Summary
Induced pluripotent stem cell (iPSC)-derived dopamine neurons provide an opportunity to model Parkinson’s disease (PD), but neuronal cultures are confounded by asynchronous and heterogeneous appearance of disease phenotypes in vitro. Using high-resolution, single-cell transcriptomic analyses of iPSC-derived dopamine neurons carrying the GBA-N370S PD risk variant, we identified a progressive axis of gene expression variation leading to endoplasmic reticulum stress. Pseudotime analysis of genes differentially expressed (DE) along this axis identified the transcriptional repressor histone deacetylase 4 (HDAC4) as an upstream regulator of disease progression. HDAC4 was mislocalized to the nucleus in PD iPSC-derived dopamine neurons and repressed genes early in the disease axis, leading to late deficits in protein homeostasis. Treatment of iPSC-derived dopamine neurons with HDAC4-modulating compounds upregulated genes early in the DE axis and corrected PD-related cellular phenotypes. Our study demonstrates how single-cell transcriptomics can exploit cellular heterogeneity to reveal disease mechanisms and identify therapeutic targets.
Keywords: Parkinson’s disease, single-cell RNA sequencing, induced pluripotent stem cells, histone deacetylase 4
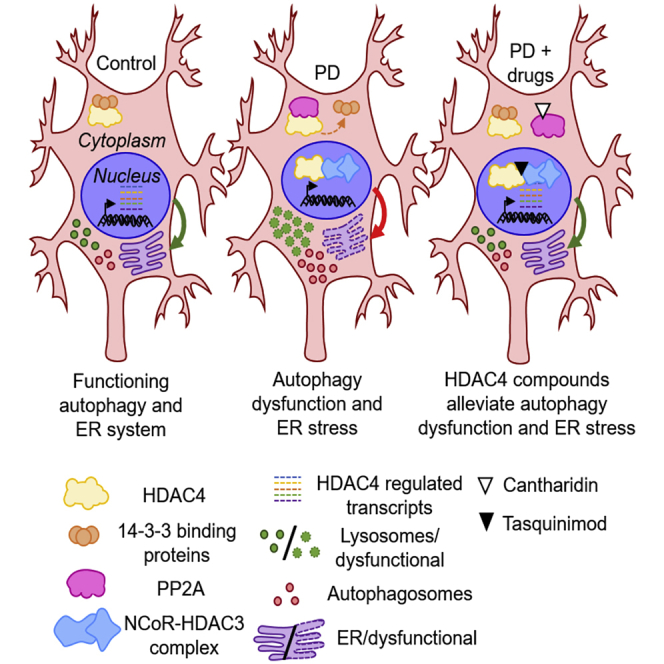
Graphical Abstract
Highlights
-
•
Single-cell RNA-seq stratifies patients with similar clinical presentation
-
•
A pseudotemporal profile aligns single cells along a control to disease axis
-
•
HDAC4 is mislocalized to the nucleus in PD patient iPSC-derived dopamine neurons
-
•
Repurposed compounds correct HDAC4 mislocalization and revert PD-related phenotypes
Bulk and single-cell RNA-seq of iPSC-derived dopamine neurons from control and PD GBA-N370S patients stratified a clinically distinct patient and revealed HDAC4 as a potential therapeutic PD target. Pharmacological modulation of HDAC4 rescued PD-related phenotypes in GBA-N370S neurons. HDAC4 perturbation was also observed in a subset of sporadic PD patients.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder affecting over 6 million people worldwide, predominantly over the age of 65 (Baker and Graham, 2004). PD is characterized by motor symptoms, including rigidity, resting tremor, bradykinesia, and postural instability, and non-motor features, including cognitive impairments, anxiety, and depression (Gonera et al., 1997). The motor symptoms are due to the progressive loss of dopamine neurons in the substantia nigra pars compacta, with approximately 50% of dopamine neurons lost in the midbrain at the onset of motor symptoms (Fearnley and Lees, 1991). The majority of PD cases are idiopathic, with only about 10% attributed to inherited PD cases. The glucocerebrosidase gene encodes the lysosomal enzyme, GCase, homozygous mutations in which cause the autosomal recessive lysosomal storage disorder, Gaucher’s disease (GD) (Hruska et al., 2008). GBA was first found to be associated with PD due to a high incidence of PD in both GD patients and heterozygous GBA carrier family members (Tayebi et al., 2003). Approximately 5%–10% of PD patients carry a heterozygous GBA mutation, making GBA variants the most common genetic risk factors for PD. The GBA-N370S mutation is the most common GBA risk variant, and patients have a clinical presentation similar to idiopathic PD (Beavan and Schapira, 2013).
Understanding the molecular basis of neurodegenerative disease has been hindered by the inaccessibility of live vulnerable human neurons from patients. The advent of induced pluripotent stem cell (iPSC) technology enables the study of patient-derived dopamine neurons from PD patients retaining genetic risk variants. Work with iPSC-derived dopamine neurons from PD patients carrying GBA (Fernandes et al., 2016, Schöndorf et al., 2014) or leucine-rich repeat kinase 2 (LRRK2) mutations (Sánchez-Danés et al., 2012) has revealed deficits in protein homeostasis via the endoplasmic reticulum (ER), autophagic, and lysosomal pathways. However, iPSC-derived neuronal cultures often contain cellular heterogeneity, confounding gene expression profiling of a specific cell type.
Recently, we developed a method to obtain pure populations of iPSC-derived dopamine neurons by fluorescence-activated cell sorting (FACS), which we used to profile gene expression in PD LRRK2-G2019S iPSC-derived dopamine neurons (Sandor et al., 2017). Nonetheless, cellular heterogeneity remains, even within a purified population, as individual cells are unlikely to experience the same gene-driven perturbation synchronously. Bulk gene expression profiling across thousands of cells provides only a population average, obscuring that cells may be at different points in one or more disease-relevant processes. By contrast, profiling gene expression within individual cells can exploit population heterogeneity, distinguishing distinct cell subpopulations and discerning the progression of cells through the disease-relevant processes being modeled (Reid and Wernisch, 2016). Our FACS-based purification method for dopamine neurons is readily applicable to plate-based deep single-cell profiling (Picelli et al., 2013).
Here, we applied bulk and deep single-cell gene expression profiling to purified populations of iPSC-derived dopamine neurons from three PD patients carrying the GBA-N370S variant. Unique to a single GBA-N370S patient, we identified increased activation of the signal recognition particle pathway. This molecular stratification was validated by clinical follow-up, which confirmed a revised diagnosis of progressive supranuclear palsy for that patient, who was removed from further downstream analysis.
Combining bulk and single-cell expression profiles, we identified a robust set of 60 genes whose expression captured an axis of variation between cells from controls and the remaining two PD GBA-N370S patients. Aligning individual cells across this axis generated a pseudotemporal profile along which the sequence of changes in the expression of individual genes could be inferred. Although variation in gene expression at the end of the pseudotemporal profile was associated with an increase in ER stress, previously characterized in PD, many early differentially expressed (DE) genes were found to be downregulated by histone deacetylase 4 (HDAC4), a class IIa histone deacetylase, which acts as a transcriptional repressor that shuttles between the nucleus and the cytoplasm. HDAC4 was found to be mislocalized to the nucleus in PD GBA-N370S iPSC-derived dopamine neurons. Modulation of HDAC4 activity or localization reversed the downregulation of the core set of DE genes and ameliorated PD-related cellular phenotypes previously described in PD GBA-N370S dopamine neurons, including ER stress, autophagic and lysosomal perturbations, and increased α-synuclein release. Finally, we demonstrated HDAC4 mislocalization and perturbation of the same core set of DE genes in iPSC-derived dopamine neurons from a subset of idiopathic PD cases. Our work demonstrates how we can exploit cellular heterogeneity to reveal disease mechanisms and therapeutic targets.
Results
Characterization and Purification of iPSC-Derived Dopamine Neurons by FACS
Previously, we reported that iPSC-derived dopamine neurons obtained from PD GBA-N370S patients exhibited increased ER stress, autophagic and lysosomal perturbations, and elevated α-synuclein release (Fernandes et al., 2016). To further investigate variation in gene expression, which may underlie disease processes, we sought to purify iPSC-derived dopamine neurons from control and GBA-N370S patients and subject them to both bulk and single-cell RNA sequencing (Figure 1A).
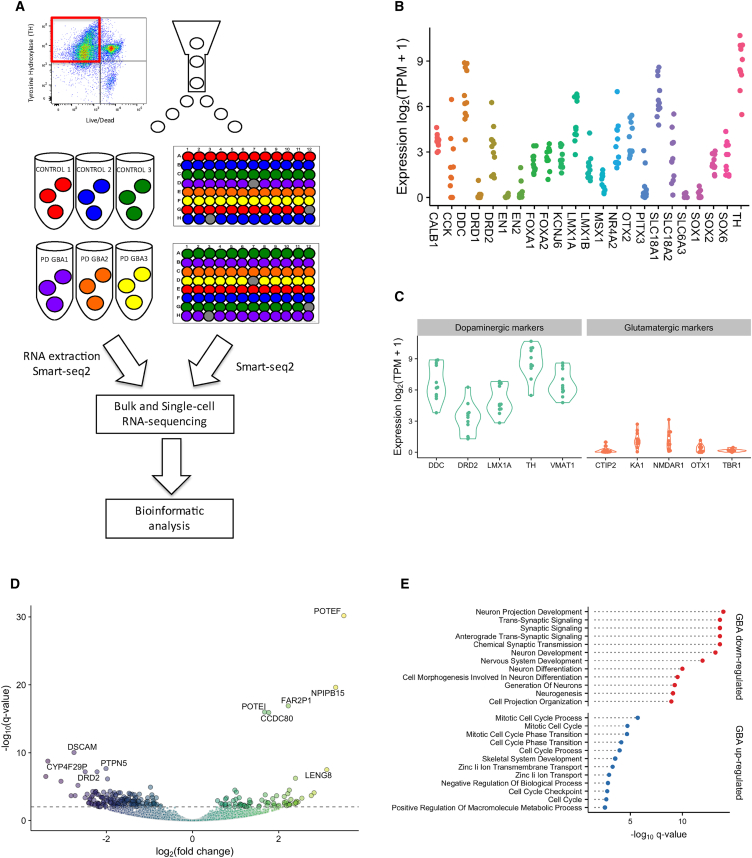
Figure 1.
Bulk RNA-Seq Analysis Confirms Purification of iPSC-Derived Dopamine Neurons and Identifies 247 DE Genes between Control and PD GBA-N370S Patients Enriched for Genes in Pathways of Neuronal Function
(A) Schematic of sorting the tyrosine hydroxylase-positive (TH+) iPSC-derived dopamine neurons from three controls and three PD GBA-N370S patients displaying a FACS plot identifying live/TH+ cells for sorting into bulk collection and into 96-well plates for single-cell RNA sequencing (gray wells indicate blank wells). Bulk and single cells went through RNA extraction, cDNA synthesis, and amplification before undergoing sequencing and bioinformatic analysis.
(B and C) Expression of dopamine neuron-specific markers (B) and the absence of glutamatergic markers (C) in the purified bulk iPSC-derived dopamine neurons.
(D) Volcano plot showing 247 genes DE between GBA-N370S PD versus control identified by DESeq2 (FDR 1%).
