The clonal nature of cancer evolution dictates that all tumor cells carry the same cancer-initiating genetic lesions. However, the latest findings have shown that the mode of action of oncogenes is not homogeneous throughout the developmental history of the tumor. Studies on different types of hematopoietic and solid tumors have shown that the contribution of some oncogenes to cancer development is mediated through the epigenetic reprogramming of the cancer-initiating target cell [1–4]. Epigenetic reprogramming is therefore a new type of interaction between oncogenes and tumor cells, in which the oncogene primes for cancer development by establishing a new pathological tumor cell identity. The current challenge is to test whether epigenetic remodeling in the absence of the driver oncogene is sufficient for tumorigenesis.
Chronic myeloid leukemia (CML) is a malignancy of hematopoietic stem cells (HSCs) that harbor the Philadelphia (Ph) chromosome [5] and leads to increased abundance of myeloid cells and other non-lymphoid lineages in the blood and bone marrow. The Ph translocation creates the BCR-ABL fusion protein, with nearly all CML patients harboring a breakpoint that results in a 210-kD protein (BCR-ABLp210). This oncogene alone is capable of transforming hematopoietic progenitors and inducing CML [5]. The BCR-ABL protein is a constitutively active kinase that can be directly targeted with ABL-specific kinase inhibitors, such as Imatinib. The introduction of these agents that specifically target the cancer-initiating oncogene was a breakthrough in the clinical management of CML. However, treatment with Imatinib or other second-generation ABL kinase inhibitors is not curative and the disease returns upon cessation of the drug or the development of resistance. This is because CML stem cells are not dependent on BCR-ABL activity [6], suggesting that there may be an oncogenic function of BCR-ABLp210 that can persist following inhibition of ABL kinase activity. In support of this notion, we have previously shown that the transient expression of BCR-ABLp210 restricted to the hematopoietic stem/progenitor cell (HSPC) compartment of mice is capable of inducing mature myeloid leukemia [7]. In addition, we have also demonstrated that the expression of other oncogenes, such as Bcl6, within the HSPC compartment can reprogram HSPCs toward malignancy by fueling epigenetic changes that can be traced from the HSPCs to the malignant cells [1]. These observations led us to hypothesize that BCR-ABLp210 may function, in part, via epigenetic mechanisms that prime the tumor stem cell for malignant myeloid differentiation.
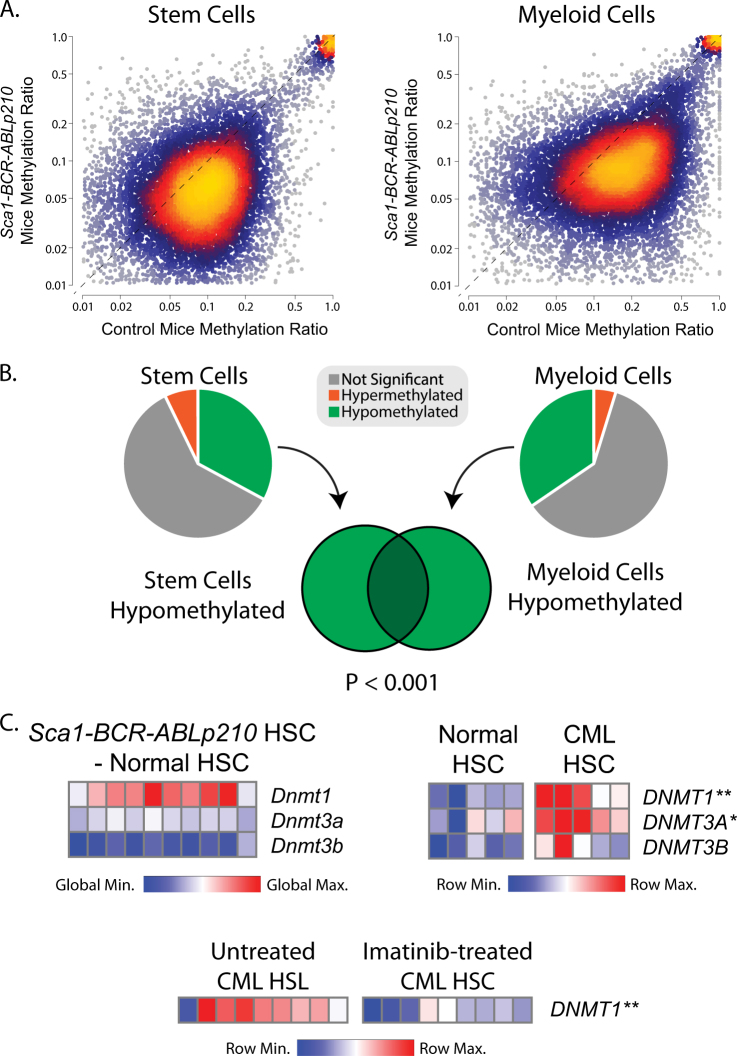
In order to investigate epigenetic reprogramming by the BCR-ABLp210 oncogene, we performed reduced-representation bisulfite sequencing to interrogate the DNA methylation landscape in HSPCs (Sca1+Lin−) from wild-type and Sca1-BCR-ABLp210 transgenic mice. The methods for all experiments are described in detail in supplementary information. This revealed a broad and significant loss of methylation at CpG islands that have a low-to-moderate level of methylation in wild-type HSPCs (Fig. 1a, Table S1). Importantly, this hypomethylation phenotype was conserved in mature myeloid cells from Sca1-BCR-ABLp210 mice, despite the absence of the BCR-ABLp210 oncogene in these cells (Fig. 1a, b, Table S2). This shows that transient HSPC-restricted expression of the BCR-ABLp210 oncogene is capable of inducing significant and lasting changes in DNA methylation that may underlie stem cell reprogramming.
