Abstract
Background
Herpes zoster (HZ) is generally thought to occur once in a lifetime and recurrence is considered to be limited to immunocompromised individuals. Although HZ recurrence rates seem to be increasing, there have been few studies exploring these rates in the general population. We investigated the recurrence rate and associated risk factors in the general population.
Methods
We used the population-based samples of the National Health Insurance Service database to identify cases of initial HZ episodes from January 1, 2002 to December 31, 2013. We also followed up on these cases through December 31, 2013 to identify recurrence.
Results
Overall, the incidence rate of HZ is 5.1 per 1,000 person years and the recurrence rate is 12.0 per 1,000 person years. There were 2,100 recurrent cases out of 39,441 initial episodes with 4.4 years of the mean follow-up period. We identified significant risk factors for recurrence such as old age (51–70 years) (hazard ratio [HR], 1.447; 95% confidence interval [CI], 1.311–1.598), women (1.476; 1.345–1.619), zoster-related pain (ZRP) longer than 30 days (cases of ZRP lasting 31–90 days [1.200; 1.042–1.383], and ZRP lasting longer than 90 days [2.293; 1.990–2.643]). Concurrent hematologic malignancies (2.864; 1.929–4.251), autoimmune diseases (1.466; 1.252–1.715), dyslipidemia (1.390; 1.263–1.530), and hypertension (1.222; 1.107–1.350) were also significant risk factors.
Conclusion
Our results suggest that the recurrence of HZ is much more common than generally expected, and that the associated risk factors can play an important role in predicting recurrence.
Keywords: Herpes Zoster, Recurrence, Risk Factors, Epidemiology, Population-based, Recurrence Rate
Graphical Abstract
INTRODUCTION
Herpes zoster (shingles, HZ) is caused by reactivation of the varicella-zoster virus (VZV), which typically remains latent after primary infection with varicella (chickenpox) during childhood. HZ is largely considered to be an once-in-a-lifetime experience and recurrence is thought to be limited to immunocompromised individuals.
Recently, a live attenuated vaccine against HZ was introduced world-wide and licensed to persons aged 50 years and older. The efficacy of the vaccine in persons over 60 years old and aged 50–59 years old is 51% and 70%, respectively.1,2 However, the indication of HZ vaccination on the persons who have already had an episode of HZ, and the appropriate withdrawal period for the vaccination after HZ have not yet been elucidated.
Although there have been several studies on HZ recurrence, these usually depended on hospital-based data with a small number of cases or a short follow-up period. General population based epidemiological data on the recurrence of HZ is necessary for treating and preventing recurrent cases. These data will also play an important role in establishing vaccination guidelines. Therefore, we aimed to assess the recurrence rate of HZ and to identify risk factors associated with recurrence using a population-based Korean database.
METHODS
Data source
In Korea, nearly all people have been enrolled obligatorily in the National Health Insurance Service (NHIS) since 1989. All demographic data, including patient's age, gender and socioeconomic status were collected along with diagnoses, physical and laboratory examination, treatment, prescription, nursing acts, and hospitalization.
This study used nationally representative random samples of the NHIS database, NHIS-National Sample Cohort (NHIS-NSC) 2002–2013. The samples were collected from the records for 1,025,340 persons, approximately 2.2% of the entire population at the beginning in 2002, and included all associated medical data of the enrolled patients from January 1, 2002 to December 31, 2013.
Study population
Among the patients included in the NHIS-NSC 2002–2013, we selected the 746,816 patients older than 20 years of age. NHIS-NSC 2002–2013 maintains the sample size by replacing the death cases or cases with loss of qualification with newborn cases every year. Of course, there is variation of follow up period in cases under 20 years old, and these cases were included in the cases under 20 years old. Therefore, we excluded those cases from the study. We followed up on all included cases until death, loss of qualification, or December 31, 2013. In addition, we set up the year 2002 as the washout period.
