Abstract
Background:
Blinatumomab is a bispecific T-cell engager (BiTE®) antibody construct targeting CD3ε on T cells and CD19 on B cells. We describe the relationship between pharmacokinetics (PK) of blinatumomab and pharmacodynamic (PD) changes in peripheral lymphocytes, serum cytokines, and tumor size in patients with Non-Hodgkin Lymphoma (NHL).
Methods:
In a phase 1 study, 76 patients with relapsed/refractory NHL received blinatumomab by continuous intravenous infusion at various doses (0.5 to 90 µg/m2/day). PD changes were analyzed with respect to dose, blinatumomab concentra-tion at steady state (Css), and cumulative area under the concentration-versus-time curve (AUCcum).
Results:
B-cell depletion occurred within 48 hours at doses ≥5 μg/m2/day, followed first-order kinetics, and was blinatumomab exposure-dependent. Change in tumor size depended on systemic blinatumomab exposure and treatment duration and could be fitted to an Emax model, which predicted a 50% reduction in tumor size at AUCcum of ≥1,340 h×μg/L and Css of ≥1,830 pg/mL, corresponding to a blinatumomab dose of 47 µg/m2/day for 28 days. The magni-tude of transient cytokine elevation, observed within 1-2 days of infusion start, was dose-dependent, with less pronounced elevation at low starting doses.
Conclusion:
B-lymphocyte depletion following blinatumomab infusion was exposure-dependent. Transient cytokine eleva-tion increased with dose; it was less pronounced at low starting doses. Tumor response was a function of exposure, suggest-ing utility for the PK/PD relationship in dose selection for future studies, including NHL and other malignant settings.
Keywords: Blinatumomab, BiTE®, non-Hodgkin lymphoma, exposure-response, pharmacokinetics, pharmacodynamics
1. INTRODUCTION
Non-Hodgkin lymphoma (NHL) is a cancer with worldwide prevalence and increasing incidence [1]. It is characterized by many histological subtypes that are mainly B-cell–derived, with malignant cells expressing common B-cell antigens, such as CD19, CD20, and CD22 [2, 3]. Although treatments with monoclonal antibodies have improved outcomes in NHL [4], many patients experience early relapse, refractory disease, or resistance to treatment [5, 6]. Blinatumomab is a bispecific T-cell engager (BiTE®) antibody construct that targets CD3ε on T cells and CD19 on normal and malignant B cells, resulting in T-cell‒mediated serial B-cell lysis and T-cell proliferation [7-9]. In a phase 1 proof-of-concept study, single-agent blinatumomab has shown antilymphoma activity in relapsed/refractory NHL [10, 11], and recent data from a phase 2 study have shown promising clinical activity of blinatumomab in diffused large B-cell lymphoma (DLBCL) [12]. Blinatumomab has been approved for the treatment of relapsed/refractory acute lymphoblastic leukemia (ALL) [13-16].
Besides evaluations of appropriate doses, adverse events, and tolerability, the main objectives during early-phase development of cancer therapeutics include obtaining a comprehensive understanding of a molecule’s mode of action, its pharmacokinetic (PK) and pharmacodynamic (PD) characteristics as well as their interrelationship(s), and how they may differ in various disease settings. Hence, PK/PD analyses provide important information for the development of phase 2 and phase 3 studies and represent a well-established tool that has gained increased attention by regulatory agencies [17-19]. An assessment of blinatumomab PK has been published [20]. Given the novel pharmacology and mode of action of blinatumomab, it is important to understand its PK/PD characteristics, and to assess how they may differ in NHL compared with other disease settings, such as ALL [9]. The objective of the current analysis was to evaluate PK/PD relationships in patients with relapsed/refractory NHL who received blinatumomab in the proof-of-concept phase 1 study. In particular, we sought to establish a quantitative relationship between blinatumomab concentrations and PD changes in peripheral lymphocytes, serum cytokines, and tumor size in lymph nodes using PK/PD modeling techniques. Furthermore, for any given novel therapeutic, selecting a dose that balances maximum clinical benefit against acceptable toxicity is critically important but continues to represent a significant challenge. Blinatumomab has a short serum half-life, mainly due to lack of an Fcγ portion compared with immunoglobulin G monoclonal antibodies [12]. Therefore, it is administered by continuous intravenous (cIV) infusion. The data from our analysis provide insight into the mode of action of blinatumomab and can ultimately contribute to selection of dosing regimens with a maximized benefit-risk ratio, thereby supporting the design of future studies in NHL, and possibly other malignant disease settings, including ALL.
2. MATERIALS AND METHODS
2.1. Study Design and Patients
The current analysis is based on the data collected in an open-label, multicenter, proof-of-concept phase 1 study in relapsed/refractory NHL (ClinicalTrials.gov, NCT00274742). The objectives of that proof-of-concept study were to investigate the adverse events associated with blinatumomab treatment in patients with relapsed/refractory NHL and to determine a dosing regimen for future studies in NHL. The phase 1 study explored various doses and administration schedules (flat and stepwise dosing; linear dose increases) in a dose escalation phase and in the follow-on extension phase (Supplemental Table 1 (149.3KB, pdf) ). The study design, patient population, and treatment have been described in detail elsewhere [11]. Adult patients with histologically confirmed relapsed or refractory NHL were enrolled from February 2004 through August 2011. Blinatumomab doses of 0.5, 1.5, 5, 15, 30, 60, or 90 µg/m2/day were administered by cIV infusion for 4 weeks using a portable mini-pump. At the end of this 4-week treatment, an additional 4-week consolidation treatment could be administered to patients without disease progression or treatment-limiting adverse events. Approval of the study protocol was obtained from the independent ethics committees at the participating institutions. All patients provided written informed consent before any study-related procedures began.
The primary study endpoint was the overall frequency of adverse events. Secondary endpoints were blinatumomab concentrations in the serum, peripheral B- and T-cell counts, cytokine concentrations in the serum, formation of anti-blinatumomab antibodies, and overall response rate per Cheson criteria [21]. Final primary and key secondary endpoint data have been reported previously elsewhere [11].
Of the 76 patients treated during the study, 11 had one (n=9) or two (n=2) additional 4-week treatments due to clinical benefits. Time between initial and additional 4-week treatments varied (1.5 to 12 months). Data from additional treatment periods were included in the analyses where indicated. For the present analysis, only patients for whom samples were available were included.
