Abstract
Warning labels on tobacco products sometimes feature images and stories of real people whose health has been affected by smoking. We examined effects of some of the design elements that may contribute to the effectiveness of these testimonial pictorial warning labels (PWLs). Beginning with a testimonial PWL that contained an image of a person and a basic warning statement (e.g., “Smoking can kill you”), we examined the impact of adding: (a) text detailing the person’s name, age and health status (identifiers); and (b) explanatory statements that elaborated on the basic warning using a testimonial or non-testimonial message. In an online experiment, 1255 adult smokers in the United States were randomly assigned to one of six experimental conditions (2 [identifier: none/identifier] x 3 [explanatory statement: none/non-testimonial/testimonial]), or a control condition (text only warning labels that currently appear on packs in the United States). In each condition, participants were exposed to multiple labels each focused on a different health effect. Effectiveness was assessed using emotional responses, engagement and behavioral intentions measured immediately post-exposure, and quit attempts measured at five-week follow-up. Testimonial PWLs were more effective than the text only labels. However, there was little evidence that adding identifiers or the explanatory statements enhanced effectiveness; rather, there was some evidence that testimonial explanatory statements reduced effectiveness. These findings suggest that the most effective design for testimonial PWLs may be to combine a basic warning statement with an image of a real person, without any additional textual components.
Keywords: warning labels, graphic warning labels, pictorial warning labels, testimonials, tobacco, United States, US Food and Drug Administration
Pictorial warning labels (PWLs) are recommended as an effective intervention for increasing public awareness about the risks of using tobacco (World Health Organization, 2011). More than 100 countries currently require PWLs on cigarette packs (Canadian Cancer Society, 2016), although there is considerable variation in the size, position, layout, amount of text, and type of images used in these PWLs (Hammond & Reid, 2012). Although a large number of studies have demonstrated the benefits of using PWLs rather than text only warning labels (WLs; Noar, Francis, et al., 2017; Noar, Francis, et al., 2016; Noar, Hall, et al., 2016), relatively few studies have assessed which PWL features contribute most to their effectiveness, and of those that have, most have focused on the relative effectiveness of different types of images (Brennan, Maloney, Ophir, & Cappella, 2017; Cameron & Williams, 2015; Hammond et al., 2012; Huang, Thrasher, Reid, & Hammond, 2016; Mutti et al., 2016; Thrasher, Arillo-Santillan, et al., 2012; Thrasher, Carpenter, et al., 2012). Our objective in this study was to strengthen the evidence base available to governments developing or revising PWL policies, by assessing whether PWL effectiveness is influenced by the inclusion of brief textual messages on the image, or expanded textual messages on the back of the pack. In particular, we examined the impact of these textual messages in the context of “testimonial PWLs”, which are PWLs that feature the image and details of a real person whose health has been affected by their own or others’ tobacco use (Brennan et al., 2017).
Testimonial PWLs currently appear on cigarette packs in several countries (Canadian Cancer Society, 2016). Furthermore, they have been identified as one approach that may help to minimize legal objections to PWLs in the United States (US), to the extent that they provide a factual account of one person’s experience with the health effects of tobacco use, rather than an opinion (Brennan et al., 2017; Goodman, 2014; Kraemer & Baig, 2013). The use of exemplars to communicate concrete and factual information about the health effects of tobacco is broadly supported by research exploring the ways in which exemplars, narratives and testimonials attract attention and facilitate message processing, comprehension, and impact (Braddock & Dillard, 2016; Durkin, Biener, & Wakefield, 2009; Durkin, Wakefield, & Spittal, 2011; Kim, Bigman, Leader, Lerman, & Cappella, 2012; Kim, Shi, & Cappella, 2016; Ophir, Brennan, Maloney, & Cappella, 2017; Shen, Sheer, & Li, 2015). However, evidence regarding the potential effectiveness of testimonial PWLs is currently mixed. Studies assessing the effectiveness of different types of PWL images have found that images depicting the “lived experiences” of sufferers of tobacco-related illnesses have either been inferior (Cameron & Williams, 2015; Hammond et al., 2012; Mutti et al., 2016; Thrasher, Arillo-Santillan, et al., 2012; Thrasher, Carpenter, et al., 2012) or equivalent to (Brennan et al., 2017) images that depict diseased organs or disfigured body parts, while they have typically outperformed symbolic and abstract images (Cameron & Williams, 2015; Huang et al., 2016; Thrasher, Carpenter, et al., 2012).
Other studies have examined whether PWL effectiveness is influenced by the amount and form of the text accompanying the image, for which there is also mixed evidence. Brown and colleagues (2013) found that graphic images garnered greater attention than non-graphic images only when accompanied by non-testimonial textual statements. Emery and colleagues (2014) compared responses to WLs carrying only a basic warning statement, or a basic warning statement plus an image, or an expanded warning statement plus an image. They found that adding images enhanced warning label effectiveness primarily by increasing feelings of worry, whereas the expanded warning statements enhanced effectiveness by increasing believability. By comparison, Evans and colleagues (2015) found that adding an expanded statement to PWLs that contained an image and a basic warning statement reduced perceived credibility. Hammond et al. (2012) found that PWLs using graphic or lived experiences images were more effective when accompanied by a quote from the person in the image, along with their name and age, than when accompanied by a non-testimonial warning statement. Furthermore, an experiment with smokers in Mexico manipulated the brief warning statements on the front and expanded warning statements on the back of packs to be either non-testimonial or testimonial in format (Thrasher, Arillo-Santillan, et al., 2012). Non-testimonial statements provided statistics and described the impact of smoking on health and quality-of-life, whereas the testimonial statements included quotes from real people about the impact of smoking on their life and their family members. PWLs with non-testimonial statements tended to outperform those with testimonial statements on measures of perceived credibility, relevance, and impact, although this effect was most pronounced among those with higher education (Thrasher, Arillo-Santillan, et al., 2012).
We built upon this previous work by further examining the unique and combined effects of two different textual elements: brief messages that appear on the image, and expanded messages that appear on the back of the pack. In general, we expected that the inclusion of additional text would increase PWL persuasiveness by increasing vividness and salience (Brown, Reidy, Weighall, & Arden, 2013; Ophir et al., 2017), facilitating understanding and learning of the key message conveyed by the image (Emery, Romer, Sheeran, Jamieson, & Peters, 2014; Strasser, Tang, Romer, Jepson, & Cappella, 2012), and in the specific case of these testimonial PWLs, by increasing the emotional depth of the case study being presented. Beginning with a testimonial PWL that contained a basic written warning statement and an image of a real person whose health had been affected in the way described by the warning statement, we tested the impact of adding a brief identifying statement detailing the person’s name, age and health status. We predicted that testimonial PWLs carrying an identifier would be more effective than those without (H1). We also tested the impact of adding an explanatory statement that elaborated on the basic warning in either a non-testimonial (general information about the prevalence, severity, and mechanisms of the health condition) or testimonial format (information about the individual’s experience with the health condition, including a direct quote). We expected that testimonial PWLs with an explanatory statement (non-testimonial or testimonial) would be more effective than those without (H2). Given mixed evidence about the relative effectiveness of using non-testimonial versus testimonial information in expanded statements, we posed a research question to examine the relative effectiveness of these two types of explanatory statements in the context of testimonial PWLs (RQ1). Furthermore, we examined whether the effectiveness of each type of explanatory statement was influenced by the presence/absence of the identifier (RQ2). In addition, all testimonial PWL conditions were compared to the text only WLs that currently appear on packs in the US. This allowed us to ensure that each PWL condition was at least more effective than the WLs to which smokers in this study were currently exposed. Consistent with a large body of research assessing the real-world impact of PWLs (Noar, Francis, et al., 2017; Noar, Francis, et al., 2016), we hypothesised that all PWL conditions would be more effective than text only WLs (H3).
Method
Sample
This study was approved by the Institutional Review Board at the University of Pennsylvania. As part of a larger experimental study conducted in 2014, 3,055 participants were randomly assigned to one of 17 experimental conditions. Seven of these conditions were relevant to the hypotheses and research questions addressed in the current study; the analytic sample is limited to the 1,255 participants randomized to one of these seven conditions. The remaining conditions were not relevant for assessing the impact of adding different textual elements to testimonial PWLs, but were used in other analyses reported by Brennan et al. (2017) and Ophir et al. (2017).
