Abstract
Background
Proficiency testing of enzymatic methods for plasminogen activator inhibitor 1 (PAI-1) showed a systematic variability indicating the aspecificity of some methods.
Methods
To define and detect specificity of enzymatic methods for PAI-1, experts of the ISTH/SSC subcommittee defined criteria and test samples to check the criteria. 16 samples were prepared to test (a) specificity in depleted plasma, (b) interference by added PAI-2 or PAI-3, (c) interference by added tissue-type plasminogen activator (t-PA), (d) performance in dose response. To exercise the test procedure participants were recruited via the subcommittee, literature and company data. Coded samples were distributed to participants. The NIBSC standard for PAI-1 activity was included for the normalisation of results. Adherence to the predetermined criteria was judged blind in the ISTH/SSC subcommittee meeting.
Results
In total 17 laboratories with 15 different assay methods participated in the study. Methods were based on four detection principles. 8 methods failed to detect sufficiently low activity in depleted plasma and were sensitive to added PAI-2. 10 methods were sensitive to interference by endogenous t-PA as revealed by additions of t-PA. Not all methods could adequately measure in acidified plasma. Two methods were fulfilling all the criteria
Conclusions
Most methods were not specific for PAI-1 (enz. procedure) in acidified plasma. Definition of criteria and test methods by experts of the ISTH/SSC proved a valuable concept, according to the exercise undertaken.
In this article.
Proficiency testing of enzymatic methods for plasminogen activator inhibitor 1 (PAI-1) showed a systematic variability indicating the aspecificity of some methods.
Introduction
Proficiency testing of assays for the fibrinolytic variable Plasminogen activator inhibitor 1 (PAI-1, imm; procedure, enz; procedure) has been executed by collaborative studies within the framework of the SSC (Scientific and Standardisation Committee) of the ISTH (International Society on Thrombosis and Haemostasis) and showed large interlaboratory and intermethod variation (1,2).
In particular the results of studies on the activity methods for PAI-1 showed a systematic variation which could not expected to be solved by harmonising with a suitable standard. It was decided that an effort was required to define criteria for specificity of enzymatic PAI-1 methods. The situation was more complex since many methods were in use both as commercial methods and in-house methods and documentation was insufficient for decisions on specificity. It was decided that in addition to defining criteria an exercise would be executed by providing a set of samples to test the methods for the specificity claimed or theoretically expected.
The present report, in addition to defining criteria and test samples describes an exercise of experimental testing and decisions based on the data and criteria. The data have thus far only been presented without the identification of the methods involved.
Materials and Methods
Samples
Sample A was a PAI-1-depleted plasma sample prepared by immunoadsorption of plasma as previously described (3); samples B, C and D were prepared by adding a defined amount of purified reactivated recombinant PAI-1 to depleted plasma (4).
Samples E and F were prepared by adding to immunodepleted plasma a defined amount of recombinant PAI-2 (Delta Biotechnology Ltd, Nottingham, UK; batch 25/7; purity >95%) (plasma dilution < 1%), or PAI-3 / Protein C inhibitor (Technoclone GMBH, Vienna, Austria) (plasma dilution 30%).
Platelet-poor plasma for sample G (see table I) was prepared as a pooled plasma from blood collected from 15 volunteers in 0.129 mol/l of sodium citrate (1:10), kept on ice and centrifuged within 30 minutes at 2000g for 20 minutes at 4_C. PAI-1 was inactivated by incubation of the pooled plasma for 24 hours at 37_C according to (5).
Table I.
| Sample code | Sample characteristics |
|---|---|
| A | PAI-1 immunodepleted |
| B | A + 4.6 U/ml r-PAI-1 |
| C | A + 17.0 U/ml r-PAI-1 |
| D | A + 55.8 U/ml r-PAI-1 |
| E | A + 50 ng/ml r-PAI-2 |
| F | A + 5.5_g/ml PAI-3 |
| G | PAI-1 inactivated by incubation at 37_C |
| H | Stabilyte plasma (PAI activity 7 U/ml) |
| I | H + 4 IU/ml tc-tPA |
| J | Stabilyte plasma (PAI activity 15 U/ml) |
| K | J + 7 IU/ml tc-t-PA |
| L | NIBSC standard 87/512 (25 U/ml) |
| M | NIBSC standard 92/654 (unlabelled) |
Stabilyte plasma for samples H,I,J,K was prepared from blood of volunteers collected in Stabilyte®tubes (6)(Biopool AB Umea, Sweden) and handled as above. PAI-activity was initially assessed using method 4, and retrospectively assigned using method 14 and expressed in U/ml (NIBSC 87/512). Two samples with 7 U/ml and 15 U/ml were used. Samples I and K were spiked with a specified amount of concentrated two-chain melanoma tissue-type plasminogen activator (t-PA) (7) (plasma dilution < 1%) with a value assigned according to t-PA activity assay using method 4.