Fig. 1.
Global hypomethylation and DNMT1 overexpression associated with BCR-ABL oncogene expression in hematopoietic stem cells (HSCs). a Heat scatter plots show RRBS methylation profiling data from the HSPCs (Sca1+Lin−) of wild-type mice compared to those from Sca1-BCR-ABLp210 transgenic mice (left) and from mature myeloid cells of wild type compared to Sca1-BCR-ABLp210 mice (right). A significant and global loss of DNA methylation can be observed in the HSPCs and myeloid cells from Sca1-BCR-ABLp210 mice. b Pie graphs and a Venn diagram illustrate that the majority of the changes in DNA methylation in each cellular compartment were hypomethylation, and the regions of hypomethylation significantly overlapped in HSPCs and mature myeloid cells. c Heat maps show the expression of DNA methyltransferases in murine and human HSCs. The relative expression of Dnmt1, but not other DNA methyltransferases, is higher in HSC from Sca1-BCR-ABLp210 transgenic mice compared to wild-type mice (top left) and in HSC from CML patients compared to those from healthy donors (top right). The expression of DNMT1 in HSC from CML patients is significantly reduced by BCR-ABL inhibition (bottom). *P < 0.05, **P < 0.01
Prior studies have shown that DNA methyltransferase (DNMT) genes, including DNMT1, are aberrantly overexpressed in myeloid leukemias [8]. In line with this, gene expression profiling (GEP) data from HSPCs of Sca1-BCR-ABLp210 mice compared to those from wild-type mice [7] showed upregulation of Dntm1 and downregulation of Dnmt3b expression, with no change in Dnmt3a expression (Fig. 1c). Higher expression of DNMT1 was also observed in GEP data from human CML stem cells compared to normal HSCs [9] (T test P value = 0.003; Fig. 1c). The expression of DNMT3A was also significantly higher (T test P value = 0.012). Furthermore, GEP data from untreated and Imatinib-treated bone marrow cells of CML patients [10] showed that inhibition of BCR-ABL significantly reduces DNMT1 expression (T test P value = 0.0004; Fig. 1c). Together these data show that BCR-ABLp210 expression is associated with overexpression of DNMT1 in both murine and human HSCs and that its expression may be linked to the activity of this oncogene.
The deregulation of DNA methylation by somatic mutations is one of the hallmarks of acute myeloid leukemia. Mutations of IDH1, IDH2, or TET2, function in the same pathway and inhibit the active and passive recycling of 5-methylcytosine to cytosine thereby inducing global hypermethylation. In contrast, mutations of the de novo methyltransferase, DNMT3A, function as dominant negative to inhibit the formation of DNMT3A homotetramers that efficiently methylate cytosine and leads to global hypomethylation. The DNMT3A protein performs its role as a de novo methyltransferase following its recruitment by other transcriptionally repressive complexes such as the polycomb repressor 2 (PRC2) complex [11]. DNMT1, DNMT3A, and DNTM3B have each been shown to interact with a similar region of the catalytic subunit of PRC2, EZH2 [11]. It is therefore plausible that overexpression of DNMT1 may sterically hinder the association between EZH2 and DNMT3A. The loss of only a single allele of Dnmt3a is sufficient to promote myeloid leukemia in mice, despite causing only modest changes in DNA methylation [12], demonstrating that slight perturbations in Dnmt function are sufficient for leukemogenesis. Human CML also shows disordered DNA methylation [13], but the mechanism for this has not been defined. Deregulation of this axis is therefore clearly important for myeloid malignancies. But there is still not a clear understanding of specific genes or pathways that contribute to myeloid transformation as a result of perturbations in DNA methylation. Our observations therefore led us to investigate whether Dnmt1 overexpression was mechanistically linked with perturbed DNA methylation and myeloid leukemogenesis.