The sample comprised 18–60 year old current established smokers (i.e., smoked at least 100 cigarettes in their lifetime and currently smoked at least some days), who had completed fewer than three online surveys about tobacco in the past three months. All participants were recruited through Survey Sampling International’s (SSI) US panel (Survey Sampling International, 2016). SSI panellists voluntarily opt-in to the panel and receive small financial incentives for completing surveys. As with other non-probability online panels, samples recruited from SSI cannot be considered representative of the broader US population. However, we found that the profile of our sample (Appendix A) was highly similar to that of 18–60 year old current established smokers in the 2013–2014 Centers for Disease Control and Prevention’s National Adult Tobacco Survey (NATS) (Centers for Disease Control and Prevention, 2016) in terms of age (M = 38.7 [SD = 11.1] cf. M = 41.7 [SD = 12.4] years) and the percentage of the sample who were female (51.5% cf. 48.8%), Hispanic (10.2% cf. 9.1%), lived in households with a total annual income <$40,000 (46.2% cf. 45.7%), were thinking about quitting (79.9% cf. 77.8%), and had made at least one quit attempt in the past 12 months (55.7% vs. 55.4%). Our sample contained fewer respondents with low education (23.8% cf. 48.4%), more respondents who were White (84.9% cf. 77.2%), fewer who were Black (7.9% cf. 14.4%), and more daily smokers (81.9% cf. 75.8%). Therefore, although we do not suggest that our parameter estimates represent the national population statistically, we expect the overall pattern of responses observed in this large and varied sample will reflect those in the population.
Experimental Design and Stimuli
The current study was conceptualized as a 2 (identifier: none/identifier) x 3 (explanatory statement: none/non-testimonial/testimonial) between-subjects experiment, with a set aside control. Participants were randomly assigned to one of seven conditions (Table 1). For each of the six intervention conditions, we created five PWLs. Each of the five PWLs had a different theme, meaning they focused on a different health effect and communicated this information using images and text relevant to that health effect. The five themes (which were the same across all six intervention conditions) were drawn from the nine warning statements prescribed for PWLs in the US under the Family Smoking Prevention and Tobacco Control Act (United States Public Laws, 2009): smoking can kill you; cigarettes cause fatal lung disease; cigarettes cause stroke and heart disease; cigarettes are addictive; and tobacco smoke causes fatal lung disease in nonsmokers. For each theme, we sourced a case study of an individual whose health had been affected in the way described. Images and stories of these case studies were used with permission from Health Canada and the Centers for Disease Control and Prevention’s (CDC), and had previously been featured either on PWLs in Canada or as part of the CDC’s Tips From Former Smokers mass media campaign. In all six intervention conditions, the testimonial PWL included a basic warning statement (e.g., WARNING: Smoking can kill you) along with an image of the case study. The basic warning statement and image covered 50% of the front and back of the pack (Table 1). In the three conditions that also included an identifier (6–8 words, M = 7.0 words across themes), this information about the person’s name, age and health status (e.g., Terrie: died from cancer at age 53) appeared as part of the image on both the front and back of the pack. In the four conditions that also included an explanatory statement, this was added to the back of the pack, such that the total area covered by the PWL on the back of the pack increased to approximately 75%. Non-testimonial explanatory statements (32–38 words, M = 34.6 words across themes) opened with a general statement about the specific health effect (e.g., Smoking kills half of all lifetime smokers.) followed by one or two sentences that further described the nature, severity and scope of the problem (e.g., In the United States, tobacco is the leading cause of preventable death. Smokers live shorter lives, and they live more years with disabling health problems.). Testimonial explanatory statements (35–48 words, M = 42.2 words across themes) opened with the same general statement about the health effect (e.g., Smoking kills half of all lifetime smokers.) but followed this with details of the case study’s experience with the health effect and a direct quote from the individual (e.g., Terrie died from cancer caused by smoking. Terrie had some advice for other smokers: “Please quit…I don’t want anyone to have to go through what I went through”.). Stimuli in the control condition were drawn from a pool comprised of the four text only WLs that currently appear on cigarette packets in the US.
Table 1.
Warning Label Content for Seven Experimental Conditions (Using Theme 1 as an Example)
| Explanatory statement | |||
|---|---|---|---|
| None | Non-testimonial | Testimonial | |
| No identifier | No identifier + No explanatory statement | No identifier + Non-testimonial explanatory statement | No identifier + Testimonial explanatory statement |
 statement statement |
 |
 |
|
| Identifier | Identifier + No explanatory statement | Identifier + Non-testimonial explanatory statement | Identifier + Testimonial explanatory statement |
 statement statement |
 |
 |
|
| Set aside control | Text only. | ||
 statement statement |
|||
Note. Images and personal details of Terrie (Theme 1) were used with permission from the United States Lefts for Disease Control and Prevention. Digital images of the warning labels and cigarette packs were created by Kyle Cassidy at the Annenberg School for Communication, University of Pennsylvania. See Appendix B for details of the text that comprised each design element for all five warning label themes.
Appendix B presents the content for each design element across the five themes, and Table 1 illustrates how these elements were combined to create the six intervention conditions. As shown in Table 1, warning label stimuli were presented via a static image that comprised a side-of-pack, front-of-pack, and back-of-pack view of the warning label. All WLs were presented on a picture of a cigarette pack, designed to be as plain looking as possible (to minimize distraction).
Procedure
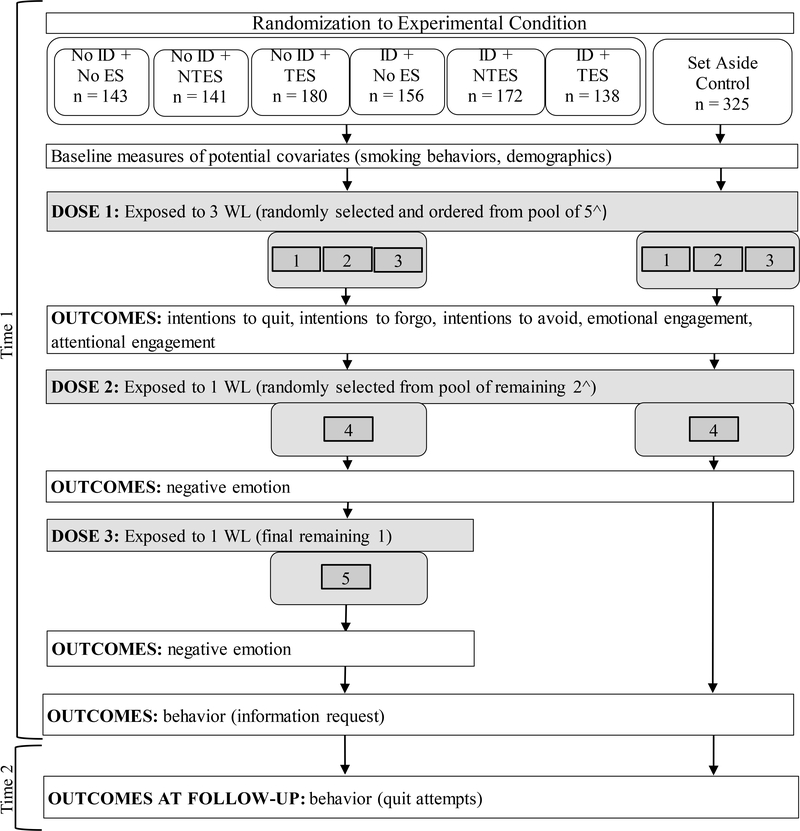
The study comprised an online experimental session (Time 1) and a five-week follow-up online survey (Time 2). Figure 1 illustrates the study procedure. One feature of this procedure is that at Time 1 participants were exposed to five WLs, one for each of the five themes, as follows: first, they were exposed to WLs for three of the themes (for each participant, three themes were randomly selected from the five themes and were presented in random order) followed by measurement of engagement and behavioral intentions; then they were exposed to one of the two remaining WLs followed by measurement of emotional reactions; and then they were exposed to the fifth WL followed by measurement of emotional reactions (in the control condition, there were only four WLs for four themes). At Time 1, this design allowed us to measure the aggregate impact of exposure to multiple WLs on engagement and intentions, while also collecting detailed assessments of emotional reactions to individual labels. This design avoided confounding our measures of the overall impact of exposure (i.e., to the first three randomly selected themes) with evaluation of the individual labels (fourth and fifth WLs), while still providing information about the individual labels in aggregate across the sample (Figure 1).
