Abstract
Since 1929, several researchers have conducted studies in relation to the nucleoside of adenosine (1) mainly distribution identifying, characterizing their biological importance and synthetic chemistry to which this type of molecule has been subjected to obtain multiple of its derivatives. The receptors that interact with adenosine and its derivatives, called purinergic receptors, are classified as A1, A2A, A2B and A3. In the presence of agonists and antagonists, these receptors are involved in various physiological processes and diseases.
This review describes and compares some of the synthetic methods that have been developed over the last 30 years for obtaining some adenosine derivatives, classified according to substitution processes, complexation, mating and conjugation. Finally, we mention that although the concentrations of these nucleosides are low in normal tissues, they can increase rapidly in pathophysiological conditions such as hypoxia, ischemia, inflammation, trauma and cancer. In particular, the evaluation of adenosine derivatives as adjunctive therapy promises to have a significant impact on the treatment of certain cancers, although the transfer of these results to clinical practice requires a deeper understanding of how adenosine regulates the process of tumorigenesis.
Keywords: Adenosine, adenosine receptors, adenosine derivatives, substitution, complexing, conjugation, glioblastoma
1. INTRODUCTION
The physiological functions of adenosine (1) are mediated by its interaction with four subtypes of G protein-coupled receptors, named A1, A2A, A2B and A3 [1]. As suggested by Cosyn, adenosine receptors are not only the main targets of caffeine, the drug most consumed worldwide every day, but they could also serve as promising therapeutic targets for a variety of conditions including cardiovascular diseases, inflammation, neurodegenerative diseases and tumors [2].
Natural adenosine (1) and its derivatives are involved in the regulation of many aspects of cellular metabolism. However, to date only adenosine in its natural form has been approved for therapeutic use in humans, this in spite of the fact that a great effort has been directed toward the discovery of potent and selective adenosine agonists [3]. In particular, high concentrations of adenosine have been reported in tumor tissues, implying a fundamental role of this nucleoside in tumor growth [1]. The anti-tumour effects of the adenosine are mediated through differents mechanisms, either dependents or independents of its receptors, and encompass the detention of the cellular cycle, inhibition of the proliferation or the induction of apoptosis [4].
The purpose of this review is to provide a summary of different concepts and applications of adenosine (1) and its derivatives. Also, we would like to compare the most common synthetic methodologies used for the synthesis of adenosine derivatives in the last 30 years, including those involving nucleophilic substitution reactions, complexation, conjugation and coupling. Further, this research describes the most important effects of adenosine (1) and its analogs in tissues and peripheral organs as well as their impact on the treatment of certain cancers [5] and pathologies of the central nervous system (CNS).
2. HISTORY, DEFINITION AND TYPES OF ADENOSINE RECEPTORS
2.1. Adenosine: Some Concepts and Biological Functions Discovered in the Course of Time
From a historical standpoint, the era of adenosine (1) begins in 1929 with the works of Drury and Gyorgyi [6]. These authors first demonstrated the effects of this nucleoside in the cardiovascular system (as a vasodilator, as an inducer of bradycardia and as a negative inotropic agent), as well as in the gastrointestinal tract [7, 8]. A decade later, Lindner and Rigler demonstrated that adenosine was a potent dilator of coronary vessels, postulating this nucleoside as a major regulator of blood flow. They also showed that this compound played a major role in regulating the cerebrospinal fluid [9, 10]. Subsequently, in 1954, Feldberg and Sherwood demonstrated the sedative effects of adenosine following its intracerebro-ventricular injection [6].
In 1963, Berne and Gerlach identified the physiological role of adenosine (1) as a mediator of coronary vasodilation in response to hypoxia or ischemia of the myocardium. The same year, Born and Cross, reported the inhibitory effects of adenosine (1) on platelet aggregation [11, 12]. Later, in 1970, Sattin and Rall showed that adenosine induces an increase in the concentration of cyclic AMP (cAMP) in the brain of mammals [3, 12]. Seven years later, Londos and Wolff demostrated that the negative chronotropic and dromotropic effects of adenosine were mediated by direct activation of specific receptors [14]. In 1978, Burnstock formulated the existence of two classes of purinergic receptors, the P1 (adenosine) and P2(ATP and ADP) receptors based on their sensitivity to nucleosides and nucleotides, respectively [10, 15]. In the 1980’s the groups of Belardinelli and Snyder focused on the role of adenosine (1) in cardiac electrophysiology and its modulatory actions on neurotransmission [6, 16]. A year later (1982), Spielman and Thomson, proposed adenosine as an intrinsic regulator of renal blood flow and glomerular filtration rate [16, 17].
A few years later, the FDA (Food and Drug Administration) approved the use of adenosine for the treatment of supraventricular tachycardia. Then, in 1991, Cohen and Downey reported that adenosine played an important role in preconditioned ischemia [18]. Subsequently, Adenoscan® (the pharmacological formulation of adenosine (1)) was marketed as a component of the stress test “myocardial perfusion imaging” aimed to visualize and evaluate the heart function [6, 9]. In 1999, adenosine derivatives were synthesized, that were assessed according to their affinity with the A3 receptor subtype present in rat cerebral cortex [19].
At the beginning of the XXI century, some researchers showed that the removal of extracellular adenosine (1) was a key mechanism in reducing extracellular levels of this nucleoside, modulating its biological actions in vascular cells increasing the levels of D-glucose [20]. Moreover, other authors reported studies about the importance of adenosine in the regulation and modulation of sleep [21, 22]. In 2005, has been extended to analogs of adenosine-3',5’-cyclic monophosphate (cAMP) for the treatment of breast cancer in mice [13, 23]. In this same period, the synthesis of N6-adenosine and N6-2’-deoxyadenosine derivatives was developed with aliphatic and aromatic amines under SNAr reactions in one-step [24]. Then, other researchers showed that adenosine played an important role in inducing pulmonary angiogenesis [25].
Afterward, in 2012, the total synthesis of stable cyclic derivatives of adenosine 5’-diphosphateribose was presented, which acted as chemical and biological tools involved in the release of intracellular Ca+2 [26].
In the latter period, a series of adenosine derivatives have been tested for the ability to inhibit forskolin-stimulated adenylyl cyclase [27] and even through an in silico receptor-driven approach the molecular bases of recognition of human purinergic receptors of type A1 and A3 [28].
2.2. Adenosine Concept and Characteristics of their Receptors
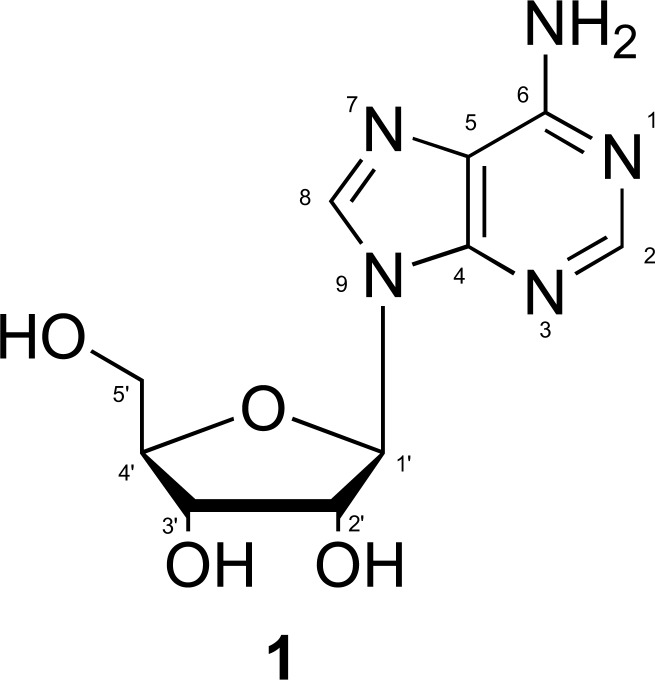
Adenosine (1) is an endogenous nucleoside consisting of an adenine molecule linked to a pentose (ribofuranose) via a β-N9-glycosidic bond (Fig. 1). Adenosine’s chemical name is 6-amino-9-β-D-ribofuranosyl-9-H-adenine, and its chemical formula is summarized as C10H13N5O4, with a molecular weight is 267,24 u.m.a [29-31].
Fig. (1).
Structure of adenosine [31].
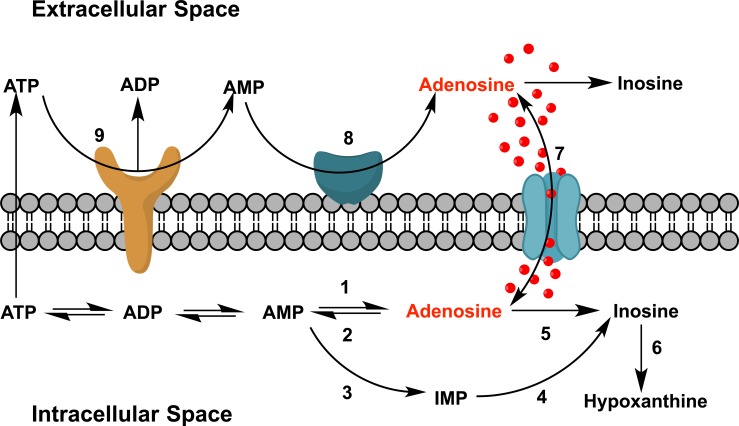
The biochemical process to obtain adenosine (1) within the cells is explained in Fig. (2). Basically, the adenosine is formed as a result of a hydrolysis of AMP by action of a type I cytosolic 5-nucleotidase (reaction 2). Once the adenosine is formed, this can be phosphorylated by an adenosine kinase (reaction 1) or converted to inosine by adenosine deaminase action (reaction 5). On the other hand, the levels of adenosine in the extracellular compartment are affected by two mechanisms mainly, it can be released from the cell inside by conveyors balancers (reaction 7) or from nucleotide extracellular adenine, mainly ATP by the action of the ecto 5'- nucleotidase (reaction 8), even will depend on the rate of hydrolysis of ATP which is released. Other enzymes involved in the metabolism of this purine are AMP deaminase (reaction 3), 5'-nucleotidase type II (reaction 4), purine nucleoside phosphorylase (reaction 6) and apyrase (reaction 9) [32-34].
Fig. (2).
Figure Schematic representation of metabolic routes adenosine levels (Source: Parkinson et al. [33]).
Functionally, adenosine (1) is involved in various physiological activities and its effects are often contrary to caffeine [14]. The nucleoside serves as the natural ligand for G protein-coupled P1 receptors, also known as Adenosine Receptors, with variable distribution in physiological systems. Based on their pharmacological, biochemical and molecular properties, these receptors are classified into four subtypes, namely A1, A2A, A2B and A3. Of these, A1, A2A and A2B have highest inter-species homology. On the other hand, the A3 receptor shows considerable differences between species [31, 34, 35].
First, the A1 is widely expressed throughout the body, with the highest expression observed in the brain, spinal cord, atria and adipose tissue [10, 36]. Following interaction with this receptor, adenosine (1) reduces the heart rate, glomerular filtration rate and renin release in the kidney. In addition, it induces bronchoconstriction and inhibits lipolysis [36]. Moreover, the activation of this receptor reduces cAMP production through an antagonistic effect on adenylate cyclase II. Furthermore, it has been found that other cardiac receptors can be influenced by adenosine [30]. Recent studies have shown that the antagonistic effects on the A1 receptor could play a potential role in the treatment of asthma [37] and even have an anti-tumor effect against glioblastomas [38].
A2 receptors are more abundant in nerve cells, mast cells, smooth muscle of the airways and circulating leukocytes [31]. These receptos have also been extensively studied in platelets [11] and shown to be expressied under conditions of oxidative stress [39-41].
