Abstract
Background:
Abnormal amyloid β (Aβ) accumulation and deposition in the hippocampus is an essential process in Alzheimer’s disease (AD).
Objective:
To investigate whether Oleanolic acid (OA) could improve memory deficit in AD model and its possible mechanism.
Methods:
Forty-five SD rats were randomly divided into sham operation group, model group, and OA group. AD models by injection of Aβ25-35 were built. Morris water maze (MWM) was applied to inves-tigate learning and memory, transmission electron microscope (TEM) to observe the ultrastructure of synapse, western blot to the proteins, electrophysiology for long-term potentiation (LTP), and Ca2+ con-centration in synapse was also measured.
Results:
The latency time in model group was significantly longer than that in sham operation group (P=0.0001); while it was significantly shorter in the OA group than that in model group (P=0.0001); compared with model group, the times of cross-platform in OA group significantly increased (P=0.0001). TEM results showed OA could alleviate neuron damage and synapses changes induced by Aβ25-35. The expressions of CaMKII, PKC, NMDAR2B, BDNF, TrkB, and CREB protein were signif-icantly improved by OA (P=0.0001, 0.036, 0.041, 0.0001, 0.0001, 0.026, respectively) compared with that in model group; the concentration of Ca2+ was significantly lower in OA group (1.11±0.42) than that in model group (1.68±0.18); and the slope rate (P=0.0001) and amplitude (P=0.0001) of f-EPSP significantly increased in OA group.
Conclusion:
The present results support that OA could ameliorate Aβ-induced memory loss of AD rats by maintaining synaptic plasticity of the hippocampus
Keywords: Alzheimer’s disease, Oleanolic acid, Aβ, Ca2+, long-term potentiation, synaptic plasticity
1. Introduction
Alzheimer’s disease (AD) is characterized by progressive memory loss, and the amyloid β (Aβ) peptide with its accumulation, due to overproduction and/or the failure of clearance mechanisms, followed by neuronal death, has been the upstream cause of AD over the past decades [1-3]. Abnormal Aβ accumulation and deposition in the hippocampus, the first regions of the brain to suffer damage which plays important roles in the consolidation of short-term memory, long-term memory, and spatial memory [4], is an essential process in the pathological events occurring in individuals with AD [5, 6].
Multiple studies reported that dysregulation of neuronal Ca2+ homeostasis also plays a pivotal role in AD pathogenesis [7, 8]. Aβ-mediated toxicity could regulate the calcium channels in the membrane to promote extracellular Ca2+ influx [9], and/or release intracellular Ca2+ pool [10, 11], to constantly enhance intracellular Ca2+ concentration, leading to calcium overload, which could increase the neuronal sensitivity to excitotoxicity and apoptosis [12], eventually leading to neuronal synaptic injury, resulting in learning and memory impairment. Therefore, it could improve learning and memory ability in AD patients by reducing Aβ-mediated toxicity and intracellular Ca2+ concentration.
The complicated and still unclear pathogenesis of AD made it hard to treat it. Though there are a few medications currently being used, no one has been clearly shown to delay or halt the progression of AD [13], even with many reported adverse effects [14-16], which drives researchers to look for new treatments to alter the course of AD. Nowadays, natural products and their extracts are promising drug candidates [17-19], and many of them, such as Huperzine A [20], Galantamine [21], and (-)-Epigallocatechin-3-gallate [22, 23], have shown an effect in treating AD. Similarly, many extracts from several traditional Chinese herbs such as Astragalus membranaceus [24], Paeonia suffruticosa [25], Magnolia ofcinalis [26], and Rhizoma anemarrhenae [27] have been reported to effectively prevent memory impairment.
Oleanolic acid (OA), a natural extract from the traditional Chinese herb Ligustrum lucidum (Nv Zhenzi), was found to exhibit weak anti-HIV [28], anti-HCV [29], anti-inflammatory [30, 31], and anti-cancer [32, 33] properties. Combined with other herbs, Ligustrum lucidum could delay memory loss of AD in our previous clinical study [34], and further study is still needed to investigate the mechanism of OA against the AD.
Therefore, in this study, we build AD model rats by right intracerebroventricular injection of Aβ25-35, after intervened by OA for four weeks, to investigate whether OA could improve learning and memory deficit and its possible mechanism.
2. Material and Methods
2.1. Animals
Eight-week-male Sprague-Dawley rats (230-250g, from Beijing Vital River Laboratory Animal Technology Co., Ltd, Beijing, China) were kept at a 12h light/dark cycle under controlled temperature (22±2°C) and humidity (50±10%), and bred with standard diet and water ad libitum, acclimatized for 7 days before the experiments. All animal procedures were approved by the local ethical committee at Tianjin University of Traditional Chinese Medicine and met the guidelines of the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Documentation 55, 2001).
2.2. Drugs and Reagents
Oleanolic acid (purity>98%, Melonepharma), 2mg/mL, was suspended in 5% sodium carboxymethyl cellulose, and amyloid β-Protein Fragment 25-35 (Aβ25-35, purity≥97%, Sigma) was dissolved in 0.9% sterile water at a concentration of 4μg/μL and incubated at 37°C for 96h to induce aggregation before usage.
The selection of OA dose in rats was calculated by equivalent dose ratio [Rat(200g):Human(70kg)=6.3], based on the clinical application of OA in patients (240mg/d/70kg), the result was 21.6mg/kg for a rat. The concentration of OA for AD rats was 2mg/mL, thus we selected the dose of 10mL/kg.
2.3. Modeling and Administration
Forty-five SD rats were randomly divided into three groups: the sham operation group, the model group, and the OA group, 15 in each group. Rats were anaesthetized by intraperitoneal injection of 10% chloral hydrate (0.3g/kg) and fixed in the rat brain stereotaxic device (Ruiwode Life Technology Co., Ltd, China). A hole was drilled on the right parietal bone (anteroposteriorly [AP], 1mm; laterally right [LR], 1.5mm; and dorsoventrally [DV], 4mm) [35]. The rats in the sham operation group received a right intracerebroventricular injection of 0.9% sterile saline 5μL (speed, 1μL/min), while the rats in the model and OA group received a right intracerebroventricular injection of Aβ25-35 solution 5μL [36] at the same speed. The rats in OA group received OA treatments intragastricly administrated (10mL/kg) seven days after Aβ25-35 infusion, once daily from then on before water maza test, for four weeks, while distilled water was administrated (10mL/kg) to rats in other groups.
2.4. Morris Water Maze (MWM) Test
Spatial learning ability was tested using the Morris water maze (MWM) test after four-week intragastric administration. The water maze was an open circular black tank (120cm in diameter and 60 cm in height) and a movable circular platform (8cm in diameter). A camera two meters above the tank was connected to the computer to record the rat’s movement, and the data was analyzed by TopScanLite (CleverSys Inc). The tank was filled with water at a height of 23cm (23-25°C) and dyed with black edible pigment [37]. The tank was divided into 4 quadrants by two imaginary perpendicular axes with four cardinal points: North (N), South (S), East (E) and West (W), and the platform was placed in the middle of SW quadrant, 2cm submerged into the water surface.