(E) GO enrichment analysis of the upregulated and downregulated genes in PD GBA-N370S patients highlights DE of genes involved in neuronal development and synaptic activity.
iPSC lines derived from three PD GBA-N370S patients and three controls (Figure S1) were differentiated into dopamine neurons, as previously (Kriks et al., 2011), with minor modifications (Beevers et al., 2017). All iPSC lines were successfully differentiated, typically yielding dopaminergic neuronal cultures 40%–60% positive for tyrosine hydroxylase (TH), a marker of dopamine neurons (Figure S2A). To isolate dopamine neurons from the heterogeneous population of differentiated cells, neurons were sorted by FACS as described (Sandor et al., 2017; Figure S2B). Approximately 35–40,000 TH+ neurons were purified and collected from each of the three control and three PD GBA-N370S samples, and RNA was extracted. There was no significant difference in the number of cells collected for each group (Figure S2C) or in extracted RNA quality by RNA integrity (RIN) values of ∼9 for the bulk-collected FACS-purified samples (Figure S2D).
Bulk RNA Sequencing of Purified iPSC-Derived Dopamine Neurons Reveals Downregulated Genes Associated with Synaptic Function and Development
Bulk RNA sequencing (RNA-seq) profiles of FACS-purified cells showed increased expression of dopamine neuron marker genes (Figure 1B), such as tyrosine hydroxylase (TH), dopa decarboxylase (DDC), solute carrier family 18 member A1 (VMAT1), LIM homeobox transcription factor 1 alpha (LMX1A), and dopamine receptor D2 (DRD2). Purified neurons lacked expression of glutamatergic neuronal markers, including COUP-TF-interacting protein 2 (CTIP2), N-methyl-D-aspartate receptor subunit NR1 (NMDAR1), orthodenticle homeobox 1 (OTX1), and T-box brain protein 1 (TBR1), confirming purification specifically of dopamine neurons (Figure 1C).
DE analysis between the PD GBA-N370S and control lines identified differences in gene expression patterns, with 247 genes DE at a 1% false discovery rate (FDR) (Figure 1D). Overall, gene ontology (GO) enrichment analysis of the upregulated and downregulated genes in PD GBA-N370S iPSC-derived dopamine neurons highlighted DE of genes involved in neuronal development, neuronal differentiation, and synaptic activity, whereas zinc ion transport functions featured in the upregulated genes (Du et al., 2017, Forsleff et al., 1999, Park et al., 2014; Figure 1E).
Single-Cell RNA-Seq Stratifies PD GBA-N370S Patients
Initial analyses of the 146 single-cell transcriptomic profiles passing quality control (QC) demonstrated the same enrichment of neuronal marker genes as the bulk transcriptional profiles, although with individual cell gene dropouts typical of single-cell data (Pierson and Yau, 2015; data not shown). Principal component analysis (PCA) found that the transcriptional profiles of the cells segregated by patient origin along both the second and third components (Figure 2A). Notably, cellular transcriptional variation attributed to dopamine neurons derived from one of the PD GBA-N370S patients (referred to as “GBA3”) was represented by the third principal component (Figure 2A). Over-dispersion analysis used to identify genes that varied more than can be expected due to the inherent technical variation in the dataset (Brennecke et al., 2013) observed 143 genes (0.6%) as significantly over-dispersed at 5% FDR (Figure 2B). A GO enrichment analysis on the over-dispersed genes identified the signal recognition particle (SRP)-dependent co-translational protein targeting to membrane pathway and related processes as driving this variation.
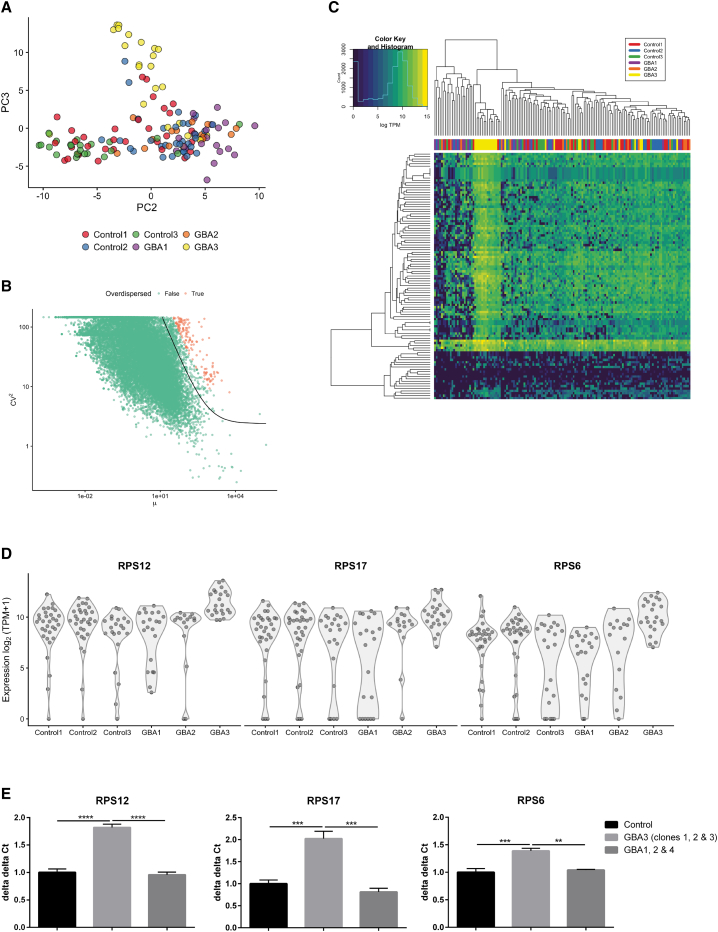
Figure 2.
Single-Cell RNA-Seq Stratification Identifies iPSC-Derived Dopamine Neurons from GBA3 as Significantly Different from Both PD Patient and Control Neurons
(A) Transcriptome PCA analysis resolves GBA3 neurons (yellow) from the remaining two PD GBA-N370S patients and three controls.
(B) Over-dispersion analysis identifies a subset of genes that vary more than expected due to technical fluctuations in the dataset alone.
(C) Heatmap of the single-cell RNA-seq samples identifies an enrichment in the endoplasmic reticulum (ER) signal recognition particle (SRP) pathway in GBA3.
(D) Expression in log2 (TPM+1) of three genes (RPS12, RPS17, and RPS6) prioritized from those significantly DE within the SRP pathway between GBA3 and controls 1, 2, and 3 and GBA1 and 2. DE analysis was performed using a two-sided Wilcoxon signed-rank test on all genes in the SRP pathway.
(E) The upregulation of the three selected genes involved in this pathway was confirmed in iPSC-derived dopamine neurons differentiated from three iPSC clones of GBA3 compared to the three original controls and two PD GBA-N370S patients (GBA1 and 2), plus a fourth PD GBA-N370S patient (GBA4). Data are represented as mean ± SD (∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
The separation of GBA3 dopamine neurons along the third principal component, and the large variation in a small set of genes belonging to one pathway, prompted concerns that a single sample could be driving the variation in gene expression between the PD GBA-N370S cases and controls. The expression of genes belonging to the SRP-dependent co-translational protein targeting to membrane pathway strikingly demonstrated increased activation in the GBA3 patient alone, who clustered apart from all other case and control samples (Figure 2C). Expression analysis of three genes in this pathway—ribosomal protein S12 (RPS12), ribosomal protein S17 (RPS17), and ribosomal protein S6 (RPS6)—confirmed the upregulation in this pathway to be specific to patient GBA3 (Figure 2D).
Expression of the same three genes was confirmed by qRT-PCR in iPSC-derived dopamine neurons generated from the original GBA3 iPSC line, two further iPSC lines from the GBA3 patient, the three controls, the two other original GBA patients, and an additional fourth PD GBA-N370S patient (GBA4; Figure 2E). Comparison of the three GBA3 patient lines with the three controls and GBA patients 1, 2, and 4 confirmed elevation of the SRP-dependent co-translational protein targeting to membrane pathway to be specific to GBA3, proposing a molecular stratification of the patients used in this study.
Although all the patients in our study fulfilled UK Brain Bank diagnostic criteria for clinically probable PD at presentation, longitudinal clinical follow-up allows the diagnosis to be reviewed in light of disease progression and subsequent medication response. GBA patients 1, 2, and 4 presented at an early stage with akinetic-rigid parkinsonism and maintained a good levodopa response for their first five years of treatment without significant falls or dementia. In contrast, patient GBA3 presented with akinetic-rigid parkinsonism, failed to respond to standard medication (600 mg levodopa with benserazide 150 mg daily), and presented with early dementia and frequent falls two years after initial PD diagnosis. A supranuclear gaze palsy with dysarthria was then noted, and the patient received a revised clinical diagnosis of progressive supranuclear palsy (PSP). The stratification of PSP from PD among these GBA-N370S carriers by single-cell profiling of their iPSC-derived dopamine neurons is therefore consistent with the clinical stratification and reveals a potential disease-relevant pathway for PSP.
A Functionally Enriched Gene Set Defines a Pseudotemporal Axis of PD GBA-N370S iPSC-Derived Dopamine Neuron Gene Expression Variation
Upon removal of GBA3 from the analysis, we observed minimal changes in the set of genes found to be DE (Figure S3). Analysis of the transcriptomes of individual dopamine neurons broadly segregated along the second principal component from a higher concentration of control cells to a higher concentration of PD GBA-N370S case cells (Figure S4A). We hypothesized that the case-control divergence along this component reflected cells that were at varying points in a common disease-related process, with GBA1 and GBA2 neurons that were more control-like being at an earlier point in the same process than GBA1 and GBA2 dopamine neurons that were less control-like. Our approach is comparable to the idea of “pseudotime” in the context of cellular differentiation (Haghverdi et al., 2016, Ji and Ji, 2016, Reid and Wernisch, 2016).
As cells segregated by case-control status along the second principal component, there was a possibility the data simply represented two distinct cell types with the apparent continuum due to transcriptional noise. To test this hypothesis, we repeated principal-component analysis on the GBA-N370S iPSC-derived dopamine neurons alone and found a remarkable correlation of the second principal component when all cells are included (Figure S4C). Therefore, the transcriptional heterogeneity at the single-cell level represents a continuous disease axis from case to control.
We next sought to identify a core set of genes consistently perturbed across both bulk RNA-seq and the single-cell transcriptomic signature. This core set was identified as the intersection of two gene sets from the analysis: (1) those DE in bulk RNA-seq using DESeq2 after the removal of GBA3 (at 5% FDR) and (2) those DE across PC2 using switchde (at 5% FDR; Campbell and Yau, 2017; Figure S4B). We further refined this set to include only those additionally identified as discriminating marker genes after clustering the single-cell RNA-seq data using SC3 (Kiselev et al., 2017). By combining genes found through both bulk and single-cell DE as well as single-cell clustering (STAR Methods), we identified a core set of 60 genes, 52 of which were consistently downregulated and 8 of which were consistently upregulated in PD GBA-N370S iPSC-derived dopamine neurons (Figure S4B).
To validate that our core set of 60 genes were functionally convergent, we assessed the functional similarity between these genes within a phenotypic linkage network, as compared to known PD genes and a random background set controlled for the relevant core set gene properties (STAR Methods; Figures S4D, S4E, and 3B). We found significant enrichment of functional similarity within the 60-gene set compared to background genes (p < 2.6e−16; Figure 3B). Strikingly, we also found a significant enrichment between the 60 genes and a set of known PD genes (Figure 3B).
