Abstract
AIM
To describe the involution patterns of vessel growth of retina through fluorescein angiography (FA) of children, who had been under treatment up to 1y previously intravitreal ranibizumab (IVR) as monotherapy for retinopathy of prematurity (ROP).
METHODS
This is a retrospective study. The medical information and FA of 17 children (34 eyes) whose area of avascular retina from the ora serrata was more than two disc diameters (DD) were analyzed.
RESULTS
Among 34 eyes, all were the presence of finger-shaped vessels and arteriolar-venular shunts (100%, 34/34 eyes). Popcorn abnormalities were found in most of the eyes (94.1%, 32/34 eyes). Furthermore, in many cases (23.5%, 8/34 eyes), there were leakage persisting in the region of the junction between avascular and vascular retina. In contrast, just 2 eyes (5.9%) showed damage of retinal capillary bed and 3 eyes (8.8%) showed large area of retinal pigment epithelium (RPE) atrophy.
CONCLUSION
Although IVR can be very effective in ROP, we should remain cautious as infants may remain avascular peripheral retinas and abnormal vessel. FA allows accurate visualization of vessel abnormalities in eyes with ROP, which will be helpful to affect assessment of disease activity and therapeutic effect.
Keywords: retinopathy of prematurity, fluorescein angiography, anti-VEGF, ranibizumab
INTRODUCTION
Retinopathy of prematurity (ROP), which currently represent about 10% of all births worldwide, is a vasoproliferative ROP[1]–[3]. With the premature survival rate improved, more infants are at risk for ROP worldwide, especially in developing countries[4]. Photocoagulation have been the gold standards of therapy for the first line treatment of ROP, which can induce the regression of neovascularization by ablating avascular retina areas and depress the overproduction of vascular endothelial growth factor (VEGF) in the retina. Nevertheless, this treatment could cause complications, such as high myopia and visual field defect[5]–[8].
Recently, intravitreal bevacizumab (IVB) has been shown to be significantly less treatment requiring recurrences than photocoagulation in the BEATROP trial[9], that showed the cure rate only once for disease in zone I alone is 94% and disease in zone II posterior alone is 95%, and vascularization after the therapy is finished in 19.5wk. And then, Zhao et al[10] also exhibited a total of 266 eyes (94.0%) after intravitreal ranibizumab (IVR) as primary treatment were positive response in China. However, in a recent study, compared with laser-treated eyes, anti-VEGF-treated eyes showed more vascular abnormalities and macular lesion[11]. Moreover, abnormal vascular patterns and large peripheral avascular areas may occur after anti-VEGF treatment for ROP[12].
In this research, we describe the involution patterns of vessel growth of retina of fluorescein angiography (FA) performed on 17 children (34 eyes), who had been under treatment up to 1y previously with IVR as monotherapy for ROP. We have assessed the involution patterns of retinal vessel growth after IVR.
SUBJECTS AND METHODS
Ethical Approval
We performed a retrospective analysis. The Peking University People's Hospital (Beijing, China) Ethics Committee approved this study, and Written informed consent was obtained from parents, who were apprised that intravitreal injection of ranibizumab is an off-label therapy for ROP.
The children who visited People's Hospital of Peking University in from March 2015 to June 2016 were included in this research. Totally 83 children (154 eyes) who were consecutively treated for type 1 ROP and aggressive posterior ROP (APROP) and followed-up until nearly 90wk postmenstrual age were included. During the follow-up, we found 120 eyes retinal vascularization were completed or avascular retina area less than 2 disc diameters (DD) from the ora serrata. Our study included 17 children (34 eyes), the rest of them, whose area of avascular retina from the ora serrata was more than 2 DD. In every case, fundal examinations and treatments were taken by two experienced ophthalmologists (Cheng Y and Liang JH), who expertise in treatment for ROP.
