Abstract
Background
Non-sustained ventricular tachycardia (NSVT) can occur asymptomatically and can be incidentally detected in the internal records of pacemakers (PM). The clinical value of NSVT in the population of PM patients is still uncertain.
Our aim was to assess the prevalence of NSVT detected by remote PM control, to describe the clinical and demographic characteristics of patients with NSVT, and to assess the prognostic significance of NSVT in terms of both overall and cardiovascular mortality.
Methods
Consecutive patients followed with PM remote interrogations from September 2010 to December 2015 were included. The transmissions pertaining to the first 12 months of remote control were analysed and the patients were divided by those presenting NSVT and those without NSVT. The two groups were compared in terms of total mortality and cardiovascular mortality based on the administrative data provided by the regional administration of the Italian National Health System.
Results
The prevalence of NSVT in 408 patients (62% males, mean age 75.6; SD 10.6 years old) was 21% in a year. During a mean follow-up duration of 44 months, NSVT did not emerge as independently associated with overall mortality, but was associated with cardiovascular mortality in a competing risk regression model with older age, male gender, diabetes, chronic renal insufficiency, ischemic cardiomyopathy and chronic obstructive pulmonary disease.
Conclusions
We show that NSVT episodes recorded by remote control in a PM population are independently associated with cardiovascular mortality with possible implications for risk stratification and therapeutic options.
Keywords: Pacemaker, Remote interrogation, Non-sustained ventricular tachycardia, Cardiovascular mortality
1. Introduction
Modern cardiac implantable electronic devices are implemented with integrated features to diagnose atrial and ventricular tachyarrhythmias. Non-sustained ventricular tachycardia (NSVT) has been investigated for its prognostic significance in patients with Implantable Cardioverter-Defibrillators (ICD), which are generally affected by structural heart disease [[1], [2], [3]]. NSVTs were detected in a percentage from 20 to 45% at ordinary out-patient clinic follow-up visits in patients treated with pacemaker (PM) therapy [[4], [5], [6]], but the clinical significance is to date not clear.
The development of remote monitoring and remote interrogation technology, which can potentially allow for the replacement of all in-clinic device checks, has also allowed for the easy storage and review of arrhythmic events [[7], [8], [9]]; therefore, knowing the clinical significance of NSVT may be of even greater interest to manage PM patients properly.
In recent years, two single-center retrospective studies have examined the prognostic role of NSVT detection at routine outpatient follow-up and in both studies NSVT did not emerge as an independent predictor of overall mortality [4,6].
Our aim was to assess the prevalence of NSVT in the first year of remote follow-up in a large population of patients treated with PM and to assess the long-term prognostic significance of NSVT on all-cause and cardiovascular mortality.
2. Methods
The study was authorized with the formal acknowledgment for retrospective studies of the Institutional Review Board of Friuli Venezia-Giulia Region (Italy) Healthcare Administration (registration number 8B.2/31.01.2017). All patients were at least 18 years old and had signed an informed consent to the use of their anonymized data for research purposes. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
We retrospectively included all consecutive patients with a Medtronic® PM implanted between February 2007 and April 2015 at the Cardiology Division of the University Hospital of Udine (Italy), who had received a Medtronic CareLink® remote transmitter, and made their first transmission from September 2010, when the CareLink® net for PM was available in Italy, to December 2015. Patients were enrolled at the first transmission and those who did not complete at least one year of remote follow-up were excluded.
Patients actively performed a scheduled transmission to the server every 6 months.
The clinical data of the patients was extracted from the clinical E-Charts, which were regularly updated.
The investigators reviewed all the ventricular high-rate episodes automatically recorded during the first 12 months of follow-up (lower ventricular detection rate of 180 bpm as programmed “on box”); patients were subsequently divided in two groups: A) those with at least one NSVT episode, and B) those without NSVT episodes.
