Abstract
AIM
To evaluate whether the level of thrombospondin-1 (TSP-1) in aqueous humor can predict the prognosis of trabeculectomy in patients with primary angle-closure glaucoma (PACG).
METHODS
This case-control study involved 26 patients with PACG who experienced a failed trabeculectomy (case group) and 78 age- and sex-matched patients with PACG who underwent successful trabeculectomy (control group). Aqueous humor was collected at the time of trabeculectomy and tested for TSP-1 and TGF-β2 levels with an enzyme-linked immunosorbent assay method. Logistic regression modeling was used to assess the risk factors for failed trabeculectomy.
RESULTS
The mean TSP-1 aqueous concentrations were significantly higher in the case group (20.67±9.79 ng/mL) than the control group (5.17±2.29 ng/mL) (P<0.001). The transforming growth factor-β2 (TGF-β2) aqueous concentrations were significantly different between the case and control group, at 3633.25 and 1090.24 pg/mL, respectively (P<0.001). Logistic regression analysis revealed TSP-1 level as an independent risk factor for a failed trabeculectomy (OR=3.540; 95%CI=1.092-11.482).
CONCLUSION
The aqueous humor TSP-1 and TGF-β2 levels are higher in PACG eyes with failed trabeculectomy than with successful trabeculectomy at one year. The aqueous humor TSP-1 level is an independent risk factor associated with failed trabeculectomy.
Keywords: thrombospondin-1, prognosis, primary angle-closure glaucoma
INTRODUCTION
The World Health Organization estimates that glaucoma is the third leading cause of blindness worldwide, after cataract and uncorrected refractive errors. In China, glaucoma most commonly occurs as primary angle-closure glaucoma (PACG). Foster and Johnson[1]. estimated that the number of blindness cases was 10 times higher in patients with PACG than with primary open angle glaucoma (POAG). Other population-based studies have estimated that about 6-7.5 million people suffer from PACG[2]–[3].
PACG patients with peripheral angle synechiae (PAS) >180 degrees are currently always recommended to undergo glaucoma filtering surgery to prevent progression of optic neuropathy[4]. A combination of trabeculectomy and the use of antifibroblastic agents, such as mitomycin-C (MMC), offers the ideal efficacy for the treatment of the glaucoma[5]. However, a fraction of glaucoma patients still fail to achieve target pressure without additional topical medication and even experience trabeculectomy failure despite continuous improvements in surgical techniques[6]. Existing evidence suggests that younger age[6], higher intraocular pressure (IOP)[7], any postoperative complication[7], and race[8] are risk factors for trabeculectomy failure. Nevertheless, other unknown risk factors most likely exist for this failure.
Trabeculectomy is carried out under the conjunctival and subconjunctival spaces; consequently, scarring of the conjunctiva and sclera after a trabeculectomy are the most important factors leading to surgical failure, as the resulting fibrosis ultimately impairs the function of the filtering bleb. One known mediator of myofibroblast transdifferentiation and fibrosis is transforming growth factor-β2 (TGF-β2), which has also been implicated in several reports in the activation of Tenon's fibroblasts[9]–[12]. More importantly, the activation of proliferation of fibroblasts by TGF-β can also influence surgical results after trabeculectomy[13]–[14].
A potent activator of TGF-β, the matricellular protein thrombospondin-1 (TSP-1), acts by binding to the TGF-β complex at the cell surface[15]. Overexpression of TSP-1 in scleroderma and keloids correlates with increased TGF-β activity[16]–[17], which suggests that TSP-1 might also play roles in tissue repair and fibrosis. In the present study, we speculated that fibrosis at the site of glaucoma surgery may also be influenced by TSP-1 levels. Following trabeculectomy, the aqueous humor drains into the subconjunctival space and percolates to the conjunctival tissue. Thus, components in the aqueous humor might affect the outcomes of glaucoma surgery. In this regard, the aim was to measure the levels of TSP-1 and TGF-β2 in the aqueous humor at the time of trabeculectomy in PACG patients and to evaluate whether any association exists between TSP-1 levels and the success rate of trabeculectomy.
SUBJECTS AND METHODS
Ethical Approval
The study protocol was approved by the Institutional Review Committee at Chenzhou First People's Hospital, and all procedures complied with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all the participants involved in the study.