MWM test was started with the place navigation trial, and each rat in all groups (n=15 in each group) underwent four trials per day with a one-minute-interval for 5 consecutive days. The rat was gently placed in the water from the starting position (location of SE, NE, SW, and NW quadrant, respectively), facing the wall of the tank. The rat was allowed to swim for 90 seconds until it found the submerged platform and stayed on the platform for a maximum of 10 seconds. If the rat failed to find the hidden platform within 90 seconds, it would be guided to the platform and remained on the platform for a maximum of 10 seconds before being removed from the pool. The escape latency was defined as the time taken to search for the platform within 90 seconds. The escape latency, swimming speed, and swimming path were recorded by the tracking system.
The spatial probe test was conducted 24 hours after the 5-day place navigation trial where the platform was removed from the pool to assess memory retention for the location of the platform. The rat was put into the pool at the NE point and allowed to swim for 90 seconds. The swimming speed, swimming path, and the frequency of rat crossing the virtual platform were recorded.
2.5. Transmission Electron Microscope
Rats (n=3 in each group) were anesthetized by intraperitoneal injection 10% chloral hydrate (0.3g/kg) after MWM test. The brain was removed rapidly and placed on ice. The CA1 region of the left hippocampus was dissected and then diced into smaller blocks of 1mm×1mm×1mm. The methods to prepare samples were as follows: the specimens were fixed by immersing in 4% buffered glutaraldehyde 4 h, and then washed in cold 1/15M phosphate buffered saline 15-30min for 3 times. The tissues were then post-fixed for 2 h in 1% osmium tetroxide, and washed in cold 1/15M phosphate buffered saline 30min for 3 times. The samples were dehydrated in ascending grades of acetone: 50%, 70%, 80%, 90%, 15 min respectively, and then in 100% acetone 15min ×2. The tissues were infiltrated in embedding medium for 3 h at room temperature, 60 min in mixture of 100% acetone/embedding medium (1:1) in 37oC, overnight in mixture of 100% acetone/embedding medium (1:3) in 37oC, 5 h in embedding medium in 37oC. All tissues were embedded and then cured in a 37oC oven for 24 h followed by 48 h in a 60oC oven. The samples were sliced into 50nm with an ultra-microtome (Leica UCT, Germany). The samples were stained with uranyl acetate for 45 min, and then stained with lead citrate for 15 min. A transmission electron microscope (TEM) (Hitachi H7500, Japan) was used to observe the neurons using.
2.6. Western Blot Analysis
Rats (n=5 in each group) were sacrificed for extracting protein samples from right hippocampi after MWM test by splitting, centrifugation, and boiling. Equal amounts (40 μg) of protein were loaded into each well of a sodium dodecyl sulfate-polyacrylamide gel (10% polyacrylamide gels) and separated by electrophoresis. The proteins were then transferred to nitrocellulose membranes (Amersham, USA), which were blocked with 5% bovine serum albumin and prepared in a Tris-buffered saline (TBS) overnight at 4°C. The membranes were incubated with antibodies to detect CaMKII (ab134041, 1:1000), PKC (ab136491, 1:1000), NMDAR2B (ab65783, 1:1000), BDNF (ab205067, 1:1000), TrkB (sc-377218, 1:1000), CREB (ab32515, 1:1000), GAPDH (ab8245, 1:1000) and beta Actin (ab184220 1:1000), followed by incubation with appropriate HRP-conjugated goat anti-rabbit (ZSGB-BIO, China; ZB-5301, 1:1,000) or HRP-conjugated goat anti-mouse secondary antibodies (ZSGB-BIO, China; ZB-5305, 1:1,000). Immunoreactive bands were visualized using the BeyoECL Plus (Beyotime Company, China), and the resulting membranes were imaged using the VersaDoc MP5000 imaging system (BIO-RAD, USA). The Quantity One was used to assess the band intensity and to carry out semi-quantitative analyses.
2.7. Measurement of Ca2+ Concentration
Rats (n=3 in each group) were intraperitoneally injected with 10% chloral hydrate (0.3g/kg) after the MWM tests, and the right hippocampi were rapidly separated. They were weighed and homogenized in an isotonic sucrose solution (0.32 mol/L) at 4°C in 1 g: 10 mL. The homogenate was centrifuged at 500g for 10 min to get the supernatant and then centrifuged at 4°C for 20 min (10000 g) to obtain the crude synaptosomal. The crude synaptosome was suspended with 0.32 mol/L sucrose solution, and then carefully spread over the density gradient of 0.8M and 1.2M sucrose. They were centrifuged with high-speed at 50000 g for 25 min to obtain the synaptosomes, and centrifuged at 2000 g for in 4°C to take precipitation [38]. The synaptosomes were quantified by BCA method and equal amounts (30 μg) of synaptosomes were added to each well of the black 96-well cell culture plate. Rhod 2-AM (1:500) probe 100 μL was added and incubated at 37°C for 30 min away from light. The culture medium was placed in a fluorescent microplate reader (BioTek FLx800, USA) to measure the fluorescence intensity at excitation wavelength of 557 nm and emission wavelength of 581 nm. Formula: [Ca2+]i=Kd(F-Fmin)/(Fmax-F).
2.8. Electrophysiology
After the MWM test, long-term potentiation was quantified with in vivo electrophysiological techniques. Rats (n=3in each group) were anaesthetized with an intraperitoneal injection of 20% urethane (1.2 g/kg), and then were positioned in a stereotaxic apparatus (Narishige, Japan) for surgery, covered with a towel to keep warm. A 3cm incision, blunt off the skin periosteum was cut to fully expose the bregma, sagittal suture, and parietal. A hole in the skull was drilled to expose CA3 region (4.2mm posterior to the bregma, 3.8mm right to the sagittal suture, 2.0 mm-3.5mm beneath the cortex) and CA1 region (3.4 mm posterior to the bregma, 2.5mm right to the sagittal suture, 1.5 mm-2.5mm beneath the cortex), coordinated according to the mouse brain atlas [35]. A bipolar stimulation electrode was placed in the CA3 region and a monopolar stainless steel recording electrode in the CA1 region. Stimulus-response was tested using a range of stimulation intensities from 0.1mA to 1 mA with 0.2ms,0.03HZ to determine the appropriate stimulating. The stimulating intensity (range 0.3-0.5 mA, stimulus pulse with 0.2 ms, at 0.03 Hz) that could evoke a response of 70% of its maximum amplitude was chosen for the following experiment.
The basal field excitatory postsynaptic potentials (fEPSP) were recorded for 20 min with a single pulse of 2 times every minute. LTP was evoked by theta burst stimulation (TBS): 10 trains at 5 Hz consisting of 10 single pulses at 100 Hz. The f-EPSP was recorded for 60 min with a single pulse of 1 time every minute. LTP analysis was carried out in ClampFit (Molecular Devices, USA) and figures were constructed in KaleidaGraph (Synergy Sofware, Reading, PA, USA) using values for the amplitude of the fEPSP.
2.9. Statistical Analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) v19.0, and all data were presented as Mean±SD. For the repeated measurement data (speed and escape latency time), repeated measurement analysis of variance (ANOVA) was applied to speed, and taking the sample size and normality, Generalized Estimating Equations analysis was applied to escape latency time; for other results, one way analysis of variance was applied with post-hoc LSD to data according to normality and homogeneity variance; or nonparametric test with Kruskal-Wallis was applied. The threshold for significance was set at P<0.05 for all analyses.