We classified our cases based on the International Committee for the Classification of ROP revisited[13]. Children with other disease except ROP, for example congenital cataract and glaucoma, and with a history of prior treatment for ROP were excluded. We defined success of treatment as structural outcomes, including regression of neovascularization and plus disease, and normal retinal vessels growing toward the peripheral retina. The recurrence of ROP was defined as the recurrence of new extraretinal fibrovascular proliferation, such as plus disease and ridge after their regression. According to the standard protocol as described in the BEAT-ROP, all infants were treated with IVR monotherapy[9]. Both initial treatment and second treatment for recurrence IVR treated for infants were the same dose (0.25 mg; 0.025 mL). children were reviewed at 24-48h after treatment and then examined every 1-2wk until regression of neovascularization and plus disease. The next follow-up examine were done weekly every four weeks, biweekly for the next eight weeks, and then monthly until 90wk postmenstrual age. RetCam photos were taken before treatment, 1wk after treatment, and every follow up.
FA was performed on infants, whose area of avascular retina from the ora serrata was more than 2 DD, to document the involution patterns of peripheral retinal vessel. FA was performed using A 10% solution of fluorescein intravenously injected at a dose of 0.1 ml/kg, followed by an isotonic saline flush[14]. None of systemic complications happened related to FA.
Descriptive statistics was done using SAS for Windows software version 9.4, (SAS Institute, Cary, NC, USA) for means, standard deviations and standard error of the mean.
RESULTS
The average gestational age (GA) was 28.8±2.9 (25-36)wk, and respectively birth weights (BW) was 1123.4±312.2 (628-1900) g. The average postmenstrual age (PMA) at first treatment was 40.3±5.1 (33-53)wk. Of all 17 infants, twelve were female (35.3%) and eleven were male (64.7%). The IVR-FA interval with avascular retina of these eyes was ranging from 32 to 99wk (median 44wk). Four eyes were classified as zone I stage 2 with plus disease, ten eyes as zone II posterior stage 3 with plus disease, eighteen eyes as zone II stage 3 with plus disease, and two eyes APROP (Table 1). None of ocular or systemic complications were found during the follow-up period.
Table 1. Patient demographics and characteristics.
| Patient No. | Gender | GA (wk+d) | BW (g) | Diagnosis | PMA at first treatment (wk) | PMA at latest follow-up (wk) | PMA at latest FA (wk) | Weeks IVR to last FA | No. of totally injection |
| 1 | M | 32+4 | 1500 | Zone II, Stage 3+ | 46 | 92 | 80 | 34 | 1 |
| 2 | M | 29 | 1000 | Zone II, Stage 3+ | 39 | 147 | 138 | 99 | 1 |
| 3 | F | 27 | 1080 | Zone I, Stage 2+ | 33 | 100 | 100 | 43 | 2 |
| 4 | F | 25+6 | 852 | Zone II posterior, Stage 3+ | 38 | 94 | 82 | 42 | 2 |
| 5 | M | 36+1 | 937 | Zone II, Stage 3+ | 53 | 129 | 129 | 74 | 1 |
| 6 | F | 30 | 1270 | Zone II, Stage 3+ | 36 | 86 | 81 | 39 | 2 |
| 7 | M | 29+5 | 1400 | Zone II posterior, Stage 3+ | 38 | 88 | 85 | 44 | 2 |
| 8 | M | 26+2 | 1000 | Zone II posterior, Stage 3+ | 39 | 96 | 88 | 32 | 2 |
| 9 | M | 28 | 1080 | Zone I, Stage 2+ | 36 | 96 | 96 | 41 | 2 |
| 10 | M | 27+5 | 1150 | Zone II, Stage 3+ | 40 | 82 | 74 | 34 | 1 |
| 11 | M | 33+3 | 1900 | APROP | 36 | 115 | 88 | 50 | 2 |
| 12 | M | 28+6 | 1250 | Zone II, Stage 3+ | 44 | 119 | 119 | 75 | 1 |
| 13 | M | 30+1 | 1145 | Zone II posterior, Stage 3+ | 38 | 151 | 124 | 86 | 1 |
| 14 | F | 31 | 1350 | Zone II, Stage 3+ | 40 | 87 | 87 | 37 | 1 |
| 15 | M | 25+6 | 675 | Zone II, Stage 3+ | 37 | 90 | 80 | 53 | 1 |
| 16 | F | 27+6 | 628 | Zone II posterior, Stage 3+ | 49 | 94 | 84 | 45 | 2 |
| 17 | F | 26+4 | 880 | Zone II, Stage 3+ | 43 | 108 | 108 | 65 | 1 |
GA: Gestational age; BW: Birth weight; PMA: Postmenstrual age; FA: Fluorescein angiography; IVR: Intravitreal ranibizumab; OS: Left eye; OD: Right eye.
