Abstract
Rodent tendons are widely used to study human pathologies such as tendinopathy and repair, and to address fundamental physiological questions about development, growth, and remodeling. However, how the gross morphology and multi‐scale hierarchical structure of rat tendons, such as the tail, plantaris, and Achilles tendons, compare with that of human tendons are unknown. In addition, there remains disagreement about terminology and definitions. Specifically, the definitions of fascicle and fiber are often dependent on diameter sizes, not their characteristic features, and these definitions impair the ability to compare hierarchical structure across species, where the sizes of the fiber and fascicle may change with animal size and tendon function. Thus, the objective of the study was to select a single species that is commonly used for tendon research (rat) and tendons with varying mechanical functions (tail, plantaris, Achilles) to evaluate the hierarchical structure at multiple length scales using histology, SEM, and confocal imaging. With the exception of the specialized rat tail tendon, we confirmed that in rat tendons there are no fascicles and the fiber is the largest subunit. In addition, we provided a structurally based definition of a fiber as a bundle of collagen fibrils that is surrounded by elongated cells, and this definition was supported by both histologically processed and unprocessed samples. In all rat tendons studied, the fiber diameters were consistently between 10 and 50 μm, and this diameter range appears to be conserved across larger species. Specific recommendations were made highlighting the strengths and limitations of each rat tendon as a research model. Understanding the hierarchical structure of tendon can advance the design and interpretation of experiments and development of tissue‐engineered constructs.
Keywords: hierarchical structure, imaging, multi‐scale, fiber, fascicle
Introduction
Rodent tendons are widely used to study human pathologies, such as tendinopathy and repair, and to address fundamental physiological questions about development, growth, and remodeling. Rat tail tendon is widely used because its structure is relatively simple, easy to isolate, and often available as waste tissue; however, rat tail tendon is highly specialized due to its relatively low load‐bearing mechanical function (Rowe, 1985; Strocchi et al. 1985; Bruneau et al. 2010; Hori et al. 2011). Rat plantaris and Achilles tendons are also popular model systems (Lambertz et al. 2000; Maffulli & Kader, 2002; Eliasson et al. 2007, 2009; Gumucio et al. 2014; Schwartz et al. 2015), and there is an increasing interest in plantaris tendon as it has recently been suggested to contribute to chronic Achilles tendinopathy (Lintz et al. 2011; van Sterkenburg et al. 2011a, 2011b). However, the gross morphology and multi‐scale hierarchical structure of these rat tendons are not fully understood.
Tendon hierarchical structure, spanning multiple length scales, is widely known, yet there remains disagreement about terminology and definitions, which hinders communication among scientists and interpretation of experimental results. For example, much of the literature uses the terms fiber and fiber bundle to arbitrarily refer to structures made of collagen. This general usage of ‘fiber’ can cause confusion when studies report fibrils (Nakagawa et al. 1989) and fascicles (Kastelic et al. 1978) as fibers. It is widely accepted that fascicle is the largest tendon subunit (Kastelic et al. 1978; O'Brien, 1997; Handsfield et al. 2016), followed by smaller subunits called fibers, and each fiber is composed of fibrils. However, the definitions of fascicle and fiber are often dependent on their size and not their characteristic features. These definitions impair the ability to compare species, where the sizes of a fiber and fascicle may change with animal sizes (Bear, 1952; Handsfield et al. 2016). In support of this notion, fascicle size in larger animals (Handsfield et al. 2016) is about the same size of an entire rat tendon. Fascicle should be defined as a bundle of fibers with distinct interfascicular matrix boundaries at the macro‐scale that is populated with round cells (Benjamin et al. 2008; Thorpe et al. 2017). We hypothesize that rat tendons do not have fascicles due to the small size, with the exception of the special case of rat tail tendon (Rowe, 1985; Strocchi et al. 1985). Precise definition and terminology of these hierarchical structures are crucial because tendon structures are directly related to physiology and mechanical function.
Another source of confusion is the existence of a fiber. While some include fiber as a part of tendon hierarchical structure schematics (O'Brien, 1997; Kannus, 2000; Wang, 2006), others exclude it (Kastelic et al. 1978; Szczesny & Elliott, 2014a), and the term fiber is used inconsistently. Moreover, when a fiber is defined, it is done by diameter size, which may be species‐dependent (Bear, 1952; Handsfield et al. 2016). This definition is problematic, and it is hard to distinguish between fibers from fascicle because they have been reported to have large and overlapping ranges of sizes (Kannus, 2000; Wang, 2006). An alternative way to define a fiber is to base it on another structural component such as cells. Elongated tendon cells reside parallel to the direction of fibrils, and a group of cells is aligned and connected by gap junction (McNeilly et al. 1996; Ralphs et al. 1998; Benjamin et al. 2008). These cells outline a bundle of collagen fibrils, and this bundle may be defined as a fiber. This definition is consistent with the developmental process of tendon, where multiple cells in contact deposit collagen between cell–cell junctions during early development (Kalson et al. 2015). At later stages of development, cells surround a bundle of collagen fibrils and grow in size (Kalson et al. 2015), supporting the hypothesis that cells may define the boundary of a fiber. In this study, we will test our hypothesis that fiber is defined by the surrounding cells.
Tendon hierarchical structures may also depend on the loading environment. For example, there are differences between low‐ and high‐stress bearing tendons in the multi‐scale mechanics (Thorpe et al. 2012, 2013a, 2015; Herod et al. 2016) and composition (Thorpe et al. 2016) in equine and bovine tendons. These differences between tendons with different mechanical functions suggest that their hierarchical organizations may be different (Handsfield et al. 2016). Thus, we selected the tail, plantaris, and Achilles tendons to represent tendons with different mechanical functions.
The objective of this study was to evaluate the hierarchical structure of rat tendons at multiple length scales to test our hypotheses that rat tendons, except for tail tendon, do not have fascicles due to the small size and that cells may define the boundary of a fiber. Because the hierarchical structure is likely to depend on the species and loading environment, we chose a single species that is widely used for tendon research (rat) and tendons with varying mechanical functions (tail, plantaris, Achilles). This study was designed to investigate the gross morphology at the macro‐scale and hierarchical structures, including fascicle and fiber, at the micro‐scale by using multiple imaging methods such as histology, scanning electron microscopy (SEM), and confocal imaging. In addition, we reconstructed a three‐dimensional (3D) structure from confocal imaging stacks. Understanding and properly defining tendon hierarchical structures are critical in research to advance the design and interpretation of experiments and development of tissue‐engineered constructs.