Case definition
HZ cases were identified through a database search for any subject with the HZ-related International Classification of Disease 10th revision code (ICD-10 code, B02) except postherpetic neuralgia (PHN) with oral antiviral therapy for more than 5 days or intravenous antiviral therapy. This definition excluded overestimation due to non-HZ cases like herpes simplex cases as we previously reported.3
Zoster-related pain (ZRP) is the most common complication of HZ and is related to HZ recurrence. We defined the duration of ZRP as that of treatment of pain associated with an initial HZ episode. We identified the duration through a database search for defined HZ cases with prescribed painkilling medications, or PHN related ICD-10 code with pain killer medication following the diagnosis of HZ. We categorized patients with ZRP by treatment duration of shorter than 31 days, 31–90 days, and longer than 90 days.
We identified initial HZ episode cases, as well as first and second recurrence cases. To meet the definition of recurrence, a minimum of 6 months must have passed from the previous HZ. From those data, we calculated recurrence rate, mean time period and time trend for recurrence following the initial episode.
Risk factors influencing recurrence
We also collected epidemiological features of initial HZ episodes and evaluated their relationship with recurrence rate. These features at the time of the initial episode included: 1) age/gender, 2) socioeconomic status, 3) residence area, 4) duration of ZRP, 5) hospitalization, and 6) immune status and comorbid diseases. Immune status was determined based on the presence of specific diseases, including solid and hematologic malignancies, autoimmune diseases, human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS), chronic renal diseases, and chronic hepatic diseases within 6 months before or after the diagnosis of HZ. Patients with one or more of these diseases were considered immunocompromised. The presence or absence of comorbid diseases, such as diabetes mellitus, hypertension and hyperlipidemia was also evaluated. Immunocompromised cases and cases with comorbid disease were identified through a database search for any subject with related ICD-10 codes as described in Table 1.
Table 1. ICD-10 codes for the operational diagnosis of immunocompromised cases or comorbid diseases.
| Risk factors | Related ICD-10 codes |
|---|---|
| Solid malignancies | C00–C80, C97 |
| Hematologic malignancies | C81–C96 |
| Autoimmune diseases | M05–M09, M30–36 |
| HIV/AIDS | B20–24, F24, O987, Z21 |
| Chronic renal diseases | E10.2, E11.2, E12.2, E13.2, E14.2, I12.0, I11.3, N18, N19, Z49, Y841 |
| Chronic hepatic diseases | K70.3, K70.4, K71.7, K72.1, K74, K76.1, K76.6, K76.7 |
| Diabetes mellitus | E11–E14 |
| Hypertension | I10–I13, I15 |
| Hyperlipidemia | E78 |
ICD-10 = International Classification of Disease 10th revision, HIV/AIDS = human immunodeficiency virus infection and acquired immune deficiency syndrome.
Statistical analysis
Recurrence rate was calculated as the number of HZ recurrent cases per 1,000 follow-up person years after initial episode. The hazard ratios (HRs) of the various epidemiological features from the initial episode to recurrence were deduced by using univariate Cox proportional hazards regression. The multivariate Cox proportional hazards regression was also used to determine the association between HZ recurrence and HZ recurrence risk factors such as duration of ZRP, immune status, and comorbid diseases to adjust for gender, age, and socioeconomic status. Cumulative recurrence rate curves were calculated using Kaplan-Meier statistics. SAS enterprise guide 4.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Ethics statement
The study protocol abided by the principles of the Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of Seoul St. Mary's Hospital (IRB No. KC14EIS0694). The need for informed consent was waived by the IRB.
RESULTS
During the study period, 39,441 initial episodes of HZ were identified with an incidence of 5.1 per 1,000 person years. Of the initial episodes, 15,638 (39.7%) were men and 23,803 (60.3%) were women. The incidence of HZ increased with age and 66.8% of cases were in patients over 50 years old. For socioeconomic status, the lowest quintile comprised 15.0% of cases. There were 5,408 (13.7%) immunocompromised patients and proportions of solid cancer, autoimmune diseases, chronic renal diseases, chronic hepatic diseases, hematologic cancer, and HIV/AIDS were 6.1%, 5.3%, 2.7%, 0.9%, 0.5%, and 0.1%, respectively. In total, 18,384 (46.6%) of patients with an initial HZ episode had either of diabetes mellitus, hypertension, or dyslipidemia. More detailed epidemiologic features are summarized in Table 2.