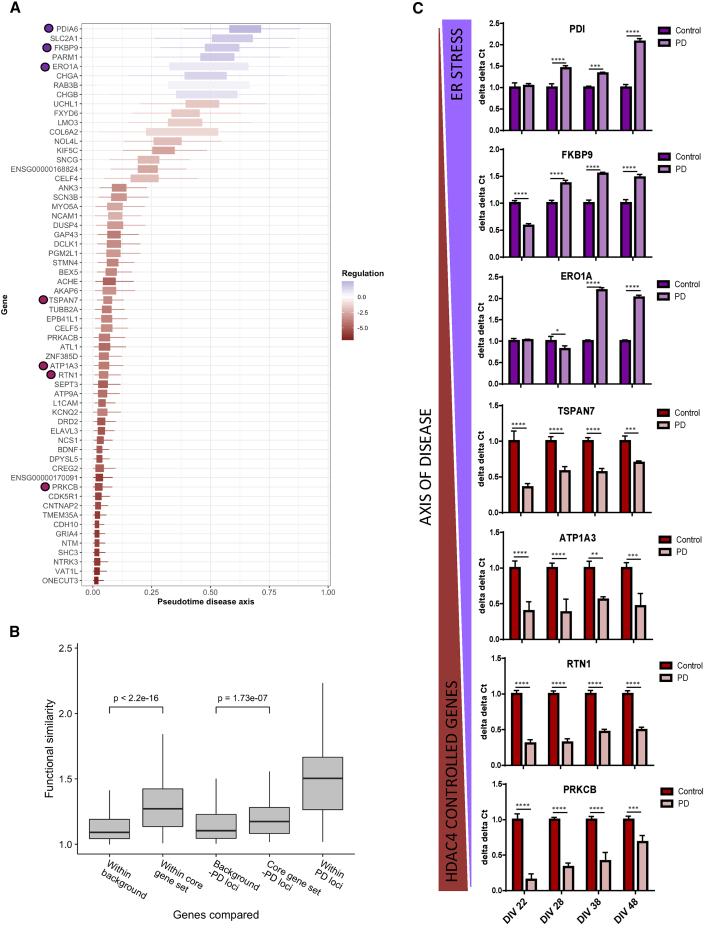
Figure 3.
Pseudotime Analysis Temporally Orders the Core Set of 60 Functionally Similar Genes DE in Both the Bulk and Single-Cell RNA-Seq between Control and PD GBA-N370S Patients
(A) Refined transcriptomic disease axis analysis of the core gene set of 60 genes. The control-disease single-cell transcriptomic axis was re-inferred with the 60 genes alone using a parametric factor analysis model that associated each gene with a point along the axis at which it was upregulated or downregulated.
(B) The phenotypic linkage network demonstrates a higher functional similarity of the 60-gene set with each other compared to a background control set (p < 2.2e−16). This higher functional similarity was also identified between the 60-gene set and a group of known PD loci, compared to a background control set (p = 8.52e−08). The high functional similarity of PD genes to each other is used as a positive control.
(C) Along the axis of disease, the downregulation of HDAC4-controlled genes (PRKCB, RTN1, ATP1A3, and TSPAN7) at 22 DIV precedes the upregulation of ER stress genes (ERO1A, FKBP9, and PDI) at 38 DIV. Data are represented as mean ± SD (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001). The locations of the HDAC4 and ER genes analyzed from the core 60 set are marked on (A).
Within the set of 60 DE genes, those downregulated early in the proposed case-control axis include genes implicated in neuronal function (γ-synuclein [SNCG], brain-derived neurotrophic factor [BDNF], and dopamine receptor D2 [DRD2]); genes involved in microtubule-associated protein tau (MAPT) splicing, microtubule function and formation, and neurite and axonal outgrowth; genes involved in protein secretion and trafficking; and protein kinase C (PKC) pathway genes. Genes identified as upregulated late in the process include the ER stress genes protein disulfide isomerase family member 6 (PDIA6), FK506 binding protein 9 (FKBP9), and ER oxidoreductase 1 alpha (ERO1A). The upregulation of ER stress genes is consistent with our previous findings, in which ER stress was increased in iPSC-derived dopamine neurons from PD GBA-N370S patients (Fernandes et al., 2016).
We further refined the single-cell transcriptomic axis of 60 genes using a recent Bayesian approach that learns transcriptomic trajectories directly from pre-specified genes using single-cell expression data. Based on nonlinear factor analysis, this approach models a small gene set in terms of “switch-like” upregulation or downregulation along the latent (pseudotime) axis, jointly inferring the pseudotimes along with all model parameters. Crucially, it probabilistically assigns a position along the axis associated with the upregulation or downregulation of each of the 60 genes, and we can anchor the direction of the axis as proceeding from those GBA1 and GBA2 iPSC-derived dopamine neurons that are most similar to controls. We hypothesize that this axis represents the continuous progression of these cells through a modeled disease-relevant process, moving from a more control-like state to a more PD-relevant disease state, and where the order of gene regulatory variation along this axis reflects this modeled disease process (Figure 3A).
Analysis of the core set of 60 DE genes using ingenuity pathway analysis (IPA) (QIAGEN) identified histone deacetylase 4 (HDAC4) as a repressor of a set of genes downregulated early in the pseudotemporal profile (Figure S5A). Although total levels of HDAC4 protein were unchanged between controls and PD GBA-N370S patients (Figure S5B), the downregulation of four of the HDAC4-regulated genes (TSPAN7, ATP1A3, RTN1, and PRKCB) in PD GBA-N370S patient-derived neurons was experimentally confirmed (Figure S5C).
We next sought to validate the proposed temporal order of gene expression events in the development of disease pathophysiology in PD GBA-N370S neurons. qRT-PCR analysis of TSPAN7, ATP1A3, RTN1, and PRKCB confirmed that these four “early” genes, predicted to be downregulated by HDAC4, are repressed early in the differentiation at 22 DIV (days in vitro). The “late” genes (ERO1A, FKBP9, and PDIA6), predicted to be upregulated as part of a subsequent ER stress response, typically increase in expression post-22 DIV, with all three increased at 38 and 48 DIV (Figure 3C).
HDAC4 Is Mislocalized to the Nucleus and Participates in the Repression of Gene Expression in PD GBA-N370S iPSC-Derived Dopamine Neurons
HDAC4, a class IIa histone deacetylase, shuttles between the cytoplasm and the nucleus, where it acts as a transcriptional repressor. We observed an increase in nuclear localization of HDAC4 in PD GBA-N370S iPSC-derived dopamine neurons compared to controls at DIV 45, consistent with the downregulation of HDAC4 controlled genes within our set of 60 genes (Figure 4A). This HDAC4 nuclear mislocalization was not observed in iPSC-derived non-dopaminergic neurons of PD GBA-N370S patients (Figure S6).
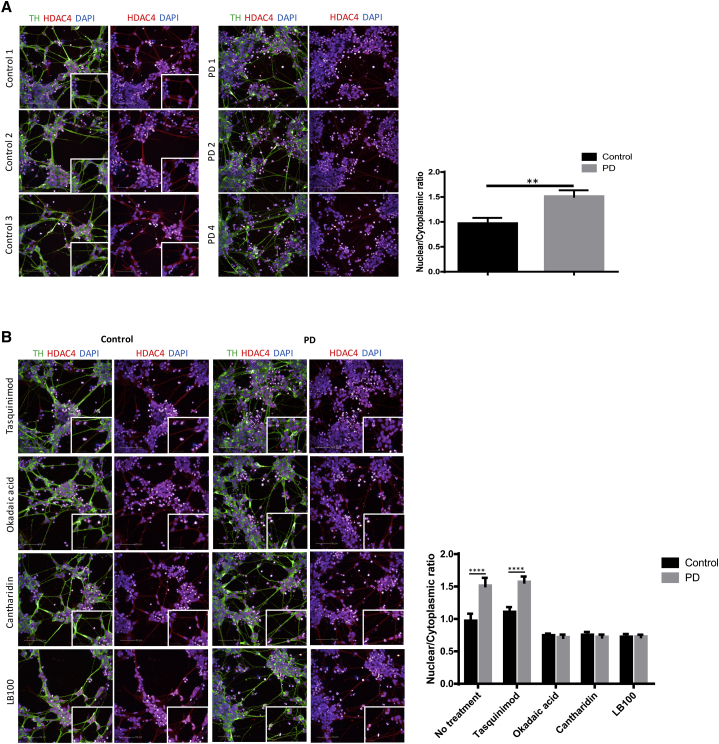
Figure 4.
Modulation of PP2A Activity Corrects HDAC4 Nuclear Mislocalization in PD GBA-N370S iPSC-Derived Dopamine Neurons
(A) Cytoplasmic and nuclear localization of HDAC4 in control and PD GBA-N370S dopamine neurons shown by immunofluorescence at 45 DIV—TH, green; HDAC4, red; DAPI, blue; HDAC4/DAPI nuclear colocalization, purple. The HDAC4 nuclear/cytoplasmic ratio is significantly increased in PD GBA-N370S patients. Data are represented as mean ± SD (∗∗p < 0.01).
(B) HDAC4 cellular localization in the presence or absence of tasquinimod (HDAC4 allosteric inhibitor) or okadaic acid, cantharidin, and LB-100 (PP2A inhibitors) at 45 DIV—TH, green; HDAC, red; DAPI, blue; and HDAC4/DAPI nuclear colocalization, purple. The three PP2A inhibitors correct HDAC4 nuclear mislocalization in PD GBA-N370S patient-derived dopamine neurons compared to no treatment. In contrast, tasquinimod, a HDAC4 allosteric inhibitor, has no effect on HDAC4 localization. Data are represented as mean ± SD (∗∗∗∗p < 0.0001).
Modulating HDAC4 Localization or Activity Corrects the Downregulation of HDAC4-Repressed Genes and Ameliorates ER Stress Phenotypes
To examine the effect of HDAC4 repression on the set of downregulated genes, we used four modulators of HDAC4 activity or localization, currently in clinical use for unrelated conditions. Tasquinimod is an allosteric inhibitor of the association of HDAC4 with the nuclear N-Cor/HDAC3-associated repressor complex (Isaacs et al., 2013), and okadaic acid (OA), cantharidin, and LB-100 (LB-100) all inhibit protein phosphatase 2 (PP2A)-mediated dephosphorylation of HDAC4, which reduces its nuclear localization (Gordon et al., 2015, Paroni et al., 2008, Pei et al., 2016).