2.2. PK and PD Sampling
Peripheral blood samples were collected at screening, prior to the start (baseline) of the treatment and every dose step, and at various time points during the blinatumomab infusion period. Intense samples were scheduled to be collected from 65 patients at 45 minutes and at 2, 6, 12, 24, and 48 hours after infusion initiation, and then once-weekly until the end of infusion. Relatively sparse samples were collected from 11 patients who received a stepwise dose of 5-15-60 µg/m2/day, with sampling specifically undertaken at 48 hours after infusion initiation and when dose was escalated, and then once-weekly until the end of infusion.
2.3. Bioanalytical Methods
2.3.1. Blinatumomab Concentrations in Serum
A validated bioassay using CD69 activation was used to measure blinatumomab concentrations in serum. The assay is based on the principle that upon dual binding of blinatumomab to T cells and B cells, the CD69 activation marker is upregulated on T cells in a blinatumomab concentration-dependent manner. HPB-ALL cells (0.75 to 1.0×106, human T-cell line; German Collection of Microorganisms and Cell Cultures [DSMZ], Braunschweig, Germany) were incubated with Raji cells (3 to 5×105, human B-cell line; DSMZ) in RPMI medium (Invitrogen, Carlsbad, CA, USA) in the presence of blinatumomab serially diluted (200 ng/mL to 0.003 ng/mL) in human serum from healthy volunteers. After incubation, T cells were labeled with fluorescence-conjugated mouse anti-CD69 FITC monoclonal antibody (BD Biosciences, San Jose, CA, USA; No. 555530) and measured in a Fluorescence-Activated Cell Sorter (FACS) unit (FACSCalibur or FACSCanto II; BD Biosciences). Data were calculated using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). The lower limit of quantitation (LLOQ) was 100 pg/mL, and the limit of detection (LOD) was 3 pg/mL [9].
2.3.2. Immunogenicity
Anti-blinatumomab antibodies in patient serum were measured using a validated sandwich enzyme-linked immunosorbent assay (ELISA) or a validated assay based on electrochemiluminescence detection (Meso Scale Discovery, Rockville, MD, USA) using biotin- and ruthenium-conjugated blinatumomab. Both assays were cross-validated to ensure comparability.
For the ELISA, 100 µL/well blinatumomab antigen (0.25 µg/mL) was coated on a microtiter plate, incubated >12 hours at 2 to 8°C, washed in phosphate-buffered saline (PBS) with 0.1% Tween 20 (PBST; Sigma, St. Louis, MO, USA) and blocked (PBST/casein) for 1 to 4 hours at room temperature (RT). After another wash step (PBST), 100 µL of sample/control was added per well and incubated for 1 hour at RT. Polyclonal goat anti-blinatumomab antibodies (MedImmune, Gaithersburg, MD, USA) were used as a positive control. Biotinylated (Thermo Scientific; No. 21425) blinatumomab (0.34 mg/mL) was added (1:1133 dilution, 100 µL/well) and incubated for 1 hour at RT. Plates were washed (PBST), incubated with streptavidin-HRP (1:5000 dilution, 100 µL/well) for 1 hour at RT, and washed again (PBST). TMB substrate was added (100 µL/well) and the reaction stopped after 10 minutes at RT by addition of 2 N H2SO4 (100 µL/well). Optical density (OD) at 450 nm was determined on a Power Wave x-select (BioTek Instruments Inc., Winooski, VT, USA) and the results calculated using KC4 software (BioTek Instruments Inc.). The presence of anti-blinatumomab antibodies was determined relative to the cutoff OD calculated from a panel of six individual blank human serum samples. This assay was designed and validated in accordance with the FDA guideline “Bioanalytical Method Validation” [22].
For the ECL assay, biotin- and ruthenium-conjugated blinatumomab were prepared using a kit from Thermo Scientific (No. 21425) and MSD SULFO-TAG (Meso Scale Discovery), respectively, following the manufacturers’ instructions. Serum samples were diluted 1:10 in PBS and incubated with biotin- and ruthenium-conjugated blinatumomab (each at 0.5 µg/mL) at RT for ≥1 hour. A streptavidin-coated 96-well microtiter plate (Meso Scale Discovery; cat. L15SA-1) was blocked with 5% bovine serum albumin (BSA; Paesel + Lorei GmbH & Co, Duisburg, Germany) in PBS at RT. Serum samples incubated with conjugated blinatumomab were then added to the plate and incubated for 0.5 to 2 hours. The plate was washed three times with PBST. Finally, 150 μL of Reading Buffer (Meso Scale Discovery) was added to each well and the ECL signals were measured immediately using a Sector Imager 2400 analyzer (Meso Scale Discovery). ECL signals were normalized against a predose serum sample tested on the same microtiter plate in order to calculate the patient-specific floating cut point [23]. Each plate also contained a high QC and a low QC positive control (purified polyclonal goat anti-blinatumomab antibodies [Biogenes, Berlin, Germany]) as well as a negative control, to ensure plate validity. Potentially positive samples were retested in a confirmatory assay, in which a drug spike was introduced to block the signal, thereby ensuring drug specificity.
2.3.3. Cytokines
Concentrations of six cytokines in serum of patients (interleukin [IL]-2, IL-4, IL-6, IL-10, interferon [IFN]-γ, and tumor necrosis factor [TNF]-α), were measured using the FACS-based Human Th1/Th2 Cytometric Bead Array Kit (BD Biosciences; No. 551809) and system. IL-8 and IL-12 were measured by ELISA (Diaclone SAS, Besançon, France; No. 850.050.096). The assays were performed according to the manufacturers’ protocols and internally validated. The LOD for cytokine determination was 20 pg/mL; the LLOQ was 125 pg/mL [9].
