Abstract
Humankind faces a plethora of environmental problems, many of which are directly influenced by individual human behaviour. To better understand pro-environmental behaviour, we here try to identify interindividual markers that explain variance in the frequency of every-day pro-environmental behaviour. So far, research on this topic has mainly relied on subjective self-report measures and has yielded mixed results. In this study, we applied a neural trait approach to assess stable, objective individual differences. Using source-localised electroencephalography, we measured cortical activation at rest and combined our neural task-independent data with an ecologically valid assessment of everyday pro-environmental behaviour. We find whole-brain-corrected evidence that task-independent baseline activation in the right lateral prefrontal cortex, a brain area known to be involved in cognitive control and self-control processes, explains individual differences in pro-environmental behaviour. The higher the cortical baseline activation in this area, the higher the frequency of everyday pro-environmental behaviour. Implications for the promotion of pro-environmental behaviour are discussed.
Introduction
It is well-established that immediate action is needed to avert a potentially catastrophic climate change1,2 and it has been argued that individual decision-making might be a promising target to reduce humankind’s negative influence on the environment3–6. However, while there is substantial interindividual variance in pro-environmental behaviour7,8, it is still not fully understood why some people exhibit pro-environmental behaviour while others do not, and how said behaviour can be fostered.
Traditional models employed in environmental psychology (like the theory of planned behaviour9 or the theory of reasoned action10, for an overview, see11) strongly focus on a person’s attitudes towards pro-environmental behaviours. It is therefore not surprising that the use of information campaigns to change attitudes and increase awareness has been a dominating approach to enhance pro-environmental behaviour. Recently however, criticism emerged from within the environmental science community2,12: Attitude change has turned out to be less effective than hoped13, and there is a mismatch between people’s pro-environmental attitudes and their pro-environmental behaviour. This “attitude-behaviour gap14,15” indicates the need to identify other factors explaining why some people behave sustainably while others do not. One possible explanation would be that there are strong environmental factors influencing behaviour. For example, sometimes there just is no infrastructure provided to recycle or to use public transport16. Additionally, and corresponding to traditional models like the norm activation theory17 and the focus theory of normative conduct18 (for an overview, see11), it has been shown that the behaviour and norms of other people in a person’s environment largely influence pro-environmental behaviour19,20.
But how can one reliably identify a “green person” (someone with a general proclivity for green behaviour across many situations)? Naturally, many researchers turned to traditional trait models like the Big Five21, typically assessed using self-reports. But while the relationship between broad personality traits and pro-environmental behaviour has sparked a considerable amount of research, no clear pattern as of which traits are related to pro-environmental behaviour has emerged, and findings are sometimes contradictory22–24.
In this study, we aimed to find a trait that explains pro-environmental behaviour using an objective measurement of individual differences. For this purpose, we applied the neural trait approach, which identifies task-independent, brain-based differences between people and links these differences to a behaviour of interest25. Specifically, we recorded task-independent resting electroencephalography (EEG) of 87 participants before measuring their everyday pro-environmental behaviour over the course of five days. Measuring an individual’s baseline cortical activation with EEG is an ideal neural trait measure because it is unique to an individual (recognising a person based on their pattern of brain electrical activity at rest is possible in up to 99% of cases) and relatively stable over time, with a retest-reliability of up to 0.80 after 5 years26–28. Prior studies could show that this measure of neural traits helps to explain variance in expertise (e.g. musicianship29), intelligence (e.g. psychometric intelligence30), and social and non-social behaviour, such as for example deception31, alcohol consumption32, cooperation33 and norm-compliance34.
Of course, the fact that we here focus on establishing a trait that explains differences in pro-environmental behaviour across different situations does not mean that situational factors can or indeed should be disregarded. Similarly, it is known that sociodemographic variables like income, level of education, or age also effect pro-environmental behaviour35. Still, exploring which traits influence inter-individual differences in pro-environmental behaviour is an equally important topic. To the best of our knowledge, this is the first study that uses a neural trait approach to explain interindividual differences in everyday pro-environmental behaviour. We therefore chose an exploratory analysis and applied a whole-brain-corrected approach (not restricting the analysis to any a priori hypothesised region).
To measure pro-environmental behaviour, we used experience-sampling36, which takes advantage of the wide-spread use of smartphones and is known to enhance ecological validity and reduce recall bias37. Typically, participants receive multiple short questionnaires a day to immediately fill out. Because they only have to remember actions from the last couple of hours and data is collected over multiple days, this method enables participants to accurately recall their behaviour38. Combining experience sampling with the neural trait approach, we aimed to establish an objective neural trait, which explains variance in pro-environmental behaviour. Additionally, participants reported their general environmental attitudes on the New Environmental Paradigm (NEP)39. This enabled us to compare the predictive power of the neural trait approach with an established measurement of environmental attitudes.