The two groups were compared in terms of baseline characteristics and in terms of clinical outcomes, which included all-cause mortality and cardiovascular mortality. Data on clinical outcomes was provided by the regional administration of Friuli-Venezia Giulia Region, which oversees all public hospitals in the area. Data consisted of death events up to January 2016 with the relative principal diagnosis expressed as International Classification of Disease 9 (ICD-9) Codes. Specifically, the death events with the following ICD-9 codes were considered as cardiovascular: from 390 to 429 and from 785.0 to 785.9. All the other death events were considered as non-cardiovascular.
For the analyses, R (ver. 3.5.1) was used with the following packages: cmprsk for the statistical analyses of competing risk and tidyverse for the data management.
The statistical test used for each variable was different according to the following rule: Wilcoxon–Kruskal–Wallis test for continuous variables, Pearson chi-square test for categorical variables, and the likelihood ratio chi-square test from the proportional odds model for ordinal variables. To determine the association between clinical events and the presence of NSVT during the first year of follow-up, multivariable Fine-Gray sub-distribution hazard models were estimated [10]. The models were validated at the median follow-up time of the non-dead patients with a follow-up time sufficient to obtain at least 10 deaths for each degree of freedom.
3. Results
Four-hundred and forty-nine patients were originally included in the database according to the above-mentioned inclusion criteria. Forty-one of them were further excluded due to the absence of a complete one-year remote follow-up: 35 (85%) patients died, of whom 9 (25%) for cardiovascular causes; the others did not have an adequate compliance to remote-monitoring or could not complete one year of follow-up before the end of the study. In only 2 of the excluded patients NSVT was reported. The remaining 408 patients were sorted into two groups: Group A included 86 patients (21.1%) with at least one detected NSVT (median number of NSVT 1 IQR 1), and Group B included 322 patients (78.9%) without detection of NSVT during remote interrogation.
Demographic, clinical data and PM data are reported in Table 1. As expected, our PM population was characterized by old mean age and by a remarkable presence of hypertension, hyperlipidaemia and atrial fibrillation.
Table 1.
Population and Device Characteristics.
| Reported NSVT group A (n = 86) | non-NSVT group B (n = 322) | Combined (n = 408) | P-value | |
|---|---|---|---|---|
| Age | 75.7 ± 9.8 | 75.6 ± 10.8 | 75.6 ± 10.6 | 0.82 |
| Male gender | 63 (73%) | 188 (58%) | 251 (62%) | 0.012 |
| Comorbidities | ||||
| Ischemic Cardiomyopathy | 24 (28%) | 88 (27%) | 112 (27%) | 0.105 |
| Non-ischemic Cardiomyop. | 3 (4%) | 12 (4%) | 15 (4%) | 0.66 |
| Aortic Valve Disease | 19 (22%) | 45 (14%) | 64 (16%) | 0.036 |
| History of Syncope | 6 (7%) | 37 (11%) | 43 (11%) | 0.226 |
| Heart Failure | 19 (22%) | 41 (13%) | 60 (15%) | 0.071 |
| LVEF | 56/61/65 | 55/62/67 | 55/62/67 | 0.368 |
| History of Atrial Fibrillation | 23 (27%) | 114 (35%) | 157 (38%) | 0.180 |
| History of Ventricular Arrhythmia | 6 (7%) | 13 (4%) | 19 (5%) | 0.250 |
| Hypertension | 51 (59%) | 195 (61%) | 246 (60%) | 0.832 |
| Diabetes Mellitus | 24 (28%) | 75 (23%) | 99 (24%) | 0.375 |
