Abstract
Background.
Among kidney transplant recipients, gender differences in medication adherence may contribute to the higher graft failure risks observed in girls and young women compared with boys and young men. Our aim was to determine whether adherence differs by gender, and whether gender differences vary by age in adolescent and young adult kidney transplant recipients.
Methods.
We examined data from the 3-month run-in period (no intervention) of the randomized TAKE-IT trial. Adherence was monitored using electronic pillboxes in 136 patients (11–24 y) followed in 8 transplant centers in Canada and USA. We used ordinal logistic regression with generalized estimating equations to estimate the association between gender and each of daily taking (proportion of prescribed doses taken) and timing (proportion of prescribed doses taken on time) adherence, considering effect modification by age (11–16 vs. 17–24 years).
Results.
No difference in taking adherence was observed by gender among participants 11–16 years (OR=0.92 (95%CI 0.55–1.54)), whereas among participants 17–24 years, women had significantly greater odds of higher taking adherence scores (OR=3.03 (95%CI 1.20–7.66)) than men. Results were similar for timing adherence, with no difference among participants 11–16 years (OR=1.03 (95%CI 0.65–1.63)) but a greater odds of higher timing adherence scores in women than in men among participants 17–24 years (OR=3.26 (95%CI 1.43–7.45)). There were no differences in adherence assessed by self-report or SD of tacrolimus trough levels.
Conclusions.
Gender differences in adherence vary by age. Whereas younger adolescents show no adherence differences by gender, young women show much better adherence than young men.
1. INTRODUCTION
Among kidney transplant recipients, girls and young women have been shown to have a higher graft failure risk than boys and young men, whereas older women have a similar or lower risk compared with older men.1,2 The reasons for these relationships are not clear. Although sex hormones may play a role in this relationship, with estrogens enhancing and androgens suppressing immune activation3,4, another possibility is that medication adherence differs by gender, and that gender differences in adherence vary by age. Several prior studies of kidney transplant recipients reported associations between gender and medication adherence. However, none specifically addressed the question of whether medication adherence differs by gender. Furthermore, past studies were restricted to either children or to adults; no prior study focused on adolescents and young adults. Some studies of adult kidney transplant recipients showed better adherence in women than in men5–9, whereas others showed no difference by gender.10–13 In contrast, most studies of pediatric kidney transplant recipients showed no difference in adherence between boys and girls; this may reflect the fact that parents take responsibility for medication adherence in children.14–16 Adolescence and young adulthood may represent a period distinct from other ages with respect to gender differences in adherence. As young people take greater responsibility for their own care throughout adolescence, the differing rates of cognitive maturation in boys versus girls17,18 may influence gender differences in adherence.19
The primary aim of this study was to determine whether medication adherence, as measured using electronic monitoring, differs by gender among adolescent and young adult kidney transplant recipients, and whether gender differences vary by age. We hypothesized that adherence would be similar for boys and girls 11–16 years old, but that women would have higher adherence than men among those 17–24 years. We also considered gender differences in medication adherence measured by self-report and by standard deviation of tacrolimus trough levels. We expected that gender differences in adherence would be similar regardless of the method used to assess adherence.
2. MATERIALS AND METHODS
This study is a secondary analysis of data from the Teen Adherence in Kidney transplant Effectiveness of Intervention Trial (TAKE-IT)20,21, which was a multicenter, prospective randomized trial of an adherence-promoting intervention for adolescent and young adult kidney transplant recipients. The present study is an observational cohort study, focusing exclusively on the 3-month run-in period between the enrollment visit and initiation of the intervention. Neither study personnel nor participants were aware of group assignment (intervention or control) during the run-in. The intervention interval was not included in this study because the impact of the intervention on adherence may have differed by gender, and we had insufficient numbers of participants to consider a gender by age by intervention interaction.
TAKE-IT was approved by the Research Ethics Boards of all sites. Written informed consent was obtained from all participants and parents (for those <18y).
2.1. Study population
Prevalent kidney-only transplant recipients 11 to 24 years old, who were ≥3 months posttransplant, had a functioning graft, and were expected to be followed in 1 of the 8 participating centers in Canada and USA for the 15-month study were eligible for TAKE-IT. Exclusion criteria included: impending graft failure, severe neurocognitive disabilities, lack of electronic pillbox connectivity, use of liquid immunosuppressive medications, having a sibling participating in the study, participating in another adherence-promoting intervention study, or inability to communicate comfortably in English (or French - Montreal site only).
Electronic adherence assessment:
All participants were given an electronic multidose pillbox in which to store all immunosuppressive medications. During the first 4–6 months of recruitment, participants received a Medminder pillbox (Medminder, Needham, MA). However, due to technical difficulties with this device for some participants, subsequent participants were given a SimpleMed device (Vaica Medical, Tel Aviv, Israel); the Medminder and SimpleMed were similar, with the same types of adherence tracking and reminder functions. The prescribed immunosuppressive medication dosing times were recorded in the web-based pillbox record for each participant. The date and time of each pillbox compartment opening was registered in the electronic record of the patient to whom the device was assigned. Participants were instructed to inform study staff if they were not using the pillbox for a period (ie, weekend or vacation travel).