Results
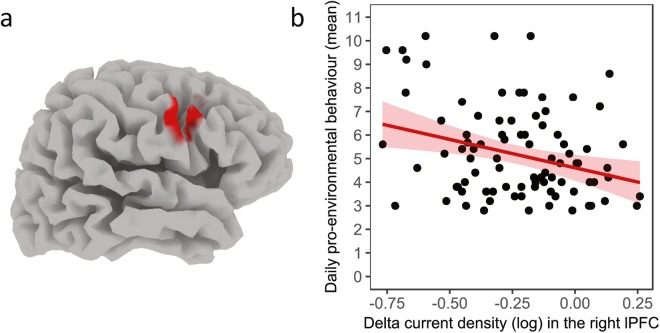
On average, participants had a pro-environmental behaviour score of 5.29 (SD: 1.97; range: 2.80–10.20). On the NEP, they showed a mean of 3.35 (SD: 0.43; range: 2.37–4.36). The whole-brain-corrected analyses showed statistically significant negative correlations between current density in the right lateral prefrontal cortex [MNI-coordinates: x = 60; y = 10; z = 30, Brodmann area 6/9] in the delta band and pro-environmental behaviour (p < 0.05, whole-brain corrected). A robust regression (M estimation with iterated re-weighted least squares) using the mean current density in a ROI (10 mm sphere around the peak voxel) resulted in a correlation coefficient of −0.30, p = 0.004, R2 = 0.09 (see Fig. 1). Partialling out participants’ gender and age did not affect the relation between current density in the delta band and pro-environmental behaviour (r = −0.29, p = 0.006, R2 = 0.084). As baseline slow wave oscillations likely reflect decreased cortical activation40–42, our findings suggests that people with higher baseline activation in the right lateral PFC at rest behave in a more environmentally friendly way.
Figure 1.
Depiction of the correlation between delta current density and pro-environmental behaviour. Panel a shows the area in the right lateral prefrontal cortex where baseline delta current density is statistically significantly correlated with pro-environmental behaviour (whole-brain corrected). Panel b shows a corresponding scatter plot with a robust regression line (which accounts for potential outliers) and 95%-confidence intervals. Depicted is the relationship between baseline delta current density in the right lateral PFC (10 mm sphere around the peak voxel) and pro-environmental behaviour. Baseline slow wave oscillations likely reflect decreased cortical activation, thereby suggesting that higher baseline activation in the right lateral PFC is related to more environmentally friendly behaviour.
Additionally, we wanted to explore whether baseline activation in the right lateral PFC can explain unique variance which cannot be explained by general attitudes towards the environment. To this end, a hierarchical regression was conducted with pro-environmental behaviour scores as dependent variable, and NEP score and delta current density in the right lateral PFC as first and second predictor, respectively. Due to the nine participants who did not fill out the NEP, these analyses have been calculated with N = 78. The NEP score correlated with daily pro-environmental behaviour on a trend level (p = 0.09). Including the second predictor into the regression raised the explained variance from 3.8% to 13.4%, and an ANOVA proofed the difference between the two models to be statistically significant, F(1, 75) = 8.31, p = 0.005, Cohen’s f2 = 0.11 (corresponding to a small effect). Hence, baseline activation in the right lateral PFC does indeed explain unique variance.
Discussion
Using an exploratory whole-brain neural trait approach, we report evidence that a specific neural trait – task-independent baseline activation in the right lateral prefrontal cortex – partly explains daily pro-environmental behaviour in the field. The higher the level of baseline activation in the lateral prefrontal cortex, the more pro-environmental behaviour was reported by the participants. We therefore propose that baseline activation in the right lateral PFC is a stable marker for every-day pro-environmental behaviour.
While one has to be careful with interpreting exploratory findings, our results fit well into the established literature on the role of the lateral PFC in decision-making. A substantial body of research shows that higher levels of both baseline and task-related activation in the lateral PFC correlates with increased self-regulation, inhibitory control, or executive functions in general32,43–47. Additionally, EEG baseline activation of the lateral PFC is also related to established task-related neural markers of self-control like the NoGo-Anteriorisation and the NoGo-P30034,48. Converging evidence comes from the study of a different neural trait approach, namely anatomical brain differences measured using structural magnetic resonance imaging (MRI), with evidence suggesting that structural MRI and resting EEG are correlated49. It has been shown that cortical thickness in the lateral PFC is positively correlated with the ability to suppress impulsive behaviour50, and as the lateral PFC matures, children are increasingly capable of suppressing egoistic impulses to act according to more complex long-term goals51,52. Finally, neuromodulation studies showed that inhibiting the lateral PFC diminishes the ability to excerpt self-control in social and non-social contexts53–58.