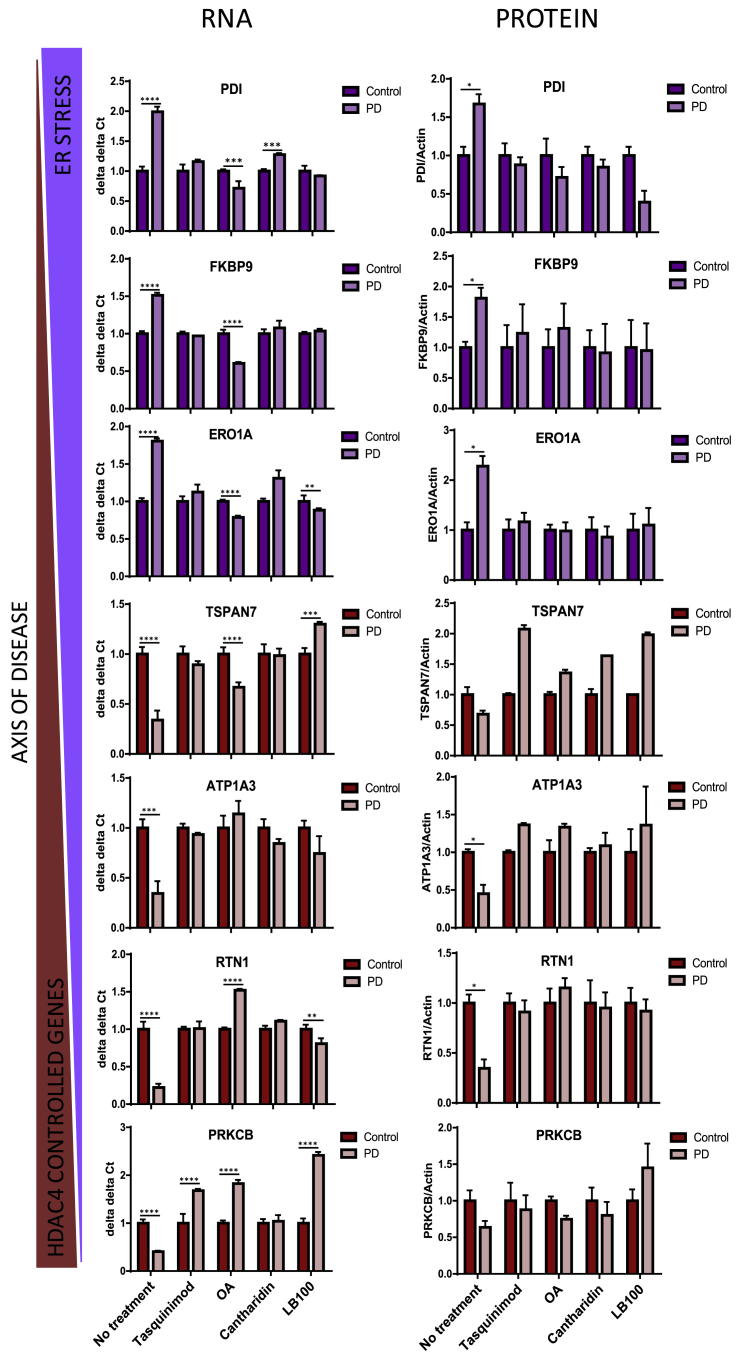
Treatment of PD GBA-N370S iPSC-derived dopamine neurons with each of the three PP2A inhibitor compounds reduced the nuclear localization of HDAC4, correcting HDAC4 mislocalization in GBA-N370S dopamine neurons to that of controls (Figure 4B). The addition of the HDAC4 allosteric inhibitor tasquinimod did not reduce the HDAC4 nuclear localization (Figure 4B) consistent with its mode of action, which does not involve HDAC4 relocalization. We next examined the impact on gene expression phenotypes of treating iPSC-derived dopamine neurons with the HDAC4 modulators. Treatment with all four compounds corrected, or even reversed, the reduction in expression of all four HDAC4-controlled genes reduced early in the pseudotemporal axis (PRKCB, RTN1, ATP1A3, and TSPAN7) in PD GBA-N370S iPSC-derived dopamine neurons at DIV 45 (Figure 5). Furthermore, compounds ameliorated the increase seen in the three ER stress genes (ERO1A, FKBP9, and PDI), late in the pseudotemporal axis, at the RNA and protein level (Figures 5 and S7A).
Figure 5.
Modulation of HDAC4 Activity or Localization Corrects the Downregulation of HDAC4-Controlled Genes in PD GBA-N370S iPSC-Derived Dopamine Neuron Cultures and Ameliorates PD GBA-N370S ER Stress Phenotypes
Expression of four HDAC4-regulated genes (TSPAN7, ATP1A3, RTN1, and PRKCB; bottom) and three ER stress genes (PDIA6, FKBP9, and ERO1A; top) at the RNA (left) and protein (right) levels in the presence and absence of HDAC4-modifying drugs tasquinimod, okadaic acid, cantharidin, and LB-100 in PD GBA-N370S and control patient-derived neurons at 45 DIV. The upregulation of HDAC4-repressed genes in PD GBA-N370S iPSC-derived dopamine neurons by all four compounds was accompanied by a decrease in ER stress. Data are represented as mean ± SEM (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001).
HDAC4 Modulation Corrects Perturbations in the Autophagy and Lysosomal Pathway in PD GBA-N370S iPSC-Derived Dopamine Neurons
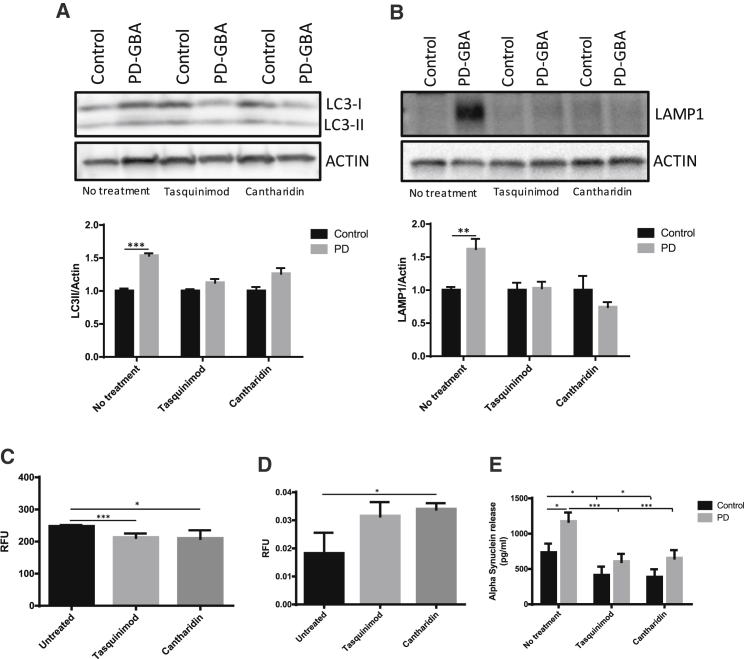
In addition to increased ER stress, we have previously observed perturbations in the autophagic and lysosomal pathway and increased release of α-synuclein in PD GBA-N370S iPSC-derived dopamine neurons (Fernandes et al., 2016). Treating PD GBA-N370S iPSC-derived dopamine neurons with the HDAC4 allosteric inhibitor tasquinimod or the representative PP2A inhibitor cantharidin corrected the increase in autophagosome number assessed by LC3-II levels (Figure 6A) through decreased autophagic induction rather than increasing flux (Figures S7B–S7D), reduced the increase in lysosomal accumulation measured by LAMP1 (Figures 6B and 6C), increased lysosomal activity (Figure 6D), and reduced the increased release of α-synuclein into the extracellular medium (Figure 6E).
Figure 6.
Modulation of HDAC4 Activity or Localization Rescues Deficits in the Autophagic and Lysosomal Pathway and Reduces α-Synuclein Release in PD GBA-N370S iPSC-Derived Dopamine Neurons
(A and B) Modulation of HDAC4 activity by allosteric inhibition of HDAC4 (tasquinimod) or inhibition of PP2A (cantharidin) rescues the increase in autophagosomal (LC3-II; A) and lysosomal (LAMP1; B) compartments seen by western blot in PD GBA-N370S patient iPSC-derived neurons compared to controls.
(C) The reduction of lysosomes in PD GBA-N370S iPSC-derived dopamine neurons treated with tasquinimod or cantharidin was confirmed by a decrease in lysosome punctae by immunofluorescence.
(D) Modulation of HDAC4 increases lysosomal activity in PD GBA-N370S iPSC-derived neurons measured by DQ-BSA cleavage.
(E) Tasquinimod or cantharidin reduces the increase in α-synuclein release seen in PD GBA-N370S patient-derived neurons compared to controls.
Data are represented as mean ± SEM (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001).
Nuclear Mislocalization of HDAC4 and Related Perturbations in Gene Expression Are Observed in Idiopathic PD Cases
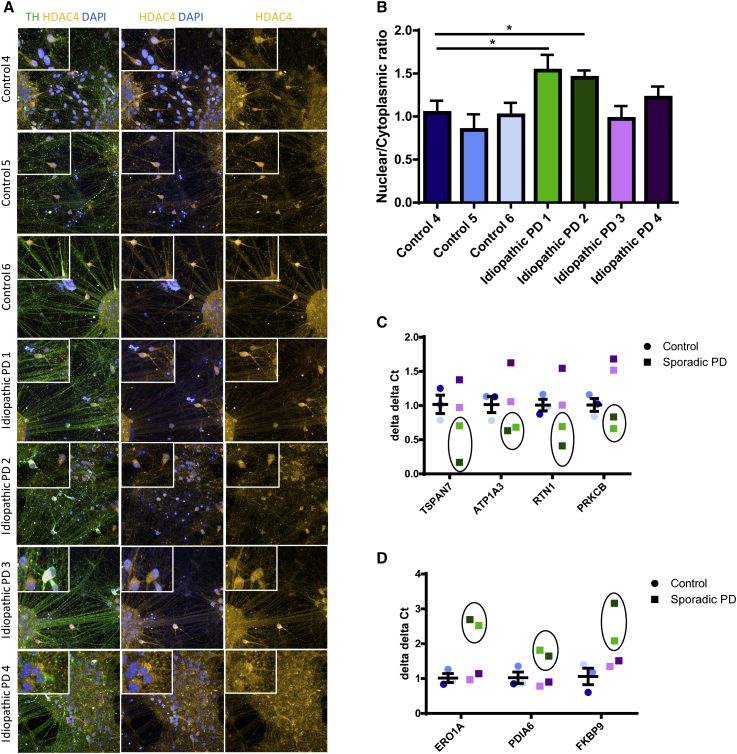
To address whether HDAC4 mislocalization is a disease mechanism relevant to PD beyond carriers of GBA mutations, we examined HDAC4 mislocalization and perturbation of gene expression in dopamine neurons differentiated from iPSC lines generated from four idiopathic PD cases and three age-matched controls. An increase in HDAC4 nuclear localization was observed in iPSC-derived dopamine neurons from two of the four idiopathic PD cases (Figures 7A and 7B). Furthermore, the reduction of expression of the HDAC4-regulated genes TSPAN7, ATP1A3, RTN1, and PRKCB, and the upregulation of the ER stress genes ERO1A, PDIA6, and FKBP9, was observed in iPSC-derived dopamine neurons from the same two idiopathic PD cases, which exhibited HDAC4 mislocalization (Figures 7C and 7D).
Figure 7.
Nuclear Mislocalization of HDAC4 and Related Perturbations in Gene Expression Are Observed in Idiopathic PD Cases
(A) Cytoplasmic and nuclear localization of HDAC4 in control and idiopathic PD iPSC-derived dopamine neurons shown by immunofluorescence at 45 DIV—TH, green; HDAC4, yellow; DAPI, blue.
(B) The HDAC4 nuclear/cytoplasmic ratio is significantly increased in two of the four idiopathic PD patients. Data are represented as mean ± SEM (∗p < 0.05).
(C and D) A (C) decrease in the expression of HDAC4-controlled genes: TSPAN7; ATP1A3; RTN1; and PRKCBI and an (D) increase in the expression of ER stress genes: ERO1A; PDIA6; and FKBP9 is observed in the same two idiopathic PD cases that display HDAC4 mislocalization.