2.3.4. Peripheral Blood Lymphocytes
Lymphocytes were measured by FACS at various time points throughout treatment and quantified as described previously [9, 10, 13]. Peripheral blood mononuclear cells were isolated using Biocoll (Biochrom GmbH, Berlin, Germany) density gradient centrifugation followed by staining with fluorescence-conjugated monoclonal antibodies (anti-CD3 APC-H7, anti-CD56 PE-Cy7, anti-CD19 APC, anti-CD13 FITC, and anti-CD14 FITC) obtained from various commercial sources. Cell acquisition was performed on either FACSCalibur or FACSCanto II instruments (BD Biosciences),
with subsequent analysis using CellQuest Pro (BD Biosciences) or FACS Express (De Novo Software, Los Angeles, CA, USA) software. Absolute cell counts were obtained by combining FACS-based percentage data and absolute lymphocyte numbers from differential blood cell counts. Samples from 65 patients were analyzed by Amgen Research (Munich) GmbH. The remaining samples were analyzed by SYNLAB International GmbH (Augsburg, Germany) using the same methods but different suppliers of reagents, instruments, and software.
2.4. Determination of Sum of Longest Perpendicular Dimensions (SPD)
In the phase 1 study, tumor response, a secondary study endpoint, was determined per Cheson criteria [21], which are based on SPD. For each patient, SPD was determined across the six largest dominant lymph nodes and nodal masses using computerized tomography. SPD was measured at screening, at study days 29 and 57, and at the end of the study. For patients with incomplete treatment cycles or who withdrew early from the study, SPD was determined at unscheduled time points. Change from baseline (screening) in SPD was used as a PD endpoint.
2.5. PK/PD Data Analysis
2.5.1. Pharmacokinetics
Average individual steady-state concentration (Css) was calculated based on concentrations collected at ≥10 hours from infusion initiation. The systemic clearance (CL) of blinatumomab was calculated for each patient according to the equation CL=Ro/Css, where Ro was the dosing rate. The cumulative area under the concentration-versus-time curve (AUCcum) was calculated as dosecum/CL, where dosecum was the cumulative dose for an individual patient over a given 4-week treatment. Non-compartmental analysis was performed using Phoenix WinNonlin version 6.2.1 software (Certara, St. Louis, MO, USA). Dose proportionality of exposure was assessed by linear regression of mean Css versus dose. For patients with doses ≤5 µg/m2/day, serum concentrations were generally below the LLOQ. Because these patients provided PD data, their Css were estimated from the relationship between exposure and dose.
2.5.2. Depletion of B-lymphocytes
The rate of B-cell depletion was determined at early time points (0 to 6 hours) after infusion initiation. The initial rate of depletion followed first-order kinetics; therefore, the depletion rate constant (λ) was estimated from linear regression of a semi-log plot of B-cell count versus time from baseline (hour 0) until 6 hours after infusion start, using three to four data points. The depletion rate constant was converted into percentage depletion per hour (B-cell–specific depletion rate, kdep) using the equation kdep=(1−e−λ)×100. The relationship between exposure to blinatumomab after first dose (Css) and B-cell‒specific depletion rate was fitted to the simple Emax model kdep=(kdep,max×C)/(EC50+C) using Phoenix WinNonlin version 6.2.1 (Certara USA, Inc.) [24], where kdep,max is the maximum specific depletion rate, C is the exposure (Css), and EC50 is the exposure that led to ½kdep,max.
2.5.3. Change in Tumor Size
SPD at the end of each initial 4-week treatment was used to explore the relationship with corresponding AUCcum over the treatment period or with Css at the highest dose tested in each patient. In patients who withdrew from study early, SPD measurement at the time closest to treatment end was used. The fold-change in SPD from screening to treatment end was used as the effect variable, and either AUCcum or Css was used as the exposure variable. Data were fitted to a sigmoidal inhibitory Emax model, with baseline E=Eo−(Emax×Cɣ)/(EC50ɣ+Cɣ) using Phoenix WinNonlin version 6.2.1 (Certara USA, Inc.) [24], where E (effect) is defined as the change in SPD from screening as a function of exposure; Eo is the baseline change in SPD without drug treatment; Emax is the maximum decrease in SPD relative to Eo; C is the exposure variable (AUCcum or Css); EC50 is the exposure parameter that lead to ½Emax; and ɣ is the sigmoidicity factor. The model fit was optimized by refining the initial estimates and applying weighting factors. The quality of model fitting was assessed by random scatter of residuals, accuracy (percent coefficient of variation, CV%) of parameter estimates, and other available diagnostic tools in Phoenix WinNonlin version 6.2.1 (Certara).
2.5.4. Cytokines
The percentages of patients per dose level who had quantifiable levels (>LLOQ) of cytokines in the serum after the first blinatumomab dose along with the observed peak levels (Cmax) of each cytokine during the first week of treatment at each first-dose level were assessed using descriptive statistics. The time courses of cytokine profiles in patients with and without dose steps were described graphically using GraphPad Prism software (GraphPad Software).
3. RESULTS
3.1. Patient Population
A total of 76 adult patients with relapsed or refractory NHL were enrolled and received treatment with blinatumomab in the phase 1 study. The median age was 65 years (range, 20−80); 75% of patients were male. Lymphoma histology included follicular lymphoma (n=28 patients), mantle cell lymphoma (n=24), diffuse large B-cell lymphoma (n=14), or other lymphomas (n=10). Complete patient demographics and baseline characteristics along with the primary analysis data are available elsewhere [11].
3.2. Pharmacokinetics
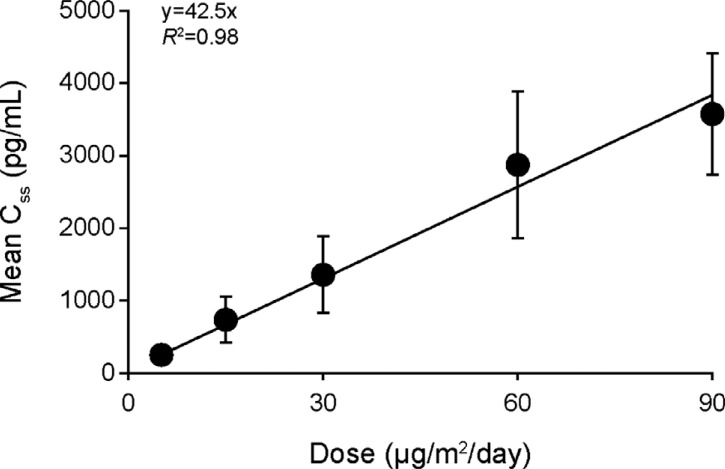
Following start of blinatumomab cIV infusion, steady- state levels of blinatumomab in serum were quickly reached (within 1 day), and exposure to blinatumomab was dose-proportional within the tested range. Mean (SD) values of blinatumomab Css at doses of 5, 15, 30, 60, and 90 µg/m2/day were 210 (85), 651 (307), 1210 (476), 2730 (985), and 3490 (904) pg/mL, respectively. The relationship between mean Css and dose was fitted to the linear regression equation y=42.5×x, with a correlation coefficient of R2=0.98 (Fig. 1). Mean Css at doses of 0.5 and 1.5 µg/m2/day were extrapolated for modeling purposes and were 21 and 63 pg/mL, respectively. PK was constant with time. The average CL (CV%) was 2.25 (52) L/h, and the average serum half-life (CV%) was 2.44 (66) hours.