In the realm of pro-environmental behaviour, a similar process might be involved. It is well possible that the implementation of pro-environmental behaviour in everyday situations requires active self-control to not succumb to temptations59. If people’s self-control is low (corresponding to less activation in the lateral PFC), they might not be able to stick to their initial intentions (e.g., conserving water) in the light of tempting behavioural alternatives (e.g., taking a bath after a long day at work), even if they do care about the environment. This might also explain why general attitudes towards the environment are not as ideal a predictor of pro-environmental behaviour as one could expect, with pro-environmental behaviour being a typical case of a “willing spirit” meeting “weak flesh”. On the other hand, people with a high baseline activation in the lateral PFC might be better at considering their original intentions when presented with a tempting behavioural alternative.
Our finding fits well into recent theorising in environmental science. Indeed, multiple researchers have proposed that it might be crucial to take self-control processes into account when trying to encourage pro-environmental behaviour2,12, but empirical evidence for this proposal is rare60. Our results, however, grant neuroscientific support to the idea that self-control is involved in everyday pro-environmental decision-making.
Resting EEG (and resting fMRI) might also have some limitations. First, performing a resting state recording might not be as straightforward as it seems; participants’ experiences and stimulus independent thoughts may vary despite similar instructions61. Controlling these spontaneous thoughts is difficult, but consistent activation patterns of resting state networks among studies suggest only a limited influence62. Second, because the sLORETA solution space is restricted to the cortical grey matter and the hippocampus, future studies could apply other methods (e.g. structural MRI, resting functional MRI) to explore whether neural trait measures of subcortical areas can also be used to predict pro-environmental behaviour.
It is important to note that while our data provide evidence for a stable neuroscientific trait, “stable” does not equal “unchangeable”: Indeed, the human brain is known to change throughout adulthood63 and techniques like self-control trainings, neurofeedback, repeated practice or meditation can have lasting effects on neural structure and functioning of the prefrontal cortex64–70. Drawing on this knowledge, our research could help to find new ways to promote pro-environmental behaviour in those who so far struggle to behave sustainably.
Methods
Participants
Participants consisted of 87 healthy students from the University of Bern (66 female; mean age ± SD: 20.9 ± 2.0 years). All participants gave written informed consent; the study was approved by the local ethics committee of the Faculty of Human Sciences (University of Bern, Switzerland) and conducted in accordance to the declaration of Helsinki. Participants received a compensation of 25 Swiss Francs (CHF; 1 CHF ≈ 1 US dollar) and an additional 2 CHF for every time they completed the experience sampling, resulting in a maximum additional amount of 30 CHF.
Procedure
Resting EEG data was acquired in the EEG laboratory, where only one participant was tested at a time. For the experience sampling part of the study, our participants were invited to the behavioural lab and registered with the online software through which they would receive the invitations to the experience sampling questions via text message. Then, they were familiarised with the use of the experience sampling method on their smartphones and the content of each item. The actual experience sampling started two days later. An online-version of the New Environmental Paradigm (NEP)39 was distributed to the participants after the experience sampling. We implemented a gap of several weeks between EEG-recording, experience sampling, and assessment of the NEP to minimise any potential carry-over effect.