The A2 receptors in turn can be subdivided into A2A and A2B receptors based on their affinity for adenosine [31]. While A2A receptors are expressed throughout the brain (CNS), they are particularly concentrated in the basal ganglia. Besides the brain, they are also expressed in vascular smooth muscle, endothelium, neutrophils, platelets, mast cells and T cells [42]. On the contrary, A2B receptors are highly expressed in vascular areas, retina and brain, with low levels of expression in platelets. Recent studies have shown that this receptor subtype has a high expression in a state of stress, inflammation and hypoxia and on those who have a high-fat diet [43].
In 1992, Zhous discovered the type A3 receptors as G protein-coupled receptors with high similarity (58%) to A1and A2 receptors but with different pharmacological properties [44]. The A3 receptors are expressed in testes, lung, kidney, placenta, heart, brain, spleen, liver, uterus, bladder, jejunum, proximal colon and rat eye, sheep and humans. However, there are marked differences in expression levels within and between species [8]. On the other hand, this type of adenosine receptor is involved in anti-inflammatory effects [42]. Furthermore, several studies, reported that the A3 adenosine receptors were responsible for cardioprotection in a variety of species and models [36].
In general, A1 and A2A receptors have a greater affinity with the adenosine as ligand, while the A2B and A3 receptors have a lower affinity with this nucleoside. Nevertheless, the role of these two last receptors would be important in physiological process or on pathological situations stress where the concentration of adenosine greatly increase [34].
The distribution of adenosine receptors in different tissues is shown in Table 1. These results were based on detection of the protein by radioligand binding or detection of its mRNA by RT-PCR [7].
Table 1.
Distribution of adenosine receptors in different tissues.
| Adenosine Receptors Distribution in Tissues | ||||
|---|---|---|---|---|
| Level of Expression | A1 | A2A | A2B | A3 |
| High up | Brain (cortex, hippocampus, cerebellum, sheet spine, eyes, atria and adrenal gland (adrenal). | Thymus, leukocytes (lymphocytes and granulocytes), platelets, spleen, GABAergic and olfactory bulb. | Cecum, colon, bladder. |
Mast cells, testis. (Rat) |
| Intermediate | Other brain regions, skeletal muscle, liver, kidney, adipose tissue, salivary glands, esophagus, colon, testis. | Heart, lung, blood vessels, peripheral nerves. | Lung, blood vessels, eyes, mast cells. | Cerebellum, hippocampus, microglial cells, lung, spleen, pineal. |
| Low | Lung (but probably high in bronchi), pancreas. | Other brain regions. | Adipose tissue, adrenal gland, brain, kidney, liver, ovary, pituitary gland. | Thyroid, other cerebral regions, adrenal gland, spleen (human), liver, kidney, heart, intestine, testes (human). |
(Source: Modified from Fredholm [8]).
3. Methods of synthesis of Adenosine Derivatives, interaction with specific receptors or targets and physiological actions
In 1930, Honey and colleagues first described properties of adenosine (1) to block conduction in the atrioventricular node in the human heart, a finding that inaugurated research on adenosine and its derivatives as cardiovascular drugs [9]. Consequently, some of the methods and/or techniques used for the synthesis of multiple adenosine derivatives are explained in the following sections [14-86].
3.1. Adenosine Derivatives by Substitution Reactions
Some researches, as Cristalli et al., reported the formation of a series of derivatives of 2-alkynyladenosine and 2-alkynyladenosine-5'-N-ethyluronamide. The first series was performed by palladium-catalyzed cross-coupling reaction; the second reaction was carried out by a similar
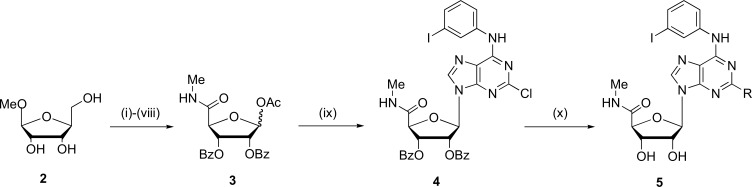
cross-coupling reaction between the terminal alkynes and a new nucleoside N-ethyl-1'-deoxy-1'-(6-amino-2-iodo -9H-purine-9-yl)-β-D-ribofuranuronamide [45]. The 5'-N-ethyluronamide derivatives show higher affinity for adenosine receptor A2 whereas the A1 receptor affinity was lower. A similar pattern was observed in the adenylate cyclase assays and platelet aggregation studies. Similarly, has been proposed a synthesis of 2-substitution of N6-benzyladenosine-5'-uronamides analogs as agonists of the A3 receptor (Fig. 3). This reaction was proposed in 10 stages using methyl-β-D-ribofuranoside (2) as precursor wich was subjected to this nucleophilic substitution reaction [46].
Fig. (3).
General synthesis of 2-substitution of N6-benzyladenosine- 5’-uronamides (5). Reagents and conditions: (i) TBDPSiCl, DMAP, DMF, r.t.; (ii) Bz2O, Py; n-Bu4NF, THF; (iv) RuO2, NaIO4, CHCl3:CH3CN:H2O (2:2:3); (v) EDAC, DMAP, MeOH; (vi) MeNH2, THF, 75ºC; (vii) BzCl, Py–CH2Cl2; (viii) Ac2O, H2SO4, AcOH; (ix) TMSOTf, (1,2)-dichloroethane; reflux.9-silylated adenine derivative; (x) NH3/MeOH, nucleophile. (Source: Modified from Kim et al., [46]).
Van Schaick et al., proposed that adenosine derivatives apply a series of 8-alkylamino of N6- cyclopentyladenosine, wich were proved to be partial agonists with the A1 receptor type cardiovascular through in vivo tests [47]. The synthesis of such series was conducted through three routes identified and described by Roelen et al. [48], which are broadly summarized as follows:
Introduction of an alkylamino on N6-derivative-8-halideadenosine.
The second synthetic route occurs through the introduction from 6-alkylamino substituent on 8-aminoalkyl-6-ribose chloropurine derivative, as key intermediates.
The last synthesis route was a selective introduction of the alkylamino substituent at 6, 8-dichloropurine riboside, producing a key intermediate radical.
Adenosine derivatives synthesized by these three routes were described and they have been grouped in Table 2.
Table 2.
C8-amino-N6-disubstituted adenosine derivatives.

|
Route | Derivative | R1 (N6) | R2 (C8) | |||
|---|---|---|---|---|---|---|---|
| I | 6 | Cyclopentyl | -CH3 | ||||
| 7 | Cyclopentyl | -CH2-CH3 | |||||
| 8 | Cyclopentyl | - (CH2)2-CH3 | |||||
| 9 | Cyclopentyl | - (CH2)3-CH3 | |||||
| 10 | Cyclopentyl | Cyclopentyl | |||||
| II | 11 | -CH3 | Cyclopentyl | ||||
| 12 | -CH2-CH3 | Cyclopentyl | |||||
| 13 | - (CH2)2-CH3 | Cyclopentyl | |||||
| 14 | - (CH2)3-CH3 | Cyclopentyl | |||||
| III | 15 | -H | -CH3 | ||||
| 16 | -H | -CH2-CH3 | |||||
| 17 | -H | - (CH2)2-CH3 | |||||
| 18 | -H | - (CH2)3-CH3 | |||||
| 19 | -H | cyclopentyl | |||||
(Source: Modified from Roelen et al. [48]).
In addition, all compounds were tested in the radioligand binding to determine their affinities for adenosine receptors types A1 and A2A in rat cerebral cortex and striatal cells, respectively [48].
Such methods applied for the synthesis of derived compounds showed advantages involving partial agonist function proving to be more potent than -C8monosubstituted derivatives series nucleoside with an additional gain in selectivity of the A1 receptor. However, while these synthetic routes give easy access to the preparation of compounds, the yields were low despite being highly selective with A1 receptor (6-10 compounds). The others showed a higher or moderate yield, but with less interaction power with that A1 receptor (11-14 compounds) [48].
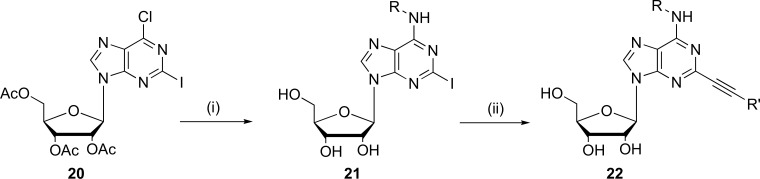
On the other hand, Volpini et al. [3] give reports on their mechanisms of synthesis for to obtain N6-adenosine derivatives-alkyl-2-alkynyl, showing potential agonist action and selectivity on A3 adenosine receptors. The synthesis was performed for two series of derivatives compounds of adenosine, which were 2-alkyladenosine and 2-alkynyl-N6-alkyladenosine (22), which was conducted from 9-(2,3,5-tri-O-acetyl-β-D-ribofuranolsyl)-6-chloro-2-iodopurine (20). In this synthesis, compounds were obtained by selective substitution of the chlorine atom in 6-position while considering protection of the sugar as it is shown in Fig. (4).
Fig. (4).
General synthesis of N6-alkyl-2-alkynyl adenosine derivatives (22). Reagents and conditions: (i) amine, (ii) R-C≡CH, CuI, [(C6H5)3P]2PdCl2, Et3N (Source: Modified from Volpini et al., [3]).
Therefore, for the formation of 5'-N-ethylcarboxami-doadenosine derivatives the 10 stages were required to obtain 2-alkynyl-(hexynyl-, phenylethinyl- (R,S)-phenylhy-droxypropynyl-) adenosine, having one methyl, ethyl or isopropyl on N6 position [3]. These showed a greater affinity for the A3 receptor and even when they were substituted by methyl and they resulted in a significant improvement over the selectivity when interacting with the receptor. Besides, according to the yields reported would be the higher the molar mass or larger the substituent, the lower the performance. In addition, those that experienced ethyl or isopropyl substitution have high affinity for A3 but presented a decrease in selectivity for A1/A3 and A2A/A3. In the proposed synthesis routes, tri-substituted compounds were also formed, which showed a reduced affinity for both subtypes A1 and A3 receptors and as well as less A2 receptor activity. Such pharmacological tests were carried out in rat ovary cells transfected with human adenosine receptors [3].
Further, researchers reported the synthesis of a series of 2-substitution-N6-methyladenosine derivatives in which affinity and selectivity were evaluated by the type A3 adenosine receptors in humans [49]. In the same way, Volpini’s studies unveiled the synthetic methodology based on 9-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-6-chloro-2-iodopurine (20); -alkynylate and 2-triazole-substituted as the starting precursors. The (1.3)-cycloaddition underwent a water/butanol as mixture solvents and a simple isolation to obtain the desired compounds that were precipitated by this reaction medium [3] (Fig. 5). Meanwhile, described the production of more 4-substituted 2-(1,2,3)-triazole-1-yl-N6-methyl adenosine analogs (26) via [3+2] Huisgen cycloaddition. Some of these derivatives showed partial agonist actions and other antagonists (those that remained intact in the molecular structure of the ribose). Then, the derivatives were tested in Chinese cells hamster ovary (CHO) [49].
Fig. (5).
Synthesis of 4-substituted-2-(1,2,3)-triazole-1-yl-N6-methyl adenosine analogues (26). Reagents and conditions: (i) CH3NH3+Cl–, Et3N, EtOH; (ii) NH3 (7N) /MeOH; (iii) CuI, (Ph3P)2PdCl2, alkyne, Et3N, DMF; (iv) CuSO4⋅5H2O, sodium ascorbate, L-proline, Na2CO3, H2O/t-BuOH (1:1), 60 ºC; (v) CuSO4⋅5H2O, sodium ascorbate, alkyne, H2O/t-BuOH (3:1), r.t. (Source: Modified from Cosyn et al., [49]).