Methods
Sample preparation
All tendons were from 4‐ to 7‐month‐old female Long Evans rats sacrificed on the day of the experiment. Rat tail (n = 13), plantaris (n = 13), and Achilles (n = 13) tendons were divided into five experimental groups, where each group was investigated with a different imaging technique. Each tendon was processed for a longitudinal PicroSirius Red staining (n = 3/tendon type), transverse H&E staining (n = 3/tendon type), SEM (n = 3/tendon type), confocal imaging with second harmonic generation (SHG) signals and cells (n = 3/tendon type), or 3D rendering of confocal image stack (n = 1/tendon type).
Gross morphology
Rat tail tendon fascicle was dissected following the same protocol from published studies (Lee & Elliott, 2017; Lee et al. 2017). Plantaris and Achilles tendons were dissected using bone‐cutting shears, scalpels, and forceps to dissect the Achilles and plantaris complex, about 4 cm in length with the calcaneus attached (Fig. 1a, b). We separated out plantaris and Achilles tendons from each other by removing excess surrounding soft tissue (Fig. 1c, d). Each tendon was randomly assigned to an imaging group after the dissection. An additional Achilles tendon was further dissected using a sharp dissection and microscope to study how Achilles tendon is fused. We separated each sub‐tendon to verify that Achilles tendon is fused tightly. This sample was discarded after taking a reference picture (Fig. 1e, f) and was not used further in the study.
Figure 1.
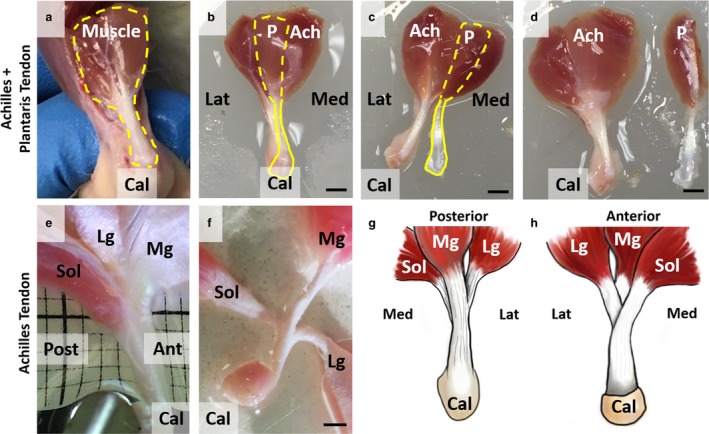
Gross morphology of plantaris and Achilles tendons. (a) Plantaris (P) and Achilles (Ach) tendons are bound together in situ (dashed yellow line). (b) Plantaris muscle is posterior to Achilles muscle complex (dashed yellow line), and plantaris tendon is visible above Achilles tendon (solid yellow line). (c) By cutting connective tissue binding plantaris and Achilles tendons from the insertion, these two tendons can be completely separated (d). (e) Achilles tendon was further dissected and is a fusion of three tendons originating from soleus (Sol), lateral gastrocnemius (Lg), and medial gastrocnemius (Mg) muscles. (f) Sub‐tendons are tightly fused, and further separation of tendons is difficult without directly cutting the tendon. Scale bars: 2 mm. We created schematics of (g) posterior and (h) anterior views to show the complex structure of Achilles tendon.
Histology
Histological analyses were performed in longitudinal and transverse directions to observe the hierarchical structure of tendons in both directions. Each tendon was fixed in 4% paraformaldehyde at room temperature. For the longitudinal histology, all tendons were fixed overnight. For the transverse histology, the tail fascicle was fixed for 30 min, plantaris tendon was fixed for 2 h, and Achilles tendon was fixed overnight. The fixed tendons were then incubated for 30 min in a 30% sucrose solution to avoid possible ice damage. To process as frozen sections, each tendon was mounted on a plastic mold with optimal cutting temperature compound (OCT) and was flash‐frozen with an indirect contact with liquid nitrogen. We used the CryoJane Tape Transfer System (Leica Biosystems) to cut 14‐μm‐thick sections for the longitudinal group and 12‐μm‐thick sections for the transverse group. We cut the mid‐plane of the tendon away from both anterior and superior planes. The longitudinal sections were stained with PicroSirius Red to visualize collagen alignment, and transverse sections were stained with H&E to visualize general organization. The staining process followed a standard staining procedure for frozen sections, and the slides were mounted to be visualized under Axio Imager 2 Pol (Carl Zeiss Inc.). Images were acquired at two different magnifications (20× and 40×).
Scanning electron microscopy
We imaged tendon structure using SEM to visualize the ultrastructure and confirm histological observations. Tendons used for SEM were processed following the same procedure as histology until sectioning. Using the cryostat, each tendon was cut once in a longitudinal direction of the mid‐plane to image the ultrastructure, and the sample was briefly placed in water to remove the remaining OCT. The section was processed for SEM using a standard processing technique consisting of fixation with 1% osmium tetroxide followed by a series of dehydration with ethanol. Each sample was dried using a critical point dryer, mounted on an SEM sample holder using carbon tabs, and coated with platinum (Leica Biosystems). The samples were visualized with a field emission SEM (Hitachi S‐4700).
Confocal imaging with second harmonic generation signals and cells
To test our hypothesis that cells outline fibers in tendon, we acquired SHG signals for the collagen alignment and fluorescence from cell for the cell placement. SHG microscopy is a nonlinear optical method that can visualize collagen without additional staining or processing (Fung et al. 2010a,b; Chen et al. 2012; Pang et al. 2017). Immediately after sacrifice and dissection, the tendon was incubated in Dulbecco's modified Eagle medium (DMEM) culture medium supplemented with 25 mm HEPES, 1% penicillin, and 1% streptomycin (Thermo Fisher Scientific) for 30 min at 37 °C. Each tendon was stained with 10 μm CellTracker Green CMFDA dye (Thermo Fisher Scientific) for 45 min at 37 °C and washed in the medium for 30 min at 37 °C. Each sample was then placed in a Petri dish for imaging with a laser‐scanning multi‐photon microscope (LSM 780, objective Plan‐Apochromat 20 × /1.0, Carl Zeiss Inc.). We used Chameleon Vision II Ti:S laser (Coherent Inc.) tuned to 810 nm and an external detector to collect SHG signals. The maximum penetration depth was ~ 100 μm. A coverglass was placed on top of the tendon to prevent it from floating. The confocal image stacks (0.42 × 0.42 × 0.67 μm pixel−1) were taken at the mid‐substance of each tendon. The SHG signals of the most superficial (closest to the skin) and deep layers (furthest from the skin) were separated from the rest of the stack to show the change of collagen alignment through the depth. To show the cell placement, an optical slice that showed the strongest cell staining in the deep plane was separated from the confocal stack.