Study Design
This retrospective case-control study was performed on all patients with PACG who underwent trabeculectomy or trabeculectomy combined with phacoemulsification at Chenzhou First People's Hospital between April 2014 and March 2017. The case group was composed of the patients with PACG who had experienced a failed trabeculectomy, conducted alone or combination with phacoemulsification, in our hospital. The controls were patients with PACG who had undergone a successful trabeculectomy, alone or combined with phacoemulsification. In order to increase the statistical power to detect a difference, we randomly selected three controls to each case ratio with the cases. If the patients received the surgery in both eyes, we only assessed the right eyes.
PACG was defined based on the following: 1) glaucomatous optic neuropathy; 2) visual field defect typical for glaucoma; 3) no pigmented trabecular meshwork visible on gonioscopy ≥180°, along with the presence of PAS[18]. In the case group, the eligibility criteria were 1) age ≥18y; 2) phakic lens status; 3) a diagnosis of PACG with uncontrolled IOP of 22 mm Hg or more on maximal antiglaucoma medication; 4) experienced a failed trabeculectomy alone or combined with phacoemulsification. In the control group, the eligibility criteria were 1) age ≥18y; 2) phakic lens status; 3) a diagnosis of PACG with uncontrolled IOP of 22 mm Hg or more on maximal antiglaucoma medication; 4) undergone a successful trabeculectomy, alone or combined with phacoemulsification. Exclusion criteria for both groups were previous intraocular surgery history or laser procedures other than laser iridotomy, no MMC application, no light perception, other disease affecting the visual field that might be confused with glaucoma, or a follow-up period <1y. All included patients were Han Chinese people.
Data Collection
Each medical record collected included the baseline clinical characteristics [IOP, best corrected visual acuity (BCVA), number of antiglaucoma medications, eye laterality and previous laser treatment], demographic data (age, sex, and presence of comorbid conditions), surgical data (the duration and concentration of MMC used, type of conjunctival flap, combined phacoemulsification, number of sutures, and releasable suture), postoperative data (IOP, BCVA, number of antiglaucoma medications, reoperation, and complications) at postoperative day 1, week 1, and months 1, 3, 6, and 12 from both the case and control groups.
Aqueous Humor and Surgical Techniques
All patients with PACG who underwent trabeculectomy surgery between April 2014 and March 2017 underwent aqueous humor collection. After performing the conjunctival and sclera flap surgery, but before the trabeculectomy, around 100 µL aqueous humor was collected by anterior chamber paracentesis with a 30-gauge needle. Each sample was immediately placed into an Eppendorf tube and stored at -80°C, protected from light, until it was assayed.
Following peribulbar anesthesia, a fornix or limbal-based conjunctival flap was prepared in the superior temporal or nasal quadrant. A rectangular (5×4 mm2) scleral flap (half of the sclera thickness) was then prepared. Prior to the trabeculectomy, MMC (0.25-0.33 mg/mL, 2-5min) was administered, with the duration and concentration of MMC being judged by the surgeon with regard to the risk for failed surgery. After 2-5min, the MMC was copiously irrigated with 200 mL of balanced salt solution. For the patients who underwent trabeculectomy combined with phacoemulsification, a 1.5 mm clear cornea side port incision at the 2-o'clock position and a 3.0 mm main clear corneal incision were made, followed by phacoemulsification of the cataract and implantation of the intraocular lens. This was followed by the trabeculectomy (2×1 mm2), basal iridectomy, suturing of the scleral flap using 10/0 nylon sutures (2-5 sutures, some patients received releasable sutures), and suturing of the conjunctiva with water-tight 10/0 nylon sutures. After the surgery, topical corticosteroids combined with antibiotics (TobraDex, Alcon, Inc.) were administered every two to three hours a day and then, based on the degree of inflammation, this was replaced with 0.1% pranoprofen eye drops.
Evaluation Criteria
Surgical success was defined as a 5 mm Hg <IOP<21 mm Hg on 2 consecutive study visits after 3mo with (qualified success) or without (complete success) additional glaucoma medications. Failure was defined as IOP more than 21 mm Hg on 2 consecutive study visits after 3mo despite the use of maximal glaucoma medication. IOP less than 5 mm Hg on 2 consecutive study visits after 3mo, reoperation for glaucoma, loss of light perception vision, or devastating complications (endophthalmitis, suprachoroidal hemorrhage, retinal detachment) were also defined as failure. Reoperation for glaucoma was defined as additional glaucoma surgery requiring a return to the operating room (not including adjustment or removal of releasable sutures[19]–[20]).