Figure 1.
Study procedure. By design, twice as many participants were assigned to the control condition as to each of the intervention conditions. ID = identifier; ES = explanatory statement; NTES = non-testimonial explanatory statement; TES = testimonial explanatory statement; WL = warning label.
^ For each participant in the Set Aside Control condition, the first three warning labels they were exposed to were randomly selected (and randomly ordered) from a pool of four warning labels (and so the fourth label was the final one remaining). For each participant in all other conditions, the first three warning labels they were exposed to were randomly selected (and randomly ordered) from a pool of five warning labels.
Measures
Negative emotional reactions.
Emotional reactions are a key mechanism through which WLs contribute to changes in smoking behavior (Emery et al., 2014; Peters, Evans, Hemmerich, & Berman, 2016; Wang, Lowen, Romer, Giorno, & Langleben, 2015; Yong et al., 2014). After exposure to the fourth and fifth WLs (Figure 1), participants were asked: While looking at the warning on this pack of cigarettes, I felt…(a) disgusted; (b) fearful; (c) guilty; (d) regretful; (e) sad; (f) worried; and (g) angry at myself for being a smoker (1 “strongly disagree” – 5 “strongly agree”; Gibson et al., 2015; Nonnemaker et al., 2015). The negative emotional reaction scale comprised the average of the seven responses (α = .91 after fourth warning label; α = .92 after fifth warning label). Responses after the fourth and fifth warning label were retained as two separate values for analyses, such that in all models predicting negative emotional reactions, we adjusted for clustering at the individual level and used robust standard errors.
Emotional and attentional engagement.
Following exposure to the first three WLs, we measured engagement. Consistent with the approach taken by Ophir et al. (2017) two engagement sub-scales were created. Emotional engagement was measured using four items that captured empathy and sympathy for the individual featured in the warning label (Moyer-Gusé, 2008): How much do each of the following statements apply to the images and words you just saw? (a) I felt concerned; (b) I felt sympathy; (c) I was affected emotionally; and (d) I was touched (1 “not at all; 2 “a little”; 3 “some”; 4 “very much”). Responses to the four items were averaged together (α = .89). Using the same four-point scale, attentional engagement was measured using five items adapted from the transportation scale (Green & Brock, 2000): (a) I was involved mentally; (b) My attention was fully captured; (d) My thoughts were about the images and words only; (d) The images and words were difficult to put out of my mind; and (e) The images and word were relevant to my everyday life (α = .86). The two engagement scales were strongly correlated (r = .80).
Intention outcomes.
Following exposure to the first three WLs, intentions to quit were measured using participants’ willingness to engage in three quitting-related behaviors in the next 30 days: (a) try to quit smoking; (b) reduce the number of cigarettes smoked per day; and (c) quit smoking completely (1 “definitely will not” – 4 “definitely will”; α = .87; Gibson et al., 2015).
Longitudinal population surveys have demonstrated that smokers who forgo cigarettes because of WLs may be more likely to make subsequent quit attempts (Li, Borland, Fong, et al., 2015; Yong et al., 2014). Following exposure to the first three WLs, intentions to forgo were measured using the item: If my usual pack of cigarettes looked like these packs of cigarettes, I would hold back from smoking a cigarette when I was about to smoke one (1 “strongly disagree” – 5 “strongly agree”; Gibson et al., 2015). Longitudinal population studies have also demonstrated that when smokers try to avoid WLs by covering them up or moving their cigarettes to a different container, they tend to experience more frequent thoughts about the harms of smoking (Yong et al., 2014) and increased quitting activity (Thrasher et al., 2016). Intentions to avoid the WLs were measured using the average of three items: If my usual pack of cigarettes looked like these packs of cigarettes, I would…(a) cover it up; (b) keep the pack out of sight; and (c) transfer the cigarettes to a different container (1 “strongly disagree” – 5 “strongly agree”; α = .85; Gibson et al., 2015).
Behavioral outcomes.
At the end of the Time 1 session, participants were offered the opportunity to read tips on how to quit smoking. We measured the proportion of participants who requested quitting information. At the beginning of Time 2, about five weeks after Time 1, participants were reminded that they recently took part in a study in which they viewed cigarette packs. They were then asked whether, since participating in that study, they had thought about changing or had made any changes to their smoking behavior. Participants could select one of six responses: (a) I have not made any changes to my smoking behavior; (b) I thought about quitting, but did not make an attempt; (c) I tried to cut down the number of cigarettes, but didn’t make an actual attempt to quit; (d) I decided to quit, but haven’t made an actual attempt yet; (e) I made an attempt to quit, but I’ve relapsed to smoking; and (f) I quit, and I’m still quit (Brennan, Durkin, Wakefield, & Kashima, 2014). We created a binary variable measuring whether participants had made a quit attempt (responses (e) and (f) combined, compared to responses (a) – (d) combined).
Potential covariates.
Participants reported their age, sex, educational attainment, race, ethnicity, parental status, annual household income, smoking status (daily vs. not daily), and how many times they had tried to quit in the past year. At the beginning of the study, we measured readiness to quit (Biener & Abrams, 1991), nicotine dependence (six questions from the Fagerstrӧm Test for Nicotine Dependence; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and cigarette cravings (two questions adapted from the brief questionnaire of smoking urges; Cox, Tiffany, & Christen, 2001; Maloney & Cappella, 2016). At the end of Time 1, we asked participants whether they smoked at any point during the study (Maloney & Cappella, 2016).
Statistical Analysis
All analyses were conducted using Stata Version 14.1. Preliminary analyses indicated that, of all potential covariates, only sex, household income, smoking status, and nicotine dependence were unevenly distributed across conditions (p < .10; Appendix A). In bivariate models (data not shown), each of these variables was significantly associated with at least two of the eight outcomes and was therefore included as a covariate in adjusted models for all outcomes.
For each outcome, we conducted an adjusted linear or logistic regression model, in which the text only control condition was the referent category and each of the six intervention conditions was entered as dummy variables. We then conducted a series of post-estimation Wald tests of various linear combinations of beta coefficients from the regression model to test the: (a) overall effect of all intervention conditions compared to the text only control condition; (b) main effect of the identifier factor; (c) main effects of the explanatory statement factor (none vs. non-testimonial explanatory statement; none vs. testimonial explanatory statement; non-testimonial explanatory statement vs. testimonial explanatory statement); (d) interaction effect between the identifier factor and the explanatory statement factor; and (e) simple effects of the identifier factor within each of the three levels of the explanatory statement factor. To facilitate control over Type 1 error (given the large number of tests conducted), main effects (b) and (c) and the interaction effect (d) were examined only if the p value for the overall effect (a) was p < .10. Similarly, simple effects (e) were examined only if the p value for the interaction effect (d) was p < .10. Results of the post-estimation Wald tests are reported as F statistics for linear regression models, and chi-square statistics for logistic regression models.
Of 1,255 participants who completed Time 1, 310 (24.7%) did not complete the Time 2 survey. The non-completion rate was similar across the conditions (range = 18.6% - 27.3%; χ2(6, N = 1,255) = 4.23, p = .645) and characteristics of those lost to follow-up did not differ between conditions (data not shown). For analyses predicting quit attempts at Time 2, we therefore conducted an intention-to-treat analysis (N = 1,255) with those lost to follow-up assumed not to have made a quit attempt. Sensitivity analyses demonstrated that the same pattern of effects was observed when limiting the sample to those who completed Time 2 (data not shown; available upon request).
Results
Overall Effectiveness of Testimonial PWLs Compared to Text Only Warning Labels
On six of eight outcomes, there was evidence that testimonial PWLs were more effective than the text only WLs. On both negative emotion and emotional engagement, the overall effect of the intervention conditions compared to the text only control condition was positive and significant, and the six PWL conditions all individually outperformed the text only condition (Table 2). On the measure of intentions to quit, there was an overall positive effect of the intervention conditions, and the three PWL conditions that included an identifier all lead to significantly higher intentions than the text only condition (Table 3). On the measures of intentions to forgo cigarettes and intentions to avoid the WLs, the overall effect of the intervention conditions was positive and significant and all six PWL conditions significantly outperformed the text only condition (Table 3). The overall effect of the intervention conditions was also positive and significant for the quit attempts measure, even though only one of the six PWL conditions (no identifier + no explanatory statement) significantly outperformed the text only control condition (Table 4).