Recording and processing of EEG data
The EEG acquisition took place in a sound and electrically shielded chamber that was dimly lit and contained an intercom connection to the experimenters. We used BrainVision Recorder (version 1.20) to record the EEG during rest with open or closed eyes; the instructions about eye opening/closing were given via intercom. The protocol consisted of 20-s eyes open followed by 40-s eyes closed, repeated 5 times (such a protocol guarantees minimal fluctuations in participants’ vigilance state). Data analysis is based on the 200-seconds eyes-closed condition. We recorded a continuous EEG from 60 Ag/AgCl electrodes placed according to the international 10–10 system71, using a BrainAmp DC amplifier with a sampling rate of 500 Hz (bandwidth: 0.1 to 250 Hz). The electrode at the position FCz was used as recording reference and the electrode at the position CPz as ground electrode. Horizontal and vertical electro-oculographic signals were recorded with two additional electrodes at the left and right outer canthi of the left and right eye and an additional electrode below the right eye. Impedances were kept below 10 kΩ. BrainVision Analyzer (version 2.1.2) was used for preprocessing the EEG data. We filtered the EEG data offline, using a high pass filter of 0.5 Hz and a low pass filter of 30 Hz, notch filter enabled at 50 Hz. EEG signals with excessive noise were replaced using a spline interpolation of the signal of adjacent electrodes. To remove eye movements artefacts, we first ran an Independent Component Analysis (ICA) and then manually removed factors related to horizontal and vertical eye movements (usually only two factors were detected and eliminated). After having removed the factors related to eye movements, the EEG was recomputed using an inverse ICA procedure. Then we applied an automatic artefact rejection with the following parameters: maximal voltage step: 15 μV; maximal amplitude: ±100 μV; minimal allowed activity in intervals of 100-ms length: 0.5 μV. After this automatic artefact rejection, data were also visually examined to eliminate residual artefacts. Further, data were recomputed against the average reference. All artefact-free 2-s EEG-epochs were extracted. On average, there were 88 (SD: 20.5) epochs per person. A Fast Fourier Transformation (using a square window) was applied to each epoch and channel to compute the power spectra with 0.5-Hz resolution. The spectra for each channel were averaged over all epochs for each participant. Absolute power values were obtained for the following seven independent frequency bands72: delta (1.5–6 Hz), theta (6.5–8 Hz), alpha1 (8.5–10 Hz), alpha2 (10.5–12 Hz), beta1 (12.5–18 Hz), beta2 (18.5–21 Hz), and beta3 (21.5–30 Hz). The intracortical electrical sources that generated the scalp-recorded activity in each of the seven frequency bands were estimated using the standardised low-resolution brain electromagnetic tomography (sLORETA, version v20160611)73. The sLORETA method is a properly standardised discrete, 3D distributed, linear, minimum norm inverse solution. The particular form of standardisation used in sLORETA endows the tomography with the property of exact localisation to test point sources, yielding images of standardised current density with exact localisation, albeit with low spatial resolution (i.e., neighbouring neural sources will be highly correlated). sLORETA has been validated in several simultaneous EEG/fMRI studies74,75 and in an EEG localisation study for epilepsy76. In the current implementation of sLORETA, computations were made in a realistic head model using the MNI152 template77, with the 3D solution space restricted to cortical grey matter, as determined by the probabilistic Talairach atlas78. The intracerebral volume is partitioned in 6239 voxels at 5 mm spatial resolution. Thus, sLORETA images represent the standardised electric activity at each voxel in neuroanatomic Montreal Neurological Institute (MNI) space as the exact magnitude of the estimated current density (unit: amperes per square meter, A/m2). Using the automatic regularisation method in the sLORETA software, we chose the transformation matrix with the signal-to-noise ratio set to 10. In order to reduce confounds that have no regional specificity, such as inter-subject variability in total power, a global normalisation and log-transformation of the sLORETA images was carried out prior to subsequent statistical analyses.
Assessment of daily pro-environmental behaviour
For five consecutive days, participants received the link to the daily pro-environmental questions three times daily (late morning, afternoon, evening). For this, they received a link via text message (using the SurveySignal online service). The online questions on daily pro-environmental behaviour were implemented using the Qualtrics survey software. After the experience sampling was completed, participants received information about their final compensation and were paid. Every set of experience sampling consisted of five questions. Rather than focusing on one specific aspect, we assessed a wide range of everyday pro-environmental behaviours (not littering in the street; separating waste; not buying products that are not environmentally friendly; paying attention to using little water; ordering coffee in a reusable cup rather than a paper cup). Participants were asked to indicate whether they had shown these behaviours since the last time they had answered the questions. The assessment of daily pro-environmental behaviour took place during the semester, so that that participants (all of whom were students) had plenty of opportunities to experience situations in which they could behave pro-environmentally, and so that all participants had a fairly similar daily routine. Additionally, we asked to report whether there were any extraordinary circumstances that prevented them from showing the behaviours in questions (e.g., whether they were sick at home). This did not occur.
On average, participants responded at 14.8 of the 15 experience sampling time points (SD: 0.7; range: 12–15). To analyse participants’ pro-environmental behaviour, we first calculated the sum of pro-environmental behaviour per day and then computed the arithmetic mean over all 5 days, so that participants could have a pro-environmental behaviour score between 0 and 15.