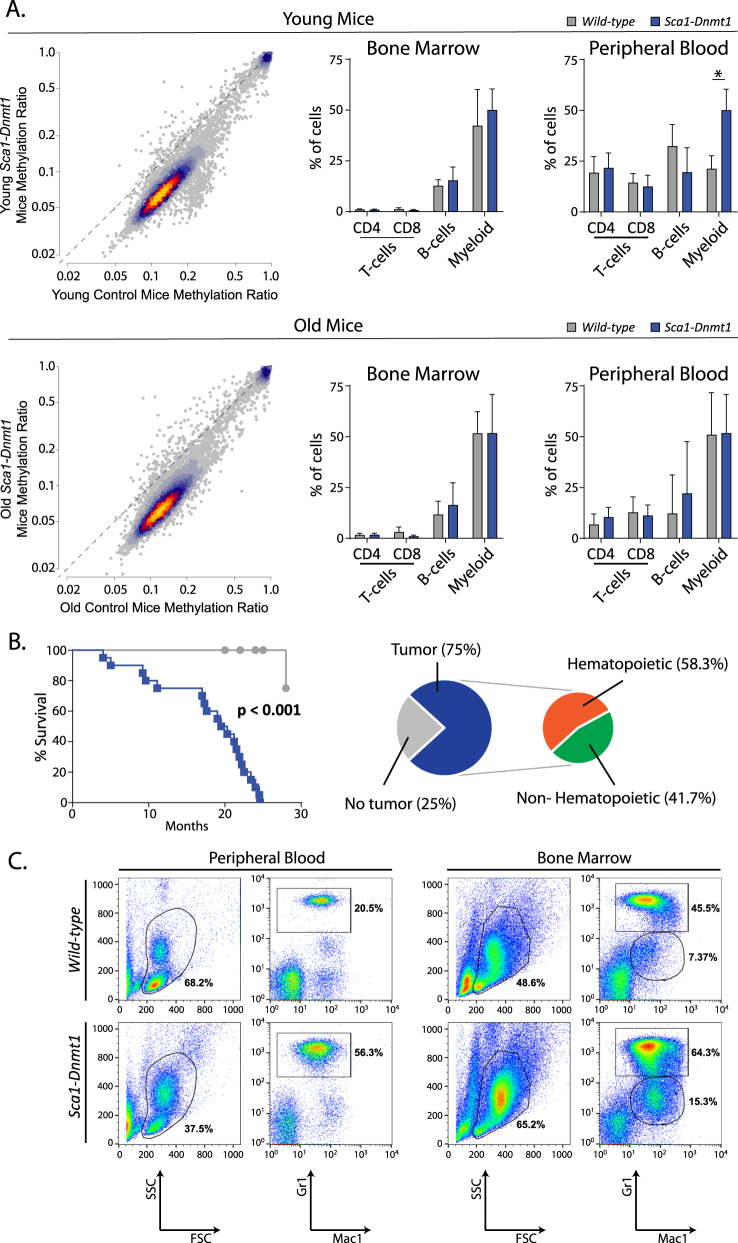
Transient expression of BCR-ABLp210 in HSPCs was sufficient to promote myeloid leukemia, and Imatinib treatment leads to downregulation of Dnmt1 but does not eradicate the disease. We therefore hypothesized that transient expression of Dnmt1 would also be sufficient for stem cell reprogramming, and we modeled this by expressing Dnmt1 under control of the endogenous Sca1 promoter for HSPC-restricted expression (Figs. S1–2). Notably, the expression of Dnmt1 resulted in DNA hypomethylation in HSPCs similar to that observed in Sca1-BCR-ABLp210 mice, with the most notable changes at loci with normally low/moderate levels of methylation (Fig. 2a, Table S3). No significant alterations were observed in the frequencies of major hematologic subsets in the bone marrow of young Sca1-Dnmt1 mice (3–7 months), but there was a significant expansion of myeloid cells in the peripheral blood compared to wild-type mice (Fig. 2a). Although the DNA hypomethylation phenotype persisted in the HSPCs of older mice (16–24 months, Fig. 2a, Table S4), there was no significant difference in myeloid cells in either the bone marrow or the peripheral blood at this stage. The HSPC-restricted expression of Dnmt1 is therefore capable of phenocopying the pattern of lasting DNA hypomethylation that was observed in BCR-ABLp210 mice.