Table 2.
Effects of Identifiers and Explanatory Statements on Negative Emotions, Emotional Engagement, and Attentional Engagement
| Negative emotiona | Emotional engagement | Attentional engagement | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Linear regression model | M (SD) | B | 95% CI | M (SD) | B | 95% CI | M (SD) | B | 95% CI |
| Text only control | 3.05 (1.02) | ref | 2.42 (0.89) | ref | 2.74 (0.78) | ref | |||
| No ID + No ES | 3.35 (1.06) | 0.32 | 0.13 – 0.51 | 2.67 (0.96) | 0.28 | 0.11 – 0.46 | 2.73 (0.89) | 0.01 | −0.14 – 0.17 |
| No ID + NTES | 3.25 (0.99) | 0.22 | 0.04 – 0.40 | 2.64 (0.87) | 0.25 | 0.07 – 0.42 | 2.72 (0.73) | 0.01 | −0.15 – 0.17 |
| No ID + TES | 3.39 (0.97) | 0.32 | 0.15 – 0.49 | 2.71 (0.93) | 0.28 | 0.12 – 0.44 | 2.73 (0.85) | −0.02 | −0.17 – 0.12 |
| ID + No ES | 3.43 (1.02) | 0.33 | 0.15 – 0.51 | 2.80 (0.89) | 0.35 | 0.18 – 0.52 | 2.86 (0.83) | 0.09 | −0.07 – 0.24 |
| ID + NTES | 3.42 (1.04) | 0.32 | 0.13 – 0.50 | 2.83 (0.93) | 0.37 | 0.21 – 0.54 | 2.89 (0.84) | 0.12 | −0.03 – 0.27 |
| ID + TES | 3.42 (1.02) | 0.38 | 0.18 – 0.57 | 2.91 (0.91) | 0.50 | 0.33 – 0.68 | 2.86 (0.85) | 0.14 | −0.02 – 0.30 |
| Post-estimation Wald Tests | |||||||||
| Overall effect of all intervention conditions (vs. text only control) | F(6, 1,249) = 4.40, p < .001 | F(6, 1,240) = 7.09, p < .001 | F(6, 1,240) = 1.12, p = .346 | ||||||
| Main effects | |||||||||
| No ID vs. ID | F(1, 1,249) = 0.73, p = .392 | F(1, 1,240) = 5.63, p = .018 | |||||||
| No ES vs. NTES | F(1, 1,249) = 0.54, p = .464 | F(1, 1,240) = 0.01, p = .930 | |||||||
| No ES vs. TES | F(1, 1,249) = 0.10, p = .750 | F(1, 1,240) = 1.08, p = .298 | |||||||
| NTES vs. TES | F(1, 1,249) = 1.14, p = .287 | F(1, 1,240) = 1.29, p = .256 | |||||||
| Interaction effectb | F(2, 1,249) = 0.16, p = .855 | F(2, 1,240) = 0.66, p = .516 | |||||||
Note. Bolded results are significant at p < .05. All models adjust for the effect of sex, household income, smoking status, and nicotine dependence, which were unevenly distributed across experimental conditions and were significantly associated with some outcome measures in bivariate models. N varied slightly for some models due to missing data, but where there was missing data, it applied to less than 1% of all cases. ID = identifier; ES = explanatory statement; NTES = non-testimonial explanatory statement; TES = testimonial explanatory statement; M = mean; SD = standard deviation; B = unstandardized beta coefficient; CI = confidence interval; ref = referent category in regression model.
Participants in each intervention condition provided two responses each: one after Dose 2 and one after Dose 3 (see Figure 1). Therefore, in all models predicting negative emotion, we adjusted for clustering at the individual level and used robust standard errors.
Given that none of the interaction effects were significant (p > .10), simple effects were not examined.
Table 3.
Effects of Identifiers and Explanatory Statements on Intentions to Quit, Intentions to Forgo Cigarettes, and Intentions to Avoid the Warning Labels
| Intentions to quit (1–4 scale) | Intentions to forgo (1–5 scale) | Intentions to avoid (1–5 scale) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Linear regression model | M (SD) | B | 95% CI | M (SD) | B | 95% CI | M (SD) | B | 95% CI |
| Text only control | 2.44 (0.75) | ref | 2.33 (1.14) | ref | 2.21 (1.04) | ref | |||
| No ID + No ES | 2.58 (0.79) | 0.13 | −0.02 – 0.27 | 3.05 (1.21) | 0.72 | 0.50 – 0.95 | 3.18 (1.02) | 0.99 | 0.79 – 1.19 |
| No ID + NTES | 2.56 (0.75) | 0.11 | −0.04 – 0.25 | 3.01 (1.17) | 0.68 | 0.46 – 0.91 | 3.30 (0.95) | 1.12 | 0.92 – 1.32 |
| No ID + TES | 2.55 (0.78) | 0.10 | −0.03 – 0.24 | 3.04 (1.14) | 0.69 | 0.48 – 0.90 | 3.09 (1.04) | 0.87 | 0.68 – 1.05 |
| ID + No ES | 2.67 (0.80) | 0.17 | 0.03 – 0.31 | 3.05 (1.22) | 0.64 | 0.42 – 0.86 | 3.32 (1.11) | 1.07 | 0.87 – 1.26 |
| ID + NTES | 2.65 (0.76) | 0.17 | 0.03 – 0.31 | 3.17 (1.20) | 0.79 | 0.58 – 1.00 | 3.20 (1.11) | 0.96 | 0.77 – 1.15 |
| ID + TES | 2.64 (0.72) | 0.20 | 0.05 – 0.34 | 3.10 (1.26) | 0.79 | 0.57 – 1.02 | 2.88 (1.03) | 0.70 | 0.50 – 0.90 |
| Post-estimation Wald Tests | |||||||||
| Overall effect of all intervention conditions (vs. text only control) | F(6, 1,240) = 1.89, p = .080 | F(6, 1,239) = 16.32, p < .001 | F(6, 1,240) = 37.80, p < .001 | ||||||
| Main effects | |||||||||
| No ID vs. ID | F(1, 1,240) = 1.82, p = .178 | F(1, 1,239) = 0.29, p = .587 | F(1, 1,240) = 1.65, p = .199 | ||||||
| No ES vs. NTES | F(1, 1,240) = 0.02, p = .882 | F(1, 1,239) = 0.41, p = .524 | F(1, 1,240) = 0.02, p = .890 | ||||||
| No ES vs. TES | F(1, 1,240) = 0.00, p = .986 | F(1, 1,239) = 0.47, p = .493 | F(1, 1,240) = 8.92, p = .003 | ||||||
| NTES vs. TES | F(1, 1,240) = 0.03, p = .867 | F(1, 1,239) = 0.00, p = .963 | F(1, 1,240) = 9.89, p = .002 | ||||||
| Interaction effecta | F(2, 1,240) = 0.11, p = .896 | F(2, 1,239) = 0.71, p = .493 | F(2, 1,240) = 1.39, p = .251 | ||||||
Note. Bolded results are significant at p < .05. All models adjust for the effect of sex, household income, smoking status, and nicotine dependence, which were unevenly distributed across experimental conditions and were significantly associated with some outcome measures in bivariate models. N varied slightly for some models due to missing data, but where there was missing data, it applied to less than 1% of all cases. ID = identifier; ES = explanatory statement; NTES = non-testimonial explanatory statement; TES = testimonial explanatory statement; M = mean; SD = standard deviation; B = unstandardized beta coefficient; CI = confidence interval; ref = referent category in regression model.
Given that none of the interaction effects were significant (p > .10) simple effects were not examined.
Table 4.