Moreover, there is a report regarding the synthesis substitution at 2-position of adenosine [50]. The processes were carried out according to the general method Ueeda et al., and it was of a nucleophilic substitution by an appropriate alkoxy ion (as 1,2-dimethoxyethane) to replace 2-chloroadenosine, which protected the ribose [51]. Thus, the products formed include derivatives of 2-alkyloxy ethers, derivatives of 2-alkylaryl, mainly including ethers, an amino derivative and a thioether, which were compared with two similar 5',8-cycle and standardized four references. In general, this modified nucleoside, which first occurs with ether groups, demonstrated the affinity for A3 adenosine receptor and these compounds were tested on CHO cells, as well as Cosyn's studies [50].
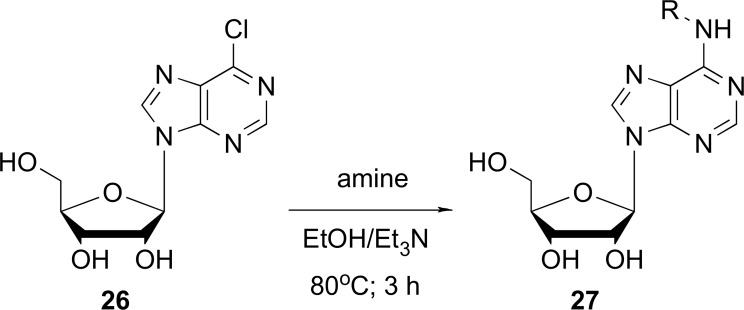
Also, there is a report of a synthesis of several series of adenosine analogs, resulting from condensation reactions between the precursor 6-chloropurine riboside (26) with a respective amine, undergoing nucleophilic substitution at the N6-position of nucleoside in absolute ethanol and triethylamine as the base acceptor [52]. These compounds were isolated and then purified by crystallization in order to be elucidated through mass spectrometric and nuclear magnetic resonance spectroscopy. The Fig. (6) shows a series of compounds that were obtained with linear chain substitutions and others with branched substituents with one or more hydroxyl groups, amino groups, unsaturated chains or cyclic substituents with aromatic rings. Such analogs were tested in cell lines of bladder carcinoma T24 and J82 types as well as colon and breast, showing anti-proliferative activities in all cases [52].
Fig. (6).
General synthesis of N6-alkyladenosine derivatives (27) (Source: Modified from Ottria et al., [52]).
Others compounds of type N6-substituted adenosines based on a regioselective 1-N-alkylation through Dimroth rearrangement were synthesized for the formation of biologically important of N6-benzyladenosine, N6-isopentenyl-adenosine, and their synthetic analogs [79, 83].
From a bioinformatic point of view, compounds 6-substituted adenosine-5'-N-ethyluronamide analogs were studied through molecular coupling (docking) with the A3 adenosine receptor [71]. Basically, these experiments of theoretical calculations filtered and selected those molecular structures that contained different substituents at the 6-position of purine ring. The molecules that presented the best interactions and contacts with the amino acid residues in the active site of receptor were the candidates to be synthesized chemically [71]. Under a similar context, other researches colleted information about of modeling-based and guided strategies that allowed the elucidation the binding of a pool of different agonists and antagonist through a extensive Site-Directed Mutagenesis (SDM) studies, in order to discuss about of the structure-activity relationships (SAR) of nucleosides binding were probed in great detail on the A3 adenosine receptor [82].
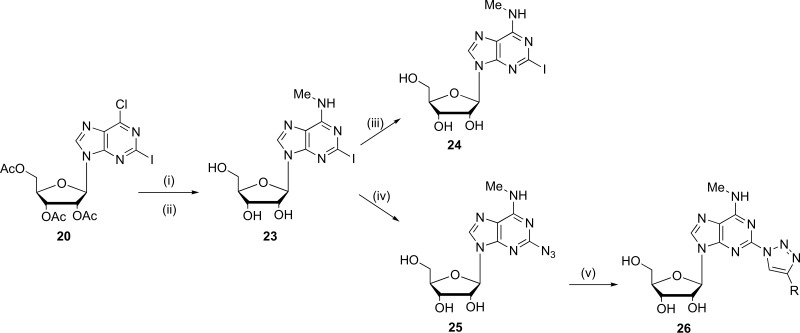
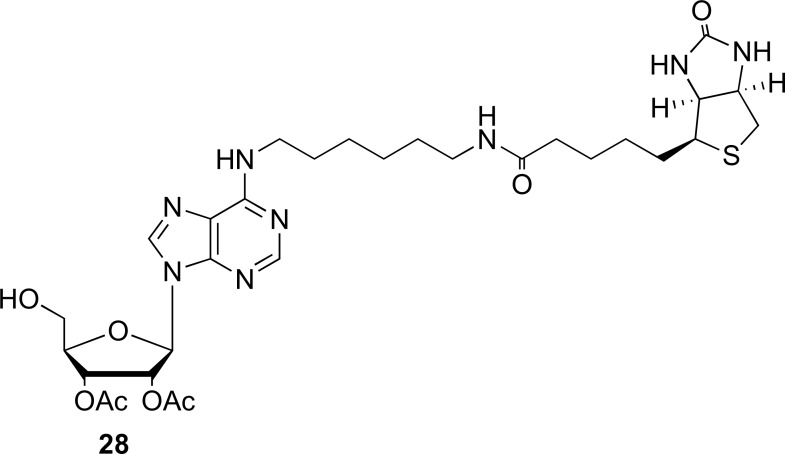
On the other hand, the synthesis of a novel N6-biotinylated-8-azido-adenosine probe (AdoR probe) (28) has being described by Mahajan et al. during the year 2015. This compound was synthesized starting from commercially available inosine, which was subjected to previous modifications in the zone of ribose. Subsequently, the substitution reactions on 6-position with mono-N-Boc-1,6-diaminohexane at -10°C gave the product. Immediately, through a reaction with CsN3 and (CH3)3SiN3 in dioxane under reflux generated the 8-azido group in the compound [74]. The chemical structure of the synthesized probe (28) is shown in Fig. (7).
Fig. (7).
Chemical structure of AdoR probe (Source: Obtained from Mahajan et al., [57]).
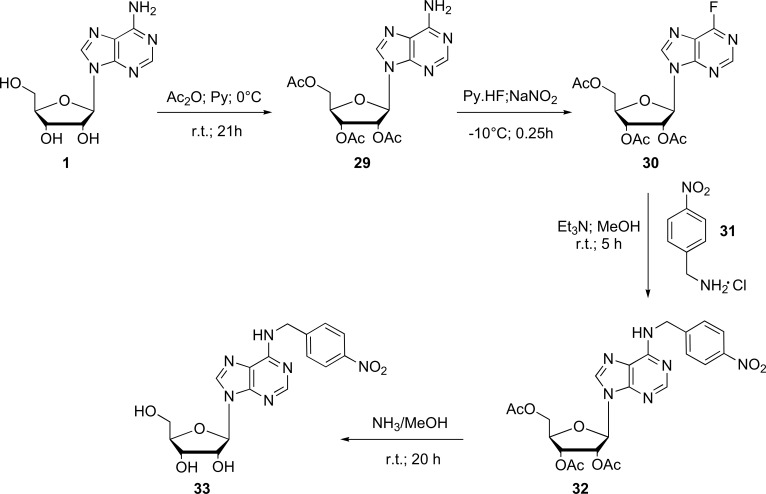
In the same period, the synthesis of novel nucleoside analogues compounds were published, using guanosine as precursor [75]. First, halogen atom was incorporated at the 2-position of this base purine. Subsequently, protection of the 3’-OH and 5’-OH groups and oxidation of the 2’- OH group were performed to the corresponding carbonyl group using oxalyl chloride as reagent under the conditions ofSwern method [76]. After, through Wittig reaction conditions the conversion of the carbonyl group to the methylene function on the 2’-keto derivatives were achieved using triphenylphosphonium bromide and butyllithium in this reaction. The final compounds were prepared by means of nonaqueous diazotization, which were subjected to in vitro antileukemic and antiviral activity upon a new L1210 cell line. It showed potent antileukemic activity because these compounds inhibited the M1 subunit of the ribonucleotide reductase enzyme [75].
Finally, in this context we can mention the synthesis of purine nucleoside and 7-deazapurine analogues of N6-(4-nitrobenzyl) adenosine (33) [78]. These compounds were prepared by two procedures. The first step involved an alkylation of the 7-deazaadenosine antibiotics at N3 position with a benzyl bromide followed by a base-promoted Dimroth rearrangement [79]. For the second step, diazotization–fluorodediazoniation followed by SNAr displacement of fluoride with a benzyl amine was used (Fig. 8). These compounds were tested on a pool of cell lines to assess inhibition of cell proliferation and nucleoside transport recombinant hENT1 produced in the Xenopus oocyte heterologous expression system. However, none of them exhibited inhibitory activity against such systems [78].
Fig. (8).
General synthesis of N6-(4-nitrobenzyl) adenosine (33) (Source: Modified from Rayala et al., [60]).
3.2. Adenosine Derivatives by Complexation Reactions
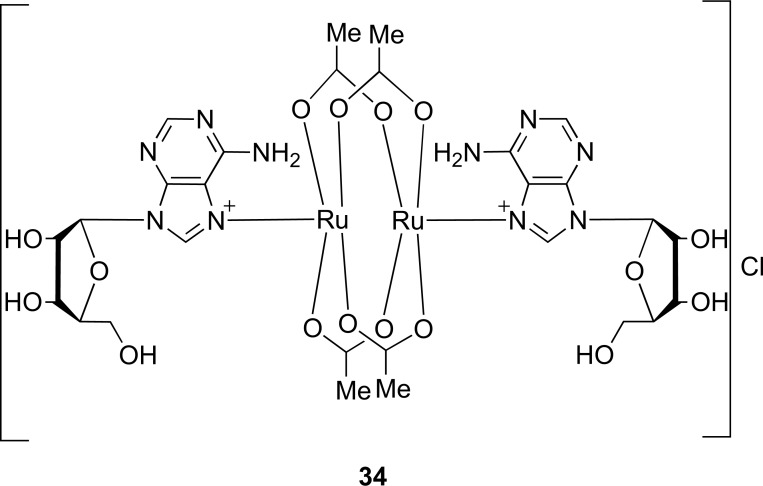
Another method that escaped from the nucleophilic substitution is one in which commercial adenosine interacts with a transition metal compound to produce a complex compound. Rather, adenosine is subjected to complexation reaction with tetraaceticdiruthenium chloride (II, III) ([Ru2(CH3CO2)4Cl]) in aqueous solution to form the derivative [Ru2(CH3CO2)4 (C10H13N5O4)2]Cl (34) [53]. In this case, the derivative of the reaction resulted from a high yield, elucidated by spectroscopic techniques (H1-NMR, UV visible and atomic absorption) and also tested in E. coli as bactericides. This reaction showed their limitations, which were the carcinogenic properties of ruthenium metal present in the complex compound (Fig. 9) [53].
Fig. (9).
Chemical structure of adenosine derivative of tetraacetatodiruthenium (II, III) chloride (Source: Gangopadhyay et al. [61]).