Three‐dimensional (3D) rendering of confocal images
To observe collagen alignment and cell placement in 3D, a separate confocal image stack from above, spanning ~ 50 μm in the z‐direction including the surface of tendons, was acquired with the same method as the previous section. The confocal image stacks were rendered by software AMIRA 6.3 (Thermo Fisher Scientific). The blood vessels and cells were selected using an automatic threshold method under a segmentation editor. Additional structures that were not captured by the automatic segmentation were selected manually. The surface of vessels and cells was rendered, and the volume of the SHG signal of collagen was rendered to show the 3D structure of tendon. The opacity of collagen was adjusted to show the 3D structure through the depth. A video of the 3D structure was created using AMIRA 6.3 animation function (Supporting Information Videos [Link], [Link], [Link]).
Results
Gross morphology of plantaris and Achilles tendons
The gross morphology of the plantaris (P) and Achilles (Ach) tendon demonstrated its structural complexity. Plantaris and Achilles tendons are bound together in vivo (Fig. 1a). The plantaris muscle originates on the anterior‐lateral side of the Achilles muscle, passes over the calcaneus (Cal), and inserts into the bottom of the foot. Once the calcaneus is cut, the plantaris and Achilles tendons can be easily distinguished from each other. The plantaris muscle is posterior to the Achilles muscle complex (Fig. 1b, dashed yellow line), and the plantaris tendon is visible anterior to the Achilles tendon (solid yellow line). We separated the two tendons by cutting connective tissue binding the plantaris and Achilles tendons together at the insertion (Fig. 1c,d). Human plantaris tendon has been previously described as a vestigial tissue that is absent in a large portion of the population (Harvey et al. 1983; Simpson et al. 1991; Freeman et al. 2008; Nayak et al. 2010). However, recent studies identified plantaris tendon in all or most cadavers with variable insertion sites (Saxena & Bareither, 2000; dos Santos et al. 2009; van Sterkenburg et al. 2011a). We observed a single insertion site for all rat plantaris tendons, unlike human plantaris tendons. The Achilles tendon was further dissected to observe its gross morphology, and we verified that the Achilles tendon is a fusion of three sub‐tendons originating from soleus (Sol), lateral gastrocnemius (Lg), and medial gastrocnemius (Mg) (Fig. 1e) muscles, similar to human (Szaro et al. 2009; Bojsen‐Møller & Magnusson, 2015). The sub‐tendons from these muscles are tightly fused for approximately half of the tendon length and cannot be further separated without directly cutting the tendon (Fig. 1f). Among the three muscle groups, the Sol tendon is the most anterior sub‐tendon, and Mg tendon is the most posterior sub‐tendon. Schematics of the posterior (Fig. 1g) and anterior (Fig. 1h) views show the complex 3D morphological structure of Achilles tendon.
Histological evaluation of tendon micro‐scale structure
To investigate the hierarchical structure and cell morphology in rat tendons, we conducted several analyses using multiple imaging methods. In longitudinal sections stained with PicroSirius Red, we observed a separation between structures with a diameter of 10–50 μm at both low (Fig. 2a‐c) and high magnifications (Fig. 2d‐f) for all three tendons. The hierarchical structure defined by this unit of separation, highlighted in white arrows, is likely to be a fiber. Supporting this notion, we also observed structures with a similar diameter in a longitudinal view of SEM in all tendons, highlighted in green (Fig. 2g‐i). In addition, the transverse sections stained with H&E also had separations with a similar diameter at low (Fig. 3a‐c) and high magnifications (Fig. 3d‐f), outlined in blue. Rat tail tendon fascicle did not have a similar pattern of separation between the subunits as seen in plantaris and Achilles tendons in the transverse direction, perhaps due to its small overall size. Nonetheless, the distance between cells of tail fascicle (blue arrows, Fig. 3d) was similar to the separation observed in plantaris and Achilles tendons (blue outlines, Fig. 3e,f). Note that the observed separation between fibers is likely due to loss of water and non‐collagenous matrix during sample processing.
Figure 2.
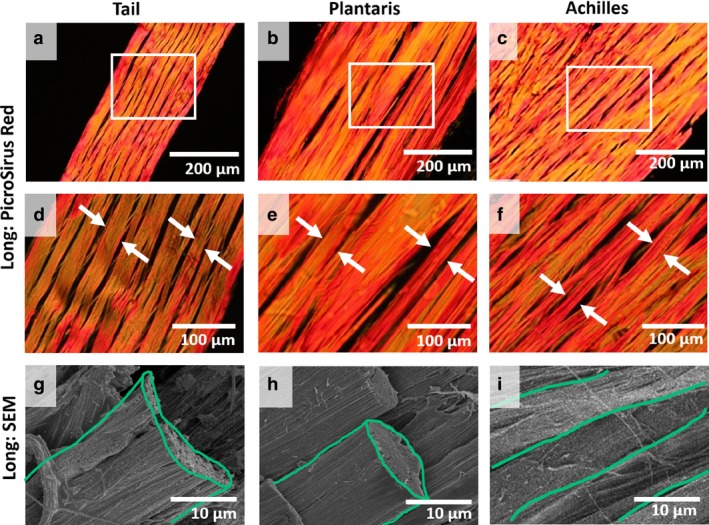
Evaluation of fiber structure in longitudinal direction. (a‐c) Rat tail, plantaris, and Achilles tendons were cut in longitudinal (Long) direction, stained with PicroSirius Red, and viewed at low magnification. White boxes represent areas observed at (d‐f) high magnifications, and fibers are highlighted (white arrows). (g‐i) Longitudinal SEM shows fibers (green) with a similar diameter as observed in histology. No fascicle boundary was observed.