Effects of Identifiers and Explanatory Statements on Requests for Quitting Information at Time 1 and Quit Attempts at Time 2
| Requested quitting information | Quit attempt | |||||
|---|---|---|---|---|---|---|
| Logistic regression model | % | OR | 95% CI | % | OR | 95% CI |
| Text only control | 33.9 | ref | 7.4 | ref | ||
| No ID + No ES | 35.0 | 1.13 | 0.74 – 1.72 | 15.4 | 2.30 | 1.23 – 4.28 |
| No ID + NTES | 26.2 | 0.70 | 0.45 – 1.10 | 13.5 | 1.88 | 0.99 – 3.58 |
| No ID + TES | 34.4 | 1.01 | 0.68 – 1.49 | 6.1 | 0.82 | 0.39 – 1.72 |
| ID + No ES | 36.5 | 1.04 | 0.69 – 1.57 | 11.5 | 1.49 | 0.77 – 2.85 |
| ID + NTES | 37.8 | 1.10 | 0.74 – 1.64 | 9.3 | 1.23 | 0.63 – 2.40 |
| ID + TES | 42.0 | 1.49 | 0.98 – 2.26 | 6.5 | 0.89 | 0.40 – 1.97 |
| Post-estimation Wald Tests | ||||||
| Overall effect of all intervention conditions (vs. text only control) | χ2(6, N = 1,251) = 8.59, p = .198 | χ2(6, N = 1,251) = 13.16, p = .041 | ||||
| Main effects | ||||||
| No ID vs. ID | χ2(1, N = 1,251) = 1.31, p = .253 | |||||
| No ES vs. NTES | χ2 (1, N = 1,251) = 0.59, p = .442 | |||||
| No ES vs. TES | χ2(1, N = 1,251) = 7.12, p = .008 | |||||
| NTES vs. TES | χ2(1, N = 1,251) = 3.84, p = .050 | |||||
| Interaction effecta | χ2 (2, N = 1,251) = 0.91, p = .633 | |||||
Note. Bolded results are significant at p < .05. All models adjust for the effect of sex, household income, smoking status, and nicotine dependence, which were unevenly distributed across the experimental conditions and were significantly associated with some outcome measures in bivariate models. N varied slightly for some models due to missing data, but where there was missing data, it applied to less than 1% of all cases. ID = identifier; ES = explanatory statement; NTES = non-testimonial explanatory statement; TES = testimonial explanatory statement; OR = odds ratio; CI = confidence interval; ref = referent category in regression model.
Given that neither of the interaction effects were significant (p > .10) simple effects were not examined.
Effectiveness of Identifiers and Explanatory Statements
The main effect of the identifying statement factor was significant for just one of eight outcomes, emotional engagement (Table 2). Mean emotional engagement across the three conditions that did not carry an identifier (M = 2.68, SD = 0.92) was significantly lower than mean emotional engagement across the three conditions that did carry an identifier (M = 2.85, SD = 0.91, F(1, 1,240) = 5.63, p = .018).
Main effects of the explanatory statement factor were significant for just two of eight outcomes. On intentions to avoid, the mean across conditions that carried a testimonial explanatory statement (M = 3.00, SD = 1.04) was significantly lower than the mean across conditions that carried a non-testimonial explanatory statement (M = 3.25, SD = 1.04, F(1, 1,240) = 9.89, p = .002) and those that did not carry an explanatory statement (M = 3.26, SD = 1.07, F(1, 1,240) = 8.92, p = .003) (Table 3). In the same way, the mean proportion of smokers who had attempted to quit was lower in conditions that carried a testimonial explanatory statement (6.3%) than in conditions that carried a non-testimonial explanatory statement (11.2%; χ2(1, N = 1,251) = 3.84, p = .050) and those that did not carry an explanatory statement (13.4%; χ2(1, N = 1,251) = 7.12, p = .008) (Table 4).
There were no significant interactions between the identifier and explanatory statement factors (Tables 2 – 4).
Discussion
Our aim in this study was to assess the impact of two different textual elements that are often employed in PWLs; namely, identifying statements and detailed explanatory statements. Overall, we found little evidence that these specific design elements contributed to the effectiveness of testimonial PWLs. Rather, the results suggest that the most effective design for testimonial PWLs is to combine a basic warning statement with an image of a real person whose health has been affected in the way described by the statement, without any additional textual components. If additional text is to be used, then these findings suggest this should be limited to non-testimonial explanatory statements. As PWLs continue to be implemented as part of comprehensive tobacco control programs around the world (Canadian Cancer Society, 2016) and are increasingly recommended for a range of other tobacco products and alternative nicotine delivery systems (Thrasher, Brewer, et al., 2018), findings such as these are critical for demonstrating that not all PWLs are created equally, such that careful attention must be directed to identifying, and employing, only those features that most contribute to PWL effectiveness.
Specifically, we found only minimal support for H1, as testimonial PWLs with a statement identifying the person in the image were more effective than those without on only one outcome, emotional engagement. Furthermore, there was no evidence that testimonial PWLs with a non-testimonial explanatory statement differed in effectiveness compared with PWLs that did not carry explanatory statements (no support for H2). However, testimonial PWLs with a testimonial explanatory statement resulted in lower intentions to avoid the WLs and a lower proportion of smokers who made a quit attempt in the weeks following exposure when compared with PWLs without an explanatory statement (contrary to H2) and those with a non-testimonial explanatory statement (RQ1). We found no evidence that the effectiveness of each type of explanatory statement was influenced by the addition of the identifying statement (RQ2). Supporting H3, as a set the testimonial PWLs outperformed text only WLs on most of the outcomes and in no case were any of the individual testimonial PWL conditions less effective than the text only WLs. That is, despite the observed variation in effectiveness between the PWL conditions, these findings indicate that any of the PWLs should be preferred over text only WLs. These findings add to the considerable body of evidence that PWLs are more effective than text only labels, including from studies evaluating the real-world impact of PWLs (Noar, Francis, et al., 2017; Noar, Francis, et al., 2016).
There are several parameters of this study that need to be kept in mind when interpreting these findings, both because of the potential impact on these results and for the guidance provided towards useful further research. First, we note the potential impact of the research methodology used and the context in which this study was conducted. In this experiment, smokers in the US were exposed only briefly to static images of the PWLs. Given that these smokers are currently exposed to text warnings that have appeared on packs since 1984, the novelty of the images used in these PWLs may have been so impactful that the mere presence of the image overshadowed any potential benefit of the additional textual elements. Indeed, in a recent qualitative study that assessed reactions to the PWLs proposed by the FDA, Bigman and colleagues (2016) found that many smokers reported directing their attention solely to the images, without reading any of the text. Persuasive benefits of the additional text may be more likely to be observed in countries where smokers have become accustomed to seeing images on their cigarette packs. Furthermore, it is possible that when PWLs like these are implemented in the real world, smokers may first attend primarily to the image, only beginning to direct their attention to other elements of the label—such as the detailed explanatory statements on the back of the pack—after several exposures. For these reasons, naturalistic studies that require smokers to carry real cigarette packets affixed with PWLs may be needed to further compare the effectiveness of PWLs with and without identifying statements and explanatory statements. Such studies, although resource intensive, have been shown to be a viable method of comparing the impact of different types of WLs (Brewer et al., 2016).
Second, it is possible that some effects may be attributable, in part, to the specific testimonial PWLs tested in this study, and various aspects of the way in which these messages were designed (besides the two manipulated textual elements). For instance, two of the five testimonial explanatory statements included a quote from the individual that comprised a direct plea for the audience to change their behavior (e.g., Terrie had some advice for other smokers: “Please quit…I don’t want anyone to have to go through what I went through.”). This may not be the most effective form for testimonial explanatory statements to take, especially if the direct plea draws the audience’s attention to the persuasive intent of the message and consequently elicits counterarguing or other forms of reactance (Kreuter et al., 2007). Such reactance may account for our finding that including a testimonial explanatory statement resulted in lower intentions to avoid the labels and lower rates of quit attempts, when compared to PWLs both without explanatory statements and with non-testimonial explanatory statements. Direct pleas may be particularly problematic when used in tobacco warning messages, given that many smokers are accustomed to receiving pressure to quit from their family and friends (Dunlop, Cotter, & Perez, 2014). Further research is required to assess whether direct pleas for behavior change from the sufferers of certain health conditions may be useful in other contexts.