Table 2. Epidemiologic features of the initial HZ episode.
| Risk factors | Initial episode of HZ, No. (%) | |
|---|---|---|
| Age, yr | ||
| 21–50 | 13,094 (33.2) | |
| 51–70 | 19,233 (48.8) | |
| > 70 | 7,114 (18.0) | |
| Gender | ||
| Men | 15,638 (39.7) | |
| Women | 23,803 (60.4) | |
| Socioeconomic status | ||
| QL | 5,906 (15.0) | |
| QO | 33,535 (85.0) | |
| Residence area | ||
| Urban | 18,772 (47.6) | |
| Rural | 20,669 (52.4) | |
| Duration of ZRP, day | ||
| < 30 | 33,222 (84.2) | |
| 31–90 | 3,950 (10.0) | |
| > 90 | 2,269 (5.8) | |
| Hospitalization | ||
| Yes | 3,022 (7.7) | |
| No | 36,419 (92.3) | |
| Immunocompromised condition | 5,408 (13.7) | |
| Solid cancer | 2,415 (6.1) | |
| Hematologic cancer | 185 (0.5) | |
| Autoimmune diseases | 2,104 (5.3) | |
| HIV/AIDS | 32 (0.1) | |
| Chronic renal diseases | 1,080 (2.7) | |
| Chronic hepatic diseases | 356 (0.9) | |
| Comorbid diseases | 18,384 (46.6) | |
| Dyslipidemia | 10,359 (26.3) | |
| Diabetes mellitus | 7,530 (19.1) | |
| Hypertension | 13,213 (33.5) | |
| Total | 39,441 (100.0) | |
HZ = herpes zoster, QL = lowest quintile, QO = higher four quintile, ZRP = zoster-related pain, HIV/AIDS = human immunodeficiency virus infection and acquired immune deficiency syndrome.
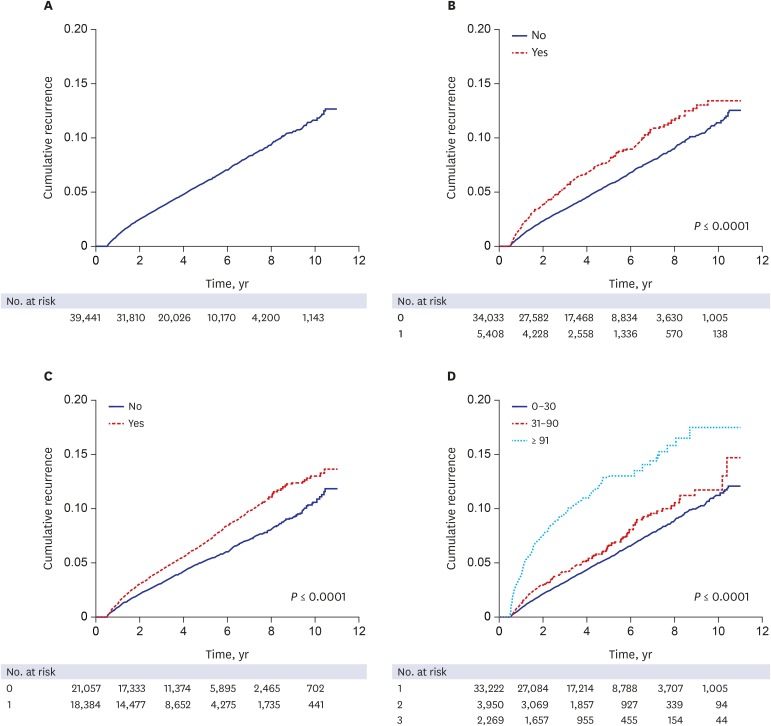
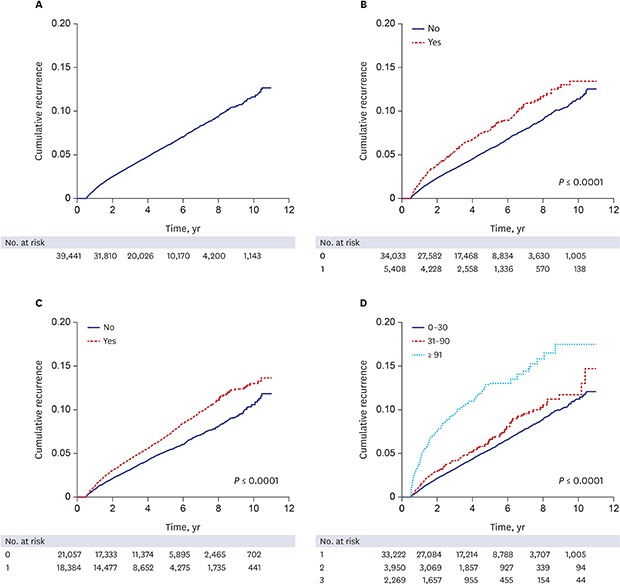
For the recurrence study, 39,441 cases were followed for a mean of 4.4 years, with a range of 1–4,014 days. In total, 2,358 HZ recurrences were observed in 2,100 cases, which included 232 cases with a second recurrence and 26 cases with a third recurrence. The overall recurrence rate was 12.0 per 1,000 person years, or 5.3%. The time between initial HZ episode and first recurrence varied from 181 to 3,815 days, with a mean of 1,062.9 days. A cumulative recurrence rate curve using Kaplan-Meier estimates shows a linear increase until 10 years of follow-up (Fig. 1A).