Samples L and M were NIBSC 87/512 and 92/654 from the National Institute for Biological Standards and Control, Potters Bar, UK and were provided as lyophilised samples. NIBSC 87/512 had a certified value of 25 IU/ml; 92/654 was certified later with 27,5 IU/ml (8).
Methods
Assay 1 is an assay based on the measurement of t-PA/PAI-1 complexes present in the sample before addition and after addition of an excess of t-PA (9). The difference between the two values reflects the amount of active PAI-1. Assay was performed by P.J. Declerck and I. Knockaert using lot MA-15H12/MA-62E8-HRP, detection limit 1 U/ml.
Assay 2 is an assay based on the measurement of t-PA/PAI-1 complexes present in the sample before addition and after addition of an excess of t-PA (10). The difference between the two values reflects the amount of active PAI-1. The study was performed by Dr W. Nieuwenhuizen and R. Laterveer using a prefinal version without addition of PPACK and not yet adapted for use on acidic plasma as in the reference (10); detection limit 0.4 ng/ml.
Assay 3 measures active PAI-1 by addition of excess single chain t-PA and measurement of t-PA-PAI-1 complex in both the original and treated samples. Active PAI-1 is determined by subtraction and expressed as ng/ml (11). N. Booth and A.M. Croll performed the study.
Assay 4 is a titration assay of PAI-1 in the sample with a series of two-chain melanoma t-PA concentrations according to Verheijen et al (12) using CNBr fragments to stimulate t-PA activity and measurement of plasmin/chromogenic substrate activity. Results are expressed in t-PA international units calibrated against NIBSC 83/517. C.Kluft and P. Meijer performed the study.
Assay 5 is Coatest® PAI from Chromogenix AB, Molndal, Sweden and measures residual single chain t-PA after incubation with plasma, by means of chromogenic substrate activity of plasmin formed in 50 minutes in the presence of a fibrin-derived stimulator (13). S. Rosen and L. Wejkum performed the study on lot X0074 employing the manual version in tubes. Results are expressed in AU/ml; 1 AU/ml PAI activity being defined as the amount required to inhibit 1 IU/ml t-PA.
Assay 6 is SpectrolyseÔ/pl Biopool AB, Umea, Sweden; a two-stage indirect enzymatic assay assessing residual melanoma single chain t-PA after incubation with plasma (14-16). t-PA is measured after an acidification step to destroy plasma plasmin inhibitors and by its ability to generate plasmin activity on a chromogenic substrate in the presence of the stimulator poly-lysine. Results are expressed in units defined as the amount of PAI that inhibits 1 IU of human sct-PA calibrated against NIBSC 83/517. A Takada and T Urano performed the study using v1-1, lot 1102025.
Assay 7 is also SpectrolyseÔ/pl (see assay 6) and performed by I. Juhan-Vague and J. Ansaldiusingv1-1, lot 1102025.
Assay 8 is a Bioimmunoassay employing anti-PAI-1 IgG coated on wells, incubation with the sample, incubation after washing with two-chain t-PA and a subsequent assay of residual t-PA activity by its ability to convert Glu-plasminogen assessed by the activity of plasmin formed on H-D-Val-Leu-Lys-pNA. One unit is defined as the amount inhibiting 1 unit of two-chain t-PA. K. Okada and O. Matsuo performed the study.
Assay 9 is the simplified version of the SOFIA-PAI assay (17) measuring residual melanoma sct-PA after incubation with plasma. Residual t-PA is bound to solid-phase fibrin. t-PA activity is assessed by plasmin activity measured on a chromogenic substrate after addition of Glu-plasminogen. PAI activity is expressed in units defined relative to single-chain t-PA calibrated against NIBCS 83/517. The assay is not designed for acidified plasma. E. Angles-Cano and S. Loyau performed the study.