Discussion
Applying cell type purification and a combination of bulk and single-cell gene expression profiling to iPSC-derived dopamine neurons from three GBA-N370S patients, our study identified disease-distinguishing molecular etiologies and revealed a temporal ordering of gene expression variation that proposed a role for the transcriptional regulator HDAC4 in upstream variation. The pharmacological modulation of HDAC4 activity or localization confirmed this finding by the rescue of downstream expression variation and correction of cellular phenotypes previously shown in this model of PD.
Our FACS-based purification method is well-suited to deep single-cell profiling. Although the cell fixation necessary for sorting creates a 3′ bias in transcript coverage, our gene level coverage was high, enabling subsequent studies. Cells can be clustered post-sequencing according to their expression profiles, but the cellular heterogeneity in these cultures would have halved our capture of dopaminergic neurons. Our robust pseudotemporal analyses require only ∼150 single-cell transcriptomic profiles to reveal disease-relevant perturbations.
Beyond cell-type heterogeneity, our study exploited significant intra-culture heterogeneity to unpick the disease processes being modeled. First, we identified a distinct molecular perturbation present in iPSC-derived dopamine neurons generated from GBA-N370S patient 3 (GBA3). Despite an initial diagnosis of PD and possessing a genetic variant strongly associated with PD, this patient’s cellular profile prompted a clinical reassessment, leading to the revised diagnosis of PSP. Although superficially similar in clinical presentation, PSP is a tauopathy with a cellular pathophysiology distinct to the α-synucleinopathy PD. Each of this patient’s single-cell profiles proved an effective technical replicate in the analyses. Profiling this patient’s cells revealed a distinct perturbation, which was further validated in two additional iPSC-derived dopamine neuron lines from the same patient.
A second source of cellular heterogeneity is the varying progression of each cell through the same disease process over time. Although a bulk expression profile averages across the cellular population, obscuring variation, a single-cell approach is able to exploit this heterogeneity and reveals insights into dynamic processes across a pseudotemporal axis. The ability to infer the temporal nature of disease progression allowed us to explore the relationship between early biological changes in gene expression and their influence on later disease phenotypes.
We identified HDAC4 as a master regulator of a number of genes downregulated early in the disease axis. Unlike class I HDACs, which reside permanently in the nucleus, HDAC4 acts as part of the HDAC4/N-CoR/HDAC3 complex that shuttles between the cytoplasm and the nucleus, repressing the expression of genes important in synaptic function and neuronal health. Under normal conditions, phosphorylated HDAC4 is retained in the cytoplasm, but upon dephosphorylation of the Ser298 residue by the catalytic subunit of PP2A, HDAC4 relocalizes to the nucleus. Although HDAC4 was not DE in PD GBA-N370S patient-derived dopamine neurons compared to controls, an increase in the nuclear-to-cytoplasmic ratio of HDAC4 was identified, consistent with the downregulation of DE genes in the core set under the transcriptional control of HDAC4.
We therefore hypothesize that downregulation of HDAC4-controlled genes due to the mislocalization of HDAC4 in the nucleus early in the disease may contribute to driving ER stress later in neurodegeneration. For example, mutations in the gene ATP1A3, which is downregulated by HDAC4, cause a rare rapid-onset dystonia-parkinsonism and is linked to altering intracellular calcium levels, which could impact on the ER, the principal intracellular store of calcium (Blanco-Arias et al., 2009). Similarly, PRKCB participates at mitochondrial-ER-associated membrane (MAM) sites, playing a crucial role in the phosphorylation of the p66Shc protein, which is involved in the regulation of calcium homeostasis between these two organelles (Pinton and Rizzuto, 2008).
Deficits in calcium signaling may also cause the increased nuclear localization of HDAC4 in PD GBA-N370S patient-derived dopamine neurons. HDAC4 is known to regulate genes involved in synaptic activity and memory and neuronal health (Sando et al., 2012). As cytoplasmic retention of class IIa HDACs requires calcium-dependent phosphorylation through calcium and/or calmodulin-dependent kinases, elevated cytoplasmic calcium caused by influx through voltage-gated ion channels in highly active neurons maintains HDAC4 cytoplasmic retention. Conversely, loss of synaptic excitation due to neurodegeneration may contribute to HDAC4 nuclear localization and repression of genes that promote neuronal survival. PD GBA-N370S patient-derived dopamine neurons are known to exhibit impaired cellular calcium homeostasis (Schöndorf et al., 2014), and low synaptic calcium levels in hippocampal and cerebellar granule cell cultures triggered the shuttling of HDAC4 from dendritic spines to the nucleus (Bolger and Yao, 2005, Chawla et al., 2003).
Pharmacological modulation of HDAC4 activity or localization corrected cellular phenotypes previously described in PD GBA-N370S patient-derived dopamine neurons, alleviating ER stress to reduce autophagic induction, suggesting HDAC4 as a therapeutic target for PD. All compounds tested are currently in clinical development for unrelated conditions, principally cancer. Decreased HDAC4 nuclear localization through increased phosphorylation and cytoplasmic retention was achieved through inhibition of PP2A. PP2A dephosphorylates multiple targets in addition to HDAC4, including the major neurodegenerative proteins tau and α-synuclein, which may prevent prolonged clinical use. More interesting is the use of the allosteric inhibitor tasquinimod to inhibit formation of the HDAC4/N-CoR/HDAC3 repression complex by locking HDAC4 in an inactive form (Isaacs et al., 2013). Tasquinimod has been tested through phase II and III clinical trials to treat prostate cancer with a good safety profile (Armstrong et al., 2013, Sternberg et al., 2016). The compound is well-tolerated in patients for up to 3 or 4 years with few dose interruptions or reductions. HDAC4 is considered to be a potential therapeutic target in Huntington’s disease (HD), as a heterozygous Hdac4+/− background rescued neuronal function in a HD mouse model (Mielcarek et al., 2013) but has yet to be explored therapeutically in PD.
To investigate whether these disease mechanisms are relevant to PD beyond carriers of GBA mutations, we extended our study to include iPSC-derived dopamine neurons from idiopathic PD cases. Remarkably, we found that increased HDAC4 nuclear localization was seen in iPSC-derived dopamine neurons from two of four idiopathic PD cases. Furthermore, the same perturbation of expression, being the downregulation of HDAC4-regulated genes TSPAN7, ATP1A3, RTN1, and PRKCB and the upregulation of ER stress genes ERO1A, PDIA6, and FKBP9, was seen in the same two idiopathic PD cases exhibiting HDAC4 mislocalization. These data show that findings from GBA PD extrapolate to a subset of idiopathic PD cases. Heterogeneity between idiopathic patients is expected in a disease with complex polygenic inheritance, leading to a variable level of cell-autonomous effects in different individuals, and one might expect the genetic contribution to be greater in some idiopathic patients than others. Our findings are consistent with recent studies (Hsieh et al., 2016, Sánchez-Danés et al., 2012, Nenasheva et al., 2017, Tolosa et al., 2018, George et al., 2018), which have found cellular phenotypes or transcriptomic perturbations in iPSC-derived dopamine neurons from idiopathic PD patients.
Overall, our work applied high-resolution single-cell analysis to iPSC-based disease models, exploiting the cellular heterogeneity present even within a purified single cell type, in this case, iPSC-derived dopamine neurons from PD patients. We have shown the disease process to be a dynamic event and identified HDAC4 as a key regulator of the early molecular changes that lead to late pathological processes. Our approach is applicable to other diseases as a means to uncover disease mechanisms and discover potential therapeutic targets.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Tyrosine hydroxylase | Millipore | RRID: AB_90755 |
| Beta-III tubulin (TUJ1) | Covance | RRID: AB_2313773 |
| HDAC4 | Abcam | RRID: AB_298903 |
| β-actin | Abcam | RRID: AB_2305186 |
| PDI | Cell signaling | RRID: AB_2156433 |
| FKBP9 | Abcam | RRID: AB_10562617 |
| Ero1-Lα | Cell signaling | RRID: AB_823683 |
| TSPAN7 | Novus biologicals | RRID: AB_11035060 |
| Na+/K+-ATPaseα3 (ATP1A3) | Santa cruz | RRID: AB_10848453 |
| Rtn1/2 | Santa cruz | RRID: AB_2183564 |
| PRKCB | ProSci | Cat#43-319 |
| LAMP1 | Santa cruz | RRID: AB_626853 |
| LC3B | Sigma | RRID: AB_796155 |
| TRA-1-60 | Biolegend | RRID: AB_1186144 |
| Nanog | Cell signaling | RRID: AB_10694485 |
| Biological Samples | ||
| OX1-19/SFC841-03-1/2 | EBiSC | UOXFi004-B/ STBCi044-B |
| JR053-1/6 | EBiSC | UOXFi005-A/ UOXFi005-B |
| AH016-3/6 | EBiSC | University of Oxford |
| SFC156-03-01 | EBiSC | STBCi101-A |
| SFC840-03-06 | EBiSC | STBCi026-D |
| SFC067-03-01 | EBiSC | STBCi105-A |
| MK088-1 | EBiSC | UOXFi003-A |
| RH058-03 | EBiSC | STBCi025-A/B/C |
| MK082-26 | EBiSC | UOXFi002-A |
| MK071-3 | EBiSC | UOXFi001-B |
| SFC077-03-04 | EBiSC | STBCi268-A |
| SFC844-03-12 | EBiSC | STBCi294-A |
| SFC120-03-04 | EBiSC | STBCi043-B |
| SFC865-03-07 | EBiSC | STBCi298-A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| ROCK inhibitor (Y27632 dihydrochloride) | Bio-Techne | Cat#1254 |
| Tasquinimod | Tocris | Cat#S7617 |
| Okadaic acid | Abcam | Cat#O7885 |
| LB100 | Tocris | Cat#S7537 |
| Cantharidin | Tocris | Cat#1548 |
| LDN-193189 | Sigma | Cat#SML0559 |
| SB-431542 | Bio-Techne | Cat#1614 |
| SHH C24II | Bio-Techne | Cat#1845-SH-500 |
| Purmorphamine | Bio-Techne | Cat#4551/10 |
| FGF8a | Stratech | Cat#16124-HNAE-SIB |
| CHIR-99021 | Bio-Techne | Cat#4423 |
| BDNF | Peprotech | Cat#450-02 |
| GDNF | Peprotech | Cat#450-10 |
| TGFb3 | Peprotech | Cat#100-36E |
| DAPT | Abcam | Cat#ab120633 |
| Ascorbic acid | Sigma | Cat#A4544 |
| (db)-cAMP | Sigma | Cat#D0627 |
| hESC-qualified Matrigel | Corning | Cat#354277 |
| DQ BSA Red | Thermo Fisher Scientific | Cat#D12051 |
| NucBlue Live ReadyProbes | Thermo Fisher Scientific | Cat#R37605 |
| Critical Commercial Assays | ||
| Cytotune v1 Sendai Reprogramming kit | Thermo Fisher Scientific | A13780-01 |
| Cytotune v2 Sendai Reprogramming kit | Thermo Fisher Scientific | A16517 |
| β-Actin qPCR Control Kit | Eurogentec | SR-CL004-005 |
| Human-HT-12-v4 expression BeadChip Kit | BD | BD-103-0204 |
| All-Prep DNA/RNA Mini kit | QIAGEN | 80204 |
| RNeasy FFPE kit | QIAGEN | Cat#73504 |
| RNA 6000 pico kit | Agilent | Cat#5067-1513 |
| Quant-iT RiboGreen RNA kit | Thermo Fisher Scientific | Cat#R11490 |
| Nextera XT DNA Library Prep Kit | Illumina | Cat#FC-131-1096 |
| RNeasy Micro kit | QIAGEN | Cat#74004 |
| Superscript III reverse transcriptase kit | Thermo Fisher Scientific | Cat#18080093 |
| Fast SYBR green master mix | Thermo Fisher Scientific | Cat#4385612 |
| αSyn extracellular release MSD kit | Meso Scale Discovery | Cat#K151TGD-2 |
| Deposited Data | ||
| Raw RNA-seq data | ArrayExpress | ArrayExpress: E-MTAB-7303 |
| Software and Algorithms | ||
| Harmony | Perkin Elmer | N/A |
| GenomeStudio | Illumina | N/A |
| Karyostudio | Illumina | N/A |
| TrimGalore v0.4.1 | (Krueger, 2015) | https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ |
| Kallisto v0.42.5 | (Bray et al., 2016) | https://pachterlab.github.io/kallisto/ |
| Picard 2.0.1 | (Picard Toolkit, 2018) | https://broadinstitute.github.io/picard/ |
| HISAT2 | (Kim et al., 2015) | https://ccb.jhu.edu/software/hisat2/index.shtml |
| Tximport 1.4.0 | (Soneson et al., 2015). | https://bioconductor.org/packages/release/bioc/html/tximport.html |
| Scater 1.8.0 | (McCarthy et al., 2017) | https://bioconductor.org/packages/release/bioc/html/scater.html |
| Cellity 1.8.0 | (Ilicic et al., 2016) | https://bioconductor.org/packages/release/bioc/html/cellity.html |
| DESeq2 | (Love et al., 2014), | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| goseq | (Young et al., 2010) | https://bioconductor.org/packages/release/bioc/html/goseq.html |
| The R project for statistical computing | R Development Core Team, 2008 | https://www.r-project.org/ |
| switchde 1.6.0 | (Campbell and Yau, 2017) | https://bioconductor.org/packages/release/bioc/html/switchde.html |
| Ouija 0.99.0 | (Campbell and Yau, 2017) | https://github.com/kieranrcampbell/ouija/ |
| scran 1.8.2 | (Lun et al., 2016) | https://bioconductor.org/packages/release/bioc/html/scran.html |
| Phenotypic Linkage Network | (Honti et al., 2014). | https://github.com/csandorfr/AP-PLN |
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contacts, Richard Wade-Martins (richard.wade-martins@dpag.ox.c.uk).