Fig. 1. The cumulative recurrence rate of HZ according to risk factors at initial episode. (A) overall, (B) immune status, (C) comorbid diseases, (D) duration of zoster-related pain.
HZ = herpes zoster.
Age and gender were significantly associated with HZ recurrence (P < 0.001). The recurrence rate in patients over 50 years was 5.8% (1,517/26,347), whereas that in the younger group was 4.5% (585/13,094). Women had more frequent recurrence than men, with a HR of 1.476 (95% CI, 1.345–1.619). In contrast, socioeconomic status and residence area were not significantly associated.
ZRP lasting longer than 30 days was significantly associated with HZ recurrence (P < 0.001). Recurrence rates of the ZRP groups were as follows: 4.9% (1,643/33,222) for ZRP lasting less than 31 days; 5.7% (225/3,950) for ZRP lasting 31–90 days; and 10.2% (232/2,269) for ZRP lasting longer than 90 days. On the other hand, hospitalization did not show a statistical significance.
Out of 2,100 patients with recurrence, 373 (17.8%) were immunocompromised and 1,727 (82.2%) were immunocompetent at the time of the first HZ episode. Some immunocompromising conditions, such as hematologic malignancies and autoimmune diseases, were significantly associated with recurrence (P < 0.001). However, solid cancer, HIV/AIDS, chronic renal diseases, and chronic hepatic disease showed no statistical significance. Among the comorbid diseases, dyslipidemia and hypertension were significantly related with recurrence of HZ (P < 0.001), but diabetes mellitus was not (Table 3).
Table 3. HR of candidate risk factors influencing HZ recurrence.