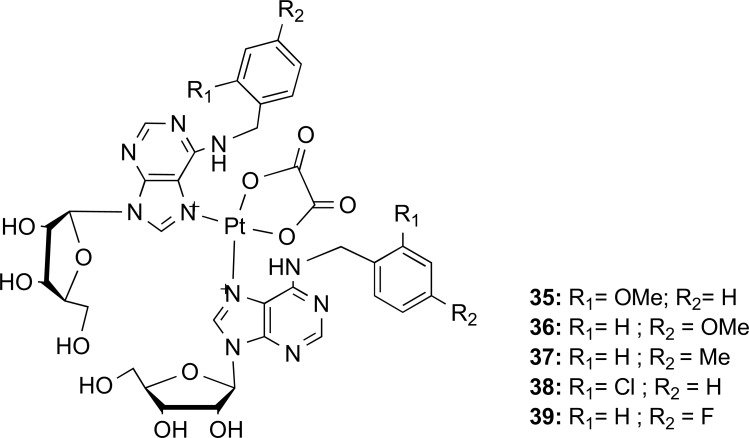
Similarly, to perform a general process for producing an appropriate interaction of a precursor as a purine base, an excess of amine in an excess of triethylamine yields compounds derived from N6-(substituted-benzyl) adenosine and 2-chloro-N6-(substituted-benzyl) adenosine, which act as ligands N-donor within the structures of trans - platinum (II) and the respective result from the complexation reaction [54]. Such complexes are elucidated on an elemental analysis (Pt-NMR, MS) and then examined on two human cancer cell lines: osteosarcoma (HOS) and breast adenocarcinoma (BCF-7). However, such complexes, although not being cytotoxic, present inactivity against tumor cell lines when applied to both [54]. Although the following year Štarha et al. performed a complex synthesis of adenosine derivatives again using the reaction of potassium bis-(oxalato)platinate (II) with the corresponding N6-benzyladenosine derivative (nL) which provided the [Pt(ox)(nL)2].1,5 H2O oxalato (ox) complexes (35-39) (Fig. 10) under a synthetic procedure “one-step”. These complex compounds were screened for their cytotoxicity in vitro against two human cancer cell lines (HOS osteosarcoma line cell and MCF-7line cell of breast adenocarcinoma). Nevertheless, the results were not so promising because they did not show any effect up to the concentration of 50.0 μM or 20.0 μM [55].
Fig. (10).
Structural formula of the prepared platinum (II) oxalato complexes [Pt(ox)(nL)2].1,5H2O (Source: Modified from Štarha et al. [62, 63]).
3.3. Adenosine Derivatives by Conjugation and Coupling Reactions
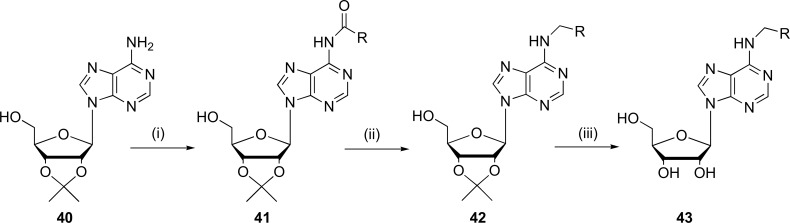
According to some studies, it has been possible to understand the synthesis of adenosine derivatives through alternative routes such as acylation, reduction and deprotection reactions. It occurs in the synthesis proposed by Lescrinier et al., which is based on a coupling reaction involving a reduction of N6-acylated 2',3'-O-isopropylidene adenosine (41) with LiAlH4 followed by the removal of the isopropylidene group protection [56]. The derivatives obtained by this group of researches are described in Fig. (11).
Fig. (11).
General synthesis of N6-alkylated adenosine derivatives (43). Reagents and conditions: (i) acylating agent-benzoyl chloride; (ii) 1,4-dioxane, LiAlH4; (iii) 80% HOAc, 75ºC. (Source: Modified from Lescrinier et al. [64]).
Under this same synthetic line, some products were identified being nucleotides obtained as adenosine derivatives and which can act as transcriptional primers. These derivatives corresponded to a series of amino adenosine compounds, which were synthesized by a method assisted from 5'-aminophosphoramidate and water-soluble carbodiimide, generating a direct coupling of diamines with AMP [57]. Fundamentally, chlorophosphorylation of 6-chloropurine riboside was carried out, followed by hydrolysis of 6-chloropurine riboside-5-monophosphate and a displacement or substitution of 6-chloride diamines later, obtaining N6-adenosine derivatives. Subsequently, two series of adenosine nucleotides were synthesized which were coupled and conjugated to groups of fluorescein and biotin at the extreme 5’ or at the N6 position, through linkers of different sizes and hydrophobicities. Both adenosine nucleotides series could act as efficient transcription initiators, producing fluorescein-labeled and biotin-labeled RNA at the extreme 5'-end under a “one-step” procedure, eliminating post-transcriptional modification [57].
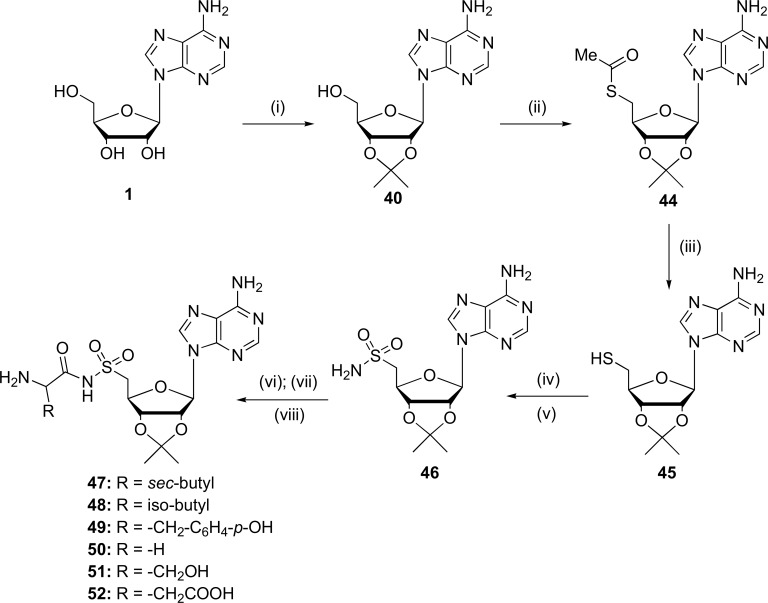
During 2015, six compounds which corresponded to the 5'-(N-aminoacyl)-sulfonamido-5'-deoxyadenosine (aaSoA) series (47-52), analogous to the aminoacyl-sulfamoyl adenosine compounds (aaSAs) and their peptidyl conjugates, were synthesized. The synthesis was based on coupling reactions (via Mitsonobu reaction [61]), ammonolysis and oxidative chlorination, for obtain the different sulfamides that were used as precursors for the condensation and hydrogenolysis reactions, obtaining 5'-O-(N-L-aminoacyl)-sulfamoyl adenosines (aaSAs) (Fig. 12). These synthetic steps were carried out with the aim of inhibiting the enzyme aminoacyl tRNAsynthetase (aaRS) on bacterias. However, these new compounds were not able to inhibit the corresponding aaRS [67, 58].
Fig. (12).
Synthesis of 5’–aminoacyl–5’–deoxy-nucleosidesulfonamide (47-52).Reagents and conditions: (i) DMK, PTSA, DMP, r.t., 24 h; (ii) thioaceticacid, PPh3, DEAD, THF, 0ºC, 1.5 h; (iii)NH3/MeOH, 0ºC to r.t., overnight; (iv) CH3CN/AcOH/H2O (40:1.5:1 v/v) DCDMH, 1h; (v) NH3, 0ºC to r.t., 1h; (vi) Boc/Cbz-aa-(tBu/Bn)-OSu, DBU, DMF; 6-8 h; (vii) TFA/H2O (5:2 v/v), r.t. 2.5 h;(viii) Pd/C, methanol,HOAc, H2, r.t. overnight. (Source: Modified of Gadakh et al., [67]).
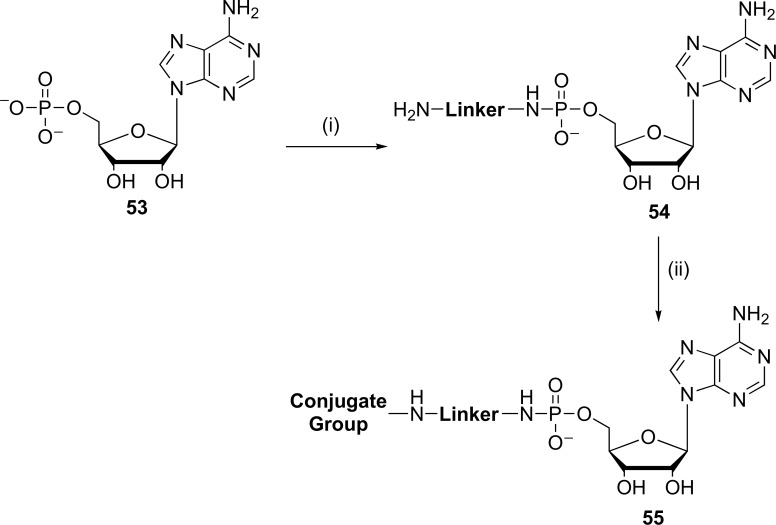
Along this same line, methods of synthesis have been described and trials methods have improved for preparing conjugates of adenosine for use as initiators of transcription in the in vitro synthesis of 5'-RNA modified by a specific type of RNA polymerase (T7). Such conjugates were of the adenosine methods described by Laing et al. These methods involved two synthetic steps: (i) preparation of a linker amino terminus of AMP and (ii) binding of the conjugate group (typically an activated ester derivative) to the free diamine linker [59]. Thus, the synthesis of compounds achieved were folate-adenosine-5'-(13-amino-4,7,10-trioxa tridecyl) phosphoramidate conjugates and folate-adenosine-5'-(6-aminohexyl) phosphoramidate (55) which were purified by HPLC (column BoronateAffi-Gel), lyophilized and elucidated by mass spectrometric positively (Fig. 13). The proposed advantage of this latter method is that it allows the rapid preparation of large amounts of conjugates in high yields. Even purity can be readily adapted for the preparation of conjugates of adenosine with a variety of linkers of diamine, giving the flexibility to choose desired length, hydrophobicity/hydrophilicity, flexibility/stiffness or other properties that may be desirable for the intended application [59].
Fig. (13).
General methods of two steps for the preparation of 5’-adenosine linker conjugate (55). Reagents and conditions: (i) methylimidazole, EDC, diamine, r.t., pH: 6.2-6.8; (ii) folic acid, NHS, EDC, r.t., pH=7.5 (Source: Modified of Laing et al., [68]).
Panda’s studies show the synthesis of adenosine derivatives based in a synthetic strategy for the desired homologated apio adenosine derivatives involved a controlled oxidative rupture of vicinal diol and condensation of the homologated glycosyl donor with a purine base under Mitsunobu condition as the key steps [60]. The reactions included couplings of glycosyl donor with 6-chloropurine giving rise to the first derivatives of such condensation. Then, these derivatives were subjected to condensation with 2,6-dichloro purine under Mitsunobu conditions [61] to produce derivatives of 2,6-dichloro purine, which were treated with 3-halobenzyl amines to give the corresponding products regioselectively substituted, resulting in the synthesis of homologated apio analogues of IB-MECA and Cl-IBMECA [60].
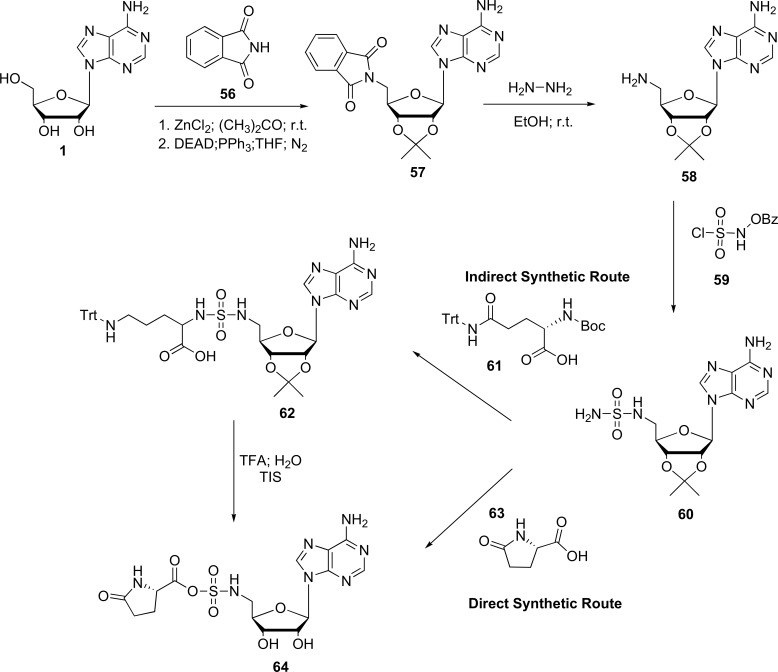
On the other hand, the synthesis of novel series of sulfamide adenosine derivatives obtaining pyroglutamyl fragment
(pGlu-SA) through direct and indirect synthetic route have been developed. First, the investigators showed that an amine (5'-amino-5'-deoxyadenosine) with L-pyroglutamic acid (63) undergoes coupling reactions resulting in a lactam adenosine conjugate (64). For the second case, the synthesis was performed through the coupling reaction of a sulfonamide adenosine derivative with Boc-L-Gln (Trt) -OH (61), involving intramolecular cyclization to form pGlu-SA (64) [70, 87]. Both methods are summarized in Fig. (14).