Fig. (1).
Dose proportionality of blinatumomab steady state concentration (Css) across 67 patients with relapsed/refractory NHL who received blinatumomab at doses from 5 to 90 µg/m2/day. Patients receiving dose steps and/or more than one treatment period provided more than one data point. Number of data points per dose was as follows: 5 μg/m2/day, n=26; 15 μg/m2/day, n=37; 30 μg/m2/day, n=8; 60 μg/m2/day, n=40; 90 μg/m2/day, n=4.
3.3. Immunogenicity
From the 76 patients treated with blinatumomab, samples from 74 patients were available for immunogenicity analysis. Paired serum samples (including screening and end-of-study visit) were available from 62 (82%) of those patients. For 11 patients no end-of-study samples were available. For one patient, no screening sample was available, and the end-of-study sample was negative. None of the analyzed patients developed any detectable anti-blinatumomab antibodies during the duration of the study. Of the 11 patients who received additional treatment with blinatumomab, four patients had samples available for testing of anti-blinatumomab antibodies after the treatment. All four samples tested negative.
3.4. Pharmacodynamics
3.4.1. B-cell Depletion
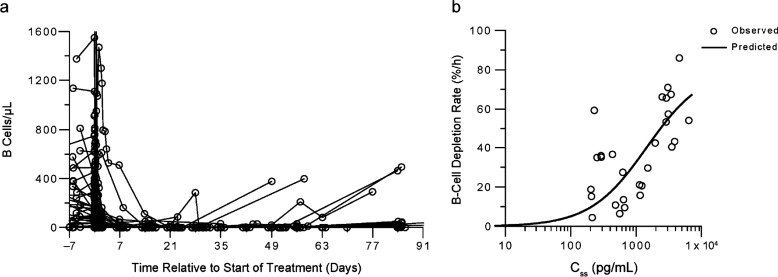
Among all 76 patients, baseline B-cell levels before the initial 4-week treatment varied between zero and approximately 5,000 cells/µL of peripheral blood. One patient who had 12,285 cells/µL at baseline was excluded from the analysis because of an erratic profile. Despite variable baseline levels, once blinatumomab infusion started, B-cell counts declined quickly in the peripheral blood. Forty-nine patients had measurable B-cells before drug administration. Patients treated with blinatumomab at doses ≥5 μg/m2/day showed complete B-cell depletion within 24 to 48 hours after treatment start (Fig. 2a); therefore, there was not a clear dose-response relationship. B-cell counts remained mostly undetectable during treatment and follow-up in all but five patients−one receiving 60 μg/m2/day and four receiving 15 μg/m2/day−who showed B-cell recovery during follow-up (≥4 weeks after end of treatment; Fig. 2a). Patients who received blinatumomab at doses of 0.5 or 1.5 μg/m2/day (n=9) did not show a complete depletion.
Fig. (2).
Peripheral B-cell depletion in patients with relapsed/refractory NHL receiving blinatumomab at doses of ≥5 μg/m2/day. a Time profiles of Peripheral B-cell counts from 49 patients with detectable baseline B cells; the effect at different doses could not be distinguished. b Initial B-cell depletion rates as a function of Css after first dose (n=28) of first treatment period.
Of the 76 patients treated in the study, 28 (37%) had measurable B cells before and during treatment so that the B-cell depletion rate could be assessed. The other patients had either undetectable or very low (<10 cells/µL) peripheral B-cell counts at baseline, or did not have sufficient data at time points between 0 and 6 hours. The estimated B-cell depletion rate constant from baseline (kdep) ranged from 4.5% to 86% per hour. The relationship of Css after the first dose and kdep was fitted to an Emax model (Fig. 2b). The estimated kdep,max was 81% per hour and the EC50 was 1,456 pg/mL. The quality of model fit was considered acceptable (CV% in parameter estimates <30%). Residuals were randomly scattered around the x-axis.
3.4.2. Exposure‒SPD Relationship
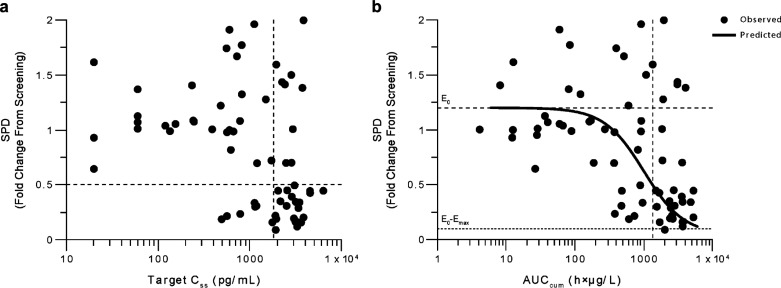
Of the 76 patients treated, data from five patients were excluded because of early blinatumomab discontinuation. For the remaining 71 patients, baseline SPD ranged from 2.3 to 261 cm2, with a mean (SD) of 45.5 (49.5) cm2. The change in SPD from screening to end of treatment ranged from 0.1-fold (90% reduction) to 2.0-fold (100% increase) and was blinatumomab exposure‒dependent (Fig. 3). Data from one patient who received stepwise blinatumomab dosing of 5‒15‒60 μg/m2/day (AUCcum=585 h×µg/L, Css=2,905 pg/mL) were considered outliers and were excluded from the exposure–SPD analysis, because the SPD increased 3.2-fold from screening. Because patients were treated with different dose schedules (flat or stepwise dosing; Supplemental Table 1 (149.3KB, pdf) ) and duration of treatment varied, two exposure metrics were used to describe the exposure‒SPD relationship (ie, AUCcum and Css). The Css‒SPD relationship did not fit a PD model due to high data variability and confounded with the step-dosing regimen. However, a group of 26 patients had a Css of ≥1,830 pg/mL, of whom 21 (70%) showed a reduction in tumor size by ≥ 50% (Fig. 3a). Therefore, Css values of ≥1,830 pg/mL were selected as target concentration range for antilymphoma activity. The AUCcum‒SPD relationship showed a greater correlation and was fitted to a sigmoidal inhibitory Emax model (Fig. 3b). There were 68 patients with AUCcum data for the model, and the model parameter estimates are shown in Table 1. The quality of fit was considered acceptable. Residuals or weighted residuals were randomly scattered around the x-axis. The AUCcum‒SPD model predicted that an AUCcum of 1,340 h×µg/L would result in a 50% (0.5-fold) reduction of SPD from baseline. Without blinatumomab treatment, the model predicted a 20% (1.2-fold) increase of SPD from baseline. Therefore, a patient may achieve a 50% reduction of SPD at an AUCcum of at least 1,340 h×µg/L and a Css of at least 1,830 pg/mL, which corresponds with a blinatumomab dose of at least 47 µg/m2/day administered for a duration (T) of 28 days using the PK dose proportionality equation Css (pg/mL)=42.5×dose (µg/m2/day) (Fig. 1) and the equation, time (day)=(AUCcum×1000)/(Css×24).