Effectiveness of the identifying statements and testimonial explanatory statements may have also been affected by the demographic characteristics of the individuals featured in the PWLs. Kim and colleagues (2016) found that anti-smoking television advertisements were perceived to be more effective when the audience matched demographically with the smoker in the advertisement. Given variation in the demographic characteristics of the five real people featured in the testimonial PWLs (see Appendix B), participants in this study would have varied in the extent to which they perceived that they demographically matched with these case studies. While participants may have responded more favourably to those PWLs to which they were matched, this may have been offset by reduced effectiveness of the non-matched PWLs. Importantly though, a mix of ‘characters’ is likely to be adopted in PWL schemes implemented in the real world (Canadian Cancer Society, 2016). Along the same lines, rather than increasing identification with the individual in the image, it is possible that the additional personal details provided in the identifiers and testimonial explanatory statements emphasised the ways in which participants differed (in sex, age, and/or health status) from the individual, making it easier to self-exempt from these messages (Kreuter et al., 2007). Participants may have also self-exempted by assigning blame to other attributes of the individual, discounting the role of their tobacco use in causing these health effects (Bigman, Nagler, & Viswanath, 2016). Future studies that specifically examine smokers’ self-exempting responses to testimonial messages will help to elucidate the extent to which such reactions undermine the impact of these messages.
There are also countless other ways in which the testimonial PWLs tested here could have been designed to be more (or less) effective, including variations in the way the text was presented (e.g., font size, color and placement; number and explicitness of arguments; use of questions; congruency between text and images) and in certain features of the images used (e.g., expression, attractiveness and gaze direction of the person). Novel methodologies, such as the use of multiphase optimization strategies (MOST) (Collins, Kugler, & Gwadz, 2016), discrete choice experiments (Gendall, Eckert, Hoek, & Louviere, 2017; Thrasher, Anshari, et al., 2018) and eye tracking studies (Lochbuehler et al., 2018), may be particularly useful in helping to determine whether small variations in the way text and images are presented in PWLs contribute to, or detract from, overall effectiveness, and there is certainly a need for continued research into the impact of such manipulations.
An additional study parameter to keep in mind is that our findings relate only to the communication of health information about combustible tobacco cigarettes. There are certain contexts or situations in which the identifying statements and explanatory statements—and testimonial PWLs in general—may be expected to more effective. For instance, efforts to correct false beliefs about tobacco and its health effects—including to address the impact of misleading brand names, variants and other descriptors (Cappella, Maloney, Ophir, & Brennan, 2015)—may benefit from using non-testimonial and testimonial explanatory statements on PWLs, as these statements may provide the information necessary for audiences to question the basis for their previously held beliefs and update their mental models, which is required for false beliefs to be effectively corrected (Cappella et al., 2015; Lewandowsky, Ecker, Seifert, Schwarz, & Cook, 2012). The detailed information provided in the identifiers and explanatory statements may also be beneficial in health domains where audiences are learning about health consequences for the first time. Although there is still the need for ongoing communication about the health risks of combustible tobacco cigarettes, the public has been exposed to a greater volume of information on this topic relative to other types of tobacco products (e.g., smokeless tobacco), alternative nicotine delivery systems (e.g., e-cigarettes), and other harmful consumer products such as alcohol. Use of case studies, and the detailed explanatory statements, may help to make this new health information more believable and convincing. There is a need for more research attention to be directed towards WLs for these other products (Hassan & Shiu, 2018; Noar, Cappella, & Price, 2017; Thrasher, Brewer, et al., 2018), and the current study provides guidance for one methodological approach that could help create effective communications in these domains. It is also possible that the impact of the textual elements, and more generally, of different types of PWLs (e.g., testimonial vs. non-testimonial), depends on the specific health consequences being communicated, although this question also requires additional research.
Another context in which identifying statements and testimonial explanatory statements may be especially effective is when PWLs are accompanied by complementary mass media campaigns that provide even more detail about the individual’s story. Past research has shown that, in general, PWLs are more effective when accompanied by complementary mass media campaigns (Brennan, Durkin, Cotter, Harper, & Wakefield, 2011; Thrasher et al., 2013), and a recent study found that smokers thought about exemplars encountered in other mediums and contexts when responding to new exemplars in PWLs (Bigman et al., 2016). In the US, the successful (McAfee, Davis, Alexander, Pechacek, & Bunnell, 2013; McAfee et al., 2017) Tips From Former Smokers campaign has featured a series of case studies, three of which provided content for the testimonial PWLs tested here. If there was an opportunity to link the content of testimonial PWLs with that of a campaign such as Tips From Former Smokers, then it is possible that the identifiers and testimonial explanatory statements on the PWLs would serve to remind smokers of the information presented in the campaign, leading to stronger effects overall (Brennan et al., 2011; Thrasher et al., 2013).
Finally, in this study all explanatory statements (non-testimonial and testimonial) were paired with a testimonial image. It is possible that the non-testimonial explanatory statements in particular may be more useful when combined with the non-testimonial images currently employed in many PWLs, which usually comprise images of diseased or disfigured body parts (Canadian Cancer Society, 2016). This textual element may be required to add depth and meaning to the non-testimonial image, increasing both cognitive processing and the emotional impact of the image. This suggestion also raises the possibility that an optimal PWL scheme may be one that includes a mix of both testimonial and non-testimonial images, and then, as appropriate, some PWLs that do not contain any text beyond the basic warning statement (so that maximum space is dedicated to the powerful image) and some that do contain additional explanatory statements, allowing those smokers who wish to learn more about the health condition and the mechanisms through which smoking leads to the condition, to do so. Increased variation in message format within PWL schemes may prolong warning label salience, reducing the rate at which impact wears out (Hitchman, Driezen, Logel, Hammond, & Fong, 2014; Li, Borland, Yong, et al., 2015).
This study was conducted in the US and was primarily intended to inform the development of new PWLs for cigarette packs in this country, after the PWLs proposed by the FDA in 2011 were deemed unconstitutional and withdrawn from consideration. However, these findings may also be useful to jurisdictions that already have PWLs in place, as these governments consider options for revising and refreshing their policies (Hammond, Wakefield, Durkin, & Brennan, 2013; World Health Organization, 2011). In some cases, PWLs in these countries already employ identifiers and explanatory statements like the ones tested in this study (Canadian Cancer Society, 2016), albeit without a strong empirical basis to support their use. These findings are also relevant for researchers and policy makers considering the development of WLs for tobacco products other than combustible cigarettes, and for other harmful products such as alcohol. Although we have outlined several reasons why different effects of the textual elements may be observed in these contexts, a key takeaway message from this study is that not all WLs are created equally. It therefore remains as critical as ever that all WLs are thoroughly pre-tested before, and evaluated after, being released to the public, and that a range of traditional and more innovative methodological approaches are employed in this work (Thrasher, Brewer, et al., 2018). There is also an ongoing need for health communication researchers to continue building the evidence base regarding the features of PWLs that most contribute to, or detract from, overall effectiveness. By ensuring that the PWLs released to the public are designed to be maximally effective, such research has the potential to have a major impact on the public health burden attributable to tobacco use (Noar, Cappella, et al., 2017).
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the contribution of Mr Kyle Cassidy at the Annenberg School for Communication, University of Pennsylvania, who created the stimuli used in this study. We thank Sijia Yang at the Annenberg School for Communication, University of Pennsylvania for his advice on statistical analyses. We also thank Professor Robert Hornik, Dr Laura Gibson, Professor Caryn Lerman, Dr Andrew Strasser, and Professor Emily Falk at the University of Pennsylvania, Professor Melanie Wakefield at Cancer Council Victoria, Associate Professor David Hammond at University of Waterloo, and Professor Ellen Goodman at Rutgers, who all provided feedback on the original study design. Thank you also to staff at Health Canada and at the Office on Smoking and Health at the Centers for Disease Control and Prevention, who arranged access to the images and stories used in the testimonial pictorial warning labels.