Assay 10 is TC® Actibind-PAI-1 from Technoclone GMBH, Vienna, Austria and is based on the immobilisation of functionally active t-PA to plates by means of a monoclonal antibody. PAI-1 in the test sample binds to t-PA and is then quantified using labelled monoclonal anti-PAI-1 antibody. Results are expressed in U/ml of a PAI standard; detection limit 4.0 U/ml. B.R. Binder and R. Beckmann performed the study with lot 593 of TC 16070. Assay 11 is TC® R Actibind-PAI-1 CL, which is a prototype kit not further developed, based on method 10, however, with t-PA cross-linked to the monoclonal antibody, using a PAI-1 standard calibrated according to NIBSC 87/512.
Assay 12 is TC® PAI activity kit from Technoclone GMBH, Vienna, Austria. And is based on the inhibition of rt-PA added to plasma. Remaining t-PA activity is determined by its Glu-plasminogen activating properties stimulated by CNBr-fragments of fibrinogen and a synthetic plasmin substrate. Plasmin inhibitor is quenched by a special reagent.(18). Results are expressed in U/ml of a PAI standard. B.R. Binder and V. Carroll performed the study with lot 593 of TC 15070. The method was not suitable for Stabilyte plasma and results on samples H-K should be interpreted accordingly. The high recording in PAI-depleted samples in this study was questioned in view of in house results and previous results (1). The mixture of single and two-chain rt-PA was later replaced by a single chain preparation to reduce sensitivity for PAI-2(19).
Assay 13 is a two stage method with incubation of plasma with an excess of single-chain t-PA followed by assay of residual t-PA enzymatic activity with a plasminogen/chromogenic plasmin substrate assay utilizing poly-D-lysine as a t-PA stimulator (16). Values are expressed in IU/ml relative to t-PA. The study was performed by E. Eriksson, P.Falk and A. Hansevi.
Assay 14 is an u-PA-PAI-1 complex ELISA (20), that assesses the u-PA complex formed after incubation with added u-PA. Assay with lot 2F5013 was performed by M.Philips and B. Lillethorup and results were reported in pmol/l active PAI-1; the experience is that when analyzing NIBSC 83/517 29 fmol equals 1 IU.
Assay 15 is similar to assay 15 with reagents obtained from Novo Nordisk, Baegsvaerd, Denmark and performed by E. Eriksson, P.Falk and A. Hansevi with lot 774-776.
Assay 16 is Stachrom® PAI, from Diagnostica Stago, Asnières-sur-Seine, France. After incubation of the plasma with u-PA, the excess u-PA is measured by its capability to generate plasmin in an environment with inhibitors for plasmin inhibitor and alpha-2-macroglobulin (21).The assay was run on a semi-automated device ST 888, by F. Nicham and N. Barat with lot 922190.
Assay 17 is Berichrom® R PAI from Behringwerke AG, Marburg, Germany, an assay which employs u-PA as the target enzyme for PAI in plasma and measurement of uninhibited u-PA via plasminogen/plasmin conversion and subsequent cleavage of a chromogenic plasmin substrate in an environment with oxidative inactivation of plasmin inhibitor (22,23). The study was performed by H. Keuper and P. Lenz with a two-point method; lot 25258 (test version). Results are expressed in u-PA IU/ml which according to the experience is 1/5 to 1/6 of the t-PA inhibiting units.
Results
Criteria for specificity of methods for active PAI-1 in plasma
In plasma we can expect different molecular forms of PAI-1, such as inactivated PAI-1 (originating from platelets, or generated spontaneously by time-dependent thermal inactivation of active PAI-1), active PAI-1 and PAI-1 in complex with proteases, notably t-PA (plasminogen activator, tissue-type) or u-PA (urokinase-type PA). Besides this complexity we can expect that other inhibitors in plasma compete for the target protease (t-PA or u-PA) used in a test principle. Another complexity was the co-occurrence of active t-PA besides PAI-1 in plasma. This t-PA could compete with the assay of activity or continue to react with PAI-1 after blood sampling. To avoid the latter, a method was introduced around 1988-1990 to acidify blood as soon as possible after its collection or to use acid anticoagulants to achieve a pH of around 6 (6,24). This “new” anticoagulation procedure complicates the situation as well, since not all assay methods were designed for or adapted to the use of acidified plasma. In figure 1 the situation in plasma is summarised and in particular it should be noted that we aim to measure active PAI-1 and not the residual activity of PAI-1.