| Risk factors | Recurrence, No. (%) | HR (95% CI) | P value | ||
|---|---|---|---|---|---|
| No (n = 37,341) | Yes (n = 2,100) | ||||
| Age, yr | < 0.001 | ||||
| 21–50 | 12,511 (33.5) | 583 (27.8) | 1 | ||
| 51–70 | 18,026 (48.3) | 1,207 (57.5) | 1.447 (1.311–1.598) | ||
| > 70 | 6,804 (18.2) | 310 (14.8) | 1.009 (0.879–1.159) | ||
| Gender | < 0.001 | ||||
| Men | 14,986 (40.1) | 652 (31.1) | 1 | ||
| Women | 22,355 (59.9) | 1,448 (69.0) | 1.476 (1.345–1.619) | ||
| Socioeconomic status | 0.123 | ||||
| QL | 5,567 (14.9) | 339 (16.1) | 1.096 (0.975–1.231) | ||
| QO | 31,774 (85.1) | 1,761 (83.9) | 1 | ||
| Residence area | 0.144 | ||||
| Urban | 17,741 (47.5) | 1,301 (49.1) | 1 | ||
| Rural | 19,600 (52.5) | 1,069 (50.9) | 0.938 (0.861–1.022) | ||
| Duration of ZRP, daya | < 0.001 | ||||
| < 30 | 31,579 (84.6) | 1,643 (78.2) | 1 | ||
| 31–90 | 3,725 (10.0) | 225 (10.7) | 1.200 (1.042–1.383) | ||
| > 90 | 2,037 (5.5) | 232 (11.1) | 2.293 (1.990–2.643) | ||
| Hospitalizationa | 0.737 | ||||
| Yes | 2,854 (7.6) | 168 (8.0) | 0.973 (0.830–1.141) | ||
| No | 34,487 (92.4) | 1,932 (92.0) | 1 | ||
| Immunocompromised conditiona | 5,035 (13.5) | 373 (17.8) | 1.361 (1.216–1.525) | < 0.001 | |
| Solid cancer | 2,265 (6.1) | 150 (7.1) | 1.246 (1.055–1.473) | 0.010 | |
| Hematologic malignancy | 160 (0.4) | 25 (1.2) | 2.864 (1.929–4.251) | < 0.001 | |
| Autoimmune disease | 1,932 (5.2) | 172 (8.2) | 1.466 (1.252–1.715) | < 0.001 | |
| HIV/AIDS | 29 (0.1) | 3 (0.1) | 1.917 (0.618–5.952) | 0.260 | |
| Chronic renal disease | 1,005 (2.7) | 75 (3.6) | 1.344 (1.066–1.695) | 0.012 | |
| Chronic hepatic disease | 335 (0.9) | 21 (1.0) | 1.192 (0.775–1.833) | 0.424 | |
| Comorbid diseasesa | 17,290 (46.3) | 1,094 (52.1) | 1.312 (1.192–1.445) | < 0.001 | |
| Dyslipidemia | 9,720 (26.0) | 639 (30.4) | 1.390 (1.263–1.530) | < 0.001 | |
| Diabetes mellitus | 7,091 (19.0) | 439 (20.9) | 1.124 (1.008–1.254) | 0.036 | |
| Hypertension | 12,416 (33.3) | 797 (38.0) | 1.222 (1.107–1.350) | < 0.001 | |
HR = hazard ratio, HZ = herpes zoster, CI = confidence interval, QL = lowest quintile, QO = high four quintile, HIV/AIDS = human immunodeficiency virus infection and acquired immune deficiency syndrome.
aThe HR was adjusted for age, gender, and socioeconomic status.
A Kaplan-Meier estimate curve of cumulative recurrence rates based on the immune status, the presence of the comorbid diseases, and the duration of ZRP during the initial HZ episode is shown in Fig. 1B-D, respectively. For all parameters except ZRP duration, cumulative recurrence rates increased linearly with time from the first year following the initial HZ episode, as shown in the overall cumulative incidence curve. In cases of ZRP lasting longer than 90 days, cumulative recurrence rate increased sharply within 4 years following the initial HZ episode and gradually increased thereafter.
DISCUSSION
In this study, the population-based recurrence rate of HZ was12.0 per 1,000 person years (5.3%) during a mean follow-up period of 4.4 years. Compared with previous studies, our recurrence rate is on the higher side, with a limited number of studies reporting the recurrence of HZ of 0.2%–12.5%.1,4,5,6,7,8,9,10,11,12 In reports with a relatively short follow-up period of 3–5 years, the recurrence rate was 0.2%–0.9% out of 457–1,075 initial cases.1,5,9 As expected, studies with a longer follow-up period tended to show a higher recurrence rate. For example, Hope-Simpson8 identified 8 recurrence cases out of 192 (4.2%) during 16 years. Similarly, in a larger study population, 31(5.3%) recurrence cases were identified from 590 initial HZ episodes from 1945 to 1959.6 Recently, Yawn et al.4 reported a relatively high recurrence rate of 6.2%, with 105 recurrence cases out of 1,669 first episodes during an average follow-up period of 7.3 years. The discrepancy in these results likely originates from the different population composition, study methods, and duration of follow-up. In addition, most studies were designed to focus on the characteristics of initial HZ episodes rather than HZ recurrence. In most studies, the whole study period was disclosed rather than the follow-up period from the initial episode to the recurrence, meaning the exact follow-up period could not be concluded from the data. Accordingly, recurrence rates could not be expressed by the number of recurrent cases per 1,000 person years, but instead by percentage of initial HZ cases. In this study, we used nationally representative random samples of 39,441 initial HZ episodes with a relatively long-term follow-up period of 4.4 years and a whole study period of 11 years. Therefore, we expect our data to reflect the actual HZ recurrence rate.