General attitudes towards the environment
In order to examine whether individual differences in cortical baseline activation are capable of explaining unique variance in pro-environmental behaviour compared to an environmental attitude measure, we also employed the New Environmental Paradigm39, the most widely used measure of environmental concern4,79. It consists of 15 items about environmental views (e.g., “If things continue on their present course, we will soon experience a major ecological catastrophe” or “Humans are seriously abusing the environment”). The NEP has an alpha of 0.8339. Dunlap et al.39 also provide a good overview over the relevant literature regarding the NEP’s validity. For example, the NEP has been shown to correlate with behavioural intentions and behaviours. Additionally, groups who would theoretically be expected to hold more pro-environmental attitudes (e.g., members of environmental groups) indeed score higher on the NEP. The content validity has been confirmed by ethnographic interviews, lending support from qualitative research as well. Participants responded on a 5-point Likert scale, and we used the mean over all items (reverse coding the anti-NEP items, so that higher scores correspond to more pro-environmental attitudes) for further analyses, resulting in a pro-environmental score between 1 and 5. Out of the 87 participants, 78 completed the NEP.
Statistical analysis
The main goal of this study was to examine whether pro-environmental behaviour can be explained based on a task-independent neural trait. For this purpose, we decided to run robust regression analyses to minimize the influence of potential outliers. Since robust regressions are not implemented in the sLORETA software, normalised and log-transformed current density values for each voxel, frequency band, and participant were exported from sLORETA to Matlab. We then used a custom made script that allows to run robust regression analyses in the whole-brain (sLORETA solution space; 6239 voxels) and to conduct non-parametric statistics in order to incorporate correction for multiple comparisons80. This approach uses a randomisation strategy that determines the values of the critical probability threshold (at p < 0.05, corrected) for the observed r-values to identify cortical voxels that significantly correlate with the measures of interest in each frequency band.
Acknowledgements
We thank Anne Saulin for her help with programming the survey and Alexander von Bergen, Peggy Kübler, Anne Saulin, Flavio Schmidig, and Gina Spizzi for their help with data collection. This work was supported by grants from the Mens Sana Foundation to D.K., the Jacobs Foundation to D.K. and R.M.M., and the Swiss National Science Foundation (grant number 100019_166006) to D.K. and L.R.R.G.
Author Contributions
D.K. and R.M.M. conceived the research, T.B. and L.R.R.G. conducted research, T.B., B.P.L. and L.R.R.G. carried out the analyses, B.P.L. and T.B. wrote the paper with inputs and edits from D.K. and L.R.R.G.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thomas Baumgartner and Benedikt P. Langenbach contributed equally.
References
- 1.Kolstad, C. et al. Social, economic and ethical concepts and methods. In Climate Change 2014: Mitigation of climate change. Contribution of working group III to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Edenhofer, O. et al.) 177–248 (Cambridge University Press 2014).
- 2.Nielsen KS. From prediction to process: A self-regulation account of environmental behavior change. J. Environ. Psychol. 2017;51:189–198. doi: 10.1016/j.jenvp.2017.04.002. [DOI] [Google Scholar]
- 3.Clayton S, et al. Psychological research and global climate change. Nat. Clim. Change. 2015;5:640–646. doi: 10.1038/nclimate2622. [DOI] [Google Scholar]
- 4.Gifford R. Environmental psychology matters. Annu. Rev. Psychol. 2014;65:541–579. doi: 10.1146/annurev-psych-010213-115048. [DOI] [PubMed] [Google Scholar]
- 5.Steg L, Vlek C. Encouraging pro-environmental behaviour: An integrative review and research agenda. J. Environ. Psychol. 2009;29:309–317. doi: 10.1016/j.jenvp.2008.10.004. [DOI] [Google Scholar]
- 6.Vandenbergh MP, Barkenbus J, Gilligan JM. Individual carbon emissions: The low-hanging fruit. UCLA Law Rev. 2008;55:08–36. [Google Scholar]
- 7.Abrahamse W, Steg L, Vlek C, Rothengatter T. The effect of tailored information, goal setting, and tailored feedback on household energy use, energy-related behaviors, and behavioral antecedents. J. Environ. Psychol. 2007;27:265–276. doi: 10.1016/j.jenvp.2007.08.002. [DOI] [Google Scholar]
- 8.Gifford, R. & Nilsson, A. Personal and social factors that influence pro-environmental concern and behaviour: A review. Int. J. Psychol. 141–157 (2014). 10.1002/ijop.12034 [DOI] [PubMed]
- 9.Ajzen I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991;50:179–211. doi: 10.1016/0749-5978(91)90020-T. [DOI] [Google Scholar]
- 10.Fishbein, M. & Ajzen, I. Belief, attitude, intention and behavior: An introduction to theory and research (Addision-Wesley 1975).
- 11.Vining, J. & Ebreo, A. Emerging theoretical and methodological perspectives on conservation behavior. In Handbook of environmental psychology (eds Betchel, R. B. & Churchman, A.) 541–558 (2002).