Fig. (3).
Exposure‒pharmacodynamic response relationship in patients with relapsed/refractory NHL. a Analysis using target Css and SPD (n=66). b Analysis using AUCcum and SPD (n=68) with model fitting. The horizontal dotted line corresponds to a Css of 1,830 pg/mL leading to a ≥50% decrease of SPD in 70% of patients. AUCcum cumulative area under the concentration-versus-time curve, Css concentration at steady state, SPD sum of the products of the greatest diameters.
Table 1.
PD model parameters of AUCcum‒SPD relationship in patients with relapsed/refractory NHL.
| All Patients (N = 68)a | ||||||
|---|---|---|---|---|---|---|
| Eo | Emax | Eo‒Emax | EC50 | ɣ | AUCcum at 0.5-fold SPD Change | |
| Parameter estimate | 1.20-fold (20% increase) |
1.15 | 0.05-fold (95% decrease) |
1,000 h×µg/L | 1.50 | 1,340 h×µg/L |
| CV% | 13% | 32% | ‒ | 45% | 60% | ‒ |
AUCcum cumulative area under the concentration-versus-time curve, Css concentration at steady state, EC50 median effective concentration, ɣ sigmoidicity factor, Emax maximum decrease in SPD relative to Eo, Eo baseline change in SPD from screening prior to blinatumomab treatment, NHL non-Hodgkin lymphoma, PD pharmacodynamics, SPD sum of the products of the largest diameters. CV% reflects accuracy in parameter estimates. aAll patients with evaluable samples.
3.4.3. Cytokine Elevation
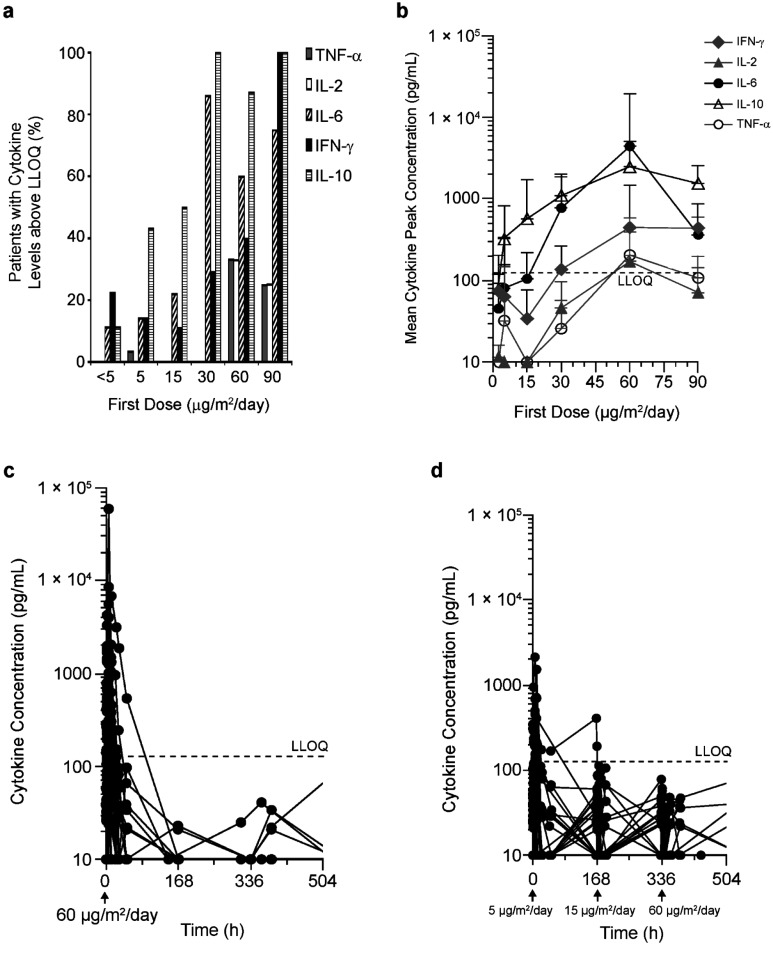
Over a blinatumomab dose range of 0.5 to 90 µg/m2/day, the proportion of patients with quantifiable cytokine levels in serum after the start of blinatumomab infusion increased with dose (Fig. 4a). Cytokine elevation was transient and occurred predominantly after the start of blinatumomab infusion, peaked within 24 hours, and then declined within 2 days. At start of subsequent 4-week treatments (n=11 patients), cytokine elevation of a lower intensity was observed. Cytokines with quantifiable levels in serum included TNF-α, IL-2, IL-6, IL-10, and IFN-γ. The most prominent cytokines were IL-6, IL-10, and IFN-γ, which were elevated in ≥50% of patients who received doses of ≥30 µg/m2/day (n=51). TNF-α and IL-2 were quantifiable in 10% and 9% of patients, respectively; IL-4, IL-8, and IL-12 were not detectable in any of the patients analyzed.