Fig. (14).
General synthetic routes of pGlu-SA (64) (Source: Modified of Kaybullin et al., [70]).
These derivatives were synthesized with the aim to inhibit the protein translation and the activity of cognate aminoacyl tRNAsynthetases, while simultaneously trying to find out new anticancer and antiviral drugs [70].
Several 8-brominated or 8-aminated adenosine derivatives with different substituents at 5’-C were biologically tested on IspE protein from the non-mevalonate pathway. These compounds were synthesized from isopropylidene-protected adenosine, then were oxidized to carboxylic acid and amide-coupling using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and p-nitrophenol. Finally, the compound was subjected to deprotection with TFA. However, these compounds when were evaluated biologically, displayed very little to no inhibitory activity on IspE against E. coli and P. falciparum [72].
Meanwhile, others authors performed a synthesis of a series of truncated N6-substituted-4′-oxo- and 4′-thioadenosine derivatives, where lithiation-mediated stannyl transfer was used and palladium-catalyzed cross coupling reactions were utilized for functionalization of the 2-C position of 6-chloropurine nucleoside forming compounds with substitution on 2-C or 8-C position. These compounds were synthesized in order to examine the structure activity relationships of this class of nucleosides as dual acting A2A and A3A receptors ligands [73].
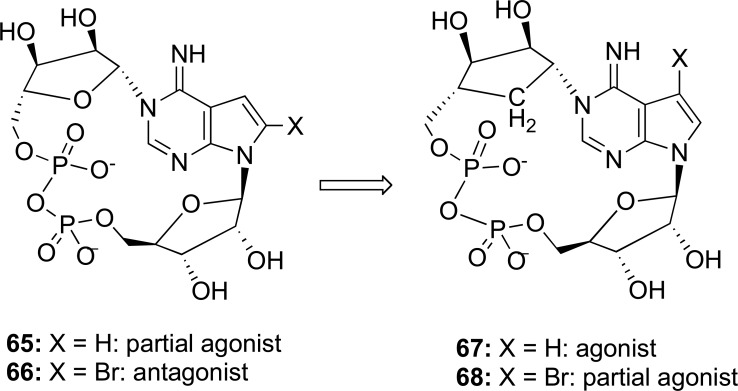
Furthermore, the synthesis of compounds 7-deaza analogues were constructed by a retrosynthetic analysis. The N1-carbocyclic ribosyl-7- deazaadenosine structure was obtained from of condensation between the2,3-disubstituted pyrrole nucleoside and the carbocyclic ribosylamine [77]. Subsequently, this allowed the synthesis of 7-deaza-cyclic adenosine-5′-diphosphate-carbocyclicribose through an Ag+-promoted intramolecular condensation to construct the 18-membered pyrophosphate ring structure (Fig. 15). Likewise, some researches synthesized its 7-bromo derivative compound. When performing a biological evaluation of these compounds, these researches determined that 7-deaza-cyclic adenosine-5′-diphosphate-carbocyclicribose and 7-deaza-7-bromo-cyclic adenosine-5′-diphosphate-carbocyclicribose acted as a full agonist and a partial agonist in Ca2+ mobilizing activity which was fluorometrically monitored with Hemicentrotus pulcherrimus sea urchin egg homogenate [77].
Fig. (15).
Structures of 7-deaza-cyclic adenosine-5′-diphosphate-carbocyclicribose and its 7-bromo derivative (Source: Takano et al., [74]).
Others studies showed the mechanism of obtainment of some adenosine etheno derivatives and other nucleosides. Basically, Jahnz-Wechmann et al., presented a literature compilation on the general method of synthesis of such derivative [80]. These researches focused on the reaction mechanism that was generated on the ring of nucleosides. In the case of etheno adenosine derivatives (1, N6-ethenoadeno-sine) were obtained by reaction with alpha-halocarbonyl group (haloketones or haloaldehydes), although other studies with reagents of a similar nature, such as bromomalonaldehyde, N-(tert-butoxycarbonyl)-2-bromoacetamide and 2-chloroketenene diethyl acetal, malonaldehyde, epoxy carbonyl compounds, 1-halooxiranes, 1,2-dicarbonyl reagents and via a copper-catalyzed domino Michael oxidative cross-coupling with nitroolefins [81].
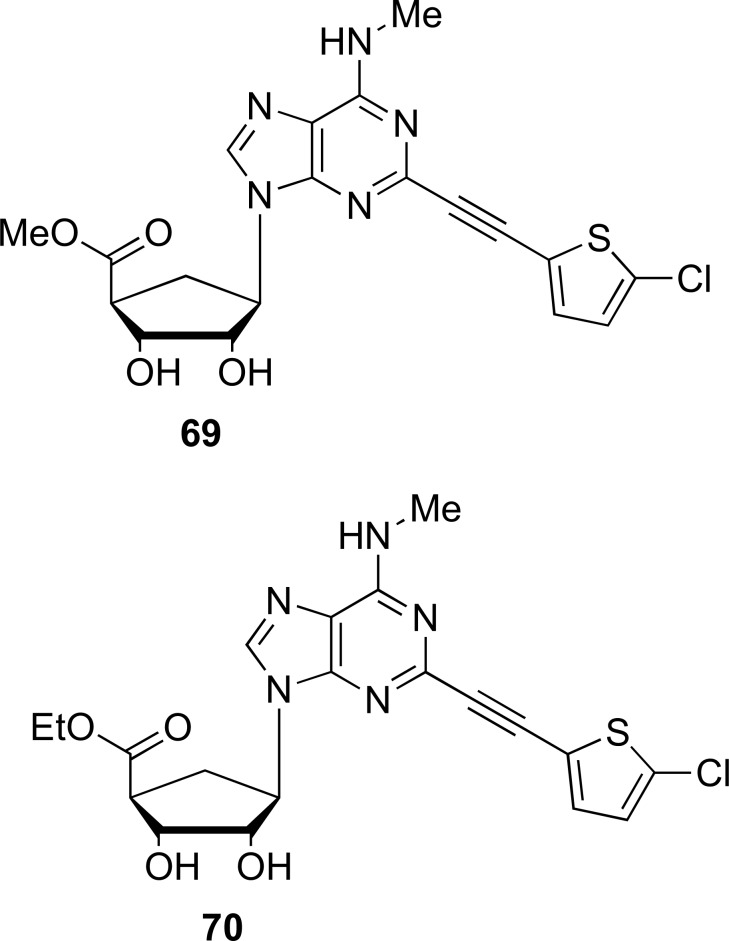
As an additional data, more recent studies have shown the application of an adenosine derivative, IFC-305, which is the aspartate salt of adenosine prepared with adenosine freebase, obtained through of coupling and substitutions reactions differents. This drug was tested on hepatocellular carcinoma (HCC) through of in vitro and in vivo assays, showing an important hepatoprotective role [84, 85]. In addition, there are researchers who have synthesized and evaluated through of structure-activity relationship (SAR) an expanded class of nucleosides. These compounds lost their affinity for A3AR, after being modified in specific areas. The general synthesis was based on different reaction stages that involving oxidation, substitution nucleophilic aromatic and coupling on precursor reagents that contained methanocarba ring and other ribose ring [86]. In Fig. (16) it is showed the compound that acted as selective and allosterically modulate to Dopamine Transporter (DAT), in micromolar range, compared to A3AR. This would be useful as research tools to study the structure and function of DAT that expect a greater effectiveness in the treatment depression and other disorders associated with dopamine (DA) deficiency [86].
Fig. (16).
Chemical structures of two N-methanocarba 5’-ester adenosine derivatives (Source: Tosh et al., [79]).
Thus, the actions of the various series of adenosine derivatives investigated in the past 30 years are summarized in Table 3, briefly describing precursors; types of reaction involved for obtaining analogs, and applied biological assays of the respective series.
Table 3.
Summary of synthetic adenosine derivatives, methods and physiological action of the last 25 years.