Contributor Information
Emily Brennan, Centre for Behavioural Research in Cancer, Cancer Council Victoria
Erin Maloney, Penn Tobacco Center of Regulatory Science, Annenberg School for Communication, University of Pennsylvania
Yotam Ophir, Penn Tobacco Center of Regulatory Science, Annenberg School for Communication, University of Pennsylvania
Joseph N. Cappella, Penn Tobacco Center of Regulatory Science, Annenberg School for Communication, University of Pennsylvania
References
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10, 360–365. doi: 10.1037/0278-6133.10.5.360 [DOI] [PubMed] [Google Scholar]
- Bigman CA, Nagler RH, & Viswanath K (2016). Representation, exemplification, and risk: Resonance of tobacco graphic health warnings across diverse populations. Health Communication, 31, 974–987. doi: 10.1080/10410236.2015.1026430 [DOI] [PubMed] [Google Scholar]
- Braddock K, & Dillard JP (2016). Meta-analytic evidence for the persuasive effect of narratives on beliefs, attitudes, intentions, and behaviors. Communication Monographs, 83, 446–467. doi: 10.1080/03637751.2015.1128555 [DOI] [Google Scholar]
- Brennan E, Durkin S, Cotter T, Harper T, & Wakefield M (2011). Mass media campaigns designed to support new pictorial health warnings on cigarette packets: Evidence of a complementary relationship. Tobacco Control, 20, 412–418. doi: 10.1136/tc.2010.039321 [DOI] [PubMed] [Google Scholar]
- Brennan E, Durkin SJ, Wakefield MA, & Kashima Y (2014). Assessing the effectiveness of antismoking television advertisements: Do audience ratings of perceived effectiveness predict changes in quitting intentions and smoking behaviours? Tobacco Control, 23, 412–418. doi: 10.1136/tobaccocontrol-2012-050949 [DOI] [PubMed] [Google Scholar]
- Brennan E, Maloney EK, Ophir Y, & Cappella JN (2017). Potential effectiveness of pictorial warning labels that feature the images and personal details of real people. Nicotine & Tobacco Research, 19, 1138–1148. doi: 10.1093/ntr/ntw319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT, Hall MG, Lee JGL, Peebles K, Noar SM, & Ribisl KM (2016). Testing warning messages on smokers’ cigarette packages: A standardised protocol. Tobacco Control, 25, 153–159. doi: 10.1136/tobaccocontrol-2014-051661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KG, Reidy JG, Weighall AR, & Arden MA (2013). Graphic imagery is not sufficient for increased attention to cigarette warnings: The role of text captions. Addiction, 108, 820–825. doi: 10.1111/add.12008 [DOI] [PubMed] [Google Scholar]
- Cameron LD, & Williams B (2015). Which images and features in graphic cigarette warnings predict their perceived effectiveness? Findings from an online survey of residents in the UK. Annals of Behavioral Medicine, 49, 639–649. doi: 10.1007/s12160-015-9693-4 [DOI] [PubMed] [Google Scholar]
- Canadian Cancer Society. (2016). Cigarette package health warnings: International status report (5th edition) Retrieved 14 June 2018, from http://www.cancer.ca/~/media/cancer.ca/CW/for%20media/Media%20releases/2016/CCS-international-cigarette-packaging-report-2016-English.pdf?la=en
- Cappella JN, Maloney E, Ophir Y, & Brennan E (2015). Interventions to correct misinformation about tobacco products. Tobacco Regulatory Science, 1, 186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2016). National Adult Tobacco Survey (NATS) Retrieved 28 October 2016, from http://www.cdc.gov/tobacco/data_statistics/surveys/nats/
- Collins LM, Kugler KC, & Gwadz MV (2016). Optimization of multicomponent behavioral and biobehavioral interventions for the prevention and treatment of HIV/AIDS. AIDS and Behavior, 20, 197–214. doi: 10.1007/s10461-015-1145-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3, 7–16. doi: 10.1080/14622200124218 [DOI] [PubMed] [Google Scholar]
- Dunlop SM, Cotter T, & Perez D (2014). When your smoking is not just about you: Anti-smoking advertising, interpersonal pressure and quitting outcomes. Journal of Health Communication, 19, 41–56. doi: 10.1080/10810730.2013.798375 [DOI] [PubMed] [Google Scholar]
- Durkin S, Biener L, & Wakefield MA (2009). Effects of different types of antismoking ads on reducing disparities in smoking cessation among socioeconomic subgroups. American Journal of Public Health, 99, 2217–2223. doi: 10.2105/AJPH.2009.161638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin S, Wakefield MA, & Spittal MJ (2011). Which types of televised anti-tobacco campaigns prompt more quitline calls from disadvantaged groups? Health Education Research, 26, 998–1009. doi: 10.1093/her/cyr048 [DOI] [PubMed] [Google Scholar]
- Emery LF, Romer D, Sheerin KM, Jamieson KH, & Peters E (2014). Affective and cognitive mediators of the impact of cigarette warning labels. Nicotine & Tobacco Research, 16, 263–269. doi: 10.1093/ntr/ntt124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AT, Peters E, Strasser AA, Emery LF, Sheerin KM, & Romer D (2015). Graphic warning labels elicit affective and thoughtful responses from smokers: Results of a randomized clinical trial. PLoS ONE, 10, e0142879. doi: 10.1371/journal.pone.0142879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall P, Eckert C, Hoek J, & Louviere J (2017). Estimating the effects of novel on-pack warnings on young adult smokers and susceptible non-smokers. Tobacco Control, Published online first. doi: 10.1136/tobaccocontrol-2017-053719 [DOI] [PubMed] [Google Scholar]
- Gibson L, Brennan E, Momjian A, Shapiro-Luft D, Seitz H, & Cappella JN (2015). Assessing the consequences of implementing graphic warning labels on cigarette packs for tobacco-related health disparities. Nicotine & Tobacco Research, 17, 898–907. doi: 10.1093/ntr/ntv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman EP (2014). Visual gut punch: Persuasion, emotion and the constitutional meaning of graphic disclosure. Cornell Law Review, 99, 513–569. [PubMed] [Google Scholar]
- Green MC, & Brock TC (2000). The role of transportation in the persuasiveness of public narratives. Journal of Personality and Social Psychology, 79, 701–721. doi: 10.1037//0022-3514.79.5.701 [DOI] [PubMed] [Google Scholar]
- Hammond D, & Reid JL (2012). Health warnings on tobacco products: International practices. Salud Publica de Mexico, 54, 270–280. [DOI] [PubMed] [Google Scholar]
- Hammond D, Thrasher J, Reid JL, Driezen P, Boudreau C, & Santillan EA (2012). Perceived effectiveness of pictorial health warnings among Mexican youth and adults: A population-level intervention with potential to reduce tobacco-related inequities. Cancer Causes & Control, 23(Suppl 1), 57–67. doi: 10.1007/s10552-012-9902-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D, Wakefield M, Durkin S, & Brennan E (2013). Tobacco packaging and mass-media campaigns: Research needs for Articles 11 and 12 of the WHO Framework Convention on Tobacco Control. Nicotine & Tobacco Research, 15, 817–831. doi: 10.1093/ntr/nts202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan LM, & Shiu E (2018). A systematic review of the efficacy of alcohol warning labels: Insights from qualitative and quantitative research in the new millennium. Journal of Social Marketing, Published online first. doi: 10.1108/JSOCM-03-2017-0020 [DOI] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström K (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Hitchman SC, Driezen P, Logel C, Hammond D, & Fong GT (2014). Changes in effectiveness of cigarette health warnings over time in Canada and the United States, 2002–2011. Nicotine & Tobacco Research, 16, 536–543. doi: 10.1093/ntr/ntt196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L-L, Thrasher JF, Reid JL, & Hammond D (2016). Predictive and external validity of a pre-market study to determine the most effective pictorial health warning label content for cigarette packages. Nicotine & Tobacco Research, 18, 1376–1381. doi: 10.1093/ntr/ntv184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Bigman CA, Leader AE, Lerman C, & Cappella JN (2012). Narrative health communication and behavior change: The influence of exemplars in the news on intention to quit smoking. Journal of Communication, 62, 473–492. doi: 10.1111/j.1460-2466.2012.01644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Shi R, & Cappella JN (2016). Effect of character–audience similarity on the perceived effectiveness of antismoking PSAs via engagement. Health Communication, 31, 1193–1204. doi: 10.1080/10410236.2015.1048421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer JD, & Baig SA (2013). Analysis of legal and scientific issues in court challenges to graphic tobacco warnings. American Journal of Preventive Medicine, 45, 334–342. [DOI] [PubMed] [Google Scholar]