Figure 3.
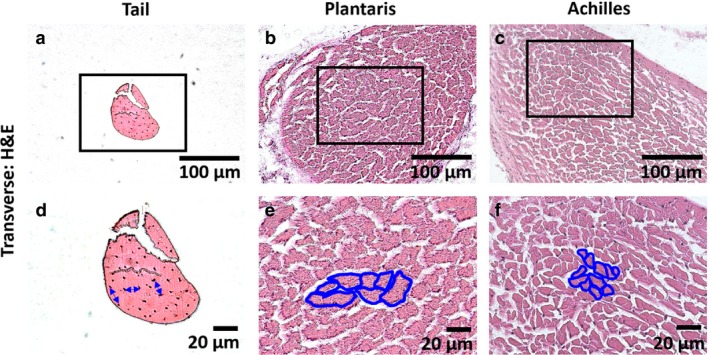
Evaluation of fiber structure in transverse direction. (a‐c) Rat tail, plantaris, and Achilles tendons were cut in the transverse direction, stained with H&E, and viewed at low magnification. Black boxes represent areas observed at (d‐f) high magnifications. The distance between cells are highlighted (blue arrow) for the rat tail tendon, and fibers in plantaris and Achilles tendons are outlined (blue). No fascicle boundary was observed.
SHG evaluation of fiber structure and cell morphology
The collagen structure and cell morphology were visualized using the confocal microscope to confirm histological findings without the potential fixation and histological artifacts. At the deep plane (away from the surface of the tendon), all tendons had aligned collagen (Fig. 4a‐c). At the superficial plane (the surface of tendon), tail tendon fascicle had no change in collagen alignment from that at the deep planes (Fig. 4d), but both plantaris and Achilles tendons had an additional layer of peritenon, a complex meshwork of collagen that was about 20 μm thick (Fig. 4e,f). In deep planes, the cells with elongated morphology aligned along collagen bundles (white arrows) that were the same diameter as observed histologically (Figs 2 and 3). Thus, rat tendons have a single hierarchical structure that is surrounded by the elongated cell, and this structure had consistent diameters between 10 and 50 μm; hereafter defined as fiber (Fig. 4g–i).
Figure 4.
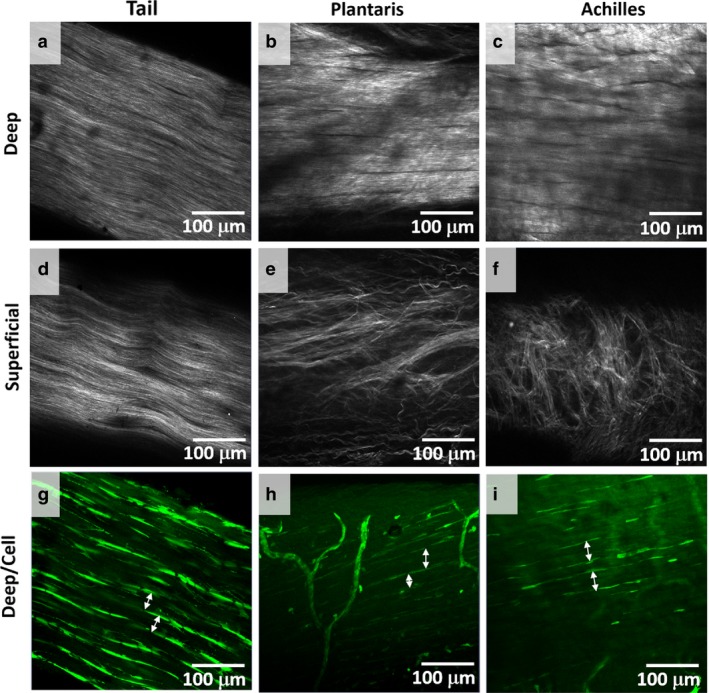
Collagen alignment and cell morphology. (a‐c) Collagen alignment was viewed with SHG signals. Collagen was aligned at deep planes, away from the skin, for tail, plantaris, and Achilles tendons. (d‐f) Whereas the collagen alignment was not different between the superficial, close to the skin, and deep planes for the tail tendon, plantaris and Achilles tendons had an additional layer of a complex meshwork of collagen or peritenon. (g,h) In deep planes, elongated cells outlined collagen bundle (white arrows), which matched the sizes of fiber observed with histology. Thus, we define fiber as a bundle of collagen with diameter 10–50 μm outlined by elongated cells. No fascicle boundary was observed.
Three‐dimensional evaluation of tendon
The confocal image stacks were reconstructed to view the 3D tendon structure (Fig. 5). The 3D reconstruction confirms that cells, shaded in green, surround the collagen bundles (gray) that match the size of the fiber that was identified by histology (Figs 2 and 3). For the tail tendon fascicle, we again observed no peritenon (Fig. 5a), but we did observe peritenon for the plantaris and Achilles tendons. The peritenon was populated with blood vessels, characterized by high fluorescent density and a web‐like network that wraps the surface, and with localized cells with different morphology than the elongated cells between fibers, shaded in red (Fig. 5b,c). In the transverse direction, the 3D structure of tendon shows that cells surround the fiber, highlighted with yellow arrows (Fig. 5d‐f). Similarly, the cells surround the fiber in an oblique view, highlighted with yellow arrows (Fig. 5g‐i). Again, we observed no additional boundary. The video of the 3D reconstructions are included as Videos [Link], [Link], [Link]. Thus, the 3D rendering of tendon confirms that the definition of a fiber is a bundle of collagen that is outlined by elongated cells, supported by both histologically processed and unprocessed samples. In all rat tendons studied, the fiber diameters were consistently 10–50 μm.
Figure 5.