Fig. (4).
a) Proportion of patients with serum cytokine levels above the lower limit of quantification (LLOQ) after blinatumomab infusion start. Number of patients per first dose level: <5 µg/m2/day, n=9; 5 µg/m2/day, n=35; 15 µg/m2/day, n=18; 30 µg/m2/day, n=7; 60 µg/m2/day, n=15; 90 µg/m2/day, n=4. b) Cytokine Cmax (mean SD) in the first week of treatment as a function of blinatumomab dose. Number of patients per first dose level: <5 µg/m2/day, n=9; 5 µg/m2/day, n=35; 15 µg/m2/day, n=18; 30 µg/m2/day, n=7; 60 µg/m2/day, n=15; 90 µg/m2/day, n=4. Data below LLOQ were included as such into the analysis; data below LOD were set at 10 pg/mL (ie, ½LOD). c) Cytokine profiles after continuous IV blinatumomab doses of 60 µg/m2/day throughout treatment (n=9); elevation of cytokines was transient and declined quickly during infusion. d) Cytokine time profiles after stepwise blinatumomab dosing of 5‒15‒60 µg/m2/day (n=21); elevation of cytokines was transient and did not increase with increasing dose. Cmax observed peak concentrations, IFN interferon, IL interleukin, LLOQ lower limit of quantitation, TNF tumor necrosis factor.
Cytokine serum Cmax generally increased with blinatumomab dose, particularly above 15 μg/m2/day (Fig. 4b). The blinatumomab target dose of 60 µg/m2/day was tested in both flat and stepwise dosing regimens. Stepwise dosing (5‒15‒60 µg/m2/day) showed less pronounced cytokine elevation after first dose and subsequent dose steps, compared with a flat dose of 60 µg/m2/day (Fig. 4c and 4d). There was a large interpatient variability in cytokine Cmax; mean (SD) values after a first dose of 60 µg/m2/day were 2,460 (2,643), 4,409 (15,177), 446 (1,013), 171 (224), and 205 (370) pg/mL for IL-10, IL-6, IFN-γ, IL-2, and TNF-α, respectively.
4. DISCUSSION
In this phase 1 study, patients with relapsed/refractory NHL received blinatumomab for 4 or 8 weeks. As reported previously [11], neurologic events were dose limiting, and 60 μg/m2/day was established as the maximum tolerated dose. Among patients treated who received the target dose of 60 μg/m2/day (n=35), the overall response rate was 69% across NHL subtypes [25-27]. In the present assessment, we explored the pharmacokinetic and pharmacodynamic relationships of blinatumomab in patients with NHL.
In this phase 1 study, the PK parameters of blinatumomab were stable over time while Css increased in a dose-proportional manner. PK data were consistent with those reported in patients with ALL [9]. Following blinatumomab cIV infusion, PD was characterized by peripheral B-cell depletion, cytokine elevation, and tumor reduction. The changes of these PD endpoints were dependent on blinatumomab exposure.
B-cell depletion was rapid, with B-cell levels dropping to zero within 48 hours in almost all patients receiving doses of ≥5 μg/m2/day, regardless of B-cell levels at baseline. The initial rate of depletion followed first-order kinetics, was exposure-dependent (Css), and could be fitted to an Emax model. This model can be used to predict how much time is required for a patient to have complete peripheral B-cell depletion at a given baseline count and a given dose. The depletion of B cells in patients with NHL enrolled in the present study was similar to that observed in studies of blinatumomab for the treatment of ALL [9] and is consistent with the in vitro pharmacology of the drug and its mode of action [8]. The variability among patients in B-cell count in the peripheral blood at baseline was high, with 17 of 67 patients treated at doses ≥5 µg/m2/day having had a zero B-cell count in blood before the first treatment period. This variability might be related to variations in disease state and previous anti-lymphoma therapies. The cIV infusion of blinatumomab is necessary for a comprehensive and sustained depletion of B cells throughout treatment and during the 4-week follow-up, because the B-cell depletion was not observed in earlier phase 1 studies in which blinatumomab was administered by repeated short-term IV infusions [28]. Peripheral CD19-positive B cells recovered slightly during follow-up in five patients; baseline lymphocyte counts and baseline tumor size were not different in these patients compared with other patients in the study. Sustained depletion of B cells, which would suppress immunoglobulin production, may also explain the absence of immunogenicity against blinatumomab in this study despite the murine origin of the molecule.
Despite complete B-cell depletion within 48 hours in peripheral blood at blinatumomab doses of ≥5 μg/m2/day, higher doses and longer treatment duration were required to achieve better treatment response. Generally, antibody penetration of tissue is mainly driven by convection and porosity of the paracellular path [29, 30]. In NHL, the accessibility of blinatumomab to B cells within the tumor might be more limited than it is in peripheral blood and bone marrow (as in ALL, for example) because of the tight, dense structure of lymph nodes and extralymphatic lymphoma tissue. Likewise, the influx of T cells into lymphoma lesions may be limited and the expansion of intralesional T cells is expected to take time before reaching a critical threshold translating into tumor shrinkage. These factors may contribute to the difference in dose and time required to achieve effects in tissues versus the blood.
Our observations suggest the involvement of two PD processes in B-cell depletion in NHL: one in the easily accessible blood compartment with immediate, intense B-cell killing associated with cytokine release; and a second, slower process in the deep compartments of lymphoid tissues and extralymphatic lymphoma tissue. In ALL, blinatumomab doses between 15 and 30 μg/m2/day resulted in antileukemia activity [14, 15, 31]. The higher accessibility to the site of action in ALL (bone marrow), compared with NHL (lymph nodes), for both blinatumomab and T cells migrating from outside to inside the target tissue is a potential reason for the difference in dose requirements between the two B cell malignancies. In both cases, B-cell depletion in the peripheral blood has been identified as a prerequisite of clinical response and is an immediate PD marker. The observation that B-cell depletion rate in blood and extent of tumor reduction in tissues are both dependent on blinatumomab exposure, suggests an association between these two PD effects. Systems pharmacology models are currently being developed to explore a possible linkage of both compartments, using broader data sets from across various studies [32].