Figure 1.
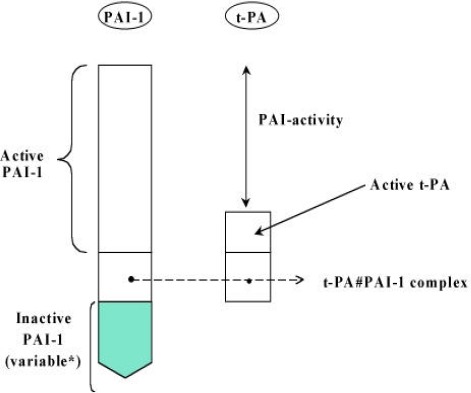
There were four test principles involved in the methods evaluated (see table II) and criteria focus on these principles. The criteria were established during the Meeting of the ISTH/SSC subcommittee on Fibrinolysis in 1992.
Table II.
| Assay Number | Name of assay or principle | Units used in the assay | Measured value in NIBSC 87/512 in units of the assay |
|---|---|---|---|
| Detection of increase int-PA-PAI-1 complex | |||
| 1 | Immunofunctional t-PA-PAI-1 | U/ml | |
| 2 | t-PA-PAI-1 complex ELISA | ng/ml | 13.6 |
| 3 | t-PA-PAI-1 complex ELISA | ng/ml | 33.5 |
| Detection of t-PA excess after reaction | |||
| 4 | 8-point titration assay with t-PA | IU/ml (t-PA) | 19.2 |
| 5 | Coatesr®PAI-1 | AU/ml | 23.7 |
| 6 | ípeclroh’so©PL, participantl | U/ml | 31.8 |
| 7 | Spectrolyse®PL, participant 2 | U/ml | 32.0 |
| 8 | Bioimmunoassry | U/ml (t-PA) | 24.8 |
| 9 | SOIA-PA1 | U/ml | 27.0 |
| 10 | TC®Actibind PAI-1 | U/ml | 22.4 |
| 11 | TC®Actibind PAI-1/CL | U/ml | 25.0 |
| 12 | TC®PAI-1 activity assay | U/ml | 13.5 |
| 13 | Single point titration with t-PA | IU/ml (t-PA | 52.2 |
| Target enzyme for PAI-1 is u-PA | |||
| Detection of u-PA-PAI-1 complex | |||
| 14 | u-PA-PAI-1 complex ELISA, participant 1 | pmol/l | 713 |
| 15 | u-PA-PAI-1 complex ELISA, participant 2 | pmol/l | 581 |
| Detection of u-PA excess after reaction | |||
| 16 | Stachrom© PAI | AU/ml | 25.9 |
| 17 | Berichrom© PAI | IU/ml (u-PA) | 5.6 |
Criteriumla and b: General: No interference by other plasma (inhibitory) components Specific 1a: Immunodepleted and thermally inactivated plasma should have an activity < 5% of that of NIBSC standard 87/512 (grading A = both < 5%; grading B = only one < 5%; grading C = both > 5%) Specific 1b: Added PAI 2 or PAI 3 added to immunodepleted plasma should have an activity < 5% of that of NIBSC standard 87/512 (grading A = both < 5%; grading C = one or two < 5%) Criterium 2: General: No interference by endogenoust PA Specific: Addedt PA to two (acid) plasmas with different PAI-1 activity level should not reduce recorded PAI-1 activity with an amount > 20% of the initial activity (Grading A = mean reduction < 20%; grading C = mean reduction > 20%).
Criterium 3: Precision / performance Specific: Dose-response of added PAI-1 to plasma (0; 4.6; 17; 55.8 U/ml) should be of high quality (grading A = r > 0.999; grading B = r > 0.9; grading C = r < 0.9)
The criteria (1a, 1b, 2, 3) were to be independently applied and any C should result in exclusion and A and B both in inclusion. The criterium on performance was limited to criteria for the dose-response curve and not intended at this stage to evaluate whether the coefficient of variation was half of that of the total, including biological variation, which should also be evaluated (25).
Samples to evaluate adherence to the criteria
The following samples were prepared and included in the set that was distributed:
r–PAI–1 = recombinant PAI–1;
r–PAI–2 = recombinant PAI–2;
tc–t–PA = two chain melanoma t–PA.