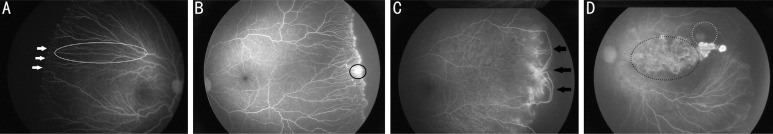
During long-term follow-up, we found after IVR treatment retinal vascularization completed or area of avascular retina from the ora serrata was less than DD were in 120 eyes (77.9%), while the rest of them, 34 eyes (22.1%), remained extensive areas of avascular retina from the ora serrata. Among 34 eyes, all were the presence of finger-shaped vessels and arteriolar-venular shunts (100%, 34/34 eyes; Figure 1A, 1C, Table 2). Popcorn abnormalities were found in most of the eyes (94.1%, 32/34 eyes) (Figure 1A, Table 2). Furthermore, in many cases (23.5%, 8/34 eyes), there were leakage persisting in the region of the junction between avascular and vascular retina (Figure 1B, Table 2). In contrast, just 2 eyes (5.9%) showed damage of retinal capillary bed and 3 eyes (8.8%) showed large area of RPE atrophy (Figure 1D, Table 2).
Figure 1. Montage of FA images of 4 different infants who was received injection of ranibizumab in this eye for type 1 ROP.
A: Vessels popcorn abnormalities, mild leakage (white arrows) at the junction between vascular and avascular retina (white arrows) and vessels stretched like finger shaped (white circles); B: Peripheral retinal neovascularization, leakage is more evident; C: Arteriolar-venular shunts between vessel arcades (black arrows); D: Retinal non-perfusion at the posterior pole with hypofluorescent areas (black dotted circles) and large area of retinal pigment epithelium (RPE) atrophy with hypofluorescent areas transmitted fluorescence (grey dotted circles).
Table 2. Number and percentage of eyes with vessels abnormalities.
| Feature | No. of latest FA | Percentage (%) |
| Finger-shaped vessels | 34 | 100 |
| Arteriolar-venular Shunt | 34 | 100 |
| Popcorn abnormalities | 32 | 94.1 |
| Retinal non-perfusion at the posterior pole | 2 | 5.9 |
| Avascular of peripheral retina. | 34 | 100 |
| Leakage of neovascularization | 8 | 23.5 |
| Large area of RPE atrophy | 3 | 8.8 |
FA: Fluorescein angiography; RPE: Retinal pigment epithelium.
DISCUSSION
Anti-VEGF is increasingly used in the management of ROP[15]–[17], but some have reported abnormal vascular findings after anti-VEGF[18]–[19]. There was creation of establishment of directional flow, small vessels, maturation of retinal vessels and adjustment of vascular density. In this research, we intended to estimate the extent of growth of peripheral retinal in the eyes with ROP that were treated up to 1y previously with IVR as monotherapy for ROP. FA was used to strictly estimate the involution patterns of retinal vessel growth in these eyes.
The normal extent of peripheral retinal non-perfusion was evaluated by Blair et al[20] in normal infants at every postnatal ages. They concluded, conservatively, area of avascular retina from the ora serrata was more than 2 DD should be affirmed abnormal. The result was helpful to document the involution patterns of vessel growth of retina when screening infants with ROP using FA. Among the 154 eyes who were consecutively treated for aggressive posterior ROP (APROP) and Type 1 ROP and followed-up until nearly 90wk postmenstrual age, we found 120 eyes retinal vascularization were completed or avascular retina from the ora serrata extending less than two DD. Therefore, the retina of peripheral area remained incomplete vascularization in 34 (22.1%) of the eyes. This result is consistent with the findings of Alyamaç et al's study[21]. They found 18% of the infants in bevacizumab treatment and 26% of the infants in ranibizumab had remaining avascular areas at 1y old. However, Tahija et al[22] reported that about 55% of the eyes with ROP treated with IVB had not achieved normal retinal vascularization. We thought the difference on the percentage of incomplete retinal vascularization was that their study contained more APROP (7/11 eyes).