Other adherence measures:
We also described tacrolimus trough levels variability and self-reported adherence. The standard deviations (SD) of all tacrolimus trough levels done for clinical care (except during hospitalizations or illnesses) during a 6-month period (including the 3 months before enrollment and the 3-month run-in period) were calculated for participants with ≥3 tacrolimus levels. Self-reported adherence was measured using the Medical Adherence Measure-Medication Module (MAM-MM)22,23 which is a semistructured interview, developed in children and adolescents with end stage kidney disease including kidney transplantation, and administered by a trained interviewer at the enrollment visit and at the end of the run-in. The MAM-MM captures adherence during the previous week.
2.2. Statistical analysis
Primary exposure:
Participant gender was the primary exposure. We considered the possibility that gender differences in adherence may be modified by age by including a gender by age interaction term.
Primary outcomes:
The primary outcomes were daily ‘taking adherence” (proportion of prescribed doses taken) and “timing adherence” (proportion of prescribed doses taken within 1 hour before to 2 hours after the prescribed dosing time), as measured using electronic monitoring. A taking adherence score and a timing adherence score were calculated for each day of the study for each participant. Therefore, each participant had repeated outcome measures. Each score was either 0%, 50%, or 100%, since immunosuppressives were prescribed a maximum of 2 times per day. No score was calculated for days that the pillbox was not in use (turned off, not communicating with the server, or participant-reported nonuse). The first 2 weeks of electronic adherence data following enrollment were deleted to allow adaptation to the pillbox.24
Association between gender and adherence:
We used ordinal logistic regression with generalized estimating equations (proportional odds model) to estimate the association between gender and each of taking and timing adherence. 21 This approach accounts for correlation between the repeated adherence scores for each day of observation within each participant. The assumption of proportional odds was assessed graphically. To consider effect modification by age, we included an age by gender interaction term. Age was categorized as 11–16 vs. 17–24 years based on assessment of plots of adherence versus age (in 1-year intervals) showing a clear gender difference starting at ~17 years of age. Figure S1 shows the results of a model including gender, age and the age by gender interaction in which age was treated as a continuous variable. This model showed odds ratios (OR) of smoothly increasing magnitude with increasing age in the comparison between girls/women and boys/men. However, the confidence intervals at older ages were very wide; therefore we opted to dichotomize age. To identify potential confounders in the association between gender and adherence, we assessed univariate associations between baseline participant characteristics and adherence (Table S1) as well as standardized differences25 in baseline participant characteristics by gender (and age category) (Table S2). Only those variables associated with both gender (the exposure) and adherence (the outcome) were considered potential confounders. Covariates were selected based on the magnitude of the associations with adherence and gender, rather than based on statistical significance of the associations. Given the small sample size, important potential confounders may not have a statistically significant association with the exposure or outcome. Covariates associated with adherence with an OR lower than 0.9 or greater than 1.1 and for which the absolute value of the standardized difference by gender was greater than 0.3 in at least 1 age category (ie, substantial imbalance by gender) were included in multivariate models. For highly correlated characteristics, we selected only 1 to avoid collinearity.
Secondary outcomes:
The SD of tacrolimus trough levels was determined for each participant with ≥3 levels available. Using the MAM-MM, self-reported taking adherence was scored as the proportion of prescribed doses taken in the previous week; self-report timing adherence was scored as the proportion of prescribed doses taken up to 2 hours after the prescribed time in the previous week. MAM-MM scores for each patient were summarized as the mean of the 2 scores determined during the run-in.
Analyses were conducted using SAS 9.4.
3. RESULTS
3.1. Participant characteristics
The characteristics of the 83 boys and men (61 11–16 years; 22 17–24 years at baseline) and 53 girls and women (35 11–16 years; 18 17–24 years at baseline) with electronic data available for analysis are summarized in Table 1. Both boys/men and girls/women were followed for a median of 2.5 months [male interquartile range (IQR) 1.8–2.7; female IQR 1.9–2.8] in the run-in (after excluding the first 2 weeks). Among the 136 participants, 68% were white. The median [IQR] time since transplant at baseline was 2.9 [0.8–7.1] years, with a median [IQR] age at transplant of 12.0 [8.3–14.9] years. 52% of participants had a living donor. Concerning treatment characteristics, 65% of participants received 3 immunosuppressive medications per day; 92% took 2 doses of immunosuppressives per day. Detailed participant characteristics by gender within each age categories are presented in Table 1.
Table 1.