Unexpectedly, the recurrence rate was higher than the incidence rate in this study, and the cumulative recurrence rate increased linearly from the initial HZ episode without a sufficient time lag. Generally, a history of previous viral disease serves as a protective factor to recurrence, although this effect may decrease with time. For example, primary infection with VZV results in life-long protection against contracting subsequent varicella after re-exposure. In the case of HZ, it has been reported that VZV-specific T-cell mediated immunity (VZV-CMI) rises and is maintained for 2 years, with higher levels than that of the pre-rash period.13 Recently, the live attenuated HZ vaccine was demonstrated to have a preventive effect against HZ.1,2 Therefore, we expected recurrence rates to be lower than that of initial episodes, and that, even if there was recurrence, there would be a considerable time lag from the initial episode to the recurrence. However, our results seem to show that there may be little protective effect of an initial HZ episode on subsequent recurrence. This may be explained, in part, by the social boosting theory that varicella vaccination during childhood results in increased HZ incidence due to decreased exposure to VZV in adulthood.14,15,16,17,18 In Korea, varicella vaccination has been designated as national immunization program since 2006. Unfortunately, exact vaccination rate over the time has not been disclosed. However, from the report by the Korea Centers for Disease Controls and Prevention, 97.3% of infants born in 2012 were completed the varicella vaccination inoculation in 2015. Based on this theory and high uptake of varicella vaccination, we hypothesize that after an initial HZ episode, VZV-CMI may rapidly decrease to a level that is insufficient to suppress the reactivation of latent HZ due to deficient exogenous booster.
On the other hand, there are some limitations to comparing incidence rate and recurrence rate. There must be some differences in the epidemiological distribution between the population for the incidence study and that of the recurrence study. The follow-up period is another factor to consider. It has been reported that the cumulative recurrence rate stabilizes rather than increases linearly after a considerable amount of time from the initial episode.4 This means that a longer follow-up period may result in a lower recurrence rate per 1,000 person years while maintaining the incidence rate if the study period is long enough. Therefore, for precise evaluation of the protective role of an initial episode, further studies with a larger population and a longer follow-up period are necessary.
From a previous study, the highest frequency of VZV-specific CD4 memory cell is observed at 34 years of age after wild-type VZV infection.19 In an elderly group over 60 years old, VZV-CMI response progressively decreased with age; a 2.7%–3.9% decline each year was reported depending on the assay method. In addition, VZV-CMI responses following zoster vaccination or HZ are lower in older persons.20,21Although there have been no direct reports of VZV-CMI response after HZ according to age, it could be assumed that immune response to initial HZ would be lower and decrease more quickly in elderly patients. Therefore, HZ recurrence could be more common in the elderly, coinciding with our results. VZV-CMI is also influenced by various diseases and medications which can induce immune suppression. For example, solid cancer, hematologic malignancies, systemic lupus erythematosus, renal failure, antineoplastic drugs, and other immune suppressants are reported to be associated with the incidence and recurrence of HZ.22,23,24
Although host immune status is the most important risk factor for HZ, concurrent chronic diseases such as diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, depression, and hypothyroidism have recently been reported to be associated with HZ incidence and recurrence.25,26,27 In cases of diabetes, a higher incidence of HZ and PNH is thought to be related to T-cell dysregulation.28,29,30 In cases of dyslipidemia, high cholesterol levels or statin use may result in VZV reactivation.31,32 In our study, both dyslipidemia and hypertension were significant risk factors of HZ recurrence.