The exposure–SPD model indicated exposure-dependent reduction in tumor size, with a higher proportion of patients showing SPD reduction from baseline at higher doses. To achieve SPD reduction of ≥50%, the minimum reduction required to qualify as a clinical response according to Cheson criteria [21], the PK/PD model predicts that Css ≥1,830 pg/mL and AUCcum ≥1,340 h×µg/L would be required, which is equivalent to a dose of ≥47 μg/m2/day for 28 days. In this phase 1 study, 60 µg/m2/day was identified as the maximum-tolerated dose [11]. Of the 26 patients who achieved the above thresholds for Css and AUCcum, 20 had an SPD reduction from baseline of at least 50% and therefore, were considered partial or complete responders per Cheson criteria. Of note, the presented analysis only included response data after the initial 4-week treatment. However, some patients achieved their best response (i.e., SPD reduction) only after 8 weeks of treatment or after the 4-week follow-up period. It might be speculated that despite efficient tumor cell eradication during the initial 4-week treatment, measurable tumor shrinkage might be delayed in NHL due to slower degradation of secondary tumor structures in the lymphoid microenvironment. Thus, tumor response rates might be underestimated. Based on the PK data from this analysis, PK/PD modeling can help in designing dosing regimens for future studies of blinatumomab in NHL. Furthermore, because relapsed/refractory NHL is more similar to bulky disease in solid tumors than to ALL, the PK/PD relationships reported here may also guide the evaluation of BiTE® antibody constructs in solid tumor settings.
The elevation of cytokines in serum was mainly a first-dose effect that occurred within the same time frame (48 hours) as peripheral B-cell depletion, and it was self-limiting under continued blinatumomab treatment. Despite high variability in cytokine levels, there was an obvious relationship between incidence and magnitude of cytokine elevation and initial blinatumomab dose; this was particularly apparent between doses of 15 and 30 μg/m2/day. The differences between doses of 5 and 15 μg/m2/day were minor. Based on in vitro data [8, 17], it could be hypothesized that the transient cytokine elevation after the first dose augments the immune response and intensifies T-cell recruitment and tumor cell killing. However, considering the high variability of cytokine levels and the small sample size of the current analysis, this hypothesis could neither be confirmed nor rejected.
CONCLUSION
In conclusion, in this phase 1 study of blinatumomab for the treatment of relapsed/refractory NHL, PK parameters were consistent with the previously published data in ALL. PD data showed rapid, complete depletion of peripheral B-lymphocytes at blinatumomab doses ≥5 μg/m2/day. Cytokine elevation was transient and dose-dependent. SPD decreased with increased exposure to blinatumomab, with the exposure–SPD model suggesting a minimum target dose of 47 µg/m2/day for 28 days to achieve activity in NHL. The observation that both B-cell depletion and tumor size reduction have been shown to be the functions of blinatumomab exposure indicates an association between these two PD effects.
Acknowledgements
The authors acknowledge Beate Quednau, PhD (Amgen Inc., Thousand Oaks, CA), and Erica Chevalier-Larsen and Diane Warner (Complete Healthcare Communications Inc., Chadds Ford, PA), as well as Ben Scott, PhD (Scott Medical Communications, LLC), whose work was funded by Amgen Inc., for assistance in editing and formatting this manuscript.
list of ABBREVIATIONS
- ALL
Acute Lymphoblastic Leukemia
- AUC
Area Under the Concentration-versus-time curve
- AUCcum
Cumulative AUC
- BiTE®
Bispecific T-cell Engager
- BSA
Bovine Serum Albumin
- cIV
Continuous Intravenous Infusion
- CL
Clearance
- Css
Steady-state concentration
- DLBCL
Diffuse Large B-cell Lymphoma
- EC50
Median effective concentration
- Eo
Baseline change in SPD from screening prior to treatment
- ECL
Electrochemiluminescence
- ELISA
Enzyme-Linked Immunosorbent Assay
- Emax
Maximum decrease in SPD relative to Eo
- FACS
Fluorescence-Activated Cell Sorter
- IFN
Interferon
- IL
Interleukin
- LLOQ
Lower Limit of Quantitation
- LOD
Limit of Detection
- NHL
Non-Hodgkin lymphoma
- PBS
Phosphate-buffered saline
- PBST
PBS Tween
- PK
Pharmacokinetic
- PD
Pharmacodynamic
- RT
Room Temperature
- SPD
Sum of the products of the largest diameters
- TNF
Tumor necrosis factor
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
AUTHOR CONTRIBUTIONS
Designed research/study: Patrick Baeuerle. Performed research/study: Matthias Klinger, Andrea Kratzer, Peter Kufer. Collected or analyzed data: Youssef Hijazi, Matthias Klinger, Andrea Kratzer, Benjamin Wu, Andreas Wolf, Dirk Nagorsen, Min Zhu. Contributed to manuscript writing: Youseff Hijazi, Matthias Klinger, Benjamin Wu, Patrick Baeuerle, Peter Kufer, Andreas Wolf, Dirk Nagorsen, Min Zhu.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study will be conducted in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice (E6) and all applicable laws and regulations.
HUMAN AND ANIMAL RIGHTS
No Animals were used in the study. All research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation including the Declaration of Helsinki, June 1964, as modified by the 48th World Medical Association, Somerset West, Republic of South Africa, October 1996 (cf. Appendix 1).
CONSENT FOR PUBLICATION
All patients provided written informed consent before any study-related procedures began.
Conflict of Interest
This work was supported by Amgen Inc. At the time this work was conducted, Youssef Hijazi and Patrick A. Baeuerle were employees of and shareholders in Amgen Research (Munich) GmbH, and Benjamin Wu and Min Zhu were employees of and shareholders in Amgen Inc. Matthias Klinger, Andrea Kratzer, Peter Kufer, and Andreas Wolf are employees of shareholders in Amgen Research (Munich) GmbH, and Dirk Nagorsen is an employee of and shareholder in Amgen Inc. Dirk Nagorsen is an inventor on blinatumomab patents. Matthias Klinger received royalties for patent US8007796B2. Peter Kufer receives royalties related to blinatumomab.