Baseline characteristics of patients during the run-in period of the TAKE-IT trial (n=136)
| Variable | Age 11–16 y (n=96) | Age 17–24 y (n=40) | ||
|---|---|---|---|---|
| Boys (n=61) | Girls (n=35) | Men (n=22) | Women (n=18) | |
| Demographics | ||||
| Age (in years) | 14.3 [12.6–15.9] | 14.8 [12.9–16.2] | 18.7 [18.1–19.6] | 18.2 [17.8–19.0] |
| Race | ||||
| White | 33 (54.1) | 25 (71.4) | 19 (86.4) | 15 (83.3) |
| Black | 10 (16.4) | 5 (14.3) | 1 (4.5) | 1 (5.6) |
| Other | 18 (29.5) | 5 (14.3) | 2 (9.1) | 2 (11.1) |
| Hispanic or Latino | 5 (8.2) | 5 (14.3) | 1 (4.5) | 1 (5.6) |
| US Study site | 37 (60.7) | 21 (60.0) | 14 (63.6) | 17 (94.4) |
| Healthcare insurer | ||||
| U.S. Public | 16 (26.2) | 10 (28.6) | 8 (36.4) | 7 (38.8) |
| U.S. Private | 21 (34.4) | 11 (31.4) | 6 (27.2) | 10 (55.6) |
| Canadian provincial | 24 (39.3) | 14 (40.0) | 8 (36.4) | 1 (5.6) |
| Medication insurer | ||||
| U.S. Public | 17 (27.9) | 11 (31.4) | 7 (31.9) | 9 (50.0) |
| Private | 29 (47.5) | 15 (42.9) | 10 (45.4) | 8 (44.4) |
| Canadian provincial | 6 (9.8) | 6 (17.1) | 3 (13.6) | 1 (5.6) |
| Other | 9 (14.8) | 3 (8.6) | 2 (9.1) | 0 (0.0) |
| Household income per year | ||||
| Less than $50 000 | 23 (37.7) | 14 (40.0) | 10 (45.4) | 10 (55.6) |
| Greater than $50 000 | 30 (49.2) | 19 (54.3) | 8 (36.4) | 5 (27.7) |
| Unknown/Prefer not to answer | 8 (13.1) | 2 (5.7) | 4 (18.2) | 3 (16.7) |
| Disease characteristics | ||||
| Years posttransplant | 2.5 [0.6–6.7] | 2.6 [0.8–7.7] | 4.3 [1.7–7.5] | 3.9 [0.8–6.0] |
| Number of prior transplants (including current one) | ||||
| 1 | 55 (90.2) | 31 (88.6) | 19 (86.4) | 18 (100.0) |
| 2 | 6 (9.8) | 4 (11.4) | 3 (13.6) | 0 (0.0) |
| Donor source | ||||
| Living | 34 (55.7) | 19 (54.3) | 11 (50.0) | 7 (38.8) |
| Deceased | 27 (44.3) | 16 (45.7) | 11 (50.0) | 11 (61.2) |
| Duration of dialysis before current transplant | ||||
| 0 month | 18 (29.5) | 8 (22.9) | 5 (22.7) | 8 (44.4) |
| > 0 month | 43 (70.5) | 27 (77.1) | 17 (77.3) | 10 (55.6) |
| Total lifetime duration of dialysis | ||||
| 0 month | 17 (27.9) | 8 (22.9) | 4 (18.2) | 8 (44.4) |
| > 0 month | 44 (72.1) | 27 (77.1) | 18 (81.8) | 10 (55.6) |
| Age at transplant (in years) | 11.6 [7.0–13.5] | 11.0 [8.2–12.8] | 13.9 [10.2–17.8] | 14.9 [12.5–17.0] |
| Primary disease | ||||
| CAKUT | 26 (42.6) | 12 (34.2) | 11 (50.0) | 6 (33.3) |
| Glomerulonephritis | 6 (9.8) | 5 (14.3) | 1 (4.5) | 1 (5.6) |
| FSGS | 7 (11.5) | 3 (8.6) | 2 (9.1) | 2 (11.1) |
| Other | 22 (36.1) | 15 (42.9) | 8 (36.4) | 9 (50.0) |
| Number of past acute rejections | ||||
| 0 | 51 (83.6) | 28 (80.0) | 18 (81.8) | 12 (66.7) |
| ≥ 1 | 10 (16.4) | 7 (20.0) | 4 (18.2) | 6 (33.3) |
| Comorbidities | ||||
| None | 32 (52.5) | 19 (54.3) | 10 (45.5) | 9 (50.0) |
| ≥ 1 | 29 (47.5) | 16 (45.7) | 12 (54.5) | 9 (50.0) |
| Treatment characteristics | ||||
| Number of immunosuppressive medications | ||||
| 1 | 2 (3.3) | 1 (2.9) | 1 (4.5) | 0 (0.0) |
| 2 | 13 (21.3) | 10 (28.6) | 10 (45.5) | 10 (55.6) |
| 3 | 46 (75.4) | 24 (68.5) | 11 (50.0) | 8 (44.4) |
| Number of doses of immunosuppressive per day | 3 (4.9) | 2 (5.7) | 3 (13.6) | 3 (16.7) |
| 1 | 3 (4.9) | 2 (5.7) | 3 (13.6) | 3 (16.7) |