Samples were coded and frozen and distributed on dry ice except for the NIBSC standards which were freeze-dried. The original set was larger including additions of r-PAI-1 to buffer, but these were later excluded.
Procedure of the evaluation and follow-up.
An invitation to participate voluntarily (by direct mail) to volunteers responding at the subcommittee meeting, to scientists of original publications of method development, method evaluation and/or modification (in total 33) and company scientists responsible for commercial methods (in total 14) resulted in 18 responses concerning 16 different methods. The general requirement and criteria were stated in a letter in advance.
Thus evaluation was confined to expert laboratories or laboratories that had designed a test and were informed about the aims and procedures.
It had been decided to perform the analysis blind in two ways (a) the test samples were coded for participants (b) the methods were coded for discussion in the SSC subcommittee meetings.
The results of the evaluation were presented coded at the SSC subcommittee on fibrinolysis meeting in 1993, New York and on the basis of the criteria and predetermined judgement, two methods only fulfilled all criteria (see below).
Subsequently, individual results were distributed to participants for comment, and to acknowledge that the single evaluation of each assay method (except in two cases) might have been subject to incidental error (misfortune) or that the engineered samples might have created unexpected problems. For instance the use of acidified plasma (pH 6) was quite recent. Additional data were welcomed. The reactions have been taken into account. Notably, the evaluation with added recombinant PAI-2 was at variance with the experience of some investigators: this issue was not resolved.
After the formal presentation of coded results and the evaluation of comments by the organisers, the results were not decoded on any occasion and were only subject to consideration by the participants for their own method / information. The selection of a potential reference method and potential recommendation within the framework of the SSC was not considered appropriate. The present manuscript is the first decoded account of the results.
Results of the exercise
a) General aspects
From_the 18 applicants, 17 continued cooperation and received 16 coded samples. From these sixteen samples, thirteen (see table I) were used for evaluation. On retrospect, the samples with PAI-1 added to buffer were excluded from evaluation due to poor and erratic recovery of added PAI-1 evident from all data.
As two independent participants participated per two methods, the whole procedure could be evaluated. For method 6 and 7 and for 14 and 15 (see table II) the data showed excellent agreement as expressed by the mutual correlation of all data r>0.97.
b) Method principles
The methods evaluated in the present study had different principles for detecting the active PAI-1. The principles involved either addition of t-PA to the samples (13 cases) or addition of u-PA (4 cases): The t-PA preparation could be a one- or two-chain molecule. The detection principle involved the measurement of the complex of PAI-1 formed with the added enzyme (7 cases). In view of the normal, endogenous presence of t-PA*PAI-1 complex the increase in the amount of complex was evaluated. In the case of u-PA addition it was assumed that normally no u-PA*PAI-1 complex was circulating, restricting the use to normal situations where this applies. The detection principle involved in 10 cases the detection of the excess of added enzyme, either t-PA (8 times) or u-PA (2 times)
In Table II, the methods are arranged and numbered according to the principles of assay as mentioned above. As can be observed from the table as well, the units in which the results are provided differ, despite the availability of standard NIBSC 87/512 (assigned value 25 U/ml). For comparison, the results of the participants for the standard in their own units is given in the table. It illustrates the variability existing at that time in reporting data on PAI-1 activity.
Assays are identified by number and described in materials and methods. AU = arbitrary units.
c) Normalization for evaluation.
For evaluation all results were normalised relative to the included sample of NIBSC 87/512 and presented in percentages of this standard throughout the manuscript, unless specified otherwise. For validation of this procedure, for all criteria, normalisation was also exercised with the other standard NIBSC 92/654 and with one of the frozen samples (Stabilyte sample H, see table I) and similar results and conclusions were obtained in all cases. For nine methods with an excellent analytical performance (dose-response curve with r> 0.999) the two NIBSC standards were compared showing close agreement on an activity of NIBSC 92/654 of 86 % of that of NIBSC 87/512 (interquartile range: 81-96, standard deviation 18%).
d) Criterium 1: absence of interference by other plasma (inhibitory) components
The evaluation on criterium I could not use the samples with buffer matrix due to poor recovery/stability of such samples as concluded retrospectively, preventing direct evaluation of matrix interferences
Interference by plasma components and other inhibitors was evaluated by 1) the results in immunodepleted plasma and a pooled citrated plasma incubated overnight at 37_C to inactivate PAI-1 (activity-depleted) 2) the results of assay in immunodepleted plasma supplemented with PAI-2 or PAI-3
Results are summarised in table III in three categories I - III. Category I concerns methods with a combination of a low recording in depleted plasmas (criteria A = both < 5%) and low interference by PAI-2 (criterium A = below 5%). Category II does not fulfil criteria A and/or B but is less clearly aberrant compared to category III Category III is clearly not adequate due to criterium C applying to depleted plasma or the addinon of PAI-2.