TGF-β2 and TSP-1 Analyses
The levels of total (active plus latent) TGF-β2 and TSP-1 in the aqueous humor were measured with a commercially available human TGF-β2 Quantikine enzyme-linked immunosorbent assay (ELISA) Kit (R&D Systems, Minneapolis, MN, USA) with a sensitivity of 15.4 pg/mL and with a human TSP-1 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA) with a sensitivity of 0.944 ng/mL, respectively, following the manufacturer's protocol. All samples were run in duplicate. In brief, the samples were pipetted into 96-well plates precoated with anti-human TGF-β2/TSP-1 antibody. After repeated incubation and washing and incubation with a second antibody and washing, the reaction was terminated with a stop solution. The total concentration of TGF-β2 was measured by treating the aqueous humor with 1 mol/L HCl to convert TGF-β2 to its active form and then neutralized by adding 1.2 mol/L NaOH/0.5 mol/L HEPES. The absorbance at 450 nm was then immediately determined using a spectrophotometer (SpectraMax Gemini UVmax; Molecular Devices, Sunnyvale, CA).
Statistical Analysis
Statistical analyses were performed with SPSS 17.0 (SPSS, Chicago, IL, USA). For continuous variables with a skewed distribution, the Mann-Whitney U test was used for comparison of the case and control groups. For continuous variables with a normal distribution, the independent t-test was used. For categorical variables, the Chi-square test was used. Potential risk factors for failure of surgery were assessed by univariate logistic regression analyses performed for each covariate. Covariates with a probability value ≤0.10 in the univariate models were included in a multivariate model to investigate confounding factors. All P values less than 0.05 were considered statistically significant.
RESULTS
Demographic and Clinical Characteristics
During the period of April 2014 and March 2017, 233 preliminarily enrolled patients with PACG (who met the inclusion and exclusion criterion) underwent trabeculectomy, alone or combined with phacoemulsification, in our hospital. In this period, we collected the aqueous humor of all PACG eyes from patients who underwent this surgery. Of these 233 patients, 65 patients were not assessed for different reasons, such as death, loss at follow up, other eye surgery, incomplete medical records, inability to contact the patient, etc. Among the remaining 168 patients, 26 patients underwent surgery failure at the one-year follow up. Interestingly, these 26 trabeculectomy patients all failed because of the bled scarring, not because of the devastating complications that occurred. We randomly selected 78 patients as controls. Ultimately, 104 patients were included in this study, and 104 aqueous humor samples were measured.
The characteristics of PACG patients in both the case and control groups are outlined in Table 1. This was an age and sex matched case-control study, with an age of 56.53±9.32y for the case group and 56.55±10.29y for the control group. The ratio of males to females was 7:19 for the case group and 22:56 for the control group, with no statistically significant difference. The average preoperative IOP was 30.38±8.57 mm Hg in the case group and 31.22±7.55 mm Hg in control groups, with no statistically significant difference between the two groups. The preoperative mean glaucoma medication used and the mean MMC concentration and duration used during the surgery were comparable between the case and control groups. In addition, no significant difference was detected between the groups for conjunctival flap type, number of sutures in the scleral flap, the eye of the surgery, and the number of patients who underwent combined phacoemulsification surgery. However, the case group had a greater incidence of shallow anterior chamber and a lower rate of releasable suture use.
Table 1. Characteristics of patients with cases and controls.