- 12.Bamberg S. Changing environmentally harmful behaviors: A stage model of self-regulated behavioral change. J. Environ. Psychol. 2013;34:151–159. doi: 10.1016/j.jenvp.2013.01.002. [DOI] [Google Scholar]
- 13.Abrahamse W, Steg L, Vlek C, Rothengatter T. A review of intervention studies aimed at household energy conservation. J. Environ. Psychol. 2005;25:273–291. doi: 10.1016/j.jenvp.2005.08.002. [DOI] [Google Scholar]
- 14.Juvan E, Dolnicar S. The attitude–behaviour gap in sustainable tourism. Ann. Tour. Res. 2014;48:76–95. doi: 10.1016/j.annals.2014.05.012. [DOI] [Google Scholar]
- 15.Kennedy EH, Beckley TM, McFarlane BL, Nadeau S. Why we don’t ‘walk the talk’: Understanding the environmental values/behaviour gap in Canada. Hum. Ecol. Rev. 2009;16:151–160. [Google Scholar]
- 16.Kollmuss A, Agyeman J. Mind the Gap: Why do people act environmentally and what are the barriers to pro-environmental behavior? Environ. Educ. Res. 2002;8:239–260. doi: 10.1080/13504620220145401. [DOI] [Google Scholar]
- 17.Schwartz, S. H. Normative influences on altruism. In Advances in Experimental Social Psychology (ed. Berkowitz, L.)10, 221–279 (Elsevier 1977).
- 18.Cialdini RB, Reno RR, Kallgren CA. A focus theory of normative conduct: Recycling the concept of norms to reduce littering in public places. J. Pers. Soc. Psychol. 1990;58:1015–1026. doi: 10.1037/0022-3514.58.6.1015. [DOI] [Google Scholar]
- 19.Steg L, Bolderdijk JW, Keizer K, Perlaviciute G. An integrated framework for encouraging pro-environmental behaviour: The role of values, situational factors and goals. J. Environ. Psychol. 2014;38:104–115. doi: 10.1016/j.jenvp.2014.01.002. [DOI] [Google Scholar]
- 20.Jachimowicz JM, Hauser OP, O’Brien JD, Sherman E, Galinsky AD. The critical role of second-order normative beliefs in predicting energy conservation. Nat. Hum. Behav. 2018;2:757–764. doi: 10.1038/s41562-018-0434-0. [DOI] [PubMed] [Google Scholar]
- 21.Costa, P. T. & McCarae, R. R. NEO five-factor inventory (NEO-FFI) (Psychological Assessment Resources 1989).
- 22.Gordon-Wilson S, Modi P. Personality and older consumers’ green behaviour in the UK. Futures. 2015;71:1–10. doi: 10.1016/j.futures.2015.05.002. [DOI] [Google Scholar]
- 23.Kvasova O. The Big Five personality traits as antecedents of eco-friendly tourist behavior. Personal. Individ. Differ. 2015;83:111–116. doi: 10.1016/j.paid.2015.04.011. [DOI] [Google Scholar]
- 24.Milfont TL, Sibley CG. The big five personality traits and environmental engagement: Associations at the individual and societal level. J. Environ. Psychol. 2012;32:187–195. doi: 10.1016/j.jenvp.2011.12.006. [DOI] [Google Scholar]
- 25.Nash, K., Gianotti, L. R. R. & Knoch, D. A neural trait approach to exploring individual differences in social preferences. Front. Behav. Neurosci. 8 (2015). [DOI] [PMC free article] [PubMed]
- 26.Dunki RM, Schmid GB, Stassen HH. Intraindividual specificity and stability of human EEG: Comparing a linear vs a nonlinear approach. Methods Inf. Med. 2000;39:78–82. doi: 10.1055/s-0038-1634249. [DOI] [PubMed] [Google Scholar]
- 27.Näpflin M, Wildi M, Sarnthein J. Test–retest reliability of resting EEG spectra validates a statistical signature of persons. Clin. Neurophysiol. 2007;118:2519–2524. doi: 10.1016/j.clinph.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Smit DJA, Posthuma D, Boomsma DI, Geus EJC. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42:691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- 29.Klein C, Liem F, Hänggi J, Elmer S, Jäncke L. The “silent” imprint of musical training. Hum. Brain Mapp. 2016;37:536–546. doi: 10.1002/hbm.23045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langer N, et al. Functional brain network efficiency predicts intelligence. Hum. Brain Mapp. 2012;33:1393–1406. doi: 10.1002/hbm.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumgartner T, Gianotti LRR, Knoch D. Who is honest and why: Baseline activation in anterior insula predicts inter-individual differences in deceptive behavior. Biol. Psychol. 2013;94:192–197. doi: 10.1016/j.biopsycho.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Friese M, Gianotti LR, Knoch D. The association between implicit alcohol attitudes and drinking behavior is moderated by baseline activation in the lateral prefrontal cortex. Health Psychol. 2016;35:837. doi: 10.1037/hea0000179. [DOI] [PubMed] [Google Scholar]
- 33.Gianotti, L. R. R., Dahinden, F. M., Baumgartner, T. & Knoch, D. Understanding individual differences in domain-general prosociality: A resting EEG study. Brain Topogr. 10.1007/s10548-018-0679-y (2018). [DOI] [PMC free article] [PubMed]
- 34.Gianotti, L. R. R., Nash, K., Baumgartner, T., Dahinden, F. M. & Knoch, D. Neural signatures of different behavioral types in fairness norm compliance. Sci. Rep. 8, (2018). [DOI] [PMC free article] [PubMed]