| 2 | 58 (95.1) | 33 (94.3) | 19 (86.4) | 15 (83.3) |
| Total number of medications | 7.0 [5.0–8.0] | 8.0 [5.0–10.0] | 7.5 [5.3–8.8] | 7.5 [6.0–9.0] |
| Total number of doses per day | 0 (0.0) | 1 (2.9) | 2 (9.1) | 1 (5.6) |
| 1 | 0 (0.0) | 1 (2.9) | 2 (9.1) | 1 (5.6) |
| 2 | 57 (93.5) | 32 (91.3) | 16 (72.7) | 15 (83.3) |
| 3 or 4 | 4 (6.5) | 2 (5.8) | 4 (18.2) | 2 (11.1) |
In columns, data given as median [interquartile range] or number (%)
3.2. Association between gender and adherence
Among participants 11–16 years, the median [IQR] overall taking adherence during the run-in was 85.6% [75.7–95.5] in girls and 88.3% [71.6–95.7] in boys; the median [IQR] overall timing adherence was 81.2% [69.1–92.0] in girls and 81.0% [62.5–92.7] in boys. Among those 17–24 years, overall taking adherence was 91.6% [76.0–95.7] in women and 70.0% [56.3–88.3] in men; the median [IQR] overall timing adherence was 87.7% [63.6–91.3] in women and 59.1% [49.5–79.4] in men. Figure 1 shows the proportion of boys/men and girls/women with 100% taking adherence on each day of follow-up, stratified by age category. Figure 2 shows the proportion of boys/men and girls/women with 100% timing adherence on each day of follow-up, stratified by age.
Figure 1. Comparison of taking adherence between males and females during the run-in period of the TAKE-IT trial, according to age (n=136).

The proportion of males (triangles) and females (circles) with 100% taking adherence on each day of observation are shown (limited to 100 days after first day of observation).
Figure 2. Comparison of timing adherence between males and females during the run-in period of the TAKE-IT trial, according to age (n=136).
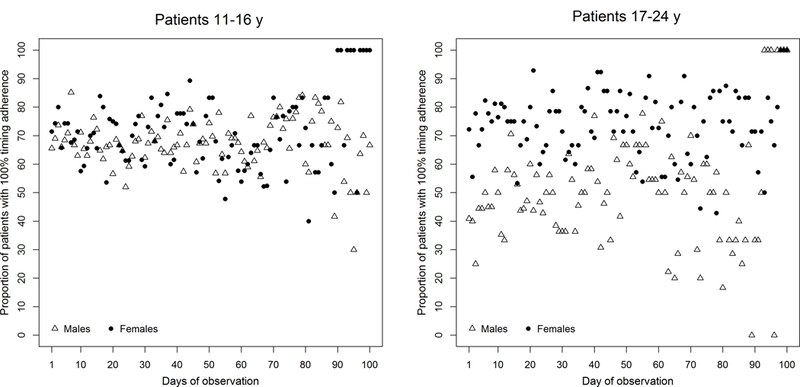
The proportion of males (triangles) and females (circles) with 100% timing adherence on each day of observation are shown (limited to 100 days after first day of observation).
Table 2 shows the results of the ordinal logistic regression models used to estimate the association between gender and each of taking and timing adherence, accounting for age. Race, healthcare insurer, dialysis duration, primary disease and number of immunosuppressive medications were identified as potential confounders. We did not adjust for number of prior transplants or number of past acute rejections because both may be markers of poor adherence. Due to the small numbers of patients, it was not possible to include all variables in a single multivariate model. Therefore, we present 3 models with different combinations of variables. Results were similar across all models. There were significant interactions between gender and age in both the taking (Model 2 p=0.019) and timing (Model 2 p=0.021) adherence models. Among participants 11–16 years, there were no significant gender differences in taking or timing adherence. In contrast, among those 17–24 years, women had significantly greater odds of higher taking adherence scores (Model 2 OR=3.06 (95%CI 1.35–6.95)) than men. Women 17–24 years also had significantly greater odds of higher timing adherence scores (Model 2 OR=3.06 (95%CI 1.48–6.35)) than men of the same age.
Table 2.
