survival28.
Our current model for nerve injury was a particularly severe phenotype involving injury to both the CN and the pudendal neurovascular bundle. Despite this severe phenotype, at least partial preservation penile hemodynamics was noted in injured rodents treated with Li-ESWT. These superior functional results were associated with improved neuronal integrity in terms of both location (i.e. MPG and dorsal penile nerves) and specific nervous tissue cell types (i.e. nNOS positivity and Schwann cell density).
To our knowledge, current work is the first investigation of 3-dimensional nerve mapping of the CN and MPG in the context of PNVI and Li-ESWT. 3DISCO is a novel imaging technology for transparent unsectioned tissues and provides excellent 3-dimensional image quality in high-power magnification15. Proper utilization of 3DISCO requires careful tissue clearance of tissues and high powered image analysis software.29 The process is very time and labor intensive, requiring between 4–8 hours per sample and producing image files on the order of 10–20 gigabytes each. For this reason, 3DISCO is a technology that should be used sparingly in contexts where 3-dimensional information is required. Additional limitations of 3DISCO include tissue shrinkage during processing and variable efficiency in different tissue types15.
Our study was not without limitations. As all animals were subjected to both pudendal and CN injury it is not possible to elucidate the relative contribution of each specific injury to the overall phenotype. Similarly, administration of Li-ESWT to both injury sites makes it difficult to assign the relative benefit of each individual Li-ESWT application. We did not assess the role of local progenitor cells; these cells have been shown to activate in response to Li-ESWT30 and may have been responsible for some of our observations. We determined that the PL was superior to PVL in terms of functional and histological outcomes. Whether PL represents the optimal dosing is unclear at this time; additional studies with higher energy transfer will be required.
CONCLUSIONS
Delayed application of Li-ESWT to the penis and pelvis was associated with restoration of penile hemodynamics in a rodent model of severe pelvic neurovascular injury. These effects appear to be mediated by Li-ESWT-enhanced restoration of angiogenesis, activation of Schwann cell, and facilitation of nerve regeneration. 3DISCO provided high-resolution images of unsectioned CN and MPG and demonstrated superior neuronal integrity in animals treated with Li-ESWT. Further explorations of Li-ESWT for ED and 3DISCO for assessment of microstructural changes are warranted.
Acknowledgments:
Research reported in this publication was supported by Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award number W81XWH-13–2-0052, and NIDDK of the National Institutes of Health under award number 1R01DK105097–01A1. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702–5014 is the awarding and administering acquisition office. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151: 54–61. [DOI] [PubMed] [Google Scholar]
- 2.Shamloul R, Ghanem H. Erectile dysfunction. Lancet 2013;381: 153–65. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano F, Amar E, Chevallier D, et al. How urologists manage erectile dysfunction after radical prostatectomy: a national survey (REPAIR) by the French urological association. J Sex Med 2008;5: 448–57. [DOI] [PubMed] [Google Scholar]
- 4.Tsertsvadze A, Fink HA, Yazdi F, et al. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: a systematic review and meta-analysis. Ann Intern Med 2009;151: 650–61. [DOI] [PubMed] [Google Scholar]
- 5.Corona G, Rastrelli G, Morgentaler A, et al. Meta-analysis of Results of Testosterone Therapy on Sexual Function Based on International Index of Erectile Function Scores. Eur Urol 2017;72: 1000–11. [DOI] [PubMed] [Google Scholar]
- 6.Linet OI, Ogrinc FG. Efficacy and safety of intracavernosal alprostadil in men with erectile dysfunction. The Alprostadil Study Group. N Engl J Med 1996;334: 873–7. [DOI] [PubMed] [Google Scholar]
- 7.Anastasiadis AG, Wilson SK, Burchardt M, et al. Long-term outcomes of inflatable penile implants: reliability, patient satisfaction and complication management. Curr Opin Urol 2001;11: 619–23. [DOI] [PubMed] [Google Scholar]
- 8.Mani-Babu S, Morrissey D, Waugh C, et al. The effectiveness of extracorporeal shock wave therapy in lower limb tendinopathy: a systematic review. Am J Sports Med 2015;43: 752–61. [DOI] [PubMed] [Google Scholar]