| Parameters | Case (n=26) | Control (n=78) | P |
| Age, y | 56.53±9.32 | 56.55±10.29 | 0.996a |
| Sex | 0.900b | ||
| M | 7 (26.9) | 22 (28.2) | |
| F | 19 (73.1) | 56 (71.8) | |
| Preoperative mean IOP (mm Hg) | 30.38±8.57 | 31.22±7.55 | 0.634a |
| Preoperative mean glaucoma medication | 2.92±0.69 | 3.09±0.54 | 0.207a |
| Mean MMC concentration (mg/mL) | 0.27±0.04 | 0.26±0.03 | 0.058a |
| Mean MMC duration (min) | 3.35±1.02 | 3.59±0.78 | 0.230a |
| Previous laser iridotomy | 0.220b | ||
| Yes | 8 (30.8) | 15 (19.2) | |
| No | 18 (69.2) | 63 (80.8) | |
| Diabetes | 0.765b | ||
| Yes | 5 (19.2) | 13 (16.7) | |
| No | 21 (80.8) | 65 (83.3) | |
| Hypertension | 0.274b | ||
| Yes | 6 (23.1) | 27 (34.6) | |
| No | 20 (76.9) | 51 (65.4) | |
| Combined phacoemulsification | 0.107b | ||
| Yes | 9 (34.6) | 15 (19.2) | |
| No | 17 (65.4) | 63 (80.8) | |
| Eye of surgery | 0.174b | ||
| Right | 10 (38.5) | 42 (53.8) | |
| Left | 16 (61.5) | 36 (46.2) | |
| Limbus-/fornix-based flap | 0.254b | ||
| Limbus | 12 (46.2) | 46 (59.0) | |
| Fornix | 14 (53.8) | 32 (41.0) | |
| Shallow anterior chamber | 0.002b | ||
| Yes | 9 (34.6) | 7 (9.0) | |
| No | 17 (65.4) | 71 (91.0) | |
| Number of sutures in the scleral flap | 0.463b | ||
| 2 | 6 (23.1) | 12 (15.4) | |
| 3 | 6 (23.1) | 27 (34.6) | |
| ≥4 | 14 (53.8) | 39 (50.0) | |
| Releasable suture | 0.023b | ||
| Yes | 4 (15.4) | 31 (39.7) | |
| No | 22 (84.6) | 47 (60.3) | |
aIndependent sample t test; bChi-square test; IOP: Introcular pressure; MMC: Mitomycin C.
n (%)
TGF-β2 and TSP-1 Levels in Aqueous Humor
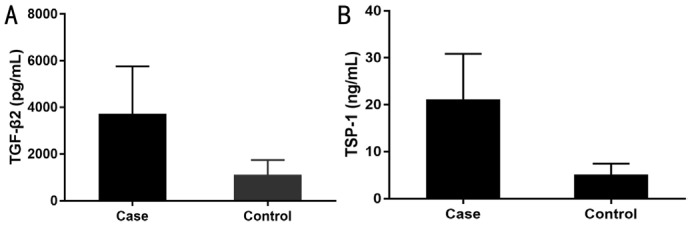
The TGF-β2 aqueous concentrations in the case and control group were 3633.25 and 1090.24 pg/mL, respectively. The level was approximately three times higher in the case group than in the control group, and the difference was statistically significant (P<0.001; Mann-Whitney U test; Figure 1A). Similarly, the mean TSP-1 aqueous concentrations were significantly higher in the case group (20.67±9.79 ng/mL) than in the control group (5.17±2.29 ng/mL; P<0.001; Mann-Whitney U test; Figure 1B).
Figure 1. TGF-β2 (A) and TSP-1 (B) levels of the aqueous humor of the groups.
They were significantly different between the groups (P<0.001).
Risk Factors for Surgery Failure
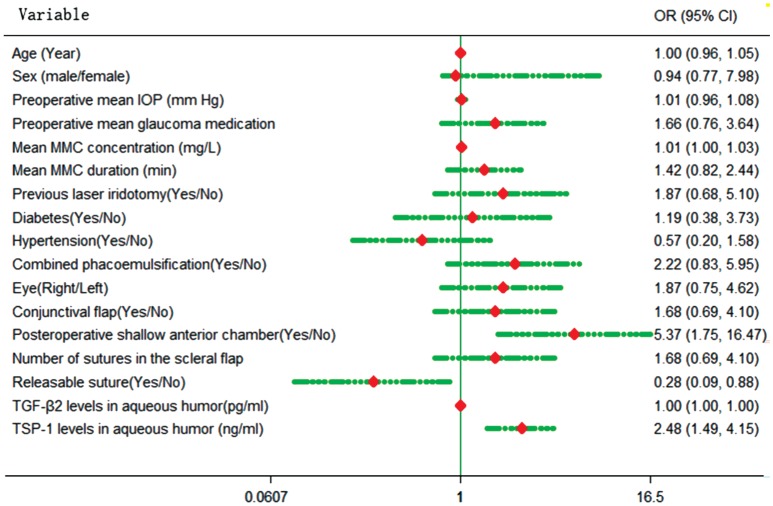
The potential risk factors for failure of surgery were first investigated by univariate logistic regression analysis, which revealed that the PACG patients with higher TGF-β2 (OR=1.002; 95%CI=1.001-1.004) and TSP-1 (OR=2.483; 95%CI=1.485-4.152) levels in aqueous humor had an increased risk of surgery failure.