In this study, ZRP lasting longer than 30 days was significantly associated with a higher recurrence rate. Long-lasting ZRP is considered to be related to a greater severity of rash and intensity of pain during the initial HZ episode.11,33 Although the reason remains unclear, patients with persistent ZRP may be more immunocompromised or have more comorbid diseases, as suggested in previous reports.34 In addition, those patients tend to seek for medical attention for recurrence symptoms more frequently than patients with mild initial episodes.
Our study had some limitations. We cannot completely exclude misdiagnosed cases, because we identified HZ cases using computerized electronic medical records without detailed descriptions. Also, HZ cases were not confirmed by laboratory tests, but rather by disease code and prescription of antiviral medication. However, to our knowledge, this is the largest study for the recurrence of HZ and its various associated risk factors. Most of all, because NHIS-NSC 2002–2013 is a nation-representative sample database, comparing with other private insurance claim database with selection bias, we presume that the results in our study are similar to the actual natural history of HZ.
In conclusion, these results suggest that the recurrence of HZ is much more common than expected, and that the associated risk factors can play an important role in predicting recurrence and establishing vaccination guidelines for patients with an initial HZ episode.
Footnotes
Funding: This study was supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. All authors have no other conflicts of interest to declare.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim YJ.
- Data curation: Lee MS, Lee JY.
- Formal analysis: Kim YJ, Lee JY, Park YM.
- Methodology: Kim YJ, Lee CN.
- Software: Han K.
- Validation: Han K.
- Writing - original draft: Kim YJ.
- Writing - review & editing: Lee CN, Lee MS, Lee JH, Lee JY, Han K, Park YM.
References
- 1.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 2.Schmader KE, Levin MJ, Gnann JW, Jr, McNeil SA, Vesikari T, Betts RF, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54(7):922–928. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS, Seo HM, Bang CH, Lee JH, Park YG, Kim YJ, et al. Validation of herpes zoster diagnosis code in the electronic medical record: a retrospective, multicenter study. Ann Dermatol. 2018;30(2):253–255. doi: 10.5021/ad.2018.30.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgason H, Sigurdsson JA, Gudmundsson S. The clinical course of herpes zoster: a prospective study in primary care. Eur J Gen Pract. 1996;2(1):12–16. [Google Scholar]
- 6.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61(5):310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Epstein E. Recurrences in herpes zoster. Cutis. 1980;26(4):378–379. [PubMed] [Google Scholar]
- 8.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58(1):9–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155(15):1605–1609. [PubMed] [Google Scholar]
- 10.Bowsher D. The lifetime occurrence of herpes zoster and prevalence of post-herpetic neuralgia: a retrospective survey in an elderly population. Eur J Pain. 1999;3(4):335–342. doi: 10.1053/eujp.1999.0139. [DOI] [PubMed] [Google Scholar]
- 11.Tseng HF, Chi M, Smith N, Marcy SM, Sy LS, Jacobsen SJ. Herpes zoster vaccine and the incidence of recurrent herpes zoster in an immunocompetent elderly population. J Infect Dis. 2012;206(2):190–196. doi: 10.1093/infdis/jis334. [DOI] [PubMed] [Google Scholar]
- 12.Weitzman D, Shavit O, Stein M, Cohen R, Chodick G, Shalev V. A population based study of the epidemiology of herpes zoster and its complications. J Infect. 2013;67(5):463–469. doi: 10.1016/j.jinf.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200(7):1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogunjimi B, Willem L, Beutels P, Hens N. Integrating between-host transmission and within-host immunity to analyze the impact of varicella vaccination on zoster. eLife. 2015;4:4. doi: 10.7554/eLife.07116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogunjimi B, Smits E, Heynderickx S, Van den Bergh J, Bilcke J, Jansens H, et al. Influence of frequent infectious exposures on general and varicella-zoster virus-specific immune responses in pediatricians. Clin Vaccine Immunol. 2014;21(3):417–426. doi: 10.1128/CVI.00818-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao DY, Chien YZ, Yeh YP, Hsu PS, Lian IB. The incidence of varicella and herpes zoster in Taiwan during a period of increasing varicella vaccine coverage, 2000–2008. Epidemiol Infect. 2012;140(6):1131–1140. doi: 10.1017/S0950268811001786. [DOI] [PubMed] [Google Scholar]