Experimental Model and Subject Details
iPSC lines and participation recruitment
Participants were recruited to the Discovery clinical cohort through the Oxford Parkinson’s Disease Centre and gave signed informed consent to mutation screening and derivation of iPSC lines from skin biopsies (Ethics committee: National Health Service, Health Research Authority, NRES Committee South Central, Berkshire, UK, REC 10/H0505/71). All the patients included in our study-fulfilled UK Brain Bank diagnostic criteria for clinically probable PD at presentation (Hughes et al., 1992). GBA-N370S PD patients 1, 2 and 4 presented with akinetic-rigid parkinsonism, and maintained a good levodopa-response for their first 5 years of treatment without significant falls or dementia. GBA-N370S patient 3 presented with akinetic-rigid parkinsonism, failed to respond to good doses of oral dopaminergic medication (600 mg levodopa, 150 mg benserazide daily), subsequently rapidly progressed more quickly with early dementia and frequent falls two years later. The patient has a revised diagnosis of Progressive Supranuclear Palsy (PSP). Patients with idiopathic Parkinson’s who met the UK Parkinson’s Disease Society Brain Bank (UKPDBB) criteria for the diagnosis of probable idiopathic PD (Hughes et al., 1992) on examination by a neurologist were recruited from ongoing cohort studies at the University of Oxford (UK) and the University of Lubeck (Germany) (Kasten et al., 2013). Patients with secondary parkinsonism due to head trauma or medication use, or features of atypical parkinsonism syndromes, were excluded (Szewczyk-Krolikowski et al., 2014).
Subject details
| Donor ID | iPSC clone | Study ID | Genotype | Age & gende | Characterization |
|---|---|---|---|---|---|
| AH016 | 03/06 | Control 1 | wt/wt | 80 M | Sandor et al., 2017 |
| JR053 | 06/01 | Control 2 | wt/wt | 68 M | This study |
| OX1 SFC841-03 | 19 01/02 | Control 3 | wt/wt | 36 M | Van Wilgenburg et al., 2013 |
| SFC156-03 | 01 | Control 4 | wt/wt | 75 M | This study |
| SFC840-03 | 06 | Control 5 | wt/wt | 67 F | Haenseler et al., 2017 |
| SFC067-03 | 01 | Control 6 | wt/wt | 72 M | This study |
| MK088 | 01 | GBA 1 | N370S/wt | 46 M | Fernandes et al., 2016 |
| MK071 | 03 | GBA 2 | N370S/wt | 81 F | Fernandes et al., 2016 |
| SFC834-03 | 03 | GBA 3 | N370S/wt | 72 M | Fernandes et al., 2016 |
| MK082 | 26 | GBA 4 | N370S/wt | 51 M | This study |
| SFC077-03 | 04 | Idiopathic PD 1 | N/A | 65 M | This study |
| SFC844-03 | 12 | Idiopathic PD 2 | N/A | 72 M | This study |
| SFC120-03 | 04 | Idiopathic PD 3 | N/A | 72 M | This study |
| SFC865-03 | 07 | Idiopathic PD 4 | N/A | 69 M | This study |
Culture, reprogramming and characterization of primary fibroblasts
Low passage fibroblast cultures were established from participant skin punch biopsies, and these were reprogrammed either by retroviral delivery or CytoTune-iPS Sendai Reprogramming kit (Thermo Fisher Scientific, version 1 or 2) as previously described (Fernandes et al., 2016). Clones were transitioned to feeder-free culture in mTeSR medium (StemCell Technologies), on hESC-qualified Matrigel-coated plates (BD), and passaged as cell clusters using 0.5 mM EDTA in PBS. Large batches were tested for mycoplasma (Mycoalert, Lonza), QCed (see below) and frozen at p15-25. When thawing for experiments, 10 μM ROCK inhibitor (Y27632, Bio-Techne) was added to promote initial survival and iPSC were passaged 1:2-3 using TryplE (Life Tech) with Y27632 during replating, culturing for maximum two weeks’ post-thaw prior to differentiation to ensure consistency.
The following iPSC lines used in this study have been previously described: OX1-19 (van Wilgenburg et al., 2013), AH016-3/6 (Sandor et al., 2017), SFC840-03, MK088-1, MK071-3, SFC834-03 (Fernandes et al., 2016) and SFC840-03-06 (Haenseler et al., 2017). iPSC PD GBA lines, iPS MK082-26 and JR053-6, and idiopathic PD lines SFC077-03-04, SFC120-03-04, SFC844-03-12 and SFC865-03-07 are characterized here (Figure S1). Control lines SFC067-03-01 and SFC156-03-01 are registered in hPSCreg, with accompanying QC reports. Briefly, fluorescence activated cell sorting (FACS) for pluripotency markers TRA-1-60 (Biolegend) and Nanog (Cell Signaling) was performed on a FACSCalibur (BD Biosciences).
Silencing of retroviral delivered reprogramming genes was assessed by quantitative RT-PCR using the following primers: pMXsAS3200v2 TTA TCG TCG ACC ACT GTG CTG GCG mNanog forward primer GCT CCA TAA CTT CGG GGA GG. The β-Actin qPCR Control Kit (Eurogentec) was used as control normalization. Clearance of Cytotune Sendai vectors was performed by RT-PCR according to the manufacturer’s instructions. Analysis of pluripotency gene expression profile was performed using the Human-HT-12-v4 expression BeadChip Kit (Illumina). Genome integrity was assessed applying the Illumina Human CytoSNP-12v2.1 beadchip array or Illumina human OmniExpress24 on genomic DNA generated using the All-Prep kit (QIAGEN) and analyzed using GenomeStudio and Karyostudio software (Illumina).
Generation and characterization of iPSC derived dopamine neurons
Six control (OX1-19/SFC841-03-01/02, JR053-6/1, AH016-3/6, SFC156-03-01, SFC840-03-06 and SFC067-03-01), four GBA-N370S (MK088-1, MK071-3, SFC834-03-03 and MK082-26) patient lines and four idiopathic (SFC077-03-04, SFC844-03-12, SFC120-03-04 and SFC865-03-07) patient lines were differentiated, as described previously (Kriks et al., 2011), with slight modifications (Beevers et al., 2017). Cells underwent 21 days of patterning and differentiation, were replated and matured for a further 5 weeks (60 DIV) when collected for flow cytometry. Control and PD GBA-N370S patient lines were successfully differentiated into dopaminergic neurons, expressing beta-tubulin III (TUJ1) a neuronal marker and Tyrosine Hydroxylase (TH) a specific dopaminergic neuronal marker by immunofluorescence (Figure S2A). Treatment of iPSC-derived dopamine neurons with the HDAC4 modifying compounds occurred at DIV 45 for 72 hours at the following concentrations: Tasquinimod (15uM), Okadaic acid (10nM), LB100 (1.25uM) and Cantharidin (250nM).
Method Details
Purification of iPSC dopaminergic neurons by flow cytometry
Purification of iPSC derived dopaminergic neurons was carried out as previously described (Sandor et al., 2017). At sorting each sample was first sorted into each row of a 96 well plate, into 2 ul of smart-seq 2 lysis buffer (0.2% triton x-100 and 2 U/ul RNase inhibitor), so that all samples were on each 96 well plate for single cell RNA-sequencing. After 96 well plate sorting, the rest of the sample was bulk collected for RNA extraction in preparation for bulk RNA-sequencing.
RNA preparation of bulk RNA-seq samples
RNA from bulk collected FACS sorted dopamine neurons was extracted using an FFPE RNA extraction kit (QIAGEN) as per manufacturer’s instructions, with minor modifications. RNA integrity analysis was analyzed using a 2100 bioanalyzer system and a RNA 6000 pico kit (Agilent). Concentration was obtained and confirmed utilizing two methods; the 2100 bioanalyzer system and a Quant-iT RiboGreen RNA kit (Invitrogen), as per manufacturer’s instructions.
Smart-seq2, RNA library construction and sequencing
Single cells and RNA extracted from bulks were processed using the Smart-seq2 protocol (Picelli et al., 2013). cDNA samples were prepared for sequencing using the Nextera XT DNA Library Prep Kit (Illumina) with our own in-house indexing primers (Lamble et al., 2013). Libraries were pooled and sequenced using Illumina HiSeq4000 75bp paired-end sequencing.
RNA-seq read alignment and expression quantification
Single-cell and bulk RNA-seq data was processed identically. FASTQ files were trimmed using TrimGalore v0.4.1 on default settings. Transcript expression levels were quantified using Kallisto v0.42.5 (Bray et al., 2016) against GRCh38 reference human transcriptome. Additional quality control (QC) metrics were compiled by Picard 2.0.1 (https://broadinstitute.github.io/picard/) on BAM files aligned to the human genome (GRCh38) using Hisat2 (Kim et al., 2015). Transcript level abundances were then summarized to gene level estimates using tximport 1.4.0 (https://bioconductor.org/packages/release/bioc/html/tximport.html) (Soneson et al., 2015).