Fig. 2.
Global hypomethylation and myeloid malignancies induced by Dnmt1 expression in hematopoietic stem cells. a Heat scatter plots show the methylation ratio of CpG islands in the HSPCs from young (top) or old (bottom) wild-type control mice compared to those from young Sca1-Dnmt1 mice. A clear loss of methylation can be observed in regions with a normally low-to-moderate level of methylation (0.1–0.2) in control mice. Bar graphs summarize the percentage of major hematological populations, interrogated by flow cytometry of bone marrow and peripheral blood of young (top) and old (bottom) wild-type and Sca1-Dnmt1 mice. No significant differences were observed in the bone marrow at either time point. A significantly increase in the percentage of myeloid cells was observed in the peripheral blood of young disease-free Sca1-Dnmt1 mice compared to their wild-type counterparts (T test P value < 0.001), but this was not observed in older mice. b Kaplan–Meier plot shows a significant reduction in survival of Sca1-Dnmt1 mice (blue) compared to their wild-type counterparts (gray). Pie graphs summarize the necropsy results from Sca1-Dnmt1 mice, with 75% of animals bearing tumors that were primarily of hematopoietic origin. c An illustrative example of flow cytometry of diseased Sca1-Dnmt1 mice compared to age-matched controls shows the expansion of Gr1+Mac1+ myeloid cells in the blood and bone marrow, as well as the appearance of an abnormal Gr1lowMac1+ population in the bone marrow
Aging Sca1-Dnmt1 mice had a shorter lifespan than wild-type mice due to the development of cancer, the majority of which were myeloid malignancies (Fig. 2b). The Sca1-Dnmt1 mice that developed myeloid malignancies showed marked expansion of Mac1+Gr1+ granulocytes in the blood and bone marrow (Fig. 2c and S3), as well as the presence of an abnormal myeloid population (Mac1+Gr1low) in the bone marrow (Fig. 2c). This was also associated with a loss of normal architecture in the spleen (Fig. S4), with tumor-bearing Sca1-Dnmt1 mice showing atrophic white pulp and hyperplasic red pulp infiltrated by myeloid cells. In the liver, tumor infiltration was accompanied by deposition of an eosinophilic hyaline substance (Fig. S4). A prior study showed that the expression of a hypomorphic Dnmt1 allele induced global DNA hypomethylation and led to tumors in mice [14], potentially via the promotion of chromosomal instability resulting from the reactivation of endogenous retroviral elements [15]. We did not observe the expression of the Cdkn2a (p19Arf) gene in bone marrow cells from Sca1-Dnmt1 mice, indicating the absence of oncogenic stress resulting from chromosome instability (data not shown). Together, these data show that HSPC-restricted expression of Dnmt1 is sufficient to phenocopy the DNA hypomethylation phenotype induced by BCR-ABLp210 expression in the same compartment and to promote the development of myeloid malignancies. The deregulation of DNA methylation alone is therefore sufficient to prime HSPCs for the development of myeloid leukemia.
In conclusion, here we have characterized epigenetic reprogramming linked to HSPC-restricted expression of BCR-ABLp210, which persists in myeloid cells despite the absence of the oncogene. We identified upregulation of Dnmt1 as a consequence of BCR-ABLp210, and we show that HSPC-restricted expression of Dnmt1 in transgenic mice is sufficient to phenocopy the BCR-ABLp210-associated DNA methylation changes and induce myeloid malignancies. This provides, to our knowledge, the first experimental evidence that epigenetic tumor stem cell reprogramming by itself is sufficient to drive cancer development and establish the tumor cell identity. These observations provide important mechanistic insight into the epigenetic reprogramming of HSC by the BCR-ABLp210 oncogene and the etiology of CML.