- 17.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 18.Kelly HA, Grant KA, Gidding H, Carville KS. Decreased varicella and increased herpes zoster incidence at a sentinel medical deputising service in a setting of increasing varicella vaccine coverage in Victoria, Australia, 1998 to 2012. Euro Surveill. 2014;19(41):16–21. doi: 10.2807/1560-7917.es2014.19.41.20926. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg A, Lazar AA, Zerbe GO, Hayward AR, Chan IS, Vessey R, et al. Influence of age and nature of primary infection on varicella-zoster virus-specific cell-mediated immune responses. J Infect Dis. 2010;201(7):1024–1030. doi: 10.1086/651199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197(6):825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142(2):291–293. [PubMed] [Google Scholar]
- 22.Yenikomshian MA, Guignard AP, Haguinet F, LaCasce AS, Skarin AT, Trahey A, et al. The epidemiology of herpes zoster and its complications in Medicare cancer patients. BMC Infect Dis. 2015;15(1):106–115. doi: 10.1186/s12879-015-0810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hata A, Kuniyoshi M, Ohkusa Y. Risk of Herpes zoster in patients with underlying diseases: a retrospective hospital-based cohort study. Infection. 2011;39(6):537–544. doi: 10.1007/s15010-011-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: population based case-control study. BMJ. 2014;348:g2911–g2930. doi: 10.1136/bmj.g2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joesoef RM, Harpaz R, Leung J, Bialek SR. Chronic medical conditions as risk factors for herpes zoster. Mayo Clin Proc. 2012;87(10):961–967. doi: 10.1016/j.mayocp.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen MH, Wei HT, Su TP, Li CT, Lin WC, Chang WH, et al. Risk of depressive disorder among patients with herpes zoster: a nationwide population-based prospective study. Psychosom Med. 2014;76(4):285–291. doi: 10.1097/PSY.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 27.Lin SY, Liu JH, Yeh HC, Lin CL, Tsai IJ, Chen PC, et al. Association between herpes zoster and end stage renal disease entrance in chronic kidney disease patients: a population-based cohort study. Eur J Clin Microbiol Infect Dis. 2014;33(10):1809–1815. doi: 10.1007/s10096-014-2143-6. [DOI] [PubMed] [Google Scholar]
- 28.Heymann AD, Chodick G, Karpati T, Kamer L, Kremer E, Green MS, et al. Diabetes as a risk factor for herpes zoster infection: results of a population-based study in Israel. Infection. 2008;36(3):226–230. doi: 10.1007/s15010-007-6347-x. [DOI] [PubMed] [Google Scholar]
- 29.Guignard AP, Greenberg M, Lu C, Rosillon D, Vannappagari V. Risk of herpes zoster among diabetics: a matched cohort study in a US insurance claim database before introduction of vaccination, 1997–2006. Infection. 2014;42(4):729–735. doi: 10.1007/s15010-014-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suaya JA, Chen SY, Li Q, Burstin SJ, Levin MJ. Incidence of herpes zoster and persistent post-zoster pain in adults with or without diabetes in the United States. Open Forum Infect Dis. 2014;1(2):ofu049. doi: 10.1093/ofid/ofu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Antoniou T, Zheng H, Singh S, Juurlink DN, Mamdani MM, Gomes T. Statins and the risk of herpes zoster: a population-based cohort study. Clin Infect Dis. 2014;58(3):350–356. doi: 10.1093/cid/cit745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung SD, Tsai MC, Liu SP, Lin HC, Kang JH. Herpes zoster is associated with prior statin use: a population-based case-control study. PLoS One. 2014;9(10):e111268. doi: 10.1371/journal.pone.0111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–S26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 34.Forbes HJ, Thomas SL, Smeeth L, Clayton T, Farmer R, Bhaskaran K, et al. A systematic review and meta-analysis of risk factors for postherpetic neuralgia. Pain. 2016;157(1):30–54. doi: 10.1097/j.pain.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]