- 35.Gatersleben B, Steg L, Vlek C. Measurement and determinants of environmentally significant consumer behavior. Environ. Behav. 2002;34:335–362. doi: 10.1177/0013916502034003004. [DOI] [Google Scholar]
- 36.Csikszentmihalyi, M. & Larson, R. Validity and reliability of the experience-sampling method. In Flow and the foundations of positive psychology: The collected works of Mihaly Csikszentmihalyi 35–54 (Springer 2014).
- 37.Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- 38.Scollon CN, Prieto C-K, Diener E. Experience sampling: Promises and pitfalls, strength and weaknesses. Assess. Well-Being. 2003;4:5–34. [Google Scholar]
- 39.Dunlap RE, Van Liere KD, Mertig AG, Jones RE. New trends in measuring environmental attitudes: Measuring endorsement of the New Ecological Paradigm: A revised NEP scale. J. Soc. Issues. 2000;56:425–442. doi: 10.1111/0022-4537.00176. [DOI] [Google Scholar]
- 40.Pizzagalli DA, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol. Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001469. [DOI] [PubMed] [Google Scholar]
- 41.Riedner, B. A., Hulse, B. K., Murphy, M. J., Ferrarelli, F. & Tononi, G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. In Progress in Brain Research193, 201–218 (Elsevier 2011). [DOI] [PMC free article] [PubMed]
- 42.Modarres MH, Kuzma NN, Kretzmer T, Pack AI, Lim MM. EEG slow waves in traumatic brain injury: Convergent findings in mouse and man. Neurobiol. Sleep Circadian Rhythms. 2017;2:59–70. doi: 10.1016/j.nbscr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diamond A. Executive Functions. Annu. Rev. Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianotti L, Figner B, Ebstein RP, Knoch D. Why some people discount more than others: Baseline activation in the dorsal PFC mediates the link between COMT genotype and impatient choice. Front. Neurosci. 2012;6:54. doi: 10.3389/fnins.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn. Sci. 2011;15:132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimura, K., Chushak, M. S., Westbrook, A. & Braver, T. S. Intertemporal decision-making involves prefrontal control mechanisms associated with working memory. Cereb. Cortex 1–12, 10.1093/cercor/bhx015 (2017). [DOI] [PMC free article] [PubMed]
- 47.Knoch D, Gianotti LRR, Baumgartner T, Fehr E. A neural marker of costly punishment behavior. Psychol. Sci. 2010;21:337–342. doi: 10.1177/0956797609360750. [DOI] [PubMed] [Google Scholar]
- 48.Schiller B, Gianotti LRR, Nash K, Knoch D. Individual differences in inhibitory control - relationship between baseline activation in lateral PFC and an electrophysiological index of response inhibition. Cereb. Cortex. 2014;24:2430–2435. doi: 10.1093/cercor/bht095. [DOI] [PubMed] [Google Scholar]
- 49.Bruder GE, et al. Relationship of resting EEG with anatomical MRI measures in individuals at high and low risk for depression. Hum. Brain Mapp. 2012;33:1325–1333. doi: 10.1002/hbm.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairchild G, et al. Brain structure abnormalities in adolescent girls with conduct disorder: Brain structure in females with conduct disorder. J. Child Psychol. Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinbeis N. Neurocognitive mechanisms of prosociality in childhood. Curr. Opin. Psychol. 2018;20:30–34. doi: 10.1016/j.copsyc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Steinbeis N, Bernhardt BC, Singer T. Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron. 2012;73:1040–1051. doi: 10.1016/j.neuron.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner T, Knoch D, Hotz P, Eisenegger C, Fehr E. Dorsolateral and ventromedial prefrontal cortex orchestrate normative choice. Nat. Neurosci. 2011;14:1468–1474. doi: 10.1038/nn.2933. [DOI] [PubMed] [Google Scholar]
- 54.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 55.Knoch D, Schneider F, Schunk D, Hohmann M, Fehr E. Disrupting the prefrontal cortex diminishes the human ability to build a good reputation. Proc. Natl. Acad. Sci. 2009;106:20895–20899. doi: 10.1073/pnas.0911619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ruff CC, Ugazio G, Fehr E. Changing social norm compliance with noninvasive brain stimulation. Science. 2013;342:482–484. doi: 10.1126/science.1241399. [DOI] [PubMed] [Google Scholar]
- 57.Strang S, et al. Be nice if you have to - the neurobiological roots of strategic fairness. Soc. Cogn. Affect. Neurosci. 2014;10:790–796. doi: 10.1093/scan/nsu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figner B, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat. Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 59.Gutsell, J. N. & Inzlicht, M. A Neuroaffective perspective on why people fail to live a sustainable lifestyle. In Encouraging sustainable behavior (ed. van Trijp, H. C. M.) 137–154 (Psychology Press 2012).