Electronic supplementary material
Acknowledgements
We are indebted to all members of our groups for useful discussions and for their critical reading of the manuscript. Research in CVD group is partially supported by FEDER, “Miguel Servet” Grant (CP14/00082 - AES 2013-2016) from the Instituto de Salud Carlos III (Ministerio de Economía y Competitividad), “Fondo de Investigaciones Sanitarias/Instituto de Salud Carlos III” (PI17/00167), and by the Lady Tata International Award for Research in Leukaemia 2016–2017. Research in ISG group is partially supported by FEDER and by MINECO (SAF2012-32810, SAF2015-64420-R and Red de Excelencia Consolider OncoBIO SAF2014-57791-REDC), Instituto de Salud Carlos III (PIE14/00066), ISCIII- Plan de Ayudas IBSAL 2015 Proyectos Integrados (IBY15/00003), by Junta de Castilla y León (BIO/SA51/15, CSI001U14, UIC-017, and CSI001U16), and by the German Carreras Foundation (DJCLS R13/26). ISG lab is a member of the EuroSyStem and the DECIDE Network funded by the European Union under the FP7 program. IGR was supported by BES-Ministerio de Economía y Competitividad (BES-2013-063789). GRH was supported by FSE-Conserjería de Educación de la Junta de Castilla y León (CSI001-15).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Michael R. Green, Email: MGreen5@mdanderson.org
Isidro Sánchez-García, Email: isg@usal.es.
Electronic supplementary material
The online version of this article (10.1038/s41375-018-0192-z) contains supplementary material, which is available to authorized users.
References
- 1.Green MR, Vicente-Duenas C, Romero-Camarero I, Long Liu C, Dai B, Gonzalez-Herrero I, et al. Transient expression of Bcl6 is sufficient for oncogenic function and induction of mature B-cell lymphoma. Nat Commun. 2014;5:3904. doi: 10.1038/ncomms4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett TE, Chindera K, McDermott J, Breeze CE, Cooke WR, Jones A, et al. Epigenetic reprogramming of fallopian tube fimbriae in BRCA mutation carriers defines early ovarian cancer evolution. Nat Commun. 2016;7:11620. doi: 10.1038/ncomms11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang D, Gan H, Lee JH, Han J, Wang Z, Riester SM, et al. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science. 2016;352:1344–8. doi: 10.1126/science.aae0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicente-Duenas C, Romero-Camarero I, Gonzalez-Herrero I, Alonso-Escudero E, Abollo-Jimenez F, Jiang X, et al. A novel molecular mechanism involved in multiple myeloma development revealed by targeting MafB to haematopoietic progenitors. EMBO J. 2012;31:3704–17. doi: 10.1038/emboj.2012.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–40. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 6.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Caro M, Cobaleda C, Gonzalez-Herrero I, Vicente-Duenas C, Bermejo-Rodriguez C, Sanchez-Beato M, et al. Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO J. 2009;28:8–20. doi: 10.1038/emboj.2008.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizuno S, Chijiwa T, Okamura T, Akashi K, Fukumaki Y, Niho Y, et al. Expression of DNA methyltransferases DNMT1, 3A, and 3B in normal hematopoiesis and in acute and chronic myelogenous leukemia. Blood. 2001;97:1172–9. doi: 10.1182/blood.V97.5.1172. [DOI] [PubMed] [Google Scholar]
- 9.Gerber JM, Gucwa JL, Esopi D, Gurel M, Haffner MC, Vala M, et al. Genome-wide comparison of the transcriptomes of highly enriched normal and chronic myeloid leukemia stem and progenitor cell populations. Oncotarget. 2013;4:715–28. doi: 10.18632/oncotarget.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benito R, Lumbreras E, Abaigar M, Gutierrez NC, Delgado M, Robledo C, et al. Imatinib therapy of chronic myeloid leukemia restores the expression levels of key genes for DNA damage and cell-cycle progression. Pharmacogenet Genomics. 2012;22:381–8. doi: 10.1097/FPC.0b013e328351f3e9. [DOI] [PubMed] [Google Scholar]
- 11.Vire E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 12.Cole CB, Russler-Germain DA, Ketkar S, Verdoni AM, Smith AM, Bangert CV, et al. Haploinsufficiency for DNA methyltransferase 3A predisposes hematopoietic cells to myeloid malignancies. J Clin Invest. 2017;127:3657–74. doi: 10.1172/JCI93041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heller G, Topakian T, Altenberger C, Cerny-Reiterer S, Herndlhofer S, Ziegler B, et al. Next-generation sequencing identifies major DNA methylation changes during progression of Ph+ chronic myeloid leukemia. Leukemia. 2016;30:1861–8. doi: 10.1038/leu.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, et al. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–92. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 15.Howard G, Eiges R, Gaudet F, Jaenisch R, Eden A. Activation and transposition of endogenous retroviral elements in hypomethylation induced tumors in mice. Oncogene. 2008;27:404–8. doi: 10.1038/sj.onc.1210631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.