3. Results
3.1. OA Improves Spatial Learning and Memory Performance in the MWM Test
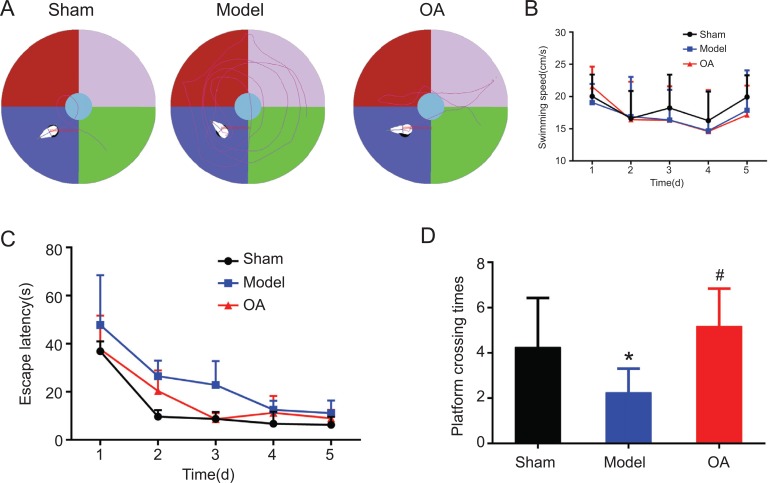
Swimming paths of rats in each group in the MWM test are presented in Fig. (1A). Repeated measurement ANOVA results showed that there was no significant difference in the average swimming speed in each group(F=0.331, P=0.721>0.05) (Fig. 1B).
Fig. (1).
Results of Morris water maze test(n=15). (A) Swimming paths of rats in each group in the Morris water maze. (B) Average swimming speed of rats in each group. Repeated measurement ANOVA results showed that there was no significant difference of the average swimming speed in each group(F=0.331, P = 0.721>0.05) (C) Escape latency time of rats in each group. Post Pairwise Comparisons using Bonferroni method showed that the latency time in model group was significantly longer than that in sham operation group (P=0.0001<0.05); while it was significantly shorter in the OA group than that in model group (P=0.0001<0.05) (D) platform crossing times of rats in each group. Post comparison with LSD method showed that compared with sham group, the times of cross-platform in model group significantly decreased (P = 0.007 <0.05); while compared with model group, the times of cross-platform in OA group significantly increased (P = 0.0001 <0.05).
Notes:*indicates compared with that in the sham operation group, P<0.05.
#indicates compared with that in the model group, P<0.05.
3.1.1. Escape Latency
In the MWM test, the escape latency was gradually shortened during the training period. Based on the Generalized Estimating Equations analysis, there was significant difference among three groups (P = 0.002<0.05), but no interaction between groups and time (P = 0.063> 0.05) (Table 1). Based on the Estimated Marginal Means, the escape latency showed sham operation group < OA group < model group (Table 2). Post Pairwise Comparisons using Bonferroni method showed that the latency time in model group was significantly longer than that in sham operation group (P=0.0001<0.05); while it was significantly shorter in the OA group than that in model group (P=0.0001<0.05).
Table 1.
Tests model effects.
| - | Wald Chi-square Value | Variance | Sig. |
|---|---|---|---|
| (Intercept) | 333.317 | 1 | 0.0001 |
| Groups | 12.827 | 2 | 0.002 |
| Time | 190.298 | 1 | 0.0001 |
| Groups×Times | 5.523 | 2 | 0.063 |
Table 2.
Estimated marginal means of escape latency.
| Groups | Mean | Standard Error | 95% Wald Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Sham | 13.95 | 2.03 | 9.97 | 17.94 |
| Model | 24.64 | 2.11 | 20.51 | 28.78 |
| OA | 16.89 | 2.29 | 12.40 | 21.37 |
3.1.2. Spatial Probe Test
One-way ANOVA analysis showed that there was a statistically significant difference among three groups (F = 9.056, P = 0.001 <0.05). Post comparison with LSD method showed that compared with sham group, the times of cross-platform in model group significantly decreased (P = 0.007 <0.05); while compared with model group, the times of cross-platform in OA group significantly increased (P = 0.0001 <0.05) (Fig. 1D).
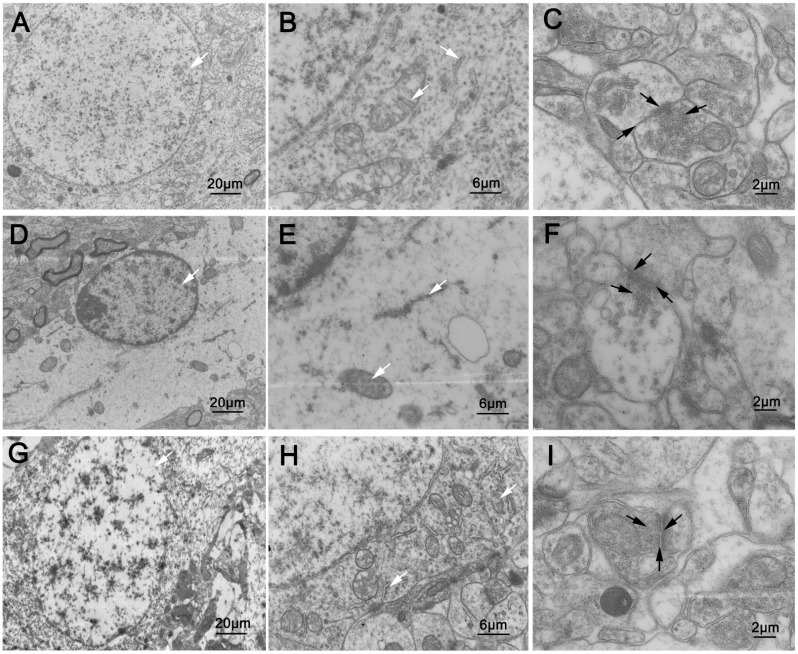
3.2. OA Alleviates Neuron Damage and Synapses Changes Induced by Aβ25-35
TEM revealed that in the sham operation group, the neurons were intact, with homogeneous nuclei, abundant organelles, oval and full mitochondria, clear and intact double layer structure of the cristal membrane, endoplasmic reticulum attached with a large number of ribosomes. The synapses were normal with abundant synaptic vesicles secreted by presynaptic elements. The synaptic cleft was narrow, and the postsynaptic density was centralized (Fig. 2A-C).
Fig. (2).
Ultrastructure of neurons and synapses (n=3). In the sham operation group: (A, B) the neurons were intact, with homogeneous nuclei, abundant organelles, oval and full mitochondria, clear and intact double layer structure of the cristal membrane, endoplasmic reticulum attached with a large number of ribosomes (white arrows). (C) the synapses were normal with many synaptic vesicles secreted by presynaptic elements. The synaptic cleft was narrow, and the postsynaptic density was centralized (black arrows). In the model group: (D, E) neurons were not intact, with shrinking nuclei, severe loss of organelles, rarely normal mitochondria in vacuolar state, ruptured endoplasmic reticulum with obvious loss of ribose (white arrows). (F) the amount of synaptic vesicles secreted by presynaptic elements decreased. The synaptic cleft clearly widened, with sparse postsynaptic density (black arrows). In the OA group: (G, H) structures of neurons were intact on the whole, with homogeneous nuclei, abundant organelles, slightly swollen mitochondria, partial fusion and fracture of cristal membrane, slight loss of ribose of endoplasmic reticulum (white arrows). (I) abundant synaptic vesicles secreted by presynaptic elements could be observed. The synaptic cleft was clear, without obvious widening, and the postsynaptic density was centralized (black arrows).