A shallow anterior chamber after surgery (OR=5.370; 95%CI= 1.751-16.467) and use of releasable sutures (OR=0.276; 95%CI=0.087-0.877) during the surgery also appeared to have a significant association with the prognosis of trabeculectomy (Figure 2). A multivariate logistic regression showed that only the variables of TGF-β2 and TSP-1 were included in the model. However, TSP-1 was the only independent risk factor for surgery failure. An increase in TSP-1 levels in the aqueous humor of 1 ng/mL increased the risk of surgery failure 3.54-fold (OR=3.540; 95%CI=1.092-11.482). Table 2 summarizes the details of the logistic regression analysis.
Figure 2. Forest figure of the risk factor surgery failure.
Table 2. Associations between covariates and surgery failure one year after trabeculectomy.
| Variable | Univariate analysis |
Multivariate analysis |
||
| OR (95%CI) | P | OR (95%CI) | P | |
| Age (change in odds per 1y increase) | 1.000 (0.957-1.046) | 0.995 | - | - |
| 40-49 | 1.0 | - | ||
| 50-59 | 2.487 (0.775-7.980) | 0.125 | ||
| >60 | 2.423 (0.824-7.119) | 0.108 | ||
| Sex (M/F) | 0.938 (0.346-2.542) | 0.900 | - | - |
| Preoperative mean IOP (change in odds per 1 mm Hg increase) | 1.014 (0.957-1.075) | 0.631 | - | - |
| Preoperative mean glaucoma medication (change in odds per 1 increase) | 1.660 (0.756-3.644) | 0.207 | - | - |
| Mean MMC concentration (change in odds per 1 mg/L increase) | 1.103 (0.999-1.026) | 0.063 | - | - |
| Mean MMC duration (change in odds per 1 min increase) | 1.420 (0.825-2.443) | 0.205 | - | - |
| Previous laser iridotomy (yes/no) | 1.867 (0.683-5.101) | 0.224 | - | - |
| Diabetes (yes/no) | 1.190 (0.380-3.732) | 0.765 | - | - |
| Hypertension (yes/no) | 0.567 (0.203-1.578) | 0.277 | - | - |
| Combined phacoemulsification (yes/no) | 2.224 (0.831-5.953) | 0.112 | - | - |
| Eye (right/left) | 1.867 (0.754-4.623) | 0.177 | - | - |
| Conjunctival flap (Limbus/fornix-based) | 1.677 (0.686-4.098) | 0.257 | - | - |
| Posteroperative shallow anterior chamber (yes/no) | 5.370 (1.751-16.467) | 0.003 | - | - |
| Number of sutures in the scleral flap (≥4/2-3) | 1.677 (0.686-4.098) | 0.257 | - | - |
| Releasable suture (yes/no) | 0.276 (0.087-0.877) | 0.029 | - | - |
| TGF-β2 levels in aqueous humor (change in odds per 1 pg/mL increase) | 1.002 (1.001-1.004) | <0.001 | 1.004 (0.999-1.009) | 0.142 |
| TSP-1 levels in aqueous humor (change in odds per 1 ng/mL increase) | 2.483 (1.485-4.152) | <0.001 | 3.540 (1.092-11.482) | 0.035 |
OR: Odds ratio; CI: Confidence interval; IOP: Intraocular pressure; MMC: Mitomycin-C; TGF-β2: Transforming growth factor-β2; TSP-1: Thrombospondin-1.
DISCUSSION
A fairly recent body of evidence has enhanced our understanding of the risk factors for trabeculectomy in treatment of glaucoma. Known factors usually include young age[6], black race[8], previous ocular surgery[21], secondary glaucoma[22], and use of multiple topical antiglaucoma drugs[22]. Patients with an increasing number of risk factors were more likely to suffer a failed surgery. On the contrary, patients who received an adjunctive antifibrotic agent and underwent releasable suture techniques were considered more likely to have a successful surgery[5],[23]. In the present case-control study, we excluded other known risks and protective factors by only including patients with PACG who had phakic lenses, no history of ocular surgery, and had undergone adjunctive MMC treatment during the surgery. In addition, this was an age and sex matched case-control study, which helped to assess the other unknown risk factors.