| Adenosine Derivatives by Substitution Reactions | |||
|---|---|---|---|
| Adenosine Derivatives Series | Methods of Synthesis and Biological Essay | Target Interaction | Refs. |
| C8-amino-N6-disubstitution adenosine |
Precursors: • N6-derivative-8-halide adenosine. • 8-aminoalkyl derivative 6-chloropurine riboside • 6,8-dichloropurine riboside. Reaction type: Double selective substitution. Essay: rat cerebral cortex cells and striated |
A1 and A2A receptors | [48] |
| • 2-alkyladenosine • 2-alkynyl-N6-alkyladenosine. • Adenosine-5'-N6-ethyluronamide |
Precursors: • 9-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-6-chloro-2-iodopurine. • 2-iodoadenosine • 2-iodine-N6-alkyladenosine Reaction type: Double and triple selective substitution. Essay: Rat ovary cells transfected with human recombinant DNA (adenosine receptors) and platelet. |
A2 and A3 receptors | [3, 45, 46] |
| 2-Substitution-N6-methyl adenosine. |
Precursor: • 9-(2,3,5-tri-O-acetyl-β-D-ribofuranosyl)-6-chloro-2-iodopurine. Reaction type: Double and triple selective substitution. |
A3 receptor | [49] |
| • 2-alkyloxy ether • 2-alkylaryl • 2-amino ether • 2-thioether |
Precursor: • 2-adenosine chlorine. Reaction type: Nucleophilic substitution. Essay: Rat ovary cells (CHO) |
A3 receptor | [50, 51] |
| • N6-alkyladenosine derivatives |
Precursor: • 6-chloropurine riboside. Reaction type: Nucleophilic substitution. Essay: Cell lines of bladder carcinoma T24 and J88 types. |
Carcinoma cell lines | [52] |
| • N6-biotinyl-8-azidoadenosine probe |
Precursor: • Inosine Reaction type: Nucleophilic substitution. Essay: Cell lines of bladder carcinoma T24 and J88 types. |
Adenosine Deaminasa (cADA) | [57] |
| • 2’-deoxymethylene nucleosides |
Precursor: • Guanosine Reaction type: Nucleophilic substitution;, oxidation by Swern method; methylenation by Wittig reaction and nonaqueous diazotization (substitution reaction) Essay: Mouse lymphocytic leukemia cell line (L1210) |
Ribonucleotide Reductase Enzyme (RNR) | [58] |
| • N-benzylated-7-deazaadenosine analogues |
Precursor: • Turbercidin Reaction type: Alkylation and SNAr Essay: Murine leukemia (L1210) cellsand human T-lymphocyte (CEM),cervix carcinoma (HeLa), prostateadenocarcinoma (PC-3), andkidney carcinoma (Caki-1) cells, and Nucleoside Transport. |
hENT1 and pool cell lines | [74] |
| Adenosine Derivatives by Complexation Reactions | |||
| [Ru2(CH3CO2)4(C10H13N5O4)2] Cl |
Precursor: • Adenosine. Reaction type: Complexation reaction Essay:Growing Escherichia coli. |
E. coli bacteria | [61] |
| Adenosine Derivatives by Complexation Reactions | |||
| Adenosine Derivatives Series | Methods of Synthesis and Biological Essay | Target Interaction | Refs. |
| • Trans-[PtCl2(N6-(substituted-benzyl) adenosine)2]•*Solv. • Trans-[PtCl2(2-chloro-N6-(substituted-benzyl) adenosine)2]•*Solv. |
Precursor: • K2[PtCl4] • 6-chloropurine-9-ribose • 2,6-dichloropurine-9-ribose. • Amine derivatives. Reaction type: Complexation reaction. Essay: Osteosarcoma (HOS) and breast adenocarcinoma. |
Carcinoma cell lines | [62, 63] |
| Adenosine Derivatives by Conjugation and Coupling Reactions | |||
| • N6-amino derivatives of adenosine 5'- monophosphate. • 5'-adenosine phosphoramidate amino derivatives • 5'-N6-fluorescein derivatives AMP • 5'- and N6 - biotin derivatives AMP |
Precursor: • Diamines • AMP • 6-chloropurine riboside-5-monophosphate. • 5(6)-carboxifluorescein-N- hydroxysuccinimide ester (FAM-SE). • Biotin N-hydroxysuccinimide ester (biotin-SE) Reaction type: Coupling and direct displacement. Essay: In vitro RNA transcription, Electrophoresis. |
RNA | [65] |
| • Folate-adenosine-5'-(13-amino-4,7, 10-trioxa tridecyl) phosphoramidate) • Folate-adenosine-5'-(6-aminohexyl) phosphoramidate |
Precursor: • AMP, N-methylimidazole and hidrochloruro N-(3-dimethylaminopropyl)-N'-Ethylcarbodiimide (EDC). • Diamine • Folic acid. • NHS Reaction type: Conjugation. Essay: RNA sequence in vitro |
RNA | |
| • Homologated apio analogues of IB-MECA and Cl-IB-MECA |
Precursor: • IB-MECA and Cl-IB-MECA • Glycosyl donor • 2,6-dichloropurine • 3-Halobenzyl amines Reactions types: • Controlled oxidative cleavage of vicinal diol. • Mitsunobu condensation reactions. Essay: Docking molecular and MM-GBSA. |
A3 receptor | [69] |
| • 2,6-diamino-8-azapurine sulfate |
Precursor: • Ribose-1-phosphate • D-aaPur • α-D-ribose-1-phosphate • 8-azaguanine Reactions types: Ribosylation Essay: Escherichia coli culture and solution purine-nucleoside phosphorylase. |
Purine-nucleoside phosphorylase (PNP) and bacteria E.coli | [87] |
| • N6-(2-methoxybenzyl)adenosine (2 OMeL) • N6-(4-methoxybenzyl)adenosine (4 OMeL) • N6-(4-methylbenzyl)adenosine (4MeL) • N6-(2-chlorobenzyl)adenosine (2 ClL) • N6-(4-fluorobenzyl)adenosine (4 FL) |
Precursor: • N6-benzyladenosine derivative • K2[Pt(ox)2]·2H2O Reactions types: • One-step synthetic procedure using the reaction of potassium bis(oxalato)platinate(II) Essay: Cancer cells (osteosarcoma and breast adenocarcinoma) |
Human cancer cell lines | [63] |
| Adenosine Derivatives by Conjugation and Coupling Reactions | |||
| Adenosine Derivatives Series | Methods of Synthesis and Biological Essay | Target Interaction | Refs. |
| • 5’-(N-aminoacyl)-sulfonamido-5’-deoxyadenosine |
Precursor: • adenosine Reactions types: Mitsunobu condensation reactions or coupling and conjugation. Essay: • E. coli, S. Lutea and C. Albicans. • In vitro aminoacylation experiments. |
Bacteria, fungi and Aminoacyl tRNA synthetases (aaRS) in vitro |
[67] |
| • Sulfamide adenosine derivatives (pGlu-SA) |
Precursor: • Adenosine. • 5'-amino-5'-deoxyadenosine • L-pyroglutamic acid Reactions types: Conjugation and coupling. Essay:Cancer cells |
Aminoacyl tRNA synthetases (aaRS) |
[70] |
| • 8-brominated adenosine derivatives • 8-aminated adenosine derivatives |
Precursor: • Isopropylidene-protected-adenosine Reactions types: Oxidation and amide-coupling Essay: Inhibitory activity against E. coli and P. Falciporum IspE. |
IspE enzyme | [72] |
| • Truncated N6-substituted 4’-oxoadenosine • Truncated N6-substituted 4’-thioadenosine |
Precursor: • 2,3-O-isopropylidene-D-erythronic-γ-lactone. Reactions types: lithiation-mediated stannyl transfer and palladium-catalyzed cross coupling reactions. Essay: • Chinese hamster ovary (CHO) cells (A1 or A3R) • Human embryonic kidney (HEK-293) cells (A2AAR). |
A3 and A2A receptors | [73] |
| • 7-deaza-cyclic adenosine-5’-diphosphate carbocyclic ribose. • 7-deaza-7-bromo-cyclic adenosine-5’-diphosphate carbocyclic ribose. |
Precursor: • 2,3-disubstituted pyrrole nucleoside • Carbocyclic ribosyl amine • Imidazole nucleoside Reactions types: Ag+ promoted intramolecular condesation and coupling reaction. Essay: Hemicentrotus pulcherrimus sea urchin egg homogenate. |
Sea urchin egg homogenate system | [77] |
| • (N)-Methanocarba-5′-ester adenosine derivatives |
Precursor: • (N)-methanocarba adenosine Reactions types: oxidation, substitution nucleophilic aromatic and coupling. Essay: Dopamine transporter (DAT) |
A3R and DAT | [79] |
(*) Solvent = H2O or MeOH.
(Source: Prepared by the author).
4. Potential therapy of compounds derived from adenosine on tumor and glioblastomas cells
During the last decade, different approaches have been developed for the treatment of cancer, mainly based on specific objectives that target tumors. It also is recognized that individualized therapy for that patient suffering from the disease, has usually been an important goal that leads to the prediction of effective agents or drugs for treatment [37].
Cancer is a serious health problem for humanity, with high rates of incidence and mortality. At present, cancer is the third leading cause of death, and it is estimated that in 2007 there were over 12 million new cases and 7.6 million people died worldwide. For the year 2030, it is estimated that there will be 26 million new cases and 17 million deaths annually [62]. However, in Chile, the cancer mortality rate is 119.4 per 100,000 people representing 23% of all deaths, for instance, a mortality rate of brain cancer that fluctuates between 1-2 patients per 100,000 people [64]. Similarly, it is important to know that the current note that currently the management of patients with brain tumors is primarily excision followed by chemotherapy, radiotherapy or both. However, an important limitation is that there are a few cases where it can be removed by surgery, which inevitably leads to relapse and metastasis, significantly reducing patient survivals, becoming a disease of poor prognosis [63, 64]. Therefore, the search for new therapeutic alternatives is currently a priority health issue that has involved both testing combinations of existing and new generational drugs based on rational approximations. Here, the evaluation of adenosine as adjunctive therapy [22, 23] promises to have a significant impact on the treatment of certain cancers, although the transfer of the results to clinical practice require a deeper understanding of how adenosine regulates the process of tumorigenesis [5].
Thus, glioblastoma (GB) is considered a diffuse infiltrative glioma with anaplastic changes. It has a high proliferative potential, areas of necrosis and high degree of vascularization [65, 66]. Rather, glioblastoma multiforme is the most common and aggressive glial tumors of the central nervous system, grade IV glioma derived from a previous grade II or III or reappears, being highly invasive, angiogenic and incurable [65]. High-grade glioma corresponds to 25% of primary tumors of the central nervous system. The overall survival of these patients is estimated between 1-2 years, half of all patients die within the first year of diagnosis [66-69].
Therefore, it has been reported that adenosine receptors (ARs) are active entities as mediators of tumor growth. Including immune cells such as lymphocytes, macrophages and natural killer cells. Some researchers also report the expression of adenosine receptors and their ability to act as cytotoxic cells against tumor cells [37]. Practically, after receptor activation, several transduction pathways signals are generated, resulting in a direct inhibitory effect on tumor growth and other types of cells such as immune cells or endothelial cells. They can respond to receptor activation by the release of cytokines and mediators that indirectly affect tumor growth [4]. Thus, Gessi and his team conclude that all adenosine receptors are potential targets for the development of new approaches to cancer treatment [1].
Moreover, the effect of adenosine and its agonists/ antagonists on tumor cells depends on their extracellular concentrations and expression of the different subtypes of this nucleoside (ARs) [5]. Therefore, this implies that adenosine, acting through A1, glioblastoma growth alters [1] or better understands how the signaling pathways of A1 receptors modulate glioblastomas development may lead to slowing the progression of this disease [38].
Regarding adenosine analogs reported to date, some of the N6- alkyladenosine derivatives have had antiproliferative activity [52]. Likewise, derivatives of 5'-N-methylcarba-moyladenosine (MECA), such as 2-chloro-N6-2-iodobenzyl)-5'-N- methylcarbamoyladenosine that are A3 receptor agonists and inhibit nanomolar tumor cell growth and even induce apoptosis [2], adenosine-3',5'-cyclic monophosphate analogs, such as 8-Cl-cAMP, also contribute to the decrease in cell proliferation [23].
However, other studies suggest that one of the strategies to optimize the treatment of brain cancer (as glioblastomas) would inhibit the function of the enzyme ecto 5'-nucleotidase CD73 (AMP hydrolyzing enzyme). Enzyme production decreases and inhibits adenosine function specific transporters, resulting in an increased bioavailability and effectiveness of antineoplastic drugs [67, 68]. Such an effect is achieved with a competitive inhibitor, adenosine derivative, called α,β-methylene-adenosine (AOPCP), obtaining as a significant result in the reduction of cell proliferation [68].
Other studies have shown that a novel N6-biotinylated-8-azido-adenosine probe (AdoR probe) was synthesized and analyzed in a lysate derived from mouse neuroblastoma N18TG2line cells, demonstrating an improvement in sensitivity for detection of AdoR-binding proteins compared to the non-labeled proteome [74].
Conclusion
In conclusion, adenosine has to be characterized as the natural metabolite and plays a role in several physiological and pathological conditions, such as inhibition of platelet aggregation, regulation of endothelial permeability, after stroke cardioprotection, vasodilation, mast cell activation processes, stimulation of epithelial chloride secretion, lipolysis, anti-inflammatory mediator, antitumor activity and angiogenesis stimulator.
This makes that adenosine and certain derivatives thereof have an inhibitory effect on the growth of tumor cells, which is mediated mainly via the A3 adenosine receptor or microglial cells in the development of glioblastomas, where pathways type receptors A1 modulates glioblastoma development, reducing the progression of this disease. Finally, the various synthetic techniques compiled from 1992 to date, allow the obtainment of multiple varieties of nucleoside analogues where those resulting substitution reactions (mono, double or triple) prove to be more effective than complexation or coupling.
ACKNOWLEDGEMENTS
This research was conducted according by professor PhD. Margarita Gutiérrez, academic professor and tutor, Instituto de Química de Recursos Naturales, Universidad de Talca. Also, the first author is grateful to the support of CONICYT through doctoral research grant Nº 21130335 and Programa de Investigación Asociativa en Cáncer Gástrico (PIA-CG, RU2107) Utalca Project.