In the model group, neurons were not intact, with shrinking nuclei, severe disruption of organelles, rarely normal mitochondria in vacuolar state, ruptured endoplasmic reticulum with obvious loss of ribose. The abundance of synaptic vesicles secreted by presynaptic elements decreased. The synaptic cleft clearly widened, with sparse postsynaptic density (Fig. 2D-F).
In the OA group, structures of neurons were intact on the whole, with homogeneous nuclei, abundant organelles, slightly swollen mitochondria, partial fusion and fracture of cristal membrane, slight loss of ribose of endoplasmic reticulum. Abundant synaptic vesicles secreted by presynaptic elements could be observed. The synaptic cleft was clear, without obvious widening, and the postsynaptic density was centralized (Fig. 2G-I).
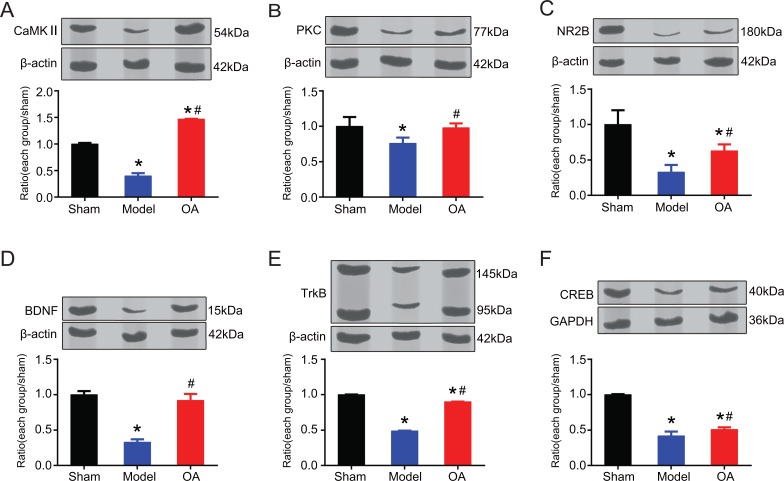
3.3. OA could Improve the Expression of CaMKII, PKC, NMDAR2B, BDNF, TrkB, and CREB Protein
We measured protein levels of CaMKII, PKC, NMDAR2B, BDNF, TrkB, and CREB of the three groups. One way ANOVA results showed that there existed inter-group difference (CaMKII, F=1054.102, P=0.0001<0.05; PKC, F=5.346, P=0.046<0.05; NMDAR2B, F=16.949, P=0.003<0.05; BDNF, F=95.467, P=0.0001<0.05; TrkB, F=40049.417, P=0.0001<0.05, CREB, F=196.703, P=0.0001 <0.05). Post hoc statistics using LSD showed that compared with that in the sham operation group, the expression of CaMKII (Fig. 3A), PKC (Fig. 3B), NMDAR2B (Fig. 3C), BDNF (Fig. 3D), TrkB (Fig. 3E), and CREB (Fig. 3F) were significantly lower in the model group, with statistically significant difference (P=0.0001, 0.026, 0.001, 0.0001, 0.0001, 0.0001, respectively). While compared with that in the model group, the expression of CaMKII (Fig. 3A), PKC (Fig. 3B), NMDAR2B (Fig. 3C), BDNF (Fig. 3D), TrkB (Fig. 3E), and CREB (Fig. 3F) were significantly higher in the OA group, with statistically significant difference (P=0.0001, 0.036, 0.041, 0.0001, 0.0001, 0.026, respectively). The levels of NR2B, Trkb, and CREB in OA group were still significantly lower than that in sham group (P=0.018<0.05, P=0.000 <0.05, P=0.000<0.05, respectively). The levels of BDNF and PKC in OA group were lower than that in sham group, but the difference was not statistically significant (P=0.165> 0.05, P=0.808>0.05, respectively). The level of CAMKII in OA group was higher than sham group, with significant difference (P=0.000<0.05).
Fig. (3).
Expression of CaMKII, PKC, NMDAR2B, BDNF, TrkB, and CREB protein (cropped blots from different gels). (A) Compared with that in the sham operation group, the expression of CaMKII was significantly lower in the model group (P=0.0001). While compared with that in the model group, the expression of CaMKII was significantly higher in the OA group (P=0.0001), and higher than that in sham group (P=0.0001); (B) compared with that in the sham operation group, the expression of PKC was significantly lower in the model group (P=0.026). While compared with that in the model group, the expression of PKC was significantly higher in the OA group (P=0.036); (C) compared with that in the sham operation group, the expression of NMDAR2B was significantly lower in the model group (P=0.001). While compared with that in the model group, the expression of NMDAR2B was significantly higher in the OA group (P=0.041), but still significantly lower than that in sham group (P=0.018); (D) compared with that in the sham operation group, the expression of BDNF was significantly lower in the model group (P=0.0001). While compared with that in the model group, the expression of BDNF was significantly higher in the OA group (P=0.0001); (E) compared with that in the sham operation group, the expression of TrkB was significantly lower in the model group (P=0.0001). While compared with that in the model group, the expression of TrkB was significantly higher in the OA group (P=0.0001), but still significantly lower than that in sham group (P=0.0001); (F) compared with that in the sham operation group, the expression of CREB was significantly lower in the model group, with statistically significant difference (P=0.0001). While compared with that in the model group, the expression of CREB was significantly higher in the OA group, with statistically significant difference (P=0.0001), but still significantly lower than that in sham group (P=0.0001).
Notes: *indicates compared with that in the sham operation group, P<0.05;
#indicates compared with that in the model group, P<0.05.
3.4. OA Could Decrease the Concentration of Ca2+
Kruskal-Wallis test showed that compared with that in the sham operation group, the concentration of Ca2+ was significantly higher in the model group, with statistically significant difference (P=0.0001). While compared with that in the model group, the concentration of Ca2+ was significantly lower in the OA group, with statistically significant difference (P=0.015) (Table 3).
Table 3.
Ca2+ concentration of each group (Mean±SD).
| Group | n | Rhod-2 Fluorescence |
|---|---|---|
| Sham operation | 3 | 0.96±0.09 |
| Model | 3 | 1.68±0.18* |
| OA | 3 | 1.11±0.42# |
Notes: *indicates compared with that in the sham operation group, P<0.05.
#indicates compared with that in the model group, P<0.05
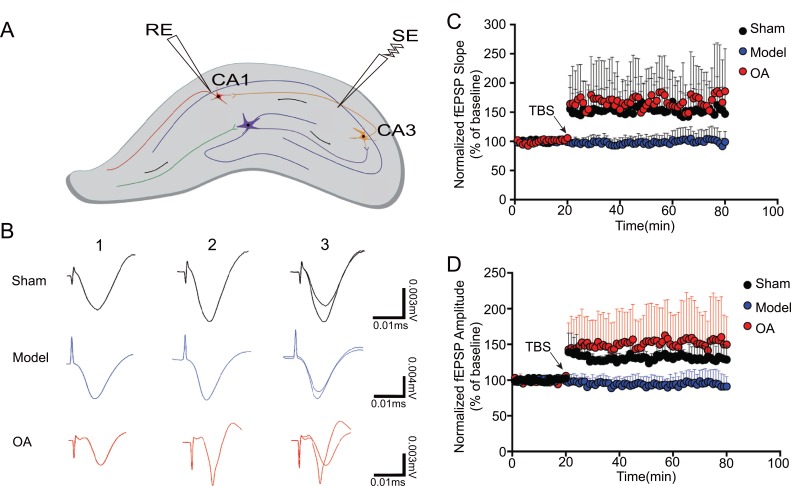
3.5. OA Could Increase the Slope and Amplitude of f-EPSP
In the LTP assay, the stimulation electrode (SE) was placed in the CA3 region and the recording electrode (RE) in the CA1 region (Fig. 4A). Following the TBS stimulation (Fig. 4B), the fEPSPs slopes were recorded. Kruskal-Wallis test showed that the slope rate significantly decreased after high frequency stimulation in the model group compared with that in the sham operation group (P=0.0001). While compared with model group, the slope rate significantly increased after high frequency stimulation in the OA group (P=0.0001) (Fig. 4C).