To the best of our knowledge, this is the first study to explore the level of TSP-1 in the aqueous humor of patients with PACG and to explore whether this factor can predict the prognosis of those patients who undergo trabeculectomy. Our use of the ELISA method allowed us to detect the expression of TSP-1 and TGF-β2 in aqueous humor, and both showed markedly higher levels in case group than in the control group. This result implied that the TGF-β2 and TSP-1 levels in the aqueous humor might affect the efficacy of trabeculectomy in patients with PACG. We clarified whether TGF-β2 and TSP-1 in the aqueous humor were independent risk factors for trabeculectomy failure by conducting a multiple regression analysis. The result showed that TSP-1 was the factor most associated with failed trabeculectomy in these patients. Furthermore, an increase in the TSP-1 level in the aqueous humor of 1 ng/mL resulted in a 3.54-fold increase in the risk for surgery failure. This implied that the TSP-1 levels in the aqueous humor might predict the prognosis of trabeculectomy in patients with PACG.
This finding is not difficult to understand based on the characteristics of TSP-1. The failed trabeculectomy is most commonly due to episcleral fibrosis and excessive scar formation[24]. TGF-β, and especially TGF-β2, has been reported as a key cytokine with high correlation to subconjunctival fibrosis and bleb formation after filtration surgery[14]. A rabbit experiment by Mead et al[25] determined that a blockage of TGF-β2 with antibodies could effectively suppress conjunctival scarring after filtration surgery. TGF-β2 appeared to be the main link to scar formation, but other regulation factors, such as TSP-1 might also be involved. Sweetwyne and Murphy-Ullrich[15] confirmed that TSP-1 could bind to the latent TGF-β complex and sub sequentially stimulate TGF-β activation. In several diseases, researchers have found that an increase in TSP-1 expression can lead to an increase in activity of TGF-β; this indicates that TSP-1 is a primary regulator of TGF-β bioactivity[26]–[27]. TSP-1 was also expressed throughout the trabecular meshwork (TM)[28] and was detected in the aqueous humor[29]. This offers an explanation as to why TSP-1 was an independent risk factor of failed trabeculectomy in the present study. The patients who suffered from a trabeculectomy failure had the highest levels of TSP-1 in the aqueous humor. This TSP-1 might activate TGF-β, and especially the TGF-β2, which would then trigger bleb fibrosis and postoperative scarring, which would ultimately result in a non-successful surgery. Therefore, it is clear that the most significant inference from this study results from the trabeculectomy, which, in combination with monoclonal antibody-based therapeutics, might be a useful treatment for glaucoma in the future.
Unlike the case for high levels of TSP-1, we did not find TGF-β2 to be an independent risk factor for the surgery failure, even though the level of TGF-β2 in the case group was almost three times that of the control group. Previous literature has proposed the overexpression of TGF-β2 as a main cause of excessive postoperative fibrosis, so this might be the main cause of the surgery failure observed in our case group. However, notably, we did not detect TGF-β2 as an independent risk factor for surgery failure in our multiple regression model. One potential explanation is that the increased TGF-β2 level occurs secondarily to the activation of the TSP-1. Another possibility is that the relatively small sample size of this study had insufficient power to detect a significant association.
Another point worth mentioning is that the baseline clinical characteristics of our patients were similar for both the case group (failure group) and the control group (success group), except for the incidence of a postoperative shallow anterior chamber and the use of releasable sutures. The univariate logistic regression analysis also showed that a postoperative shallow anterior chamber is a negative indicator for prognosis of trabeculectomy in patients with PACG, whereas the use of releasable sutures during the surgery is beneficial to this surgery. We propose the following as a possible mechanism to explain our findings: the postoperative shallow anterior chamber is usually accompanied by hypotony, which will cause a reduction in the flow of aqueous humor through the sclerostomy and into the bleb. This, in turn, would accelerate the adhesion process between the sclera and conjunctiva[30]. Another possibility is that the postoperative shallow anterior chamber would cause an increase in the inflammatory response, which would ultimately increase the risk for bleb fibrosis and scarring.