Quality control of single-cell RNA-seq
Quality-control, visualization, and handling of single-cell data was performed using Scater (McCarthy et al., 2017). Cells belonging to plates 3-6 were removed due to distinct clustering on reduced-dimensionality representations and low expression of otherwise constitutively expressed genes. Further outliers were removed using Cellity (Ilicic et al., 2016) and subsequently any cell expression GAPDH at a level below the maximum GAPDH expression in blank wells was further removed, leaving a total of 146 cells for analysis.
Differential expression analysis
Differential expression analysis on bulk RNA-seq was performed using DESeq2 (Love et al., 2014), including a covariate to account for technical replicates. GO enrichment was performed using goseq (Young et al., 2010) and over-represented p values were multiple test corrected using the Benjamini-Hochberg procedure.
Single-cell pseudotime analysis
A PCA representation of the cells was computed using the prcomp function in the stats package in R using the 500 most variable genes (in log expression space), the default in Scater. Single-cell differential expression analysis along PC2 was performed using the R package switchde (Campbell and Yau, 2017). A further refined trajectory using the combined gene list along was computed using Ouija.
Identification of pathway activation in GBA 3
Over-dispersion analysis was performed in the method identical to Brennecke et al. (2013) using the R package scran (Lun et al., 2016) using ERCC spike-ins. A gene was designated as over-dispersed if the reported q-value < 0.05. GO analysis was performed using the R package GOSeq (Young et al., 2010). Genes from the SRP-dependent co-translational protein targeting to membrane pathway were selected for validation by performing a Wilcoxon rank-sum test for log2(TPM+1) expression in GBA3 cells compared to controls and prioritized based on p value.
Phenotypic linkage network construction
To assess functional similarity and convergence of the core gene set we constructed a phenotypic linkage network (Honti et al., 2014). We wished to assess the functional similarity of genes within the core set compared to a randomly sampled background distribution. The genes selected for the background distribution should match the overall expression pattern of the core set in these iPSCs in order to account for the increased likelihood of functional similarities between genes randomly selected from the same cell type. We first noted that the core set of genes exhibited high mean expression than average among the whole transcriptome.
We then fitted a gamma distribution to the mean log2(TPM+1) values of both the core gene set and all other genes in the transcriptome (the core distribution and background distribution). Then for each gene not in the core gene set we calculated the probability of observing the mean expression level under both models, and formed an unnormalized inclusion probability of the ratio of the density of the observed expression given the core gene set distribution to the density of the observed expression given the background distribution. This can loosely be thought of as “how many times more likely is it that a gene fits the core set distribution compared to the background distribution.” To choose the background set of genes we then sampled 1000 genes from the transcriptome excluding the core gene set, where the probability of a gene being selected was proportional to the un-normalized inclusion probability. The resulting empirical distribution fitted well with the fitted core gene set distribution (Figure S4D). We subsequently constructed a phenotypic linkage network as per Honti et al. (2014) using 1) the core set of genes, 2) the 1000 sampled background genes, and 3) the genes SNCA, PARK2, PARK7, LRRK2, UCHL1, GBA, PINK1, ATP13A2, HTRA2, PLA2G6, VPS35, and EIF4G1. Links were compared between different classes of genes using a one-sided Wilcoxon rank-sum test.
qRT-PCR, immunocytochemistry and western blot
For qRT-PCR experiments to validate RNA-Seq findings RNA was extracted from 12 well plates using Trizol (life technologies) and purified using the RNeasy Micro kit (QIAGEN) as per manufacturer’s instructions. Quality and concentration were quantified using a Nanodrop 1000 (Thermo Scientific). cDNA was synthesized using a superscript III reverse transcriptase kit (Life technologies) as per manufacturer’s instructions. qRT-PCR was carried out using fast SYBR green mastermix and a StepOnePlus thermal cycler (Life technologies). Primers used in this study can be found in Table S2.
For immunocytochemistry cells were fixed in 4% paraformaldehyde in 96 well plates (microClear 96 well plates, Greiner). They were then blocked with 10% donkey serum (PBS/0.5% triton x) for 1 hour, incubated with the following antibodies; Tyrosine hydroxylase (1:500 Millipore AB1542), Beta-III tubulin (1:500 Covance MMS 435P), HDAC4 (1:500 Abcam ab12171) and DAPI in 1% donkey serum (PBS/0.5% triton x). Secondary antibodies were added in 1% donkey serum (PBS) for 1 hour. Cells were washed and kept in 1x PBS for imaging on the Opera Phenix (Perkin Elmer).
For western blotting cells were extracted from 12 well plates in RIPA buffer (Tris [50 mM, pH 8], sodium chloride [150 mM], sodium dodecyl sulfate [SDS; 0.1% w/v], sodium deoxycholate [0.5% w/v] and nonidet-P40 [1% w/v]). Samples were denatured for 5 minutes at 100°C. Protein separation was achieved using SDS polyacrylamide gel electrophoresis and transferred onto PVDF membrane. Antibodies used as follows: Tyrosine hydroxylase (1:500 Millipore AB1542), β-actin (1:10,000 Abcam ab8227), PDI (1:500 Cell signaling 3501), FKBP9 (1:500 Abcam ab91219), Ero1-Lα (1:500 Cell signaling 9956), TSPAN7 (1:250 Novus Biologicals NBP1-90310), Na+/K+-ATPase α3 (1:500 Santa cruz sc-365744), Rtn1/2 (1:500 Santa cruz sc-71981), PRKCB (1:250 ProSci 43-319), LAMP1 (1:500 Santa cruz sc-20011), LC3B (1:500 Sigma L7543), HDAC4 (1:500 Abcam ab12171).
DQ-BSA
DQ-BSA Red reagent was prepared according to manufacturer’s instructions. While remaining under treatment of the selected compounds, iPSC-derived dopaminergic neurons were incubated with 30mg/mL DQ BSA Red and NucBlue Live ReadyProbes reagents for 4 hours at 37°C. Cells were washed with DPBS, replaced into fresh media and imaged on the Opera Phenix High Content Screening System (Perkin Elmer).
α-synuclein release
Extracellular α-synuclein release was quantified as previously described (Fernandes et al., 2016). Briefly, conditioned media from treated iPSC-derived neuronal cultures (100 μl) was collected at D45 and stored at −80°C prior to analysis. α-synuclein release was quantified relative to a standard curve using an electrochemiluminescent assay (Meso Scale Discovery, MD, USA, Cat# K151TGD-2) and a MESO QuickPlex SQ 120 instrument (Meso Scale Discovery). Extracellular α-synuclein release was normalized relative to total protein content of cells, using a BCA assay.
Quantification and Statistical Analysis
For differences between more than two groups a two way-ANOVA analysis was used to test for the significance. Mean values ± SEM are shown unless otherwise stated. P value for comparisons were adjusted for multiple comparisons using a Bonferroni correction. Data was presented of 3 independent controls and patients, over three differentiations unless otherwise stated.
Acknowledgments
The work was supported by the Monument Trust Discovery Award from Parkinson's UK. C.W. was supported by the Medical Research Council, UK. The Oxford Martin School (LC0910-004) and the Wellcome Trust (WTISSF121302) provide core support to the James Martin Stem Cell Facility within the Sir William Dunn School of Pathology (S.A.C.). The OPDC Discovery cohort was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford and the Dementia and Neurodegenerative Diseases Research Network (DeNDRoN). Single-cell transcriptomics at the Oxford Genomics Centre was supported by a Wellcome Trust core grant to the Wellcome Centre for Human Genetics, reference 090532/Z/09/Z. We also thank Christine Klein (Lübeck) for clinical expertise in PD and Uroosa Chughtai (Oxford) for valuable technical assistance in cell culture. The research leading to these results has received support from the Innovative Medicines Initiative Joint Undertaking (IMIJU) under grant agreement n_115439, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies’ in kind contribution. This publication reflects only the author’s views and neither the IMI JU nor EFPIA nor the European Commission are liable for any use that may be made of the information contained therein. Funding to pay the Open Access publication charges for this article was provided by Parkinson's UK (COAF) (J-1403) and Wellcome Trust (092762/Z/10/Z).
Author Contributions
C.L. differentiated iPSC lines and generated experimental data; K.R.C. and C.W. undertook bioinformatics analysis; B.J.R. performed IPA analysis and generated experimental data; P.C. generated experimental data; M.A. and R.B. performed the RNA-seq; J.V., O.V.P., and S.A.C. generated and banked the iPSC lines; and M.T.H., F.B., and M.K. provided clinical patient assessment. R.W.-M. supervised the experimental work; R.W.-M. and C.W. devised the study.
Declaration of Interests
The authors declare no competing interests.
Published: November 29, 2018
Footnotes
Supplemental Information includes seven figures and two tables and can be found with this article online at https://doi.org/10.1016/j.stem.2018.10.023.
Contributor Information
Caleb Webber, Email: webberc4@cardiff.ac.uk.
Richard Wade-Martins, Email: richard.wade-martins@dpag.ox.ac.uk.
Supporting Citations
The following reference appears in the Supplemental Information: Takahashi et al. (2007).