REFERENCES
- 1.Ferlay J., Bray F., Pisani P., Parkin D.M. Globocan 2000. Cancer Incidence, mortality and prevalence worldwide. Lyon, France: IARC Press; 2001. [Google Scholar]
- 2.Armitage J.O., Weisenburger D.D. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’slymphoma classification project. J. Clin. Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 3.Harris N.L., Nadler L.M., Bhan A.K. Immunohistologic characterization of two malignant lymphomas of germinal center type (centroblastic/centrocytic and centrocytic) with monoclonal antibodies. Follicular and diffuse lymphomas of small-cleaved-cell type are related but distinct entities. Am. J. Pathol. 1984;117:262–272. [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson B.D., Leonard J.P. Monoclonal antibody therapy for B-cell non-Hodgkin’s lymphoma. N. Engl. J. Med. 2008;359:613–626. doi: 10.1056/NEJMra0708875. [DOI] [PubMed] [Google Scholar]
- 5.Fisher R.I., LeBlanc M., Press O.W., Maloney D.G., Unger J.M., Miller T.P. New treatment options have changed the survival of patients with follicular lymphoma. J. Clin. Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 6.Komrokji R.S., Al Ali N.H., Beg M.S., et al. Outcome of diffuse large B-cell lymphoma in the United States has improved over time but racial disparities remain: review of SEER data. Clin. Lymphoma Myeloma Leuk. 2011;11:257–260. doi: 10.1016/j.clml.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Dreier T., Baeuerle P.A., Fichtner I., et al. T cell costimulus-independent and very efficacious inhibition of tumor growth in mice bearing subcutaneous or leukemic human B cell lymphoma xenografts by a CD19-/CD3- bispecific single-chain antibody construct. J. Immunol. 2003;170:4397–4402. doi: 10.4049/jimmunol.170.8.4397. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann P., Hofmeister R., Brischwein K., et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int. J. Cancer. 2005;115:98–104. doi: 10.1002/ijc.20908. [DOI] [PubMed] [Google Scholar]
- 9.Klinger M., Brandl C., Zugmaier G., et al. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood. 2012;119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 10.Bargou R., Leo E., Zugmaier G., et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 11.Goebeler M.E., Knop S., Viardot A., et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-hodgkin lymphoma: final results from a phase I study. J. Clin. Oncol. 2016;34:1104–1111. doi: 10.1200/JCO.2014.59.1586. [DOI] [PubMed] [Google Scholar]
- 12.Viardot A., Goebeler M.E., Hess G., et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–1416. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topp M.S., Kufer P., Gokbuget N., et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 14.Topp M.S., Gokbuget N., Stein A.S., et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16:57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 15.Topp M.S., Gokbuget N., Zugmaier G., et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J. Clin. Oncol. 2014;32:4134–4140. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 16.BLINCYTO®. Full prescribing information. Thousand Oaks, CA: Amgen Inc.; 2017. [Google Scholar]
- 17.Breimer D.D., Danhof M. Relevance of the application of pharmacokinetic-pharmacodynamic modelling concepts in drug development. The “wooden shoe’ paradigm. Clin. Pharmacokinet. 1997;32:259–267. doi: 10.2165/00003088-199732040-00001. [DOI] [PubMed] [Google Scholar]
- 18.Derendorf H., Meibohm B. Modeling of pharmacokinetic/ pharmacodynamic (PK/PD) relationships: concepts and perspectives. Pharm. Res. 1999;16:176–185. doi: 10.1023/a:1011907920641. [DOI] [PubMed] [Google Scholar]
- 19.Peck C.C. Quantitative clinical pharmacology is transforming drug regulation. J. Pharmacokinet. Pharmacodyn. 2010;37:617–628. doi: 10.1007/s10928-010-9171-3. [DOI] [PubMed] [Google Scholar]
- 20.Zhu M., Wu B., Brandl C., et al. Blinatumomab, a bispecific T-cell engager (BiTE®) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin. Pharmacokinet. 2016;55:1271–1288. doi: 10.1007/s40262-016-0405-4. [DOI] [PubMed] [Google Scholar]
- 21.Cheson B.D., Horning S.J., Coiffier B., et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. NCI Sponsored International Working Group. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 22. http: //www.fda.gov/ucm/ groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm 070107.pdf
- 23.Shankar G., Devanarayan V., Amaravadi L., et al. Recommendations for the validation of immunoassays used for detection of host antibodies against biotechnology products. J. Pharm. Biomed. Anal. 2008;48:1267–1281. doi: 10.1016/j.jpba.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Gabrielsson J., Weiner D. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications. 5th ed. Stockholm: Swedish Pharmaceutical Press; 2017. [Google Scholar]
- 25.Pishko A., Nasta S.D. The role of novel immunotherapies in non-Hodgkin lymphoma. Transl. Cancer Res. 2017;6:93–103. doi: 10.21037/tcr.2017.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders S., Stewart D.A. Targeting non-Hodgkin lymphoma with blinatumomab. Expert Opin. Biol. Ther. 2017;17:1013–1017. doi: 10.1080/14712598.2017.1334053. [DOI] [PubMed] [Google Scholar]
- 27.Wilke A.C., Gokbuget N. Clinical applications and safety evaluation of the new CD19 specific T-cell engager antibody construct blinatumomab. Expert Opin. Drug Saf. 2017;16:1191–1202. doi: 10.1080/14740338.2017.1338270. [DOI] [PubMed] [Google Scholar]
- 28.Nagorsen D., Kufer P., Baeuerle P.A., Bargou R. Blinatumomab: a historical perspective. Pharmacol. Ther. 2012;136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Baxter L.T., Zhu H., Mackensen D.G., Jain R.K. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. [PubMed] [Google Scholar]
- 30.Lobo E.D., Hansen R.J., Balthasar J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004;93:2645–2668. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 31.Topp M.S., Gökbuget N., Stein A.S., et al. Confirmatory open-label, single-arm, multicenter phase 2 study of the BiTE antibody blinatumomab in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL). J. Clin. Oncol. 2014;32:7005. [Google Scholar]
- 32.Singh I., Yuraszeck T., Klinger M., et al. A systems pharmacology model to characterize the effect of blinatumomab in patients with adult B-precursor acute lymphoblastic leukemia (B-ALL). Clin. Pharmacol. Ther. 2014;95:BP-007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.