| Hyperlipidaemia | 35 (41%) | 145 (45%) | 180 (44%) | 0.458 |
| History of Stroke | 4 (5%) | 19 (6%) | 23 (6%) | 0.655 |
| Chronic Renal Insufficiency | 10 (12%) | 33 (10%) | 43 (11%) | 0.711 |
| COPD | 14 (16%) | 21 (7%) | 35 (9%) | 0.004 |
| Hyperthyroidism | 4 (5%) | 8 (2%) | 12 (3%) | 0.293 |
| Hypothyroidism | 7 (8%) | 35 (11%) | 42 (10%) | 0.454 |
| Cancer | 11 (13%) | 38 (12%) | 49 (12%) | 0.809 |
| Connective Tissue Disease | 3 (3%) | 19 (6%) | 22 (5%) | 0.376 |
| Indication to implantation | ||||
| Sinus Node Disease | 18 (21%) | 155 (48%) | 173 (43%) | 0.002 |
| Brady-Tachy Syndrome | 5 (6%) | 69 (21%) | 74 (18%) | <0.001 |
| II or III degree AVB | 68 (79%) | 167 (52%) | 235 (57%) | <0.001 |
| Replacement | 18 (21%) | 87 (27%) | 105 (26%) | 0.251 |
| Pacemaker characteristics | ||||
| Pacing Mode | 0.745 | |||
| VVIR | 11 (13%) | 32 (10%) | 43 (11%) | |
| DDDR | 48 (56%) | 204 (63%) | 252 (62%) | |
| DDD | 26 (30%) | 82 (27%) | 108 (27%) | |
| Bipolar Sensing | 64 (75%) | 216 (68%) | 280 (69%) | 0.190 |
| VP percentage < 2% | 10 (12%) | 82 (25%) | 92 (23%) | 0.004 |
| VP percent. 2–40% | 10 (12%) | 56 (17%) | 66 (16%) | 0.134 |
| VP percentage > 40% | 66 (76%) | 184 (57%) | 250 (61%) | 0.001 |
Data are expressed as mean value ± standard deviation, first/second/third quartile or as count and percentage as appropriate.
Median left ventricle ejection fraction (LVEF) was within normal limits in the two subgroups.
The main indication for PM implantation was advanced atrioventricular block (57%) and the clear majority of patients were treated with a dual chamber pacemaker.
At baseline, anticoagulation and antiplatelet therapies did not differ between the two groups. On the contrary, beta-blocker therapy was more prevalent in patients in group A (46%) in comparison to patients in group B (36%, p = 0.07), while amiodarone was significantly more prescribed in patients in group B (8%) in comparison to patients in group A (1%, p = 0.022). Patients were followed for a median time of 44.4 months (IQR 22.8) and during this period new cardiovascular drugs were prescribed in the same proportion to the patients of the two groups except for amiodarone, which was prescribed significantly more in group A (16% vs 7% of patients in group B; p = 0.004).
Of note, ventricular pacing percentage, during the first year of remote interrogation, was significantly higher in group A (VP was over 40% in 76% of patients vs 57% of patients in group B; p = 0.001).
During follow-up, 153 patients (38%) died, 59 of them (38.5%) from a cardiovascular cause.
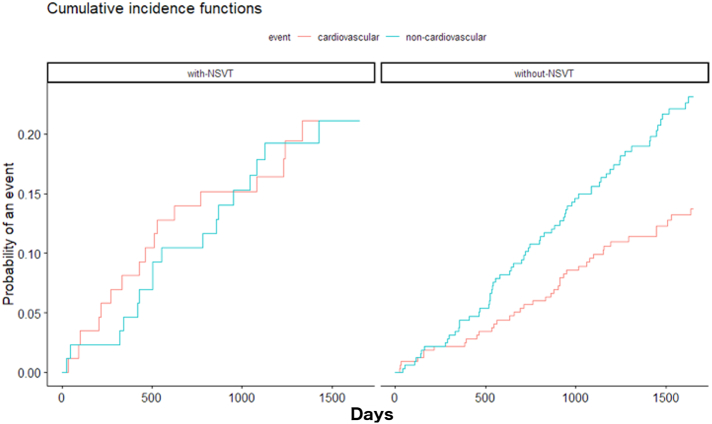
The Cumulative Incidence Function for non-cardiovascular and cardiovascular mortality stratified by the presence of NSVT in the study population are shown in Fig. 1. The incidence of non-cardiovascular mortality was clearly not negligible in both the groups. For non-cardiovascular mortality, diabetes mellitus emerged as the strongest independent predictor of mortality, followed by advanced age. Conversely, the presence of NSVT is not predictive of non-cardiovascular mortality. On the other hand, the competing risk regression analysis for the cardiovascular mortality sub-distribution shows that the presence of NSVT in the first year of follow-up, history of ischemic cardiomyopathy and older age are independently associated with cardiovascular mortality (Table 2).