Results of ordinal logistic regressions with taking and timing adherence scores as outcomes - Odds ratio associated with gender (run-in period of the TAKE-IT trial, n=136)
| Taking adherence | Timing adherence | |||
|---|---|---|---|---|
| Age 11–16y Odds ratio (95%CI) |
Age 17–24y Odds ratio (95%CI) |
Age 11–16y Odds ratio (95%CI) |
Age 17–24y Odds ratio (95%CI) |
|
| Model 1 | ||||
| Girls/women (vs. boys/men) | 1.02 (0.60–1.71) | 2.87 (1.34–6.16) | 1.12 (0.71–1.77) | 2.92 (1.47–5.81) |
| Model 2 | ||||
| Girls/women (vs. boys/men) | 0.97 (0.61–1.56) | 3.06 (1.35–6.95) | 1.12 (0.74–1.70) | 3.06 (1.48–6.35) |
| Model 3 | ||||
| Girls/women (vs. boys/men) | 0.88 (0.51–1.51) | 2.53 (1.15–5.59) | 1.00 (0.63–1.61) | 2.60 (1.31–5.18) |
Model 1: unadjusted model including only gender, age, and a gender by age interaction term
Model 2: Model 1 + race + healthcare insurer + dialysis duration
Model 3: Model 1 + primary disease + number of immunosuppressive medications
An OR < 1 means that odds of higher taking or timing adherence scores are lower for girls/women compared to boys/men.
When we compared adherence by age category, stratified on gender, we found that, compared to boys 11–16 years, men 17–24 years had significantly lower odds of taking prescribed medication on time (Model 2 OR=0.46 (95%CI 0.27–0.78)). Results were similar but not significant for taking adherence. Girls/women showed no significant difference by age category for either taking or timing adherence.
In the multivariate models, only race and dialysis duration were significantly associated with taking and timing adherence (Table 3). Black participants had lower taking (OR=0.48 (95%CI 0.28–0.95)) and timing (OR=0.51 (95%CI 0.31–0.84)) adherence scores compared to white participants. Participants without dialysis before their current transplant had higher taking (OR=1.71 (95%CI 1.09–2.66)) and timing (OR=1.80 (95%CI 1.21–2.68)) adherence scores compared to participants with dialysis.
Table 3.
Results of ordinal logistic regressions with taking and timing adherence scores as outcomes – Odds ratio associated with covariates in multivariate models (run-in period of the TAKE-IT trial, n=136)
| Taking adherence | Timing adherence | |
|---|---|---|
| Odds ratio (95%CI) | Odds ratio (95%CI) | |
| Demographics | ||
| Race | ||
| White (ref.) | - | - |
| Black | 0.48 (0.28–0.85) | 0.51 (0.31–0.84) |
| Other | 0.82 (0.47–1.42) | 0.96 (0.59–1.56) |
| Healthcare insurer | ||
| U.S. Public (ref.) | - | - |
| U.S. Private | 0.91 (0.55–1.49) | 0.99 (0.65–1.50) |
| Canadian provincial | 1.23 (0.66–2.27) | 1.34 (0.80–2.25) |
| Disease characteristics | ||
| Duration of dialysis before current transplant | ||
| 0 month | 1.71 (1.09–2.66) | 1.80 (1.21–2.68) |
| > 0 month (ref.) | - | - |
| Primary disease | ||
| CAKUT (ref.) | - | - |
| Glomerulonephritis | 1.96 (0.97–3.99) | 1.28 (0.69–2.38) |
| FSGS | 0.67 (0.38–1.16) | 0.69 (0.42–1.15) |
| Other | 1.04 (0.59–1.84) | 0.99 (0.61–1.61) |
| Treatment characteristics | ||
| Number of immunosuppressive medications | ||
| 1 | 1.83 (0.79–4.25) | 1.34 (0.35–5.15) |
| 2 | 0.84 (0.53–1.31) | 0.82 (0.55–1.22) |
| 3 (ref.) | - | - |
An OR < 1 means that odds of higher taking or timing adherence scores are lower for the compared group compared to the reference.
To consider a potential confounding effect of participants living with versus without a parent on the relationship between gender and adherence, we repeated the analysis on a sample restricted to the 127 participants not living on their own (Table S3). Among participants 11–16 years, there were no significant gender differences in taking or timing adherence. Among participants 17–24 years, results were similar to the primary analyses, with women having significantly greater odds of higher taking and timing adherence scores than men.
3.3. Secondary adherence outcomes
Self-reported adherence:
Self-reported taking and timing adherence scores were similar across both gender and age categories among the 134 patients with self-reported adherence data (Table 4).
Table 4.