Of all 34 eyes, we found all were the presence of finger-shaped vessels and arteriolar-venular shunts (100%, 34/34 eyes). Popcorn abnormalities were found in most of the eyes (94.1%, 32/34 eyes). What's more, in many cases (23.5%, 8/34 eyes), there were leakage persisting in the region of the junction between avascular and vascular retina. Wallance et al[18] also reported among the 20 eyes with ROP treated by bevacizumab available at 4y old, there were noted vessel leakage in 65% eyes. Both vascular tangles and abnormal vessel branching persist in more than 80%. In contrast to the 19 laser-treated eyes, leakage was persisted in one eye, tangles and shunts were persisted in three eyes. Whether the prevalence of abnormalities may be related to therapy of anti-VEGF, more related research is needed to confirm the mechanism. Only 2 eyes (5.9%) showed loss of retinal capillary bed and 3 eyes (8.8%) showed large area of RPE atrophy. It may be related to severity of disease. These eyes are zone I ROP and APROP.
This study was limited by small sample size and retrospective design. Nonetheless, our study demonstrates that although IVR can be very effective in ROP, we should remain cautious as infants may remain avascular peripheral retinas and abnormal vessel. Careful examination using FA allows accurate visualization of vessel abnormalities, which are not obvious based on the appearance of the fundus using the indirect ophthalmoscope or digital fundus photographs. Therefore, additional modalities such as FA, to describe abnormalities in eyes with ROP will be helpful to affect assessment of disease activity and therapeutic effect.
Acknowledgments
Conflicts of Interest: Cheng Y, None; Liu TG, None; Li WY, None; Zhao MW, None; Liang JH, None.
REFERENCES
- 1.Luk ASW, Yip WWK, Lok JYC, Lau HHW, Young AL. Retinopathy of prematurity: applicability and compliance of guidelines in Hong Kong. Br J Ophthalmol. 2017;101(4):453–456. doi: 10.1136/bjophthalmol-2016-308900. [DOI] [PubMed] [Google Scholar]
- 2.Zhang GM, Yang MM, Zeng J, Vakros G, Su KJ, Chen MH, Li HL, Tian RY, Li N, Tang S, He HH, Tan WJ, Song XM, Zhuang RS. Comparison of intravitreal injection of ranibizumab versus laser therapy for zone ii treatment-requiring retinopathy of prematurity. Retina. 2017;37(4):710–717. doi: 10.1097/IAE.0000000000001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillier RJ, Connor AJ, Shafiq AE. Ultra-low-dose intravitreal bevacizumab for the treatment of retinopathy of prematurity: a case series. Br J Ophthalmol. 2018;102(2):260–264. doi: 10.1136/bjophthalmol-2017-310408. [DOI] [PubMed] [Google Scholar]
- 4.Li QP, Wang ZH, Wang RJ, Tang HY, Chen HH, Feng ZC. A prospective study of the incidence of retinopathy of prematurity in China: evaluation of different screening criteria. J Ophthalmol. 2016;2016:1–8. doi: 10.1155/2016/5918736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoara SD, Cristian C, Irimescu I, Stefanut AC, Zaharie G. Diode laser photocoagulation for retinopathy of prematurity: outcomes after 7 years of treatment. J Pediatr Ophthalmol Strabismus. 2014;51(1):39–45. doi: 10.3928/01913913-20131112-02. [DOI] [PubMed] [Google Scholar]
- 6.Roohipoor R, Karkhaneh R, Riazi Esfahani M, Alipour F, Haghighat M, Ebrahimiadib N, Zarei M, Mehrdad R. Comparison of refractive error changes in retinopathy of prematurity patients treated with diode and red lasers. Ophthalmologica. 2016;235(3):173–178. doi: 10.1159/000443844. [DOI] [PubMed] [Google Scholar]