Supplemental Information
References
- Armstrong A.J., Häggman M., Stadler W.M., Gingrich J.R., Assikis V., Polikoff J., Damber J.E., Belkoff L., Nordle Ö., Forsberg G. Long-term survival and biomarker correlates of tasquinimod efficacy in a multicenter randomized study of men with minimally symptomatic metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013;19:6891–6901. doi: 10.1158/1078-0432.CCR-13-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M.G., Graham L. The journey: Parkinson’s disease. BMJ. 2004;329:611–614. doi: 10.1136/bmj.329.7466.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavan M.S., Schapira A.H. Glucocerebrosidase mutations and the pathogenesis of Parkinson disease. Ann. Med. 2013;45:511–521. doi: 10.3109/07853890.2013.849003. [DOI] [PubMed] [Google Scholar]
- Beevers J.E., Lai M.C., Collins E., Booth H.D.E., Zambon F., Parkkinen L., Vowles J., Cowley S.A., Wade-Martins R., Caffrey T.M. MAPT genetic variation and neuronal maturity alter isoform expression affecting axonal transport in iPSC-derived dopamine neurons. Stem Cell Reports. 2017;9:587–599. doi: 10.1016/j.stemcr.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Arias P., Einholm A.P., Mamsa H., Concheiro C., Gutiérrez-de-Terán H., Romero J., Toustrup-Jensen M.S., Carracedo A., Jen J.C., Vilsen B., Sobrido M.J. A C-terminal mutation of ATP1A3 underscores the crucial role of sodium affinity in the pathophysiology of rapid-onset dystonia-parkinsonism. Hum. Mol. Genet. 2009;18:2370–2377. doi: 10.1093/hmg/ddp170. [DOI] [PubMed] [Google Scholar]
- Bolger T.A., Yao T.P. Intracellular trafficking of histone deacetylase 4 regulates neuronal cell death. J. Neurosci. 2005;25:9544–9553. doi: 10.1523/JNEUROSCI.1826-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray N.L., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
- Brennecke P., Anders S., Kim J.K., Kołodziejczyk A.A., Zhang X., Proserpio V., Baying B., Benes V., Teichmann S.A., Marioni J.C., Heisler M.G. Accounting for technical noise in single-cell RNA-seq experiments. Nat. Methods. 2013;10:1093–1095. doi: 10.1038/nmeth.2645. [DOI] [PubMed] [Google Scholar]
- Campbell K.R., Yau C. switchde: inference of switch-like differential expression along single-cell trajectories. Bioinformatics. 2017;33:1241–1242. doi: 10.1093/bioinformatics/btw798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S., Vanhoutte P., Arnold F.J., Huang C.L., Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J. Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Du K., Liu M.Y., Zhong X., Wei M.J. Decreased circulating Zinc levels in Parkinson’s disease: a meta-analysis study. Sci. Rep. 2017;7:3902. doi: 10.1038/s41598-017-04252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley J.M., Lees A.J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fernandes H.J., Hartfield E.M., Christian H.C., Emmanoulidou E., Zheng Y., Booth H., Bogetofte H., Lang C., Ryan B.J., Sardi S.P. ER stress and autophagic perturbations lead to elevated extracellular α-synuclein in GBA-N370S Parkinson’s iPSC-derived dopamine neurons. Stem Cell Reports. 2016;6:342–356. doi: 10.1016/j.stemcr.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsleff L., Schauss A.G., Bier I.D., Stuart S. Evidence of functional zinc deficiency in Parkinson’s disease. J. Altern. Complement. Med. 1999;5:57–64. doi: 10.1089/acm.1999.5.57. [DOI] [PubMed] [Google Scholar]
- George G., Singh S., Lokappa S.B., Varkey J. Gene co-expression network analysis for identifying genetic markers in Parkinson’s disease - a three-way comparative approach. Genomics. 2018;7543:30282–30289. doi: 10.1016/j.ygeno.2018.05.005. [DOI] [PubMed] [Google Scholar]
- Gonera E.G., van’t Hof M., Berger H.J., van Weel C., Horstink M.W. Symptoms and duration of the prodromal phase in Parkinson’s disease. Mov. Disord. 1997;12:871–876. doi: 10.1002/mds.870120607. [DOI] [PubMed] [Google Scholar]
- Gordon I.K., Lu J., Graves C.A., Huntoon K., Frerich J.M., Hanson R.H., Wang X., Hong C.S., Ho W., Feldman M.J. Protein phosphatase 2A inhibition with LB100 enhances radiation-induced mitotic catastrophe and tumor growth delay in glioblastoma. Mol. Cancer Ther. 2015;14:1540–1547. doi: 10.1158/1535-7163.MCT-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenseler W., Zambon F., Lee H., Vowles J., Rinaldi F., Duggal G., Houlden H., Gwinn K., Wray S., Luk K.C. Excess α-synuclein compromises phagocytosis in iPSC-derived macrophages. Sci. Rep. 2017;7:9003. doi: 10.1038/s41598-017-09362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghverdi L., Büttner M., Wolf F.A., Buettner F., Theis F.J. Diffusion pseudotime robustly reconstructs lineage branching. Nat. Methods. 2016;13:845–848. doi: 10.1038/nmeth.3971. [DOI] [PubMed] [Google Scholar]
- Honti F., Meader S., Webber C. Unbiased functional clustering of gene variants with a phenotypic-linkage network. PLoS Comput. Biol. 2014;10:e1003815. doi: 10.1371/journal.pcbi.1003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska K.S., LaMarca M.E., Scott C.R., Sidransky E. Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA) Hum. Mutat. 2008;29:567–583. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- Hsieh C.H., Shaltouki A., Gonzalez A.E., Bettencourt da Cruz A., Burbulla L.F., St Lawrence E., Schüle B., Krainc D., Palmer T.D., Wang X. Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic parkinson’s disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A.J., Daniel S.E., Kilford L., Lees A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilicic T., Kim J.K., Kolodziejczyk A.A., Bagger F.O., McCarthy D.J., Marioni J.C., Teichmann S.A. Classification of low quality cells from single-cell RNA-seq data. Genome Biol. 2016;17:29. doi: 10.1186/s13059-016-0888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs J.T., Antony L., Dalrymple S.L., Brennen W.N., Gerber S., Hammers H., Wissing M., Kachhap S., Luo J., Xing L. Tasquinimod is an allosteric modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013;73:1386–1399. doi: 10.1158/0008-5472.CAN-12-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Ji H. TSCAN: pseudo-time reconstruction and evaluation in single-cell RNA-seq analysis. Nucleic Acids Res. 2016;44:e117. doi: 10.1093/nar/gkw430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M., Hagenah J., Graf J., Lorwin A., Vollstedt E.-J., Peters E., Katalinic A., Raspe H., Klein C. Cohort profile: a population-based cohort to study non-motor symptoms in parkinsonism (EPIPARK) Int. J. Epidemiol. 2013;42 doi: 10.1093/ije/dys202. 128–128k. [DOI] [PubMed] [Google Scholar]
- Kim D., Langmead B., Salzberg S.L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev V.Y., Kirschner K., Schaub M.T., Andrews T., Yiu A., Chandra T., Natarajan K.N., Reik W., Barahona M., Green A.R., Hemberg M. SC3: consensus clustering of single-cell RNA-seq data. Nat. Methods. 2017;14:483–486. doi: 10.1038/nmeth.4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, F. (2015). Trim Galore. A wrapper tool around Cutadapt and FastQC to consistently apply quality and adapter trimming to FastQ files. Babraham Bioinformatics, https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/.
- Lamble S., Batty E., Attar M., Buck D., Bowden R., Lunter G., Crook D., El-Fahmawi B., Piazza P. Improved workflows for high throughput library preparation using the transposome-based Nextera system. BMC Biotechnol. 2013;13:104. doi: 10.1186/1472-6750-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lun A.T., McCarthy D.J., Marioni J.C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 2016;5:2122. doi: 10.12688/f1000research.9501.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D.J., Campbell K.R., Lun A.T., Wills Q.F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics. 2017;33:1179–1186. doi: 10.1093/bioinformatics/btw777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielcarek M., Landles C., Weiss A., Bradaia A., Seredenina T., Inuabasi L., Osborne G.F., Wadel K., Touller C., Butler R. HDAC4 reduction: a novel therapeutic strategy to target cytoplasmic huntingtin and ameliorate neurodegeneration. PLoS Biol. 2013;11:e1001717. doi: 10.1371/journal.pbio.1001717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenasheva V.V., Novosadova E.V., Makarova I.V., Lebedeva O.S., Grefenshtein M.A., Arsenyeva E.L., Antonov S.A., Grivennikov I.A., Tarantul V.Z. The transcriptional changes of trim genes associated with parkinson’s disease on a model of human induced pluripotent stem cells. Mol. Neurobiol. 2017;54:7204–7211. doi: 10.1007/s12035-016-0230-7. [DOI] [PubMed] [Google Scholar]
- Park J.S., Koentjoro B., Veivers D., Mackay-Sim A., Sue C.M. Parkinson’s disease-associated human ATP13A2 (PARK9) deficiency causes zinc dyshomeostasis and mitochondrial dysfunction. Hum. Mol. Genet. 2014;23:2802–2815. doi: 10.1093/hmg/ddt623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroni G., Cernotta N., Dello Russo C., Gallinari P., Pallaoro M., Foti C., Talamo F., Orsatti L., Steinkühler C., Brancolini C. PP2A regulates HDAC4 nuclear import. Mol. Biol. Cell. 2008;19:655–667. doi: 10.1091/mbc.E07-06-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y., Liu K.W., Wang J., Garancher A., Tao R., Esparza L.A., Maier D.L., Udaka Y.T., Murad N., Morrissy S. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma. Cancer Cell. 2016;29:311–323. doi: 10.1016/j.ccell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard Toolkit (2018). Picard Toolkit. Broad Institute, http://broadinstitute.github.io/picard/.
- Picelli S., Björklund A.K., Faridani O.R., Sagasser S., Winberg G., Sandberg R. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods. 2013;10:1096–1098. doi: 10.1038/nmeth.2639. [DOI] [PubMed] [Google Scholar]
- Pierson E., Yau C. ZIFA: dimensionality reduction for zero-inflated single-cell gene expression analysis. Genome Biol. 2015;16:241. doi: 10.1186/s13059-015-0805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P., Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7:304–308. doi: 10.4161/cc.7.3.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team . R Foundation for Statistical Computing; 2008. R: A language and environment for statistical computing. [Google Scholar]
- Reid J.E., Wernisch L. Pseudotime estimation: deconfounding single cell time series. Bioinformatics. 2016;32:2973–2980. doi: 10.1093/bioinformatics/btw372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Danés A., Richaud-Patin Y., Carballo-Carbajal I., Jiménez-Delgado S., Caig C., Mora S., Di Guglielmo C., Ezquerra M., Patel B., Giralt A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R., 3rd, Gounko N., Pieraut S., Liao L., Yates J., 3rd, Maximov A. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell. 2012;151:821–834. doi: 10.1016/j.cell.2012.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor C., Robertson P., Lang C., Heger A., Booth H., Vowles J., Witty L., Bowden R., Hu M., Cowley S.A. Transcriptomic profiling of purified patient-derived dopamine neurons identifies convergent perturbations and therapeutics for Parkinson’s disease. Hum. Mol. Genet. 2017;26:552–566. doi: 10.1093/hmg/ddw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöndorf D.C., Aureli M., McAllister F.E., Hindley C.J., Mayer F., Schmid B., Sardi S.P., Valsecchi M., Hoffmann S., Schwarz L.K. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Soneson C., Love M.I., Robinson M.D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res. 2015;4:1521. doi: 10.12688/f1000research.7563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg C., Armstrong A., Pili R., Ng S., Huddart R., Agarwal N., Khvorostenko D., Lyulko O., Brize A., Vogelzang N. Randomized, double-blind, placebo-controlled phase III study of tasquinimod in men with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2016;34:2636–2643. doi: 10.1200/JCO.2016.66.9697. [DOI] [PubMed] [Google Scholar]
- Szewczyk-Krolikowski K., Tomlinson P., Nithi K., Wade-Martins R., Talbot K., Ben-Shlomo Y., Hu M.T. The influence of age and gender on motor and non-motor features of early Parkinson’s disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat. Disord. 2014;20:99–105. doi: 10.1016/j.parkreldis.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Tayebi N., Walker J., Stubblefield B., Orvisky E., LaMarca M.E., Wong K., Rosenbaum H., Schiffmann R., Bembi B., Sidransky E. Gaucher disease with parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol. Genet. Metab. 2003;79:104–109. doi: 10.1016/s1096-7192(03)00071-4. [DOI] [PubMed] [Google Scholar]
- Tolosa E., Botta-Orfila T., Morató X., Calatayud C., Ferrer-Lorente R., Martí M.J., Fernández M., Gaig C., Raya Á., Consiglio A. MicroRNA alterations in iPSC-derived dopaminergic neurons from Parkinson disease patients. Neurobiol. Aging. 2018;69:283–291. doi: 10.1016/j.neurobiolaging.2018.05.032. [DOI] [PubMed] [Google Scholar]
- Van Wilgenburg B., Browne C., Vowles J., Cowley S.A. Efficient, long term production of monocyte-derived macrophages from human pluripotent stem cells under partly-defined and fully-defined conditions. PLoS One. 2013;12:e71098. doi: 10.1371/journal.pone.0071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.D., Wakefield M.J., Smyth G.K., Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.