Self-reported adherence and variability in tacrolimus trough levels
| Age 11–16 y | Age 17–24 y | |||
|---|---|---|---|---|
| Girls | Boys | Women | Men | |
| Mean of the 2 MAM-MM scores a | ||||
| Mean taking adherence (SD) | 98.8% (4.4) | 98.7% (4.6) | 96.4% (6.0) | 97.7% (6.4) |
| Mean timing adherence (SD) | 95.4% (8.2) | 94.2% (12.4) | 90.4% (13.9) | 92.4% (9.9) |
| Tacrolimus SD (median [IQR]) b | 1.5 [0.8–2.2] | 1.6 [0.9–2.2] | 1.8 [1.1–3.3] | 1.7 [1.0–2.3] |
| Proportion of participants with tacrolimus SD > 2.0 b | 30 | 36 | 50 | 33 |
Abbreviations: MAM-MM, Medical Adherence Medication Module; SD, standard deviation
In the 1-week reporting periods at both the start and the end of the 3-month observation
In patients taking twice per day tacrolimus (Prograf) and having at least 3 tacrolimus levels
For the vast majority, self-reported adherence was higher than adherence measured electronically during the same week as the reporting period – but the magnitude of the difference between measurement methods differed by age and gender. Among those 11–16 years, the median [IQR] absolute difference between self-reported and electronic taking adherence was 14.3% [3.6–21.4] for girls and 7.1% [0.0–21.4] for boys. Similarly, the median [IQR] absolute difference between self-reported and electronic timing adherence was 14.3% [0.0–23.2] for girls and 7.1% [0.0–25.0] for boys 11–16 years. In contrast, among those 17–24 years, the median [IQR] absolute difference between self-reported and electronically-measured adherence was larger for men (taking 17.9% [1.8–42.9]; timing 21.4% [1.8–48.2]) than women (taking 7.1% [0.0–15.7]; timing 7.1% [−1.8–20.5]).
Variability in tacrolimus trough levels:
Among the 136 patients with electronic monitoring data in the run-in period, there were 100 with at least 3 tacrolimus trough blood levels (median number of levels [IQR] = 8.0 [5.0 – 16.0], similar across gender and age categories), allowing calculation of a standard deviation. In participants 11–16 years taking twice per day tacrolimus (Prograf), the median [IQR] tacrolimus level SD was 1.6 [0.9–2.2] for boys and 1.5 [0.8–2.2] for girls (Table 4). Among participants 17–24 years, the median [IQR] tacrolimus level SD was 1.7 [1.0–2.3] for men and 1.8 [1.1–3.3] for women. Tacrolimus SD was >2.0 in 30% of girls and 36% of boys 11–16 years, and in 50% of women and 33% of men 17–24 years. The number of patients taking once per day tacrolimus (Advagraf) was insufficient (n=14) to allow comparisons by gender and age.
4. DISCUSSION
Among adolescent and young adult kidney transplant recipients, we found a significant impact of age on the association between gender and electronically-measured adherence. Whereas there were no gender differences in adherence measured by electronic monitoring among those 11–16 years, both taking and timing adherence were higher in women than men 17–24 years old. These results are consistent with previous studies showing no difference in adherence by gender in pediatric kidney transplant recipients14,15 and better adherence in adult women than men.5,8 Our results underline the important modifying effect of age on gender differences in adherence.
There are several possible explanations for our findings. The lack of gender differences in younger adolescents likely reflects the fact that parents take some or all responsibility for medication adherence in this age group. The better adherence observed among young women than young men may be related to earlier cognitive maturation in females than males17 resulting in a greater capacity for self-care in young women than men. A greater influence of social desirability (a wish to comply with social expectations) among women than men26,27 may also contribute to the differences observed. Although self-reported adherence was not higher in women than men, this may reflect the fact that participants knew that their adherence was being monitored electronically; young women may have been more concerned that their reports accurately reflect the electronic record than young men. We must also consider the possibility that the observed gender differences represent different reactions to being observed between boys/men and girls/women in the context of a relatively brief study period.
Our findings also highlight possible age- and gender-based differences in the accuracy of different adherence measures. Self-report has been previously reported to overestimate adherence.6,28 We observed a large difference between self-reported and electronically measured taking adherence. We also observed what appeared to be a greater overestimation among girls than boys 11–16 years, and a greater overestimation among men than women 17–24 years. However, we cannot exclude the possibility that there are also gender differences in adherence to using the electronic monitoring pillbox. Failure to detect significant differences in self-reported adherence by gender may reflect a ceiling effect, whereby variability is not well captured, diminishing the possibility of finding differences.
We found no gender differences in either age group in adherence assessed using standard deviation (SD) of tacrolimus trough levels, which is often considered an objective measure of adherence. 29 In fact, a slightly higher proportion of women than men had a SD of tacrolimus levels >2.0, which is opposite to what one would expect based on the electronic monitoring data. There may be several explanations for this. First, patients may have better adherence in the days preceding a planned blood level30, masking gender differences. This observation also raises the question as to whether tacrolimus metabolism may be influenced by sex hormones, with changes in metabolism depending on stage of the menstrual cycle.31-33 If this were the case, tacrolimus trough levels in women may vary with the menstrual cycle, despite good adherence to a constant dose, leading to a higher SD. This has not been studied, but deserves investigation. Finally, given the small numbers of participants 17–24 years, only a few participants with SD of tacrolimus levels >2.0 may make a substantial difference to the proportions with SD of tacrolimus levels >2.0.