Fig. (4).
OA rescues Aβ-induced deficits in long-term potentiation (n=3). (A) Position of stimulation electrode (SE) and recording electrode (RE); (B) 1: potential of resting state; 2: 60 min after TBS; 3: combination of (1) and (2) in each group; (C) slope change in each group. Kruskal-Wallis test showed that the slope rate significantly decreased after high frequency stimulation in the model group compared with that in the sham operation group (P = 0.0001). While compared with model group, the amplitude significantly increased after high frequency stimulation in the OA group (P=0.0001) (D) amplitude change in each group. Kruskal-Wallis test showed that the amplitude significantly decreased after high frequency stimulation in the model group compared with that in the sham operation group (P=0.0001). While compared with model group, the amplitude significantly increased after high frequency stimulation in the OA group (P=0.0001)
Kruskal-Wallis test showed that the amplitude significantly decreased after high frequency stimulation in the model group compared with that in the sham operation group (P=0.0001). While compared with model group, the amplitude significantly increased after high frequency stimulation in the OA group (P=0.0001) (Fig. 4D).
4. Discussion
AD is the most common form of dementia in the elderly, and it is characterized clinically by progressive cognitive impairment, memory loss, and altered behavior [39-41]. Several hypotheses have been proposed, and it is widely accepted that Aβ plays a central role [42, 43]. In our study, we build the AD model by injecting Aβ25-35 aggregate into right ventricle, which is a widely used model to study AD [44, 45]. It has been reported that Aβ injection would cause memory impairment [46, 47], and the results in our study that the escape latency increased and the frequency of platform crossing decreased in MWM test of rats in model group also proved that. While the lower escape latency and higher frequency of platform crossing in OA group represent that OA could ameliorate Aβ-induced spatial learning and memory loss of AD rats.
It is widely accepted that the synapses play an important role in the formation of memory [48-50]. In the nervous system, synapses are esseential to neuronal functions:a synapse is a structure where a neuron could pass an electrical or chemical signal to another neuron. Evidences show that synapse loss or synaptic dysfunction is strongly correlated with memory impairment in AD, suggesting a causal role for dwindling synaptic integrity in the etiology of AD [51, 52]. Synapse is formed by presynaptic axon ending and by postsynaptic dendritic spine, and a similar loss of both pre-synaptic and post-synaptic components [53] as well as significant decreases in synaptic density of the hippocampus could occur in AD brains [54-56]. Synapse loss, suppressed presynaptic vesicle release [57, 58], and changes in the shape and size of dendritic spines [59] caused by Aβ deposition are the pathological hallmarks of AD, which was also found in our rats of model group by TEM. Our research showed that OA exhibited a positive effect on preserving neuron injury, improving the arrangements and the ultrastructure of neurons, and maintaining synaptic integrity, which suggested a protection and improvement of memory of AD rats.
Change in pre-synaptic and post-synaptic components by a high local concentration of Aβ oligomers could affect neuronal Ca2+ homeostasis [60], which is a popular hypothesis of AD in recent years [61, 62]. The imbalance of Ca2+ homeostasis leading to extracellular Ca2+ flow into the cytoplasm, could cause Ca2+ overload. A persistent elevation of Ca2+ concentration could not only constantly disrupt memories but also contribute to neuronal cell death. Furthermore, it actually occurs prior to cognitive decline and extensive neuronal death in AD [63]. In our study, the injection of Aβ25-35 led to synaptic Ca2+ overload in model group, then causing neurotoxicity; while OA could effectively reduce synaptic Ca2+ concentration, and exhibited the role of neuroprotection to improve learning and memory deficit.
Meanwhile, both pre-synaptic and post-synaptic mechanisms can contribute to the expression of synaptic plasticity, one of the important neurochemical foundations of learning and memory [64, 65]. Presynaptic vesicle could release neurotransmitters which are directly proportional to synaptic transmission strength. There are a lot of proteins such as N-methyl-Daspartate receptor (NMDARs), calmodulin-dependent-protein kinase II (CaMKII), and protein kinase C (PKC) in postsynaptic density (PSD), and the destruction of PSD could inactivate synaptic proteins, influence synaptic homeostasis, leading to negative synaptic formation and synaptic loss and dysfunction in AD [66, 67]. NMDAR, CaMKII, and PKC in PSD play essential roles in synaptic plasticity. The accumulated evidences demonstrated NMDAR was dysfunctional, and the protein activation of CaMKII and PKC was suppressed in AD. In our study, NMDAR2B, CaMKII and PKC protein expression, all significantly decreased in model group. We also found that OA could increase the NMDAR2B, CaMKII and PKC protein expression, to restore and maintain synaptic plasticity.
Long-term potentiation (LTP), a main form of long-term synaptic plasticity, is an increase in synaptic response following potentiating pulses of electrical stimuli, and the induction and maintenance of it could reflect the strength of synaptic transmission efficiency, which was widely considered as one of the major cellular mechanisms underlying learning and memory [68-70]. It has been reported that LTP of hippocampal CA1 area was significantly inhibited in Aβ25-35 induced learning-and-memory-impairment rats [71]. Moreover, when the way to form LTP was blocked, learning and memory would be inhibited, suggesting that the cognitive decline in individuals with AD might result from impaired LTP [72, 73]. In our study, the slope and amplitude of fEPSP were taken as reference values for LTP (fEPSP to reflect the amount of excitatory synapse, slope to reflect the speed of synaptic response to stimuli, and amplitude to the intensity of synaptic stimulation). Before TBS, the amplitude and slope of fEPSP in the model group and sham operation group were consistent; while after TBS, the amplitude and slope of fEPSP in the model group were significantly lower than those in the sham operation group, suggesting that Aβ25-35 does not affect the basic function of synaptic transmission, but it inhibits TBS induced LTP. Our results also showed that OA could increase the amplitude and slope of fEPSP in AD models, indicating it could enhance the synaptic transmission.
In the maintenance and induction of LTP, brain-derived neurotrophic factor (BDNF), as a major regulator of synaptic structure and function, through tyrosine kinase B (TrkB) plays an essential role [74, 75], and reduced BDNF/TrkB leads to impaired spatial memory in AD. The increased BDNF/TrkB expression could activate Cyclic AMP response element binding protein (CREB), a constitutively expressed nuclear transcription factor that regulates the expression of genes involved in cognition and neuronal survival [76]. CREB shows a well-documented role in the formation and retention of memory by mediating gene expression in the hippocampus during LTP [77, 78]. CREB down-regulation is implicated in AD and increasing the expression of CREB is being considered as a possible therapeutic target for AD [79]. In our study, the expression of BDNF, TrkB, CREB proteins were all significantly suppressed in model group, while OA showed an up-regulating effect on them to enhance LTP.