The application of releasable sutures during the surgery was favorable to the outcome of trabeculectomy. This was not unexpected, as releasable sutures could decrease the frequency of hypotony and the formation of a flat anterior chamber, as reported in the Meta-analysis by Zhou et al[31]. The flat anterior chamber gives rise to bleb fibrosis and scarring, as previously mentioned. In addition, the IOP is elevated postoperatively and decreases after massage; therefore, timely removal of the releasable sutures could increase the aqueous outflow, thereby decreasing the opportunity for adhesion between the conjunctiva and sclera and ultimately preventing bleb fibrosis and scarring.
Several potential limitations of this case-control study should be considered. The first limitation is the retrospective study design, which was adopted because of the relatively low incidence of surgery failure following trabeculectomy. The retrospective study design had its own limitations, such as recall bias or information missing from the medical records. A second limitation was the limited number of surgery failure patients, which meant that the study had a small sample size and restricted our ability to obtain sufficient power to detect a statistically significant difference. However, despite these sample size limitations, we still found a positive association between the TSP-1 level and the prognosis of a trabeculectomy in patients with PACG. The third limitation, selection bias, is common to all case-control studies. The controls were selected from those patients who had experienced a successful trabeculectomy. However, representing all the true source patients is impossible, and the controlled selection may thus bias the study results. Fourth, in the present study, we only included the PACG patients. As another major subtype of glaucoma, POAG patients were not included in this study. In the future, larger and more rigorous studies included both PACG and POAG patients are required to provide a more comprehensive clinical guiding significance.
In conclusion, this study indicated a tendency for the aqueous humor TSP-1 and TGF-β2 levels to be higher in eyes that would eventually experience a failed trabeculectomy. The aqueous humor TSP-1 level was an independent risk factor associated with failed trabeculectomy. In the future, anti-TSP-1 monoclonal antibody-based therapeutics might be useful as therapy for prevention of bleb fibrosis and scarring. However, because of the limitations of the present study, the results should be interpreted with caution. Further studies with a larger number of subjects are needed to confirm the role of TSP-1 in bleb fibrosis and scarring following trabeculectomy in patients with PACG.
Acknowledgments
Conflicts of Interest: Zhu JD, None; Xie LL, None; Li ZY, None; Lu XH, None.
REFERENCES
- 1.Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem? Br J Ophthalmol. 2001;85(11):1277–1282. doi: 10.1136/bjo.85.11.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang YB, Friedman DS, Zhou Q, Yang XH, Sun LP, Guo LX, Chang DS, Lian LY, Wang NL, Handan Eye Study Group Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2011;52(12):8672–8679. doi: 10.1167/iovs.11-7480. [DOI] [PubMed] [Google Scholar]
- 3.Wang YX, Xu L, Yang H, Jonas JB. Prevalence of glaucoma in north China: the Beijing eye study. Am J Ophthalmol. 2010;150(6):917–924. doi: 10.1016/j.ajo.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 4.Liang YB, Wang NL, Rong SS, Thomas R. Initial treatment for primary angle-closure glaucoma in China. J Glaucoma. 2015;24(6):469–473. doi: 10.1097/IJG.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 5.Singab AAS, Mohammed OA, Saleem MIH, Abozaid MA. A comparative study: the use of collagen implant versus mitomycin-c in combined trabeculotomy and trabeculectomy for treatment of primary congenital glaucoma. J Ophthalmol. 2017;2017:9241459. doi: 10.1155/2017/9241459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landers J, Martin K, Sarkies N, Bourne R, Watson P. A twenty-year follow-up study of trabeculectomy: risk factors and outcomes. Ophthalmology. 2012;119(4):694–702. doi: 10.1016/j.ophtha.2011.09.043. [DOI] [PubMed] [Google Scholar]
- 7.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 11. risk factors for failure of trabeculectomy and argon laser trabeculoplasty. Am J Ophthalmol. 2002;134(4):481–498. doi: 10.1016/s0002-9394(02)01658-6. [DOI] [PubMed] [Google Scholar]
- 8.Fernández Argones L, Padilla González CM, Obret Mendive I, Piloto Díaz I, Fumero González FY. Prognostic factors for trabeculectomy failure in a Cuban population. Archi Soc Esp Oftalmol. 2016;91(1):27–33. doi: 10.1016/j.oftal.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Seong GJ, Hong SM, Jung SA, Lee JJ, Lim E, Kim SJ, Lee JH. TGF-beta-induced interleukin-6 participates in transdifferentiation of human Tenon's fibroblasts to myofibroblasts. Mol Vis. 2009;15:2123–2128. [PMC free article] [PubMed] [Google Scholar]