Fig. 1.
Cumulative Incidence Functions for cardiovascular (red) and non-cardiovascular (blue) death in the group with Non-Sustained Ventricular Tachycardia (NSVT) - on the left – and without NSVT during the first year of follow-up – on the right.
Table 2.
Competing risk regression model for cardiovascular and non-cardiovascular mortality.
| Covariates | Cardiovascular mortality |
Non-cardiovascular mortality |
||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Age | <0.001 | 1.094 (1.062, 1.126) | <0.001 | 1.066 (1.038, 1.096) |
| Male gender | 0.160 | 1.509 (0.856, 2.661) | 0.500 | 0.854 (0.541, 1.349) |
| Diabetes Mellitus | 0.089 | 0.514 (0.238, 1.107) | 0.001 | 2.326 (1.405, 3.849) |
| Chronic Renal Insuff. | 0.390 | 1.442 (0.621, 3.345) | 0.140 | 1.535 (0.867, 2.718) |
| Chr. Obstr. Pulm. Disease | 0.510 | 1.306 (0.595, 2.868) | 0.160 | 1.657 (0.825, 3.328) |
| Ischemic Cardiomyop. | 0.002 | 1.677 (1.205, 2.335) | 0.780 | 1.045 (0.765, 1.429) |
| Reported NSVT | 0.047 | 1.792 (1.007, 3.189) | 0.930 | 0.976 (0.566, 1.683) |
4. Discussion
In our single center study, including an unselected population of consecutive patients treated by PMs of a single company and followed using Carelink remote control, the prevalence of NSVT in one year was 21% and its detection was independently associated with cardiovascular mortality. Only three other studies have evaluated the prevalence and/or prognostic role of NSVT in patients treated by PM; however, in these studies the follow-up was performed only by means of conventional outpatient-clinic controls [[4], [5], [6]]. In the study by Seth et al. [4], the prevalence of NSVT was lower in comparison to our study (19.8% over 2.8 years), but in the study by Gabriels et al. [6], (45% over 29.2 months) and in the data from the EVENTS registry (25.7% over 15 months) [5], the prevalence of NSVT was consistent with our results. The clinical and demographic characteristics of the patients enrolled in the studies by Seth et al. [4] and Gabriels et al. [6] were only partially similar to our population, as they presented a higher burden of cardiovascular risk factors and abnormalities, in particular hypertension and ischemic cardiomyopathy.
In our study, advanced atrioventricular block was more frequently the main indication to PM implantation in patients with reported NSVT in the first year of follow-up, as compared to those without NSVT. In our opinion, a possible explanation could be that patients with high degree AV blocks have a higher percentage of ventricular pacing that could be related to the appearance of NSVT. Indeed, it is well known that in patients with an arrhythmogenic substrate such as those implanted with an ICD, right ventricular pacing over 2% is a predictor of VT and ventricular fibrillation [11], thus suggesting a similar and possible proarrhythmic role of right ventricular pacing in some patients treated by PM.
Amiodarone was prescribed more often at baseline for patients in group B, but there were no significant differences between the two groups in the prevalence of atrial fibrillation. Also, in the work by Seth et al. [4] antiarrhythmic drugs were less, though not significantly, prescribed in patients with NSVT.
Our data on outcome shows the independent association of NSVT detection with cardiovascular mortality, but not with all-cause mortality. Consistently, the studies by Seth et al. [4], and Gabriels et al. [6], excluded a prognostic role of NSVT in all-cause mortality, but they did not test its predictive value in terms of cardiovascular mortality. In our series, patients with NSVT had a higher prevalence of significant aortic valve disease and heart failure, which may partially justify higher cardiac mortality in this subgroup of the PM population.
4.1. Study limitations
1) Baseline treatment with amiodarone in a relevant proportion of patients could have reduced the prevalence of NSVT in our population; 2) The “on box” programmed criteria for automatic NSVT detection were strict, determining the exclusion of NSVT with a frequency lower than 180 bpm; 3) Because of the study's design, the outcome events during the remote-monitoring period could not be counted and the patients who died during this period were excluded from the outcome analysis, thus potentially introducing a selection bias. However, it is plausible that the patients who died during the first months were the most globally compromised, with important senescence (the mortality rate for cardiovascular causes in this subgroup was lower than in the rest of the population).