Conclusion
In conclusions, we found that OA could ameliorate Aβ-induced memory loss of AD rats, which has never been reported before. And during the treatment, OA showed a protective effect in maintaining synaptic integrity to restore synaptic plasticity by increasing the NMDAR2B, CaMKII and PKC protein expression in PSD, reducing synaptic Ca2+ concentration, enhancing the inhibited LTP after Aβ25-35 injection by up-regulating the expression of BDNF, TrkB, CREB proteins, which might be its mechanism in improving learning and memory deficit.
Acknowledgements
Kai Wang, Weiming Sun, Wei Guo, Linlin Zhang,and Jiachun Xu made substantial contributions to conception, design, and performing of the experiment. Shuang Liu, Zhen Zhou, and Yulian Zhang analyze the data and are involved in drafting the manuscript. All authors read and approved the final manuscript.
This work is supported by National Natural Science Foundation of China (grant numbers: 81473490, 81273940), and Science and Technology Program of Tianin (grant number: 15ZXLCSY00020).
We acknowledge the contributions by Yang Cao, Congcong Bi, Yifu Wang, Xueyan Wang, Yan Ma, Hui Qin, Qijing Qin during the work. We would like to thank the Department of Electron Microscopy Center Hebei Medical University for our work, and we would be grateful to all the teachers for their help in making EM sample, and taking EM images.
List of abbreviations
- AD
Alzheimer’s Disease
- Aβ
Amyloid β
- OA
Oleanolic Acid
- MWM
Morris Water Maze
- fEPSP
Field Excitatory Postsynaptic Potentials
- TBS
Theta Burst Stimulation
Ethics Approval and Consent to Participate
All animal procedures were approved by the local ethical committee at Tianjin University of Traditional Chinese Medicine.
Human and Animal Rights
No human were used in this study the guidelines for animals were guided from the Care and Use of Laboratory Animals published by the National Institutes of Health (Documentation 55, 2001).
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Leissring M.A. Abeta-Degrading proteases: Therapeutic potential in alzheimer disease. CNS Drugs. 2016;30(8):667–675. doi: 10.1007/s40263-016-0364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fyfe I. Alzheimer disease: Anti-Abeta antibody treatment shows promise in Alzheimer disease. Nat. Rev. Neurol. 2016;12(10):554–555. doi: 10.1038/nrneurol.2016.144. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto K., Tanei Z., Hashimoto T., et al. Chronic optogenetic activation augments abeta pathology in a mouse model of Alzheimer disease. Cell Reports. 2015;11(6):859–865. doi: 10.1016/j.celrep.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav. Brain Res. 2001;127(1-2):199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 5.Mancini S., Minniti S., Gregori M., et al. The hunt for brain Abeta oligomers by peripherally circulating multi-functional nanoparticles: Potential therapeutic approach for Alzheimer disease. Nanomedicine (Lond.) 2016;12(1):43–52. doi: 10.1016/j.nano.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Meisl G., Yang X., Frohm B., et al. Quantitative analysis of intrinsic and extrinsic factors in the aggregation mechanism of Alzheimer-associated Abeta-peptide. Sci. Rep. 2016;6:18728. doi: 10.1038/srep18728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berridge M.J. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion. 2013;7(1):2–13. doi: 10.4161/pri.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daschil N., Kniewallner K.M., Obermair G.J., et al. L-type calcium channel blockers and substance P induce angiogenesis of cortical vessels associated with beta-amyloid plaques in an Alzheimer mouse model. Neurobiol. Aging. 2015;36(3):1333–1341. doi: 10.1016/j.neurobiolaging.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rovira C., Arbez N., Mariani J. Abeta(25-35) and Abeta(1-40) act on different calcium channels in CA1 hippocampal neurons. Biochem. Biophys. Res. Commun. 2002;296(5):1317–1321. doi: 10.1016/s0006-291x(02)02072-7. [DOI] [PubMed] [Google Scholar]
- 10.Ferreiro E., Oliveira C.R., Pereira C. Involvement of endoplasmic reticulum Ca2+ release through ryanodine and inositol 1, 4, 5-triphosphate receptors in the neurotoxic effects induced by the amyloid-beta peptide. J. Neurosci. Res. 2004;76(6):872–880. doi: 10.1002/jnr.20135. [DOI] [PubMed] [Google Scholar]
- 11.Lopez J.R., Lyckman A., Oddo S., et al. Increased intraneuronal resting [Ca2+] in adult Alzheimer’s disease mice. J. Neurochem. 2008;105(1):262–271. doi: 10.1111/j.1471-4159.2007.05135.x. [DOI] [PubMed] [Google Scholar]
- 12.Mattson M.P. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birks J.S., Grimley Evans J. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst. Rev. 2015;(4):CD001191. doi: 10.1002/14651858.CD001191.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Dysken M.W., Sano M., Asthana S., et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA. 2014;311(1):33–44. doi: 10.1001/jama.2013.282834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murgai A.A., LeDoux M.S. Memantine-induced Myoclonus in a Patient with Alzheimer Disease. Tremor Other Hyperkinet. Mov. (N. Y.) 2015;5:337. doi: 10.7916/D8ZG6RD9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pilotto A., Franceschi M., D’Onofrio G., et al. Effect of a CYP2D6 polymorphism on the efficacy of donepezil in patients with Alzheimer disease. Neurology. 2009;73(10):761–767. doi: 10.1212/WNL.0b013e3181b6bbe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dey A., Bhattacharya R., Mukherjee A., et al. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017;35(2):178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Wei S. Potential therapeutic action of natural products from traditional Chinese medicine on Alzheimer’s disease animal models targeting neurotrophic factors. Fundam. Clin. Pharmacol. 2016;30(6):490–501. doi: 10.1111/fcp.12222. [DOI] [PubMed] [Google Scholar]
- 19.Hielscher-Michael S., Griehl C., Buchholz M., et al. Natural products from Microalgae with potential against Alzheimer’s Disease: Sulfolipids are potent Glutaminyl Cyclase Inhibitors. Mar. Drugs. 2016;14(11):203. doi: 10.3390/md14110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damar U., Gersner R., Johnstone J.T., et al. Huperzine A: A promising anticonvulsant, disease modifying, and memory enhancing treatment option in Alzheimer’s disease. Med. Hypotheses. 2017;99:57–62. doi: 10.1016/j.mehy.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa R., Ohnishi T., Kobayashi H., et al. Long-term effect of galantamine on cognitive function in patients with Alzheimer’s disease versus a simulated disease trajectory: An observational study in the clinical setting. Neuropsychiatr. Dis. Treat. 2017;13:1115–1124. doi: 10.2147/NDT.S133145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang X., Rong C.P., Chen Y.B., et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimer’s disease model mice by upregulating neprilysin expression. Exp. Cell Res. 2015;334(1):136–145. doi: 10.1016/j.yexcr.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Dragicevic N., Smith A., Lin X., et al. Green Tea Epigallocatechin-3-Gallate (EGCG) and other flavonoids reduce Alzheimer’s Amyloid-Induced Mitochondrial Dysfunction. J. Alzheimers Dis. 2011;26(3):507–521. doi: 10.3233/JAD-2011-101629. [DOI] [PubMed] [Google Scholar]