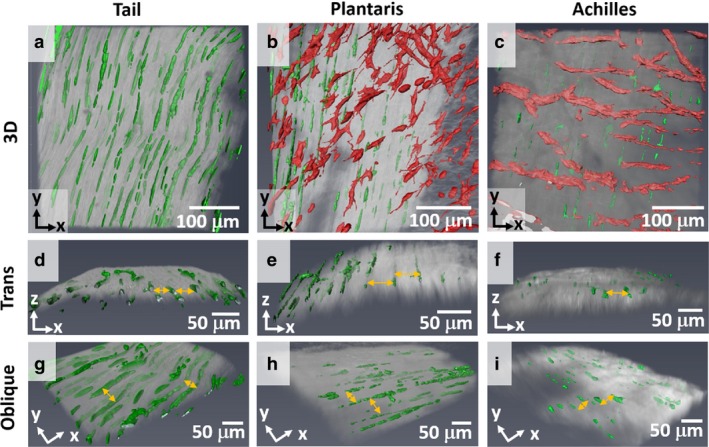
3D structure of tendon. (a‐c) 3D structure of tendon was rendered from a confocal imaging stack (50 μm deep in z‐direction) to show the overall cell morphology and collagen alignment. All tendons show that cells in the deep region (green) outline collagen (gray). For plantaris and Achilles, blood vessels and cells on peritenon (red) were observed, unlike rat tail tendon. (d‐f) Transverse view, x–z plane, without blood vessels further illustrates that elongated cells are inbetween adjacent fibers (yellow arrows) with a consistent diameter. (g‐i) Oblique view, x–y plane, shows a spacing between collagen (yellow arrows). The opacity of collagen was adjusted to show the 3D structure.
There were no additional boundaries that further divided any of the rat tendons. We confirmed that in rat tendons there are no fascicles and the fiber is the largest subunit. Whereas we call the sub‐structure in rat tail tendon a fascicle, rat tail tendon is a specialized tendon. Fiber may be the largest subunit in rat tendons, and this diameter was consistent in transverse and longitudinal histology and SEM.
Discussion
In this study, we evaluated the hierarchical structure of rat tendons at multiple length scales for tendons with varying mechanical functions, including the tail, plantaris, and Achilles tendons, and created tendon‐specific schematics based on our observations (Fig. 6). Using multiple imaging methods, we confirmed that rat tendons do not contain fascicles, with the exception of the specialized rat tail tendon, and thus fiber is the largest tendon subunit in the rat. In addition, we provided a structurally based definition of a fiber as a bundle of collagen fibrils that is surrounded by elongated cells, and this definition was supported by both histologically processed and unprocessed samples. In all rat tendons studied, the fiber diameters were consistently 10–50 μm.
Figure 6.
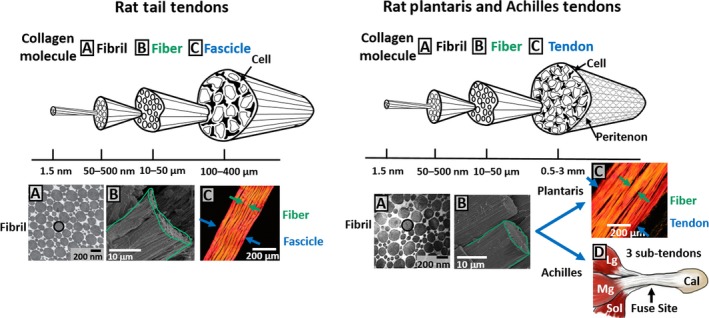
Schematic of hierarchical structure of rat tendons. Hierarchical structure of rat tail and plantaris and Achilles tendons were recreated based on the evidence from this study. Data incorporated in the schematic were adapted from Starborg et al. (2013), Szczesny & Elliott (2014b), and de Almeida Mdos et al. (2015).
Structure‐based definitions of the fascicle and fiber
This study provides hierarchical schematics of rat tendons that clarify structural‐based definitions of fibers and fascicles (Fig. 6). A fascicle is defined by the surrounding interfascicular matrix, which is populated with round cells (Benjamin et al. 2008; Thorpe et al. 2017) and is not simply a bundle of fibers. With the exception of the specialized rat tail tendon, we confirmed that there are no fascicles and the fiber is the largest subunit in rat tendons. In addition, no additional boundary was observed in a supplementary observation of SHG signals of rat patellar tendon in this study (Supporting Information Appendix S1) and a previously published study (Fung et al. 2010b), further verifying that rat tendons do not have fascicles. Considering the size of rat tendon, it is not surprising that the structural complexity is reduced with the scale of the animal (Handsfield et al. 2016). In large animals, the fascicle and interfascicular matrix are easily identified and have diameters that are larger than the entire rat tendons (Kastelic et al. 1978; O'Brien, 1997; Kannus, 2000; Wang, 2006; Handsfield et al. 2016). In the rat tendons studied here, the fiber diameter was 10–50 μm. Although this is not the first paper to identify the fiber in rat tendons (Rowe, 1985), this study provides evidence and a definition of a fiber that is based on characteristic features, confirmed by multiple imaging methods. Importantly, this definition is consistent with the developmental process of tendon. At later stages of development, as cells deposit more collagen, the tendon fiber is delineated as cells are pushed to the periphery (Kalson et al. 2015). To avoid confusion, the term fiber should be reserved specifically for this hierarchical structure of tendon, and not to generally refer to a bundle of collagen.
As a point of clarification, the visualizations of cell density in confocal images and 3D rendering can be misleading because they depend on staining intensity. Because blood vessels and cells on the peritenon can also take up fluorophore, less fluorophore penetrated to the deeper planes of the plantaris and Achilles tendons. In addition, the diameters of plantaris and Achilles tendons are larger than that of the tail tendon fascicle, contributing to the lack of fluorophore penetration. While it may appear that cell density in the tail fascicle is higher than in the plantaris and Achilles tendons, this is strictly due to the penetration of fluorophore, and is the cause for the reduced number of cells visualized in these tendons.
Comparative anatomy across species
We considered whether the fiber and fascicle sizes can be conserved across species. Tendons from larger animals such as horse and human have a subunit that falls within the fiber diameter and is outlined by cells, confirming these units as fibers (Franchi et al. 2007; Benjamin et al. 2008; Ali et al. 2018). For example, the equine tendon fiber diameter is 1–20 μm (Thorpe et al. 2012; Handsfield et al. 2016) and the human tendon primary fiber bundle diameter is 15 μm (Kannus, 2000), both similar to the fiber diameter of 10–50 μm reported here. Thus, the definition of fiber appears to be applied across species, and this supports the idea that the organization of rat tendon is not a simple downscaled version of larger animals, but that the fiber size is conserved across species. It is likely that mouse tendon only has fibers, not fascicles, similar to rat tendon, due to its small size. Tendon cell volumes (Savage et al. 2007) also appear to be consistent across species, which suggests that cells and fibers are the key units in growing aligned collagen region during the developmental process. It is unclear if the fascicle diameters are consistent across species, and it is possible that the number of fascicles or the size of fascicles may scale with the size of the animal.