The lack of concordance between the different adherence assessment methods may also reflect gender differences in willingness to consistently use an electronic monitoring device. However, we found no literature suggesting that use of electronic monitors differs by gender.
This study has limitations. First, the period of observation was relatively short. Although the first 2 weeks of electronic data were excluded from analyses (as is conventional with electronic monitoring), it is possible that participants’ behaviour continued to be influenced by the fact that they were under observation or that their medication-taking routine had been altered. Second, the short period of observation meant that we were unable to observe changes in adherence with changing age; rather we observed adherence among individuals of different ages. A third limitation is the lack of concordance in results when different methods were used to assess adherence. Although we have outlined possible explanations for these disparities, it is not possible to know which method most accurately represents behaviour. There is no consensus regarding the best method to use to measure adherence. 34–36 Although imperfect, electronic monitoring is generally considered the gold standard, with good accuracy,24,37 reflecting participants’ behavior.36 Residual confounding, due to factors such as gender differences in education, cannot be excluded. Finally, the relatively small sample, despite being derived from 8 centers in 2 countries, may limit generalizability.
Based on electronic monitoring, there are no apparent differences in medication adherence by gender in adolescents 11–16 years old, but poorer adherence in young men 17–24 years than young women of the same age. These findings may help with interpretation of studies showing differences in graft outcomes by sex.2 It may be important to take these gender differences into consideration when targeting patients for adherence-promoting interventions. This study also highlights potentially important gender differences in self-reporting of medication adherence, as well as the possibility that variability in tacrolimus trough levels may depend on factors other than adherence. Larger cohort studies using multiple methods to assess adherence are needed to confirm our findings, but also to further explore gender differences in reporting of adherence. Pharmacokinetic studies to identify biologic factors that may contribute to variability in tacrolimus trough levels would also be helpful. A longitudinal study following younger adolescents into young adulthood would also allow assessment of the evolution of adherence behavior over time.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the devotion of the study coaches and the generous participation of patients and families without whom this study would not have been possible.
Funding
The study was funded by the American National Institutes of Health, National Institutes of Diabetes, Digestive and Kidney diseases (NIDDK; R01DK092977). The funder had no role in study design, data collection, analysis, interpretation of data, writing the report, or and the decision to submit the report for publication. Dr. Boucquemont, who was a postdoctoral fellow at the Research Institute of the McGill University Health Centre when this study was done, was supported by an RI MUHC – Desjardins Studentship in Child Health Research. Dr. Foster, a member of the Research Institute of the McGill University Health Centre, was supported by a Fonds de recherche du Quebec Santé Chercheur-boursier clinicien award.
Footnotes
Authorship
All authors participated in research design, in the writing of the paper and in data analysis.
Disclosure
The authors of this manuscript have no relevant conflicts of interest to disclose, except Dr. Foster, who is a co-investigator on 2 investigator-initiated studies funded by Astellas Canada.
TAKE-IT registration number: ClinicalTrials.gov registration: NCT01356277 (May 17, 2011)
References
- 1.Kabore R, Couchoud C, Macher MA, et al. Age-Dependent Risk of Graft Failure in Young Kidney Transplant Recipients. Transplantation . 2017;101(6):1327–1335. [DOI] [PubMed] [Google Scholar]
- 2.Lepeytre F, Dahhou M, Zhang X, et al. Association of Sex with Risk of Kidney Graft Failure Differs by Age. J Am Soc Nephrol . 2017;28(10):3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update . 2005;11(4):411–423. [DOI] [PubMed] [Google Scholar]
- 4.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol . 2008;8(9):737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisholm-Burns MA, Spivey CA, Tolley EA, Kaplan EK. Medication therapy management and adherence among US renal transplant recipients. Patient Prefer Adherence . 2016;10:703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denhaerynck K, Steiger J, Bock A, et al. Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant . 2007;7(1):108–116. [DOI] [PubMed] [Google Scholar]
- 7.Prihodova L, Nagyova I, Rosenberger J, et al. Adherence in patients in the first year after kidney transplantation and its impact on graft loss and mortality: a cross-sectional and prospective study. J Adv Nurs . 2014;70(12):2871–2883. [DOI] [PubMed] [Google Scholar]