- 24.Li W.Z., Wu W.Y., Huang D.K., et al. Protective effects of astragalosides on dexamethasone and Aβ25-35 induced learning and memory impairments due to decrease amyloid precursor protein expression in 12-month male rats. Food & Chemical Toxicology An International Journal Published for the British Industrial Biological Research Association. 2012;50(6):1883–1890. doi: 10.1016/j.fct.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 25.Su SY, Cheng CY, Tsai TH, et al. Paeonol Protects Memory after Ischemic Stroke via Inhibiting β-Secretase and Apoptosis. . Evidence- based complementary and alternative medicine : eCAM, . 2011;2012(10): 932823. . doi: 10.1155/2012/932823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y.J., Choi D.Y., Sang B.H., et al. Inhibitory effect of ethanol extract of magnolia officinalis on memory impairment and amyloidogenesis in a transgenic mouse model of alzheimer’s disease via regulating β-secretase activity. Phytother. Res. 2012;26(12):1884–1892. doi: 10.1002/ptr.4643. [DOI] [PubMed] [Google Scholar]
- 27.Huang J.F., Lei S., Pei L., et al. Timosaponin-BII inhibits the up-regulation of BACE1 induced by Ferric Chloride in rat retina. BMC Complement. Altern. Med. 2012;12(1):189. doi: 10.1186/1472-6882-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mengoni F., Lichtner M., Battinelli L., et al. In vitro anti-HIV activity of oleanolic acid on infected human mononuclear cells. Planta Med. 2002;68(2):111–114. doi: 10.1055/s-2002-20256. [DOI] [PubMed] [Google Scholar]
- 29.Ma C.M., Wu X.H., Masao H., et al. HCV protease inhibitory, cytotoxic and apoptosis-inducing effects of oleanolic acid derivatives. J. Pharm. Pharm. Sci. 2009;12(3):243–248. doi: 10.18433/j3dw2d. [DOI] [PubMed] [Google Scholar]
- 30.Bednarczyk-Cwynar B., Wachowiak N., Szulc M., et al. Strong and long-lasting antinociceptive and anti-inflammatory conjugate of naturally occurring oleanolic acid and aspirin. Front. Pharmacol. 2016;7:202. doi: 10.3389/fphar.2016.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashyap D., Sharma A., Tuli H.S., et al. Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with promising anti-inflammatory activities. Recent Pat. Inflamm. Allergy Drug Discov. 2016;10(1):21–33. doi: 10.2174/1872213x10666160711143904. [DOI] [PubMed] [Google Scholar]
- 32.Amara S., Zheng M., Tiriveedhi V. Oleanolic Acid inhibits high salt-induced exaggeration of warburg-like metabolism in breast cancer cells. Cell Biochem. Biophys. 2016;74(3):427–434. doi: 10.1007/s12013-016-0736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu D.W., Guo H.Q., Zhou G.B., et al. Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling. Int. J. Clin. Exp. Pathol. 2015;8(11):13864–13870. [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Lin C., Zhang L., et al. Cognitive improvement during treatment for mild alzheimer’s disease with a chinese herbal formula: A randomized controlled trial. PLoS One. 2015;10(6):e0130353. doi: 10.1371/journal.pone.0130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates - The New Coronal Set, Fifth Edition2004 . 2004. [Google Scholar]
- 36.Galoyan A.A., Sarkissian J.S., Chavushyan V.A., et al. Neuroprotection by hypothalamic peptide proline-rich peptide-1 in Abeta25-35 model of Alzheimer’s disease. Alzheimers Dement. 2008;4(5):332–344. doi: 10.1016/j.jalz.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Vorhees C.V., Williams M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray E.G., Whittaker V.P. The isolation of nerve endings from brain: An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- 39.Brookmeyer R., Kawas C.H., Abdallah N., et al. Impact of interventions to reduce Alzheimer’s disease pathology on the prevalence of dementia in the oldest-old. Alzheimers Dement. 2016;12(3):225–232. doi: 10.1016/j.jalz.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiest K.M., Roberts J.I., Maxwell C.J., et al. The prevalence and incidence of dementia due to Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2016;43(Suppl. 1):S51–S82. doi: 10.1017/cjn.2016.36. [DOI] [PubMed] [Google Scholar]
- 41.Kojima G, Liljas A, Iliffe S, et al. Prevalence of frailty in mild to moderate Alzheimer's Disease: A systematic review and meta-analysis. [DOI] [PubMed]
- 42.Armijo E., Gonzalez C., Shahnawaz M., et al. Increased susceptibility to Abeta toxicity in neuronal cultures derived from familial Alzheimer’s disease (PSEN1-A246E) induced pluripotent stem cells. Neurosci. Lett. 2017;639:74–81. doi: 10.1016/j.neulet.2016.12.060. [DOI] [PubMed] [Google Scholar]
- 43.Li K., Wei Q., Liu F.F., et al. Synaptic dysfunction in Alzheimer’s Disease: Abeta, Tau, and epigenetic alterations. Mol. Neurobiol. 2017 doi: 10.1007/s12035-017-0533-3. [DOI] [PubMed] [Google Scholar]
- 44.Schmid S., Jungwirth B., Gehlert V., et al. Intracerebroventricular injection of beta-amyloid in mice is associated with long-term cognitive impairment in the modified hole-board test. Behav. Brain Res. 2017;324:15–20. doi: 10.1016/j.bbr.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Kim HY, Lee DK, Chung BR, et al. Intracerebroventricular injection of amyloid-beta peptides in normal mice to acutely induce Alzheimer-like cognitive deficits. J Vis Exp . 2016 doi: 10.3791/53308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi J.G., Moon M., Kim H.G., et al. Gami-Chunghyuldan ameliorates memory impairment and neurodegeneration induced by intrahippocampal Abeta 1-42 oligomer injection. Neurobiol. Learn. Mem. 2011;96(2):306–314. doi: 10.1016/j.nlm.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Christensen R., Marcussen A.B., Wortwein G., et al. Abeta(1-42) injection causes memory impairment, lowered cortical and serum BDNF levels, and decreased hippocampal 5-HT(2A) levels. Exp. Neurol. 2008;210(1):164–171. doi: 10.1016/j.expneurol.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Chai G.S., Feng Q., Wang Z.H., et al. Downregulating ANP32A rescues synapse and memory loss via chromatin remodeling in Alzheimer model. Mol. Neurodegener. 2017;12(1):34. doi: 10.1186/s13024-017-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trettenbrein P.C. The demise of the synapse as the locus of memory: a looming paradigm shift? Front. Syst. Neurosci. 2016;10:88. doi: 10.3389/fnsys.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y., Liu Y., Zhu C., et al. Minocycline enhances hippocampal memory, neuroplasticity and synapse-associated proteins in aged C57 BL/6 mice. Neurobiol. Learn. Mem. 2015;121:20–29. doi: 10.1016/j.nlm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Xie J., Wang H., Lin T., et al. Microglia-Synapse Pathways: Promising therapeutic strategy for alzheimer’s disease. BioMed Res. Int. 2017;2017:2986460. doi: 10.1155/2017/2986460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sell G.L., Schaffer T.B., Margolis S.S. Reducing expression of synapse-restricting protein Ephexin5 ameliorates Alzheimer’s-like impairment in mice. J. Clin. Invest. 2017;127(5):1646–1650. doi: 10.1172/JCI85504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nimmrich V., Ebert U. Is alzheimer’s disease a result of presynaptic failure? synaptic dysfunctions induced by oligomeric beta-amyloid. Rev. Neurosci. 2009;20(1):1–12. doi: 10.1515/revneuro.2009.20.1.1. [DOI] [PubMed] [Google Scholar]