The absence of fascicles in rat tendons should impact a researcher's animal model choice; studies that are focused on the structure and mechanics of the interfascicular matrix should exclude rat models. Interestingly, age‐related tendinopathy is mostly attributed to the degeneration of interfascicular matrix (Thorpe et al. 2013b; Godinho et al. 2017) and animals with the interfascicular matrix, such as horses and human, develop naturally occurring age‐related tendinopathy (Patterson‐Kane et al. 2012), whereas rats do not appear to do so. While this difference may be due to the animal's life‐span and activity level, studies of age‐related tendinopathy should be aware that rats lack fascicles, and thus interfascicular matrix. Nonetheless, the rat tendon is a useful model system when studying tendon structure and mechanics, because rat tendon is a simpler model system, especially as the fiber structure and diameter is conserved across species.
Comparative anatomy of rat tendons
We demonstrated that the anatomies of the rat tail, plantaris, and Achilles tendons have distinct features that are related to their mechanical functions, and each tendon can be used to answer specific research questions. The structure of tail fascicle is simple, and its mechanics are less variable, making it a suitable model to address fundamental research questions. However, the tail fascicle is limited in that it bears low stresses and lacks peritenon, cells on peritenon, and blood vessels. Thus, it may not be a suitable model for studying research questions related to high‐stress tendon or healing processes.
The plantaris tendon, on the other hand, bears a high‐stress in vivo (Rijkelijkhuizen et al. 2005) and has a physiological structure that includes the muscle‐tendon junction and blood vessels. In addition, synergist ablation, an established rat model applied to study overuse‐induced muscle hypertrophy (Goodman et al. 2011; Kirby et al. 2016; Terena et al. 2017), may be a useful model to study tendinopathy. For example, the synergist ablation model where the Achilles is removed to overload the synergist plantaris tendon, leads to increased matrix production and thickened plantaris tendon after 4 weeks (Gumucio et al. 2014; Schwartz et al. 2015). This suggests that this model may represent tendinopathic changes.
The Achilles tendon also bears high stress, and we observed a similarity between the rat and human Achilles tendons. The major similarity is that both tendons are composed of three sub‐tendons extending from medial and lateral gastrocnemius and soleus muscles with a complex arrangement (Szaro et al. 2009; Bojsen‐Møller & Magnusson, 2015). Due to its complex structure, the Achilles tendon is difficult to study at multiple length scales. Because of the fusion of three tendons, there is likely an inter‐tendon sliding (Handsfield et al. 2017) in addition to the micro‐scale sliding. This likely contributes to the inhomogeneous loading observed in vivo (Slane & Thelen, 2014; Franz et al. 2015; Handsfield et al. 2017). This structural complexity should be addressed when using the Achilles tendon as a model system.
While we and others have referred to the dissected substructure from a rat tail tendon as a ‘fascicle’, we want to stress that the rat tail fascicle is different from the fascicle in larger animal tendons. The tail tendon has a sheath that divides individual fascicles populated with round cells (Rowson et al. 2016); fascicles in larger animal tendons have interfascicular matrix that is populated with round cells (Benjamin et al. 2008; Thorpe et al. 2017). However, this sheath in the tail tendon and the interfascicular matrix of larger animals are distinctly different. In large animals, surrounding interfascicular matrix boundaries are easily visible at the macro‐scale (Thorpe et al. 2015), yet the sheath around rat tail tendon is only observable at the micro‐scale (Rowe, 1985; Strocchi et al. 1985). The fascicles of larger animals are bound tightly to each other and must be cut to be separated (Thorpe et al. 2015). The interfascicular matrix bears a small but measurable load and can resist deformation (Thorpe et al. 2012), whereas the sheath in rat tail tendon can be easily peeled off with no resistance. Thus, the rat tail fascicle is a specialized tendon with a unique hierarchical structure and should not be directly compared with the fascicle in larger animals.
Multi‐scale tendon structure‐function and damage mechanics
Tendon structure and mechanics are related at multiple scales, and the fiber scale is likely to be an important contributor to tendon mechanics and damage. For example, the micro‐scale structural damage in the tail tendon is related to the changes in macro‐scale mechanical parameters (Lee et al. 2017). Micro‐scale sliding, which represents shear between micro‐scale structures, is related to both loading (Szczesny & Elliott, 2014b) and damage (Lee et al. 2017) mechanisms. Because these studies used photobleached lines and optical microscopy, which cannot distinguish between fibrils and fibers, both substructures are captured in the term ‘micro‐scale’ (Szczesny & Elliott, 2014b; Lee et al. 2017). Other studies have shown damage that is localized within fibers. Rat tail tendon that is loaded and then labeled with collagen hybridizing peptide (CHP), which binds to denatured collagen, showed a localization of CHP staining in a structure that had the same diameter as fiber observed here (Zitnay et al. 2017). A similar observation was made in rat flexor carpi ulnaris tendon after mechanical loading, where CHP was localized in a structure with the same diameter as fiber (Szczesny et al. 2018). In addition, the 3D reconstruction of SHG signals showed that the space between fibers increased in loaded rat patellar tendon, suggesting that damage is related to structural changes in fiber (Fung et al. 2010b). Therefore, the fiber is likely to be a key substructure affected during tendon damage.
In summary, this study evaluated the hierarchical structure of rat tail, plantaris, and Achilles tendons at multiple length scales. Structurally based definitions of fascicles and fibers were established using multiple imaging methods. With the exception of the rat tail tendon, we confirmed that rat tendons do not contain fascicles, and fiber is defined as a bundle of collagen fibrils that is surrounded by elongated cells. In all rat tendons studied, the fiber diameters were consistently between 10 and 50 μm, and this diameter range appears to be conserved across larger species. Specific recommendations were made highlighting the strengths and limitations of each rat tendon as a research model.
Authors’ contributions
A.H.L. and D.M.E. designed experiments. A.H.L. performed experiments and analyzed data. A.H.L. and D.M.E. wrote the manuscript.
Supporting information
Appendix S1 SHG signal of rat patellar tendon.