- 10.Sapitro J, Dunmire JJ, Scott SE, Sutariya V, Geldenhuys WJ, Hewit M, Yue BY, Nakamura H. Suppression of transforming growth factor-β effects in rabbit subconjunctival fibroblasts by activin receptor-like kinase 5 inhibitor. Mol Vis. 2010;16:1880–1892. [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer-Ter-vehn T, Han H, Grehn F, Schlunck G. Extracellular matrix elasticity modulates TGF-β-induced P38 activation and myofibroblast transdifferentiation in human tenon fibroblasts. Invest Opthalmol Vis Sci. 2011;52(12):9149–9155. doi: 10.1167/iovs.10-6679. [DOI] [PubMed] [Google Scholar]
- 12.Meyer-Ter-vehn T, Katzenberger B, Han H, Grehn F, Schlunck G. Lovastatin inhibits TGF-β-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Opthalmol Vis Sci. 2008;49(9):3955–3960. doi: 10.1167/iovs.07-1610. [DOI] [PubMed] [Google Scholar]
- 13.Park HY, Kim JH, Park CK. Lysyl oxidase-like 2 level and glaucoma surgical outcomes. Invest Ophthalmol Vis Sci. 2014;55(5):3337–3343. doi: 10.1167/iovs.14-14027. [DOI] [PubMed] [Google Scholar]
- 14.Picht G, Welge-Luessen U, Grehn F, Lütjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239(3):199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 15.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. 2012;31(3):178–186. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen YL, Leask A, Abraham DJ, Kennedy L, Shi-Wen X, Denton CP, Black CM, Verjee LS, Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor β-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4(1):9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimura Y, Ihn H, Jinnin M, Asano Y, Yamane K, Tamaki K. Constitutive thrombospondin-1 overexpression contributes to autocrine transforming growth factor-β signaling in cultured scleroderma fibroblasts. Am J Pathol. 2005;166(5):1451–1463. doi: 10.1016/s0002-9440(10)62362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaram H, Scawn R, Pooley F, Chiang M, Bunce C, Strouthidis NG, Khaw PT, Papadopoulos M. Long-term outcomes of trabeculectomy augmented with mitomycin c undertaken within the first 2 years of life. Ophthalmology. 2015;122(11):2216–2222. doi: 10.1016/j.ophtha.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Barton K, Feuer WJ, Budenz DL, Schiffman J, Costa VP, Godfrey DG, Buys YM. Three-year treatment outcomes in the ahmed baerveldt comparison study. Ophthalmology. 2014;121(8):1547–1557.e1. doi: 10.1016/j.ophtha.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugimoto Y, Mochizuki H, Ohkubo S, Higashide T, Sugiyama K, Kiuchi Y. Intraocular pressure outcomes and risk factors for failure in the collaborative bleb-related infection incidence and treatment study. Ophthalmology. 2015;122(11):2223–2233. doi: 10.1016/j.ophtha.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 22.Broadway DC, Chang LP. Trabeculectomy, risk factors for failure and the preoperative state of the conjunctiva. J Glaucoma. 2001;10(3):237–249. doi: 10.1097/00061198-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Raina UK. Trabeculectomy with releasable sutures. Arch Ophthalmol. 1998;116(10):1288–1293. doi: 10.1001/archopht.116.10.1288. [DOI] [PubMed] [Google Scholar]
- 24.Holló G. Wound healing and glaucoma surgery: modulating the scarring process with conventional antimetabolites and new molecules. Dev Ophthalmol. 2017;59:80–89. doi: 10.1159/000458488. [DOI] [PubMed] [Google Scholar]
- 25.Mead AL, Wong TTL, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-β2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Opthalmology Vis Sci. 2003;44(8):3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- 26.Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-β bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157(4):1353–1363. doi: 10.1016/s0002-9440(10)64649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-β activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339(2):633–641. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 28.Flügel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lüssen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79(5):649–663. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267(1):257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 30.Khaw PT, Chang L, Wong TTL, Mead A, Daniels JT, Cordeiro MF. Modulation of wound healing after glaucoma surgery. Curr Opin Ophthalmol. 2001;12(2):143–148. doi: 10.1097/00055735-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Zhou MW, Wang W, Huang WB, Zhang XL. Trabeculectomy with versus without releasable sutures for glaucoma: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2014;14:41. doi: 10.1186/1471-2415-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]