- 7.Yoon JM, Shin DH, Kim SJ, Ham DI, Kang SW, Chang YS, Park WS. Outcomes after laser versus combined laser and bevacizumab treatment for type 1 retinopathy of prematurity in zone I. Retina. 2017;37(1):88–96. doi: 10.1097/IAE.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Wang AG, Sung CS, Hsu WM, Lee FL, Lee SM. Long-term visual outcomes of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye (Lond) 2010;24(1):14–20. doi: 10.1038/eye.2009.63. [DOI] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364(7):603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang QJ, Zhang Q, Fei P, Xu Y, Lyu J, Ji XD, Peng J, Li YA, Zhao PQ. Ranibizumab injection as primary treatment in patients with retinopathy of prematurity. Ophthalmology. 2017;124(8):1156–1164. doi: 10.1016/j.ophtha.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 11.Lepore D, Quinn GE, Molle F, Baldascino A, Orazi L, Sammartino M, Purcaro V, Giannantonio C, Papacci P, Romagnoli C. Intravitreal bevacizumab versus laser treatment in type 1 retinopathy of prematurity: report on fluorescein angiographic findings. Ophthalmology. 2014;121(11):2212–2219. doi: 10.1016/j.ophtha.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Lepore D, Quinn GE, Molle F, Orazi L, Baldascino A, Ji MH, Sammartino M, Sbaraglia F, Ricci D, Mercuri E. Follow-up to age 4 years of treatment of type 1 retinopathy of prematurity intravitreal bevacizumab injection versus laser: fluorescein angiographic findings. Ophthalmology. 2018;125(2):218–226. doi: 10.1016/j.ophtha.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 13.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–999. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 14.Wagner RS. Fundus fluorescein angiography in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2006;43(2):78. doi: 10.3928/0191-3913-20060301-04. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Castellanos MA, Schwartz S, Hernández-Rojas ML, Kon-Jara VA, García-Aguirre G, Guerrero-Naranjo JL, Chan RVP, Quiroz-mercado H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina. 2013;33(2):329–338. doi: 10.1097/IAE.0b013e318275394a. [DOI] [PubMed] [Google Scholar]
- 16.Vavvas DG. Anti-VEGF in retinopathy of prematurity, need to titrate. Invest Ophthalmol Vis Sci. 2013;54(3):pii: 2027. doi: 10.1167/iovs.13-11948. [DOI] [PubMed] [Google Scholar]
- 17.Pertl L, Steinwender G, Mayer C, Hausberger S, Pöschl EM, Wackernagel W, Wedrich A, El-Shabrawi Y, Haas A. A systematic review and meta-analysis on the safety of vascular endothelial growth factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS One. 2015;10(6):e0129383. doi: 10.1371/journal.pone.0129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace DK, Kraker RT, Freedman SF, Crouch ER, Hutchinson AK. Assessment of lower doses of intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol. 2017;135(6):654–656. doi: 10.1001/jamaophthalmol.2017.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henaine-Berra A, Garcia-Aguirre G, Quiroz-Mercado H, Martinez-Castellanos MA. Retinal fluorescein angiographic changes following intravitreal anti-VEGF therapy. J AAPOS. 2014;18(2):120–123. doi: 10.1016/j.jaapos.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Blair MP, Shapiro MJ, Hartnett ME. Fluorescein angiography to estimate normal peripheral retinal nonperfusion in children. J AAPOS. 2012;16(3):234–237. doi: 10.1016/j.jaapos.2011.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alyamaç Sukgen E, Çömez A, Koçluk Y, Cevher S. The process of retinal vascularization after anti-VEGF treatment in retinopathy of prematurity: a comparison study between ranibizumab and bevacizumab. Ophthalmologica. 2016;236(3):139–147. doi: 10.1159/000449530. [DOI] [PubMed] [Google Scholar]
- 22.Tahija SG, Hersetyati R, Lam GC, Kusaka S, McMenamin PG. Fluorescein angiographic observations of peripheral retinal vessel growth in infants after intravitreal injection of bevacizumab as sole therapy for zone I and posterior zone II retinopathy of prematurity. Br J Ophthalmol. 2014;98(4):507–512. doi: 10.1136/bjophthalmol-2013-304109. [DOI] [PMC free article] [PubMed] [Google Scholar]