- 8.Spivey CA, Chisholm-Burns MA, Damadzadeh B, Billheimer D. Determining the effect of immunosuppressant adherence on graft failure risk among renal transplant recipients. Clin Transplant . 2014;28(1):96–104. [DOI] [PubMed] [Google Scholar]
- 9.Weng LC, Yang YC, Huang HL, Chiang YJ, Tsai YH. Factors that determine self-reported immunosuppressant adherence in kidney transplant recipients: a correlational study. J Adv Nurs . 2017;73(1):228–239. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm MA, Lance CE, Mulloy LL. Patient factors associated with adherence to immunosuppressant therapy in renal transplant recipients. Am J Health Syst Pharm . 2005;62(17):1775–1781. [DOI] [PubMed] [Google Scholar]
- 11.Marsicano EO, Fernandes NS, Colugnati FA, Fernandes NM, De Geest S, Sanders-Pinheiro H. Multilevel Correlates of Non-Adherence in Kidney Transplant Patients Benefitting from Full Cost Coverage for Immunosuppressives: A Cross-Sectional Study. PLoS One . 2015;10(11):e0138869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vlaminck H, Maes B, Evers G, et al. Prospective study on late consequences of subclinical non-compliance with immunosuppressive therapy in renal transplant patients. Am J Transplant . 2004;4(9):1509–1513. [DOI] [PubMed] [Google Scholar]
- 13.Weng FL, Israni AK, Joffe MM, et al. Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol . 2005;16(6):1839–1848. [DOI] [PubMed] [Google Scholar]
- 14.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant . 2010;14(5):603–613. [DOI] [PubMed] [Google Scholar]
- 15.Chisholm-Burns MA, Spivey CA, Rehfeld R, Zawaideh M, Roe DJ, Gruessner R. Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. Am J Transplant . 2009;9(11):2497–2504. [DOI] [PubMed] [Google Scholar]
- 16.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation . 2009;88(5):736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex . 2001;11(6):552–557. [DOI] [PubMed] [Google Scholar]
- 18.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry . 2006;47(3–4):296–312. [DOI] [PubMed] [Google Scholar]
- 19.Stilley CS, Lawrence K, Bender A, Olshansky E, Webber SA, Dew MA. Maturity and adherence in adolescent and young adult heart recipients. Pediatr Transplant . 2006;10(3):323–330. [DOI] [PubMed] [Google Scholar]
- 20.Foster BJ, Pai A, Zhao H, Furth S, TAKE-IT Study Group. The TAKE-IT study: aims, design, and methods. BMC Nephrol . 2014;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster BJ, Pai ALH, Zelikovsky N, et al. A randomized trial of a multicomponent intervention to promote medication adherence: The Teen Adherence in Kidney Transplant Effectiveness of Intervention Trial (TAKE-IT). Am J Kidney Dis . 2018;72:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zelikovsky N, Schast AP. Eliciting accurate reports of adherence in a clinical interview: development of the Medical Adherence Measure. Pediatr Nurs . 2008;34(2):141–146. [PubMed] [Google Scholar]
- 23.Zelikovsky N, Schast AP, Palmer J, Meyers KE. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant . 2008;12(3):300–308. [DOI] [PubMed] [Google Scholar]
- 24.Denhaerynck K, Schafer-Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol . 2008;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. SAS Global Forum 2012 - statistics and data analysis, Orlando, FL: 2012. [Google Scholar]
- 26.Bernardi RA. Associations between Hofstede’s Cultural Constructs and Social Desirability Response Bias. J Bus Ethics . 2006;65(1):43–53. [Google Scholar]
- 27.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol . 1995;24(2):389–398. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med . 2001;134(10):968–977. [DOI] [PubMed] [Google Scholar]
- 29.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant . 2010;14(8):940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med . 1990;150(7):1509–1510. [PubMed] [Google Scholar]
- 31.Kuypers DR, Claes K, Evenepoel P, et al. Time-related clinical determinants of long-term tacrolimus pharmacokinetics in combination therapy with mycophenolic acid and corticosteroids: a prospective study in one hundred de novo renal transplant recipients. Clin Pharmacokinet . 2004;43(11):741–762. [DOI] [PubMed] [Google Scholar]
- 32.Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet . 2009;48(3):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fadiran EO, Zhang L Effects of Sex Differences in the Pharmacokinetics of Drugs and Their Impact on the Safety of Medicines in Women In Harrison-Woolrych M, ed. Medicines For Women. Cham: Springer International Publishing; 2015:41–68. [Google Scholar]
- 34.Lieber SR, Helcer J, Shemesh E. Monitoring drug adherence. Transplant Rev (Orlando) . 2015;29(2):73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams A, Low JK, Manias E, Dooley M, Crawford K. Trials and tribulations with electronic medication adherence monitoring in kidney transplantation. Res Social Adm Pharm . 2016;12(5):794–800. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann A, Aslani P, Ahmed R, et al. Assessing medication adherence: options to consider. Int J Clin Pharm . 2014;36(1):55–69. [DOI] [PubMed] [Google Scholar]
- 37.De Bleser L, De Geest S, Vandenbroeck S, Vanhaecke J, Dobbels F. How accurate are electronic monitoring devices? A laboratory study testing two devices to measure medication adherence. Sensors (Basel) . 2010;10(3):1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