- 54.Adlard P.A., Bica L., White A.R., et al. Metal ionophore treatment restores dendritic spine density and synaptic protein levels in a mouse model of Alzheimer’s disease. PLoS One. 2011;6(3):e17669. doi: 10.1371/journal.pone.0017669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Potter P.E., Rauschkolb P.K., Pandya Y., et al. Pre- and post-synaptic cortical cholinergic deficits are proportional to amyloid plaque presence and density at preclinical stages of Alzheimer’s disease. Acta Neuropathol. 2011;122(1):49–60. doi: 10.1007/s00401-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scheff S.W., Price D.A. Alzheimer’s disease-related alterations in synaptic density: neocortex and hippocampus. J. Alzheimers Dis. 2006;9(3) Suppl.:101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- 57.Sze C.I., Bi H., Kleinschmidt-DeMasters B.K., et al. Selective regional loss of exocytotic presynaptic vesicle proteins in Alzheimer’s disease brains. J. Neurol. Sci. 2000;175(2):81–90. doi: 10.1016/s0022-510x(00)00285-9. [DOI] [PubMed] [Google Scholar]
- 58.Tiwari S.S., d’Orange M., Troakes C., et al. Evidence that the presynaptic vesicle protein CSPalpha is a key player in synaptic degeneration and protection in Alzheimer’s disease. Mol. Brain. 2015;8:6. doi: 10.1186/s13041-015-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koffie R.M., Hyman B.T., Spires-Jones T.L. Alzheimer’s disease: Synapses gone cold. Mol. Neurodegener. 2011;6(1):63. doi: 10.1186/1750-1326-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15(3):89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Popugaeva E., Bezprozvanny I. Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front. Mol. Neurosci. 2013;6:29. doi: 10.3389/fnmol.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeiger W., Vetrivel K.S., Buggia-Prevot V., et al. Ca2+ influx through store-operated Ca2+ channels reduces Alzheimer disease beta-amyloid peptide secretion. J. Biol. Chem. 2013;288(37):26955–26966. doi: 10.1074/jbc.M113.473355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giannakopoulos P., Kovari E., Gold G., et al. Pathological substrates of cognitive decline in Alzheimer’s disease. Front Neurol. Neurosci. 2009;24:20–29. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- 64.de Bartolomeis A., Latte G., Tomasetti C., et al. Glutamatergic postsynaptic density protein dysfunctions in synaptic plasticity and dendritic spines morphology: Relevance to schizophrenia and other behavioral disorders pathophysiology, and implications for novel therapeutic approaches. Mol. Neurobiol. 2014;49(1):484–511. doi: 10.1007/s12035-013-8534-3. [DOI] [PubMed] [Google Scholar]
- 65.Takei Y., Kikkawa Y.S., Atapour N., et al. Defects in synaptic plasticity, reduced NMDA-receptor transport, and instability of postsynaptic density proteins in mice lacking microtubule-associated protein 1A. J. Neurosci. 2015;35(47):15539–15554. doi: 10.1523/JNEUROSCI.2671-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gong Y., Lippa C.F. Review: Disruption of the postsynaptic density in Alzheimer’s disease and other neurodegenerative dementias. Am. J. Alzheimers Dis. Other Demen. 2010;25(7):547–555. doi: 10.1177/1533317510382893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou J., Jones D.R., Duong D.M., et al. Proteomic analysis of postsynaptic density in Alzheimer’s disease. Clin. Chim. Acta. 2013;420:62–68. doi: 10.1016/j.cca.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li F., Wu X., Li J., et al. Ginsenoside Rg1 ameliorates hippocampal long-term potentiation and memory in an Alzheimer’s disease model. Mol. Med. Rep. 2016;13(6):4904–4910. doi: 10.3892/mmr.2016.5103. [DOI] [PubMed] [Google Scholar]
- 69.Qi X., Zhang K., Xu T., et al. Sex differences in long-term potentiation at Temporoammonic-CA1 synapses: Potential implications for memory consolidation. PLoS One. 2016;11(11):e0165891. doi: 10.1371/journal.pone.0165891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andrade-Talavera Y., Benito I., Casanas J.J., et al. Rapamycin restores BDNF-LTP and the persistence of long-term memory in a model of Down’s syndrome. Neurobiol. Dis. 2015;82:516–525. doi: 10.1016/j.nbd.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Yang Q., Zhu G., Liu D., et al. Extrasynaptic NMDA receptor dependent long-term potentiation of hippocampal CA1 pyramidal neurons. Sci. Rep. 2017;7(1):3045. doi: 10.1038/s41598-017-03287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huh S., Baek S.J., Lee K.H., et al. The reemergence of long-term potentiation in aged Alzheimer’s disease mouse model. Sci. Rep. 2016;6:29152. doi: 10.1038/srep29152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu Z., Guo Z., Gearing M., et al. Tonic inhibition in dentate gyrus impairs long-term potentiation and memory in an Alzheimer’s [corrected] disease model. Nat. Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang S.H., Wang J., Sui W.H., et al. BDNF-dependent recycling facilitates TrkB translocation to postsynaptic density during LTP via a Rab11-dependent pathway. J. Neurosci. 2013;33(21):9214–9230. doi: 10.1523/JNEUROSCI.3256-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panja D., Kenney J.W., D’Andrea L., et al. Two-stage translational control of dentate gyrus LTP consolidation is mediated by sustained BDNF-TrkB signaling to MNK. Cell Reports. 2014;9(4):1430–1445. doi: 10.1016/j.celrep.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 76.Motaghinejad M., Motevalian M., Babalouei F., et al. Possible involvement of CREB/BDNF signaling pathway in neuroprotective effects of topiramate against methylphenidate induced apoptosis, oxidative stress and inflammation in isolated hippocampus of rats: Molecular, biochemical and histological evidences. Brain Res. Bull. 2017;132:82–98. doi: 10.1016/j.brainresbull.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Alzoubi K.H., Alkadhi K.A. Chronic Nicotine Treatment Reverses Hypothyroidism-Induced Impairment of L-LTP Induction Phase: Critical Role of CREB. Mol. Neurobiol. 2014;49(3):1245–1255. doi: 10.1007/s12035-013-8594-4. [DOI] [PubMed] [Google Scholar]
- 78.Bridi M.S., Hawk J.D., Chatterjee S., et al. Pharmacological activators of the NR4A nuclear receptors enhance LTP in a CREB/CBP-dependent manner. Neuropsychopharmacology. 2017;42(6):1243–1253. doi: 10.1038/npp.2016.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corbett G.T., Roy A., Pahan K. Sodium phenylbutyrate enhances astrocytic neurotrophin synthesis via protein kinase C (PKC)-mediated activation of cAMP-response element-binding protein (CREB): Implications for Alzheimer disease therapy. J. Biol. Chem. 2013;288(12):8299–8312. doi: 10.1074/jbc.M112.426536. [DOI] [PMC free article] [PubMed] [Google Scholar]