- 60.Redondo I, Puelles M. The connection between environmental attitude–behavior gap and other individual inconsistencies: A call for strengthening self-control. Int. Res. Geogr. Environ. Educ. 2016;26:107–120. doi: 10.1080/10382046.2016.1235361. [DOI] [Google Scholar]
- 61.Diaz, B. A. et al. The Amsterdam Resting-State Questionnaire reveals multiple phenotypes of resting-state cognition. Front. Hum. Neurosci. 7, (2013). [DOI] [PMC free article] [PubMed]
- 62.Damoiseaux JS, et al. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuchs E, Flügge G. Adult neuroplasticity: More than 40 years of research. Neural Plast. 2014;2014:1–10. doi: 10.1155/2014/541870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards, B. G., Barch, D. M. & Braver, T. S. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Front. Hum. Neurosci.4, 32, 10.3389/fnhum.2010.00032 (2010). [DOI] [PMC free article] [PubMed]
- 65.Ghaziri J, et al. Neurofeedback training induces changes in white and gray matter. Clin. EEG Neurosci. 2013;44:265–272. doi: 10.1177/1550059413476031. [DOI] [PubMed] [Google Scholar]
- 66.Kang D-H, et al. The effect of meditation on brain structure: Cortical thickness mapping and diffusion tensor imaging. Soc. Cogn. Affect. Neurosci. 2013;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lazar SW, et al. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liechti MD, et al. First clinical trial of tomographic neurofeedback in attention-deficit/hyperactivity disorder: Evaluation of voluntary cortical control. Clin. Neurophysiol. 2012;123:1989–2005. doi: 10.1016/j.clinph.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi H, et al. Training of working memory impacts structural connectivity. J. Neurosci. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taren AA, et al. Mindfulness meditation training and executive control network resting state functional connectivity: A randomized controlled trial. Psychosom. Med. 2017;79:674–683. doi: 10.1097/PSY.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nuwer MR, et al. IFCN standards for digital recording of clinical EEG. Electroencephalogr. Clin. Neurophysiol. 1998;106:259–261. doi: 10.1016/S0013-4694(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 72.Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmacopsychiatry. 1979;12:237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 73.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12. [PubMed] [Google Scholar]
- 74.Mobascher A, et al. Fluctuations in electrodermal activity reveal variations in single trial brain responses to painful laser stimuli - A fMRI/EEG study. NeuroImage. 2009;44:1081–1092. doi: 10.1016/j.neuroimage.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 75.Mobascher A, et al. Laser-evoked potential P2 single-trial amplitudes covary with the fMRI BOLD response in the medial pain system and interconnected subcortical structures. NeuroImage. 2009;45:917–926. doi: 10.1016/j.neuroimage.2008.12.051. [DOI] [PubMed] [Google Scholar]
- 76.Rullmann M, et al. EEG source analysis of epileptiform activity using a 1 mm anisotropic hexahedra finite element head model. NeuroImage. 2009;44:399–410. doi: 10.1016/j.neuroimage.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazziotta J, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos. Trans. R. Soc. B Biol. Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lancaster JL, et al. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dunlap RE. The New Environmental Paradigm scale: From marginality to worldwide use. J. Environ. Educ. 2008;40:3–18. doi: 10.3200/JOEE.40.1.3-18. [DOI] [Google Scholar]
- 80.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.