Video S1 Rat tail tendon 3D structure rendered and sliced to show collagen and cell morphology.
Video S2 Plantaris tendon 3D structure rendered and sliced to show collagen and cell morphology.
Video S3 Plantaris tendon 3D structure rendered and sliced to show collagen and cell morphology.
Acknowledgement
This research was supported and made possible by National Institutes of Health grant No. R01EB002425, National Institute of General Medical Sciences grant No. P20 GM103446, the National Science Foundation grant No. IIA‐1301765, National Institutes of Health shared instrumentation grant No. S10 RR027273, and the State of Delaware. We thank the Bioimaging Center at the Delaware Biotechnology Institute, Dr. Jeff Caplan, and Ryan Locke for supporting data acquisition. We thank Francis Karani for providing a post‐mortem rats.
References
- Ali O, Comerford E, Clegg P, et al. (2018) Variations during ageing in the three‐dimensional anatomical arrangement of fascicles within the equine superficial digital flexor tendon. Eur Cell Mater 35, 87–102. [DOI] [PubMed] [Google Scholar]
- de Almeida Mdos S, de Freitas KM, Oliveira LP, et al. (2015) Acupuncture increases the diameter and reorganisation of collagen fibrils during rat tendon healing. Acupunct Med 33, 51–57. [DOI] [PubMed] [Google Scholar]
- Bear RS (1952) The structure of collagen fibrils. Adv Protein Chem 7, 69–160. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Kaiser E, Milz S (2008) Structure‐function relationships in tendons: a review. J Anat 212, 211–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojsen‐Møller J, Magnusson SP (2015) Heterogeneous loading of the human Achilles tendon in vivo. Exerc Sport Sci Rev 43, 190–197. [DOI] [PubMed] [Google Scholar]
- Bruneau A, Champagne N, Cousineau‐Pelletier P, et al. (2010) Preparation of rat tail tendons for biomechanical and mechanobiological studies. J Vis Exp 41, e2176–e2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Nadiarynkh O, Plotnikov S, et al. (2012) Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat Protoc 7, 654–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson P, Fahlgren A, Pasternak B, et al. (2007) Unloaded rat Achilles tendons continue to grow, but lose viscoelasticity. J Appl Physiol 103, 459–463. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Andersson T, Aspenberg P (2009) Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol 107, 399–407. [DOI] [PubMed] [Google Scholar]
- Franchi M, Trirè A, Quaranta M, et al. (2007) Collagen structure of tendon relates to function. Sci World J 7, 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Slane LC, Rasske K, et al. (2015) Non‐uniform in vivo deformations of the human Achilles tendon during walking. Gait Posture 41, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman AJ, Jacobson NA, Fogg QA (2008) Anatomical variations of the plantaris muscle and a potential role in patellofemoral pain syndrome. Clin Anat 21, 178–181. [DOI] [PubMed] [Google Scholar]
- Fung DT, Wang VM, Andarawis‐Puri N, et al. (2010a) Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech 43, 274–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung DT, Sereysky JB, Basta‐Pljakic J, et al. (2010b) Second harmonic generation imaging and Fourier transform spectral analysis reveal damage in fatigue‐loaded tendons. Ann Biomed Eng 38, 1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godinho MSC, Thorpe CT, Greenwald SE, et al. (2017) Elastin is localised to the interfascicular matrix of energy storing tendons and becomes increasingly disorganised with ageing. Sci Rep 7, 9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Mabrey DM, Frey JW, et al. (2011) Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J 25, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Korn MA, Saripalli AL, et al. (2014) Aging‐associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg 23, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handsfield GG, Slane LC, Screen HRC (2016) Nomenclature of the tendon hierarchy: an overview of inconsistent terminology and a proposed size‐based naming scheme with terminology for multi‐muscle tendons. J Biomech 49, 3122–3124. [DOI] [PubMed] [Google Scholar]
- Handsfield GG, Inouye JM, Slane LC, et al. (2017) A 3D model of the Achilles tendon to determine the mechanisms underlying nonuniform tendon displacements. J Biomech 51, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey FJ, Chu G, Harvey PM (1983) Surgical availability of the plantaris tendon. J Hand Surg Am 8, 243–247. [DOI] [PubMed] [Google Scholar]
- Herod TW, Chambers NC, Veres SP (2016) Collagen fibrils in functionally distinct tendons have differing structural responses to tendon rupture and fatigue loading. Acta Biomater 42, 296–307. [DOI] [PubMed] [Google Scholar]
- Hori H, Fukutani T, Nakane H, et al. (2011) Participation of ventral and dorsal tail muscles in bending movements of rat tail. Anat Sci Int 86, 194–203. [DOI] [PubMed] [Google Scholar]
- Kalson NS, Lu Y, Taylor SH, et al. (2015) A structure‐based extracellular matrix expansion mechanism of fibrous tissue growth. Elife 4, e05958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannus P (2000) Structure of the tendon connective tissue. Scand J Med Sci Sports 10, 312–320. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E (1978) The multicomposite structure of tendon. Connect Tissue Res 6, 11–23. [DOI] [PubMed] [Google Scholar]
- Kirby TJ, McCarthy JJ, Peterson CA, et al. (2016) Synergist ablation as a rodent model to study satellite cell dynamics in adult skeletal muscle. Methods Mol Biol 1460, 43–52. [DOI] [PubMed] [Google Scholar]
- Lambertz D, Pérot C, Almeida‐Silveira MI, et al. (2000) Changes in stiffness induced by hindlimb suspension in rat Achilles tendon. Eur J Appl Physiol 81, 252–257. [DOI] [PubMed] [Google Scholar]
- Lee AH, Elliott DM (2017) Freezing does not alter multiscale tendon mechanics and damage mechanisms in tension. Ann N Y Acad Sci 1409, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Szczesny SE, Santare MH, et al. (2017) Investigating mechanisms of tendon damage by measuring multi‐scale recovery following tensile loading. Acta Biomater 57, 363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintz F, Higgs A, Millett M, et al. (2011) The role of plantaris longus in Achilles tendinopathy: a biomechanical study. Foot Ankle Surg 17, 252–255. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Kader D (2002) Tendinopathy of Tendo Achillis. Bone Joint J 84, 1–8. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, et al. (1996) Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat 189, 593–600. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Totsuka M, Sato T, et al. (1989) Effect of disuse on the ultrastructure of the achilles tendon in rats. Eur J Appl Physiol Occup Physiol 59, 239–242. [DOI] [PubMed] [Google Scholar]