LIST OF ABBREVIATIONS
- aaRS
Aminoacyl Synthetase RNA Transfer
- ADP
Adenosine Diphosphate
- AMP
Adenosine Monophosphate
- cAMP
Cyclic Adenosine Monophosphate
- A1
A1 Adenosine Receptor
- A2A
A2A Adenosine Receptor
- A2B
A2B Adenosine Receptor
- A3 or A3AR
A3 Adenosine Receptor
- ATP
Adenosine triphosphate
- Cl-IBMECA
2-chloro-N6-(3-iodobencyl)adenosine-5’-N-methyluronamide
- 8-Cl-cAMP
8-Chloro-cyclic AMP
- CNS
Central Nervous System
- DMF
N,N-Dimethylformamide
- hENT1
Human Equilibrative Nucleoside Transporter 1
- HPLC
High Performance Liquid Chromatography
- IB-MECA
N6-(3-iodobencyl)adenosine-5’-N-Methyluronamide
- IspE
4-Diphosphocytidyl-2C-methyl-D-Erythritol Kinase
- mRNA
Messenger RNA
- MS
Mass Spectroscopy
- pGlu-SA
Sulfamide Adenosine Derivatives with Pyroglutamyl Fragment
- Pt-NMR
Platinum Nuclear Magnetic Resonance
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- TFA
Trifluoroacetic Acid
- u.m.a.
Unit of Atomic Mass
CONFLICT OF INTEREST
Financial contributions and any potential conflict of interest must be clearly acknowledged under the heading ‘Conflict of Interest’. Authors must list the source(s) of funding for the study. This should be done for each author.
REFERENCES
- 1.Gessi S., Merighi S., Sacchetto V., Simioni C., Borea P.A. Adenosine receptors and cancer. Biochim. Biophys. Acta. 2011;1808(5):1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Cosyn L. Thesis submitted to the faculty of pharmaceutical sciences to obtain the degree of doctor in pharmaceutical sciences. Ghent University, 2008. Synthesis and biological evaluation of purine and pyrimidine ligands for the a3 andp2y2 purinergic receptors. . [Google Scholar]
- 3.Volpini R., Costanzi S., Lambertucci C., Taffi S., Vittori S., Klotz K-N.N. 6-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A3 receptor and a starting point for searching A2B ligands. J. Med. Chem. 2002;45(15):3271–3279. doi: 10.1021/jm0109762. [DOI] [PubMed] [Google Scholar]
- 4.Hasko G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonioli L., Blandizzi C., Pacher P., Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer. 2013;13(12):842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 6.Contreras E. Adenosine: Physiological and pharmacological actions]. Arch. Biol. Med. Exp. (Santiago) 1990;23(1):1–12. [PubMed] [Google Scholar]
- 7.Schulte G. Adenosine receptor signaling and the activation of mitogen-activated protein kinases. 2002. [Google Scholar]
- 8.Fredholm B.B. Adenosine receptors. Med. Biol. 1982;60(6):289. [PubMed] [Google Scholar]
- 9.Silva J.F. Adenosina para el tratamiento de taquicardia supraventricular. 2001. [Google Scholar]
- 10.Alférez F.C. Interacción funcional entre las proteínas implicadas en el reconocimiento molecular de adenosina. Memoria para optar al grado de doctor en biología. Publicacions Universitat de Barcelona; 1996. pp. 16–80. [Google Scholar]
- 11.Dionisotti S., Zocchi C., Varani K., Borea P.A., Ongini E. Effects of adenosine derivatives on human and rabbit platelet aggregation. Correlation of adenosine receptor affinities and antiaggregatory activity. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346(6):673–676. doi: 10.1007/BF00168741. [DOI] [PubMed] [Google Scholar]
- 12.Miras M.T.M. Importancia de los modelosdeanimales en el estudio del sistema purinérgico. Discursos; 2012. pp. 23–25. [Google Scholar]
- 13.Sattin A., Rall T.W. The effect of adenosine and adenine nucleotides on the cyclic adenosine-3′,5′-phosphate content of guinea pig cerebral cortex slices. Mol. Pharmacol. 1970;6(1):13–3. [PubMed] [Google Scholar]
- 14.Galduf J., Monte E., Escrivá J. Adenosina y derivados en el tratamiento de las taquicardias supraventriculares. Farm. Hosp. 1995;20(5):279–288. [Google Scholar]
- 15.Tavares C., Sakata R.K. Caffeine in the treatment of pain. Braz. J. Anesthesiol. 2012;62(3):387–401. doi: 10.1016/S0034-7094(12)70139-3. [DOI] [PubMed] [Google Scholar]
- 16.Snyder S.H., Katims J.J., Annau Z., Bruns R.F., Daly J.W. Adenosine receptors and behavioral actions of methylxanthines. Proc. Natl. Acad. Sci. USA. 1981;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosmes P.G., Macías J.F., Aubia J., Martín-Malo A., Quereda C., Refoyo A. Adenosina y autorregulación renal. Nefrol. Madr. 1988;8(2):98–100. [Google Scholar]
- 18.Cohen M.V., Downey J.M. Precondicionamiento isquemico: De los mecanismos basicos a las aplicaciones clinicas. Rev. Argent. Cardiol. 2000;68(2) [Google Scholar]
- 19.Van Tilburg E.W., Von Frijtag D., Kunzel J., De Groote M., Vollinga R.C., Lorenzen A., IJzerman A.P.N. 6,5′-Disubstituted adenosine derivatives as partial agonists for the human adenosine A3 receptor. J. Med. Chem. 1999;42(8):1393–1400. doi: 10.1021/jm981090+. [DOI] [PubMed] [Google Scholar]
- 20.Parodi J., Flores C., Aguayo C., Rudolph M.I., Casanello P., Sobrevia L. Inhibition of nitrobenzylthioinosine-sensitive adenosine transport by elevated. Circ. Res. 2002;90(5):570–577. doi: 10.1161/01.res.0000012582.11979.8b. [DOI] [PubMed] [Google Scholar]
- 21.Morairty S., Rainnie D., McCarley R., Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123(2):451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 22.Pascual R.J.S. Hipocretinas y adenosina en la regulación del sueño. Rev. Neurol. 2004;39(4):354–358. [PubMed] [Google Scholar]
- 23.Actis A., Croci M., Bergoc R. 2005.
- 24.Wan Z-K., Binnun E., Wilson D.P., Lee J. A Highly Facile and Efficient One-Step Synthesis of N6-Adenosine and N6-2′-Deoxyadenosine Derivatives. Org. Lett. 2005;7(26):5877–5880. doi: 10.1021/ol052424+. [DOI] [PubMed] [Google Scholar]
- 25.Valdivia-Silva J., López-Molina G., González-Altamirano J. 2007. [Google Scholar]
- 26.Swarbrick J.M., Potter B.V. Total Synthesis of a cyclic adenosine 5′-diphosphate ribose receptor agonist. J. Org. Chem. 2012;77(9):4191–4197. doi: 10.1021/jo202319f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dal Ben D., Buccioni M., Lambertucci C., Kachler S., Falgner N., Marucci G. Different efficacy of adenosine and NECA derivatives at the human A3 adenosine receptor: insight into the receptor activation switch. Biochem. Pharmacol. 2014;87(2):321–331. doi: 10.1016/j.bcp.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Petrelli R., Torquati I., Kachler S., Luongo L., Maione S., Franchetti P. 5′-C-Ethyl-tetrazolyl-N6-Substituted Adenosine and 2-Chloro-adenosine Derivatives as Highly Potent Dual Acting A1 Adenosine Receptor Agonists and A3 Adenosine Receptor Antagonists. J. Med. Chem. 2015;58(5):2560–2566. doi: 10.1021/acs.jmedchem.5b00074. [DOI] [PubMed] [Google Scholar]
- 29.Conti C.R. Adenosine: Clinical pharmacology and applications. Clin. Cardiol. 1991;14(2):91–93. [PubMed] [Google Scholar]
- 30.De Micheli A., Domínguez R.C., Torres P.I., Pastelín G., Medrano G.A. Early and late effects of adenosine in experimental ventricular tachycardia. Rev. Esp. Cardiol. (Engl. Ed.) 2005;58(2):159–166. [PubMed] [Google Scholar]
- 31.Fogli E. 2010 http://eprints.unife.it/292/1/Tesi_Eleonora_Fogli.pdf
- 32.Kaster M.P., Rosa A.O., Rosso M.M., Goulart E.C., Santos A.R., Rodrigues A.L.S. Adenosine administration produces an antidepressant-like effect in mice: Evidence for the involvement of A1 and A2A receptors. Neurosci. Lett. 2004;355(1):21–24. doi: 10.1016/j.neulet.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 33.Parkinson F.E., Ferguson J., Zamzow C.R., Xiong W. Gene expression for enzymes and transporters involved in regulating adenosine and inosine levels in rat forebrain neurons, astrocytes and C6 glioma cells. J. Neurosci. Res. 2006;84(4):801–808. doi: 10.1002/jnr.20988. [DOI] [PubMed] [Google Scholar]
- 34.Castillo C.A. 2009. [Google Scholar]
- 35.Nascimento F.P., Santos A.R.S., Macedo S.J. The involvement of purinergic system in pain: Adenosine receptors and inosine as pharmacological tools in future treatments. INTECH Open Access Publisher; 2012. pp. 627–640. [Google Scholar]
- 36.Sacchetto V. The A3 adenosine receptor: A link between inflammation and cancer. Pubblicazioni Dello IUSS. 2009;3(1):1–149. [Google Scholar]
- 37.Fishman P., Bar-Yehuda S., Synowitz M., Powell J.D., Klotz K.N., Gessi S. Adenosine receptors in health and disease. Springer; 2009. Adenosine receptors and cancer. pp. 399–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Synowitz M., Glass R., Färber K., Markovic D., Kronenberg G., Herrmann K.A. 1 adenosine receptors in microglia control glioblastoma-host interaction. Cancer Res. 2006;66(17):8550–8557. doi: 10.1158/0008-5472.CAN-06-0365. [DOI] [PubMed] [Google Scholar]
- 39.Olah M.E., Stiles G.L. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35(1):581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- 40.Moreau J-L., Huber G. Central adenosine A2A receptors: an overview. Brain Res. Brain Res. Rev. 1999;31(1):65–82. doi: 10.1016/s0165-0173(99)00059-4. [DOI] [PubMed] [Google Scholar]
- 41.Johnston-Cox H.A., Ravid K. Adenosine and blood platelets. Purinergic Signal. 2011;7(3):357–365. doi: 10.1007/s11302-011-9220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gessi S., Varani K., Merighi S., Cattabriga E., Iannotta V., Leung E.A. 3 adenosine receptors in human neutrophils and promyelocytic HL60 cells: A pharmacological and biochemical study. Mol. Pharmacol. 2002;61(2):415–424. doi: 10.1124/mol.61.2.415. [DOI] [PubMed] [Google Scholar]
- 43.Johnston-Cox H.A., Koupenova M., Ravid K.A. 2 adenosine receptors and vascular pathologies. Arterioscler. Thromb. Vasc. Biol. 2012;32(4):870–878. doi: 10.1161/ATVBAHA.112.246181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baraldi P.G., Cacciari B., Romagnoli R., Merighi S., Varani K., Borea P.A.A. 3 adenosine receptor ligands: history and perspectives. Med. Res. Rev. 2000;20(2):103–128. doi: 10.1002/(sici)1098-1128(200003)20:2<103::aid-med1>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 45.Cristalli G., Eleuteri A., Vittori S., Volpini R., Lohse M.J., Klotz K.N. 2-Alkynyl derivatives of adenosine and adenosine-5′-N-ethyluronamide as selective agonists at A2 adenosine receptors. J. Med. Chem. 1992;35(13):2363–2368. doi: 10.1021/jm00091a003. [DOI] [PubMed] [Google Scholar]