5. Conclusion
In our single-center retrospective study, we demonstrated that NSVT is frequently encountered during one-year remote follow-up of a large PM population and that the detection of NSVT is independently associated with cardiovascular mortality. The detection of NSVT after PM implantation should induce a deeper risk-stratification and a better definition of the cardiac substrate and antiarrhythmic drug therapy strategy.
Conflict of interest
SI and JC are currently employed by Medtronic Italy.
Acknowledgments
We thank Dr. Loris Zanier of the Epidemiologic Services of Friuli Venezia Giulia Region for having provided the outcome data and Prof. Dario Gregori of the Department of Cardiac Thoracic Vascular Sciences and Public Health of the University of Padova for the precious contribution of the statistical analysis.
Footnotes
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
References
- 1.Chen J., Johnson G., Hellkamp A.S. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial) J. Am. Coll. Cardiol. 2013;61:2161–2168. doi: 10.1016/j.jacc.2013.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiménez-Candil J., Hernández J., Perdiguero P. Prognostic significance of nonsustained ventricular tachycardia episodes occurring early after implantable cardioverter-defibrillator implantation among patients with left ventricular dysfunction. Am. J. Cardiol. 2016;118:1503–1510. doi: 10.1016/j.amjcard.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Katritsis D.G., Zareba W., Camm A.J. Nonsustained ventricular tachycardia. J. Am. Coll. Cardiol. 2012;60:1993–2004. doi: 10.1016/j.jacc.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 4.Seth N., Kaplan R., Bustamante E. Clinical significance of nonsustained ventricular tachycardia on routine monitoring of pacemaker patients. Pacing Clin. Electrophysiol. 2015;38:980–988. doi: 10.1111/pace.12632. [DOI] [PubMed] [Google Scholar]
- 5.Faber T.S., Gradinger R., Treusch S. Incidence of ventricular tachyarrhythmias during permanent pacemaker therapy in low-risk patients results from the German multicentre EVENTS study. Eur. Heart J. 2007;28:2238–2242. doi: 10.1093/eurheartj/ehm242. [DOI] [PubMed] [Google Scholar]
- 6.Gabriels J., Wu M., Rosen L., Patel A., Goldner B. Clinical significance of nonsustained ventricular tachycardia on stored Electrograms in permanent pacemaker patients. Pacing Clin. Electrophysiol. 2016;39:1335–1339. doi: 10.1111/pace.12968. [DOI] [PubMed] [Google Scholar]
- 7.Ricci R.P., Morichelli L., Varma N. Remote monitoring for follow-up of patients with cardiac implantable electronic devices. Arrhythmia Electrophysiol Rev. 2014;3:123–128. doi: 10.15420/aer.2014.3.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comoretto R.I., Facchin D., Ghidina M., Proclemer A., Gregori D. Remote control improves quality of life in elderly pacemaker patients versus standard ambulatory-based follow-up. J. Eval. Clin. Pract. 2017;23:681–689. doi: 10.1111/jep.12691. [DOI] [PubMed] [Google Scholar]
- 9.Facchin D., Baccillieri M.S., Gasparini G. Findings of an observational investigation of pure remote follow-up of pacemaker patients: is the in-clinic device check still needed? Int. J. Cardiol. 2016;220:781–786. doi: 10.1016/j.ijcard.2016.06.162. [DOI] [PubMed] [Google Scholar]
- 10.Fine J., Gray R. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999;94:496–509. [Google Scholar]
- 11.Gardiwal A., Yu H., Oswald H. Right ventricular pacing is an independent predictor for ventricular tachycardia/ventricular fibrillation occurrence and heart failure events in patients with an implantable cardioverter-defibrillator. Europace. 2008;10:358–363. doi: 10.1093/europace/eun019. [DOI] [PubMed] [Google Scholar]