- Kreuter MW, Green MC, Cappella J, Slater MD, Wise ME, Storey D, . . . Woolley S (2007). Narrative communication in cancer prevention and control: A framework to guide research and application. Annals of Behavioral Medicine, 33, 221–235. [DOI] [PubMed] [Google Scholar]
- Lewandowsky S, Ecker UKH, Seifert CM, Schwarz N, & Cook JA (2012). Misinformation and its correction: Continued influence and successful debiasing. Psychological Science in the Public Interest, 13, 106–131. doi: 10.1177/1529100612451018 [DOI] [PubMed] [Google Scholar]
- Li L, Borland R, Fong GT, Jiang Y, Yang Y, Wang L, . . . Thrasher JF (2015). Smoking-related thoughts and microbehaviours, and their predictive power for quitting: findings from the International Tobacco Control (ITC) China Survey. Tobacco Control, 24, 354–361. doi: 10.1136/tobaccocontrol-2013-051384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Borland R, Yong H, Cummings KM, Thrasher JF, Hitchman SC, . . . Bansal-Travers M (2015). Longer term impact of cigarette package warnings in Australia compared with the United Kingdom and Canada. Health Education Research, 30, 67–80. doi: 10.1093/her/cyu074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochbuehler K, Mercincavage M, Tang KZ, Tomlin CD, Cappella JN, & Strasser AA (2018). Effect of message congruency on attention and recall in pictorial health warning labels. Tobacco Control, 27, 266–271. doi: 10.1136/tobaccocontrol-2016-053615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney EK, & Cappella JN (2016). Does vaping in e-cigarette advertisements affect tobacco smoking urge, intentions, and perceptions in daily, intermittent, and former smokers? Health Communication, 31, 129–138. doi: 10.1080/10410236.2014.993496 [DOI] [PubMed] [Google Scholar]
- McAfee T, Davis KC, Alexander RL, Pechacek TF, & Bunnell R (2013). Effect of the first federally funded US antismoking national media campaign. The Lancet, 382, 2003–2011. doi: 10.1016/S0140-6736(13)61686-4 [DOI] [PubMed] [Google Scholar]
- McAfee T, Davis KC, Shafer P, Patel D, Alexander R, & Bunnell R (2017). Increasing the dose of television advertising in a national antismoking media campaign: Results from a randomised field trial. Tobacco Control, 26, 19–28. doi: 10.1136/tobaccocontrol-2015-052517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer-Gusé E (2008). Toward a theory of entertainment persuasion: Explaining the persuasive effects of entertainment-education messages. Communication Theory, 18, 425–407. doi: 10.1111/j.1468-2885.2008.00328.x [DOI] [Google Scholar]
- Mutti S, Reid JL, Gupta PC, Pednekar MS, Dhumal G, Nargis N, . . . Hammond D (2016). Perceived effectiveness of text and pictorial health warnings for smokeless tobacco packages in Navi Mumbai, India, and Dhaka, Bangladesh: Findings from an experimental study. Tobacco Control, 25, 437–443. doi: 10.1136/tobaccocontrol-2015-052315 [DOI] [PubMed] [Google Scholar]
- Noar SM, Cappella JN, & Price S (2017). Communication regulatory science: Mapping a new field. Health Communication, Published online first. doi: 10.1080/10410236.2017.1407231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM, Francis DB, Bridges C, Sontag JM, Brewer NT, & Ribisl KM (2017). Effects of strengthening cigarette pack warnings on attention and message processing: A systematic review. Journalism & Mass Communication Quarterly, 94, 416–442. doi: 10.1177/1077699016674188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM, Francis DB, Bridges C, Sontag JM, Ribisl KM, & Brewer NT (2016). The impact of strengthening cigarette pack warnings: Systematic review of longitudinal observational studies. Social Science & Medicine, 164, 118–129. doi: 10.1016/j.socscimed.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noar SM, Hall MG, Francis DB, Ribisl KM, Pepper JK, & Brewer NT (2016). Pictorial cigarette pack warnings: A meta-analysis of experimental studies. Tobacco Control, 25, 341–354. doi: 10.1136/tobaccocontrol-2014-051978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonnemaker JM, Choiniere CJ, Farrelly MC, Kamyab K, & Davis KC (2015). Reactions to graphic health warnings in the United States. Health Education Research, 30, 46–56. doi: 10.1093/her/cyu036 [DOI] [PubMed] [Google Scholar]
- Ophir Y, Brennan E, Maloney EK, & Cappella JN (2017). The effects of graphic warning labels’ vividness on message engagement and intentions to quit smoking. Communication Research, Published online first. doi: 10.1177/0093650217700226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters E, Evans AT, Hemmerich N, & Berman M (2016). Emotion in the law and the lab: The case of graphic cigarette warnings. Tobacco Regulatory Science, 2, 404–413. doi: 10.18001/trs.2.4.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Sheer VC, & Li R (2015). Impact of narratives on persuasion in health communication: A meta-analysis. Journal of Advertising, 44, 105–113. doi: 10.1080/00913367.2015.1018467 [DOI] [Google Scholar]
- Strasser AA, Tang KZ, Romer D, Jepson C, & Cappella JN (2012). Graphic warning labels in cigarette advertisements: Recall and viewing patterns. American Journal of Preventive Medicine, 43, 41–47. doi: 10.1016/j.amepre.2012.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Survey Sampling International. (2016). Consumer online panel. Retrieved 28 October 2016, from https://www.surveysampling.com/audiences/consumer-online/
- Thrasher JF, Anshari D, Lambert-Jessup V, Islam F, Mead E, Popova L, . . . Lindblom EN (2018). Assessing smoking cessation messages with a discrete choice experiment. Tobacco Regulatory Science, 4, 73–87. doi: 10.18001/trs.4.2.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Arillo-Santillan E, Villalobos V, Perez-Hernandez R, Hammond D, Carter J, . . . Regalado-Pineda J (2012). Can pictorial warning labels on cigarette packages address smoking-related health disparities? Field experiments in Mexico to assess pictorial warning label content. Cancer Causes & Control, 23(Suppl 1), 69–80. doi: 10.1007/s10552-012-9899-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Brewer NT, Niederdeppe J, Peters E, Strasser AA, Grana R, & Kaufman AR (2018). Advancing tobacco product warning labels research methods and theory: A summary of a grantee meeting held by the US National Cancer Institute. Nicotine & Tobacco Research, Published online first. doi: 10.1093/ntr/nty017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Carpenter MJ, Andrews JO, Gray KM, Alberg AJ, Navarro A, . . . Cummings KM (2012). Cigarette warning label policy alternatives and smoking-related health disparities. American Journal of Preventive Medicine, 43, 590–600. doi: 10.1016/j.amepre.2012.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher JF, Murukutla N, Pérez-Hernández R, Alday J, Arillo-Santillán E, Cedillo C, & Gutierrez JP (2013). Linking mass media campaigns to pictorial warning labels on cigarette packages: A cross-sectional study to evaluate effects among Mexican smokers. Tobacco Control, 22, e57–e65. doi: 10.1136/tobaccocontrol-2011-050282 [DOI] [PubMed] [Google Scholar]
- Thrasher JF, Swayampakala K, Borland R, Nagelhout G, Yong H-H, Hammond D, . . . Hardin J (2016). Influences of self-efficacy, response efficacy, and reactance on responses to cigarette health warnings: A longitudinal study of adult smokers in Australia and Canada. Health Communication, 31, 1517–1526. doi: 10.1080/10410236.2015.1089456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Public Laws. Family Smoking Prevention and Tobacco Control Act. Public Law; 111–31 [H.R. 1256]. 2009. [Google Scholar]
- Wang A-L, Lowen SB, Romer D, Giorno M, & Langleben DD (2015). Emotional reaction facilitates the brain and behavioural impact of graphic cigarette warning labels in smokers. Tobacco Control, 24, 225–232. doi: 10.1136/tobaccocontrol-2014-051993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2011). WHO Framework Convention on Tobacco Control. Guidelines for implementation Article 5.3; Article 8; Articles 9 and 10; Article 11; Article 12; Article 13; Article 14. Retrieved 14 June 2018, from http://apps.who.int/iris/bitstream/10665/80510/1/9789241505185_eng.pdf
- Yong HH, Borland R, Thrasher JF, Thompson ME, Nagelhout GE, Fong GT, & Hammond D (2014). Mediational pathways of the impact of cigarette warning labels on quit attempts. Health Psychology, 33, 1410–1420. doi: 10.1037/hea0000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.