- 46.Kim H.O., Ji X., Siddiqi S.M., Olah M.E., Stiles G.L., Jacobson K.A. 2-Substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J. Med. Chem. 1994;37(21):3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Schaick E.A., Mathôt R.A.A., Gubbens-Stibbe J.M., Langemeijer M.W.E., Roelen H., IJzerman A.P. 8-Alkylamino-substituted analogs of N6-cyclopentyladenosine are partial agonists for the cardiovascular adenosine A1 receptors in vivo. J. Pharmacol. Exp. Ther. 1997;283(2):800–808. [PubMed] [Google Scholar]
- 48.Roelen H., Veldman N., Spek A.L., Von Frijtag J., Mathôt R.A., Ijzerman A.P.N. 6, C8-Disubstituted adenosine derivatives as partial agonists for adenosine A1 receptors. J. Med. Chem. 1996;39(7):1463–1471. doi: 10.1021/jm950267m. [DOI] [PubMed] [Google Scholar]
- 49.Cosyn L., Jacobson K.A., Van Calenbergh S. 2-(1,2,3-Triazol-1-yl) N6-CH3-substituted adenosine derivatives: Highly potent and selective A3 receptor ligands. Collect. Czech. Chem. Commun. 2015;7:393–394. [Google Scholar]
- 50.Gao Z-G., Mamedova L.K., Chen P., Jacobson K.A. 2-Substituted adenosine derivatives: Affinity and efficacy at four subtypes of human adenosine receptors. Biochem. Pharmacol. 2004;68(10):1985–1993. doi: 10.1016/j.bcp.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueeda M., Thompson R.D., Arroyo L.H., Olsson R.A. 2-Aralkoxyadenosines: potent and selective agonists at the coronary artery A2 adenosine receptor. J. Med. Chem. 1991;34(4):1340–1344. doi: 10.1021/jm00108a015. [DOI] [PubMed] [Google Scholar]
- 52.Ottria R., Casati S., Baldoli E., Maier J.A., Ciuffreda P.N. 6-Alkyladenosines: Synthesis and evaluation of in vitro anticancer activity. Bioorg. Med. Chem. 2010;18(23):8396–8402. doi: 10.1016/j.bmc.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 53.Dimroth O. Ueber intramolekulare Umlagerungen. Umlagerungen in der Reihe des 1, 2, 3-Triazols. Eur. J. Org. Chem. 1909;364(2):183–226. [Google Scholar]
- 54.Oslovsky V.E., Drenichev M.S., Mikhailov S.N. Regioselective 1-N-alkylation and rearrangement of adenosine derivatives. Nucleosides Nucleotides Nucleic Acids. 2015;34(7):475–499. doi: 10.1080/15257770.2015.1016169. [DOI] [PubMed] [Google Scholar]
- 55.Valdés F., Luna V., Arévalo B., Brown N., Gutiérrez M. Docking’s study of 6-substituted adenosine-5′-N-ethyluronamide analogs as selective agonists at A3 adenosine receptor and selection of compounds for to be synthesized later. Pharma Chem. 2015;7(69):212–225. [Google Scholar]
- 56.Ciancetta A., Jacobson K.A. Structural probing and molecular modeling of the A3 adenosine receptor: A Focus on agonist binding. Molecules. 2017;22(3):449. doi: 10.3390/molecules22030449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahajan S., Manetsch R., Merkler D.J. Stevens, Jr SM. Synthesis and evaluation of a novel adenosine-ribose probe for global-scale profiling of nucleoside and nucleotide-binding proteins. PLoS One. 2015;10(2):1–17. doi: 10.1371/journal.pone.0115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Youssef K.M., Lien E.J. Design and synthesis of potential ribonucleotide reductase enzyme (RNR) inhibitors as antileukemic and/or antiviral 2′-deoxymethylene nucleosides. Future J. Pharm. Sci. 2015;1(2):42–49. [Google Scholar]
- 59.Mancuso A.J., Huang S-L., Swern D. Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide “activated” by oxalyl chloride. J. Org. Chem. 1978;43(12):2480–2482. [Google Scholar]
- 60.Rayala R., Theard P., Ortiz H., Yao S., Young J.D., Balzarini J. Synthesis of purine and 7-Deazapurine Nucleoside Analogues of 6-N-(4-Nitrobenzyl) adenosine; Inhibition of nucleoside transport and proliferation of cancer cells. ChemMedChem. 2014;9(9):2186–2192. doi: 10.1002/cmdc.201402047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gangopadhyay S., Gangopadhyay P.K. Adenine and adenosine derivatives of tetraacetatodiruthenium (II, III) cation. J. Inorg. Biochem. 1997;66(3):175–178. [Google Scholar]
- 62.Štarha P., Popa I., Trávníček Z., Vančo J.N. 6-Benzyladenosine derivatives as novel N-donor ligands of platinum (II) dichlorido complexes. Molecules. 2013;18(6):6990–7003. doi: 10.3390/molecules18066990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Štarha P., Popa I., Trávníček Z. Platinum (II) oxalato complexes involving adenosine-based n-donor ligands: Synthesis, characterization and cytotoxicity evaluation. Molecules. 2014;19(3):3832–3847. doi: 10.3390/molecules19033832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lescrinier E., Pannecouque C., Rozenski J., Van Aerschot A., Kerremans L., Herdewijn P. Synthesis of N6-alkylated adenosine derivatives. Nucleosides Nucleotides Nucleic Acids. 1996;15(11-12):1863–1869. [Google Scholar]
- 65.Huang F., Wang G., Coleman T., Li N.A. Synthesis of adenosine derivatives as transcription initiators and preparation of 5′-fluorescein and biotin-labeled RNA through one-step in vitro transcription. RNA. 2003;9(12):1562–1570. doi: 10.1261/rna.5106403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitsunobu O., Yamada M. Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 1967;40(10):2380–2382. [Google Scholar]
- 67.Gadakh B., Smaers S., Rozenski J., Froeyen M., Van Aerschot A. 5′-(N-aminoacyl)-sulfonamido-5′-deoxyadenosine: Attempts for a stable alternative for aminoacyl-sulfamoyl adenosines as aaRS inhibitors. Eur. J. Med. Chem. 2015;93:227–236. doi: 10.1016/j.ejmech.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 68.Laing B.M., Guo P., Bergstrom D.E. Optimized method for the synthesis and purification of adenosine–Folic acid conjugates for use as transcription initiators in the preparation of modified RNA. Methods. 2011;54(2):260–266. doi: 10.1016/j.ymeth.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panda A., Satpati S., Dixit A., Pal S. Novel homologated-apio adenosine derivatives as A3 adenosine receptor agonists: Design, synthesis and molecular docking studies. RSC Advances. 2016;6(14):11233–11239. [Google Scholar]
- 70.Khaybullin R.N., Liang X., Qi X. Molbank. 2015;1(2):M864. [Google Scholar]
- 71.Hampton A. Nucleotides II. A New Procedure for the Conversion of Ribonucleosides to 2′, 3′-O-Isopropylidene Derivatives. J. Am. Chem. Soc. 1961;83(17):3640–3645. [Google Scholar]
- 72.Harder M., Schäfer E., Kümin T., Illarionov B., Bacher A., Fischer M. 8-Substituted, syn-Configured adenosine derivatives as potential inhibitors of the enzyme ispe from the non-mevalonate pathway of isoprenoid biosynthesis. Eur. J. Org. Chem. 2015;1(33):7276–7286. [Google Scholar]
- 73.Hou X., Majik M.S., Kim K., Pyee Y., Lee Y., Alexander V. Structure–activity relationships of truncated C2-or C8-substituted adenosine derivatives as dual acting A2A and A3 adenosine receptor ligands. J. Med. Chem. 2011;55(1):342–356. doi: 10.1021/jm201229j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takano S., Tsuzuki T., Murayama T., Sakurai T., Fukuda H., Arisawa M. Synthesis of 7-Deaza-cyclic adenosine-5′-diphosphate-carbocyclic-ribose and Its 7-Bromo Derivative as Intracellular Ca2+-Mobilizing Agents. J. Org. Chem. 2015;80(13):6619–6627. doi: 10.1021/acs.joc.5b00723. [DOI] [PubMed] [Google Scholar]
- 75.Jahnz-Wechmann Z., Framski G.R., Januszczyk P.A., Boryski J. Base-modified nucleosides: Etheno derivatives. Front Chem. 2016;4:19. doi: 10.3389/fchem.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie M-S., Chu Z-L., Niu H-Y., Qu G-R., Guo H-M. A copper-catalyzed domino route toward purine-fused tricyclic derivatives. J. Org. Chem. 2014;79(3):1093–1099. doi: 10.1021/jo4025489. [DOI] [PubMed] [Google Scholar]
- 77.Velasco-Loyden G., Pérez-Carreón J.I., Agüero J.F.C., Romero P.C., Vidrio-Gómez S., Martínez-Pérez L. Prevention of in vitro hepatic stellate cells activation by the adenosine derivative compound IFC-305. Biochem. Pharmacol. 2010;80(11):1690–1699. doi: 10.1016/j.bcp.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 78.Velasco-Loyden G., Pérez-Martínez L., Vidrio-Gómez S., Pérez-Carreón J.I., Chagoya de Sánchez V. Cancer chemoprevention by an adenosine derivative in a model of cirrhosis-hepatocellular carcinoma induced by diethylnitrosamine in rats. Tumour Biol. 2017;39(2):1–11. doi: 10.1177/1010428317691190. [DOI] [PubMed] [Google Scholar]
- 79.Tosh D.K., Janowsky A., Eshleman A.J., Warnick E., Gao Z.G., Chen Z., Jacobson K.A. Scaffold repurposing of nucleosides (Adenosine Receptor Agonists): Enhanced activity at the human dopamine and norepinephrine sodium symporters. J. Med. Chem. 2017;60(7):3109–3123. doi: 10.1021/acs.jmedchem.7b00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vega Rodríguez A.M., Rodríguez Vázquez M.I., Wilkinson Brito B., Fernández Lorente A. Tumores astrocíticos: Un acercamiento a una patología. Rev. Méd. Electrón. 2012;34:362–372. [Google Scholar]
- 81.Storstein A., Helseth E., Johannesen T.B., Schellhorn T., Mørk S., Van Helvoirt R. High-grade gliomas in adults. Tidsskr Den Nor Laegeforening Tidsskr Prakt Med Ny Raekke. 2011;131(3):238–241. doi: 10.4045/tidsskr.09.1362. [DOI] [PubMed] [Google Scholar]
- 82.Quezada C., Peigñan L., Segura R., Riquelme F., Melo R., Rojas Z.D. Glioblastoma multiforme y estudio de la resistencia a la quimioterapia mediada por transportadores ABC. Rev. Med. Chil. 2011;139:415–424. [PubMed] [Google Scholar]
- 83.Guerrero R., Ingeborg L., Cartier L. Glioma multifocal multicéntrico. Rev. Chil. Neuro-Psiquiat. 2010;48(3):213–218. [Google Scholar]
- 84.Ramirez Y.P., Weatherbee J.L., Wheelhouse R.T., Ross A.H. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals. 2013;6(12):1475–1506. doi: 10.3390/ph6121475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merighi S., Benini A., Mirandola P., Gessi S., Varani K., Leung E. Hypoxia inhibits paclitaxel-induced apoptosis through adenosine-mediated phosphorylation of bad in glioblastoma cells. Mol. Pharmacol. 2007;72(1):162–172. doi: 10.1124/mol.106.031849. [DOI] [PubMed] [Google Scholar]
- 86.Salinas J. Efecto de la inhibición del transportador MRP1 sobre la Quimioresistencia en Glioblastoma Multiforme Humano. Valdivia, Chile: Undergrade. Facultad de Ciencias – Escuela de Química y Farmacia. Universidad Austral de Chile; 2011. [Google Scholar]
- 87.Stachelska-Wierzchowska A., Wierzchowski J., Wielgus-Kutrowska B., Mikleušević G. Enzymatic synthesis of highly fluorescent 8-Azapurine ribosides Using a purine nucleoside phosphorylase reverse reaction: Variable ribosylation sites. Molecules. 2013;18(10):12587–12598. doi: 10.3390/molecules181012587. [DOI] [PMC free article] [PubMed] [Google Scholar]