- 9.Al-Abbad H, Simon JV. The effectiveness of extracorporeal shock wave therapy on chronic achilles tendinopathy: a systematic review. Foot Ankle Int 2013;34: 33–41. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann R, Cumpanas A, Hoeltl L, et al. Extracorporeal shock-wave therapy for treating chronic pelvic pain syndrome: a feasibility study and the first clinical results. BJU Int 2008;102: 976–80. [DOI] [PubMed] [Google Scholar]
- 11.Qiu X, Lin G, Xin Z, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med 2013;10: 738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardi Y, Appel B, Kilchevsky A, et al. Does low intensity extracorporeal shock wave therapy have a physiological effect on erectile function? Short-term results of a randomized, double-blind, sham controlled study. J Urol 2012;187: 1769–75. [DOI] [PubMed] [Google Scholar]
- 13.Lu Z, Lin G, Reed-Maldonado A, et al. Low-intensity Extracorporeal Shock Wave Treatment Improves Erectile Function: A Systematic Review and Meta-analysis. Eur Urol 2017;71: 223–33. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Matheu MP, Sun F, et al. Low-energy Shock Wave Therapy Ameliorates Erectile Dysfunction in a Pelvic Neurovascular Injuries Rat Model. J Sex Med 2016;13: 22–32. [DOI] [PubMed] [Google Scholar]
- 15.Erturk A, Bradke F. High-resolution imaging of entire organs by 3-dimensional imaging of solvent cleared organs (3DISCO). Exp Neurol 2013;242: 57–64. [DOI] [PubMed] [Google Scholar]
- 16.Erturk A, Mauch CP, Hellal F, et al. Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury. Nat Med 2011;18: 166–71. [DOI] [PubMed] [Google Scholar]
- 17.Qiu X, Fandel TM, Ferretti L, et al. Both immediate and delayed intracavernous injection of autologous adipose-derived stromal vascular fraction enhances recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol 2012;62: 720–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erturk A, Lafkas D, Chalouni C. Imaging cleared intact biological systems at a cellular level by 3DISCO. J Vis Exp 2014. [DOI] [PMC free article] [PubMed]
- 19.Yahata K, Kanno H, Ozawa H, et al. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J Neurosurg Spine 2016;25: 745–55. [DOI] [PubMed] [Google Scholar]
- 20.Wang HS, Oh BS, Wang B, et al. Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates Diabetic Underactive Bladder in Streptozocin-Induced Diabetic Rats. BJU Int 2018. [DOI] [PMC free article] [PubMed]
- 21.Ohtori S, Inoue G, Mannoji C, et al. Shock wave application to rat skin induces degeneration and reinnervation of sensory nerve fibres. Neurosci Lett 2001;315: 57–60. [DOI] [PubMed] [Google Scholar]
- 22.Murata R, Ohtori S, Ochiai N, et al. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci 2006;128: 96–100. [DOI] [PubMed] [Google Scholar]
- 23.Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia 2008;56: 1518–31. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Ning H, Reed-Maldonado AB, et al. Low-Intensity Extracorporeal Shock Wave Therapy Enhances Brain-Derived Neurotrophic Factor Expression through PERK/ATF4 Signaling Pathway. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed]
- 25.Yamaya S, Ozawa H, Kanno H, et al. Low-energy extracorporeal shock wave therapy promotes vascular endothelial growth factor expression and improves locomotor recovery after spinal cord injury. J Neurosurg 2014;121: 1514–25. [DOI] [PubMed] [Google Scholar]
- 26.Myojo M, Ando J, Uehara M, et al. Feasibility of Extracorporeal Shock Wave Myocardial Revascularization Therapy for Post-Acute Myocardial Infarction Patients and Refractory Angina Pectoris Patients. Int Heart J 2017;58: 185–90. [DOI] [PubMed] [Google Scholar]
- 27.Harwood AE, Green J, Cayton T, et al. A feasibility double-blind randomized placebo-controlled trial of extracorporeal shockwave therapy as a novel treatment for intermittent claudication. J Vasc Surg 2018;67: 514–21 e2. [DOI] [PubMed] [Google Scholar]
- 28.Mittermayr R, Hartinger J, Antonic V, et al. Extracorporeal shock wave therapy (ESWT) minimizes ischemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Ann Surg 2011;253: 1024–32. [DOI] [PubMed] [Google Scholar]
- 29.Vigouroux RJ, Belle M, Chedotal A . Neuroscience in the third dimension: shedding new light on the brain with tissue clearing. Mol Brain 2017;10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin G, Reed-Maldonado AB, Wang B, et al. In Situ Activation of Penile Progenitor Cells With Low-Intensity Extracorporeal Shockwave Therapy. J Sex Med 2017;14: 493–501. [DOI] [PubMed] [Google Scholar]