- Nayak SR, Krishnamurthy A, Ramanathan L, et al. (2010) Anatomy of plantaris muscle: a study in adult Indians. Clin Ter 161, 249–252. [PubMed] [Google Scholar]
- O'Brien M (1997) Structure and metabolism of tendons. Scand J Med Sci Sports 7, 55–61. [DOI] [PubMed] [Google Scholar]
- Pang X, Wu JP, Allison GT, et al. (2017) Three dimensional microstructural network of elastin, collagen, and cells in Achilles tendons. J Orthop Res 35, 1203–1214. [DOI] [PubMed] [Google Scholar]
- Patterson‐Kane JC, Becker DL, Rich T (2012) The pathogenesis of tendon microdamage in athletes: the horse as a natural model for basic cellular research. J Comp Pathol 147, 227–247. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Benjamin M, Waggett AD, et al. (1998) Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat 193, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijkelijkhuizen JM, Baan GC, de Haan A, et al. (2005) Extramuscular myofascial force transmission for in situ rat medial gastrocnemius and plantaris muscles in progressive stages of dissection. J Exp Biol 208, 129–140. [DOI] [PubMed] [Google Scholar]
- Rowe RW (1985) The structure of rat tail tendon. Connect Tissue Res 14, 9–20. [DOI] [PubMed] [Google Scholar]
- Rowson D, Knight MM, Screen HRC (2016) Zonal variation in primary cilia elongation correlates with localized biomechanical degradation in stress deprived tendon. J Orthop Res 34, 2146–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos MA, Bertelli JA, Kechele PR, et al. (2009) Anatomical study of the plantaris tendon: reliability as a tendo‐osseous graft. Surg Radiol Anat 31, 59–61. [DOI] [PubMed] [Google Scholar]
- Savage VM, Allen AP, Brown JH, et al. (2007) Scaling of number, size, and metabolic rate of cells with body size in mammals. Proc Natl Acad Sci USA 104, 4718–4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Bareither D (2000) Magnetic resonance and cadaveric findings of the incidence of plantaris tendon. Foot Ankle Int 21, 570–572. [DOI] [PubMed] [Google Scholar]
- Schwartz AJ, Sarver DC, Sugg KB, et al. (2015) p38 MAPK signaling in postnatal tendon growth and remodeling. PLoS ONE 10, e0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SL, Hertzog MS, Barja RH (1991) The plantaris tendon graft: an ultrasound study. J Hand Surg Am 16, 708–711. [DOI] [PubMed] [Google Scholar]
- Slane LC, Thelen DG (2014) Non‐uniform displacements within the Achilles tendon observed during passive and eccentric loading. J Biomech 47, 2831–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starborg T, Kalson NS, Lu Y, et al. (2013) Using transmission electron microscopy and 3View to determine collagen fibril size and three‐dimensional organization. Nat Protoc 8, 1433–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sterkenburg MN, Kerkhoffs GMMJ, Kleipool RP, et al. (2011a) The plantaris tendon and a potential role in mid‐portion Achilles tendinopathy: an observational anatomical study. J Anat 218, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sterkenburg MN, Kerkhoffs GMMJ, van Dijk CN (2011b) Good outcome after stripping the plantaris tendon in patients with chronic mid‐portion Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc 19, 1362–1366. [DOI] [PubMed] [Google Scholar]
- Strocchi R, Leonardi L, Guizzardi S, et al. (1985) Ultrastructural aspects of rat tail tendon sheaths. J Anat 140(Pt 1), 57–67. [PMC free article] [PubMed] [Google Scholar]
- Szaro P, Witkowski G, Śmigielski R, et al. (2009) Fascicles of the adult human Achilles tendon – An anatomical study. Ann Anat 191, 586–593. [DOI] [PubMed] [Google Scholar]
- Szczesny SE, Elliott DM (2014a) Incorporating plasticity of the interfibrillar matrix in shear lag models is necessary to replicate the multiscale mechanics of tendon fascicles. J Mech Behav Biomed Mater 40, 325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny SE, Elliott DM (2014b) Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater 10, 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny SE, Aeppli C, David A, et al. (2018) Fatigue loading of tendon results in collagen kinking and denaturation but does not change local tissue mechanics. J Biomech 71, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terena SML, Fernandes KPS, Bussadori SK, et al. (2017) Systematic review of the synergist muscle ablation model for compensatory hypertrophy. Rev Assoc Med Bras 63, 164–172. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, et al. (2012) Specialization of tendon mechanical properties results from interfascicular differences. J R Soc Interface 9, 3108–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Klemt C, Riley GP, et al. (2013a) Helical sub‐structures in energy‐storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomater 9, 7948–7956. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Udeze CP, Birch HL, et al. (2013b) Capacity for sliding between tendon fascicles decreases with ageing in injury prone equine tendons: a possible mechanism for age‐related tendinopathy? Eur. Cell Mater. 25, 48–60. [DOI] [PubMed] [Google Scholar]
- Thorpe CT, Godinho MSC, Riley GP, et al. (2015) The interfascicular matrix enables fascicle sliding and recovery in tendon, and behaves more elastically in energy storing tendons. J Mech Behav Biomed Mater 52, 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Karunaseelan KJ, Ng Chieng Hin J, et al. (2016) Distribution of proteins within different compartments of tendon varies according to tendon type. J Anat 229, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CT, Riley GP, Birch HL, et al. (2017) Fascicles and the interfascicular matrix show adaptation for fatigue resistance in energy storing tendons. Acta Biomater 56, 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH‐C (2006) Mechanobiology of tendon. J Biomech 39, 1563–1582. [DOI] [PubMed] [Google Scholar]
- Zitnay JL, Li Y, Qin Z, et al. (2017) Molecular level detection and localization of mechanical damage in collagen enabled by collagen hybridizing peptides. Nat Commun 8, 14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 SHG signal of rat patellar tendon.
Video S1 Rat tail tendon 3D structure rendered and sliced to show collagen and cell morphology.
Video S2 Plantaris tendon 3D structure rendered and sliced to show collagen and cell morphology.
Video S3 Plantaris tendon 3D structure rendered and sliced to show collagen and cell morphology.