Table III.
| RESULTS in % relative to NIBSC 87/512 | ||||
|---|---|---|---|---|
| Assay Number | ||||
| Category 1 | Immunodepleted Sample A | Activity Depleted Sample G | Sample E = r-PAI-2 enriched Simple A | Sample F = PAI-3 enriched sample A |
| 3 | 0.4 | 0.9 | 0.7 | 0.6 |
| 12 | 0 | 4.5 | 0 | 0 |
| 14 | <3 | <3 | 2.8 | <3 |
| Category II | ||||
| 1 | 0 | 10 | 0 | 0 |
| 2 | 8.8 | 0 | 13 | 2.9 |
| 10 | 0 | <30 | 0 | 0 |
| 13 | 5.7 | 11 | - | 3.8 |
| 15 | 6.9 | 11.5 | 11 | 8.4 |
| Category III | ||||
| 4 | 27 | 26 | >781 | 12 |
| 5 | -30 | -15 | 390 | -35 |
| 6 | 23 | 2.7 | 121 | 0 |
| 7 | 17 | 7.5 | 142 | 7.5 |
| 8 | 41 | 125 | 25 | 0 |
| 9 | 59 | 59 | >185 | 41 |
| 11 | 51 | 37 | 158 | 41 |
| 16 | 3.5 | 6.6 | 165 | 0 |
| 17 | 14 | 3.6 | 14464 | 0 |
There was no sensitivity to PAI-3; only occasional values did not fulfil criterium A.
e) Criterium 2: Absence of interference by endogenous free t-PA
To test absence of interference by endogenous active t-PA, the procedure was chosen to add to Stabilyte plasma (where added t-PA is preserved and does not react with endogenous PAI-1 in the sample) an additional amount of t-PA. This extra amount should not interfere with the assay of the PAI-1 activity in the sample. The addition to plasma H of 4 IU/ml t-PA and to plasma J of 7 IU/ml t-PA would be comparable to about 50% potential neutralisation of the endogenous PAI-1 amount.
As shown in Table IV, most methods show significant reduction in the recording of PAI-1 activity following the addition of t-PA. According to the criteria of change <20% only 4 methods fulfilled the criterium, and out of these only two methods from category I and II from table III.
Table IV:
Interference by added t-PA and grades for dose response curves
| Assay Number | mean decrease | Grade for dose-response |
|---|---|---|
| due to t-PA addition | ||
| relative to pre-value | ||
| Category I | ||
| 3 | 44% | A |
| 12 | 60% | C |
| 14 | 13% | B |
| Category II | ||
| 1 | 34% | A |
| 2 | 83% | A |
| 10 | 0% | B |
| 13 | 34% | A |
| 15 | 42% | A |
| Category III | ||
| 16 | –11 % | B |
| 5 | 35 % | A |
| 6 | 39 % | C |
| 7 | 42 % | B |
| 8 | -13 % | C |
| 9 | - | B |
| 11 | 93 % | C |
| 16 | 9 % | B |
| 17 | 11 % | A |
The decrease in PAI-1 activity by added t-PA is recorded as a percentage of the initial value. Average, theoretical maximal decrease relative to the pre-value is~ 50%. The value shown is the mean of the data of added t-PA to sample H and J. Bold values fulfil the criterium grade A.
f) Criterium 3: Dose-response curve quality
As shown in Table IV, 7 assays showed an excellent dose-response (r>0.999) while 4 performed poorly (r< 0.9). The amount of sample provided did not allow for dilution experiments, causing in some cases a poor performance for the higher value of PAI-1 in sample D.
g) Overall scoring
When using either exclusion or inclusion as an approach only methods 10 and 14 were not excluded. Grading C on criterium C did not contribute independently to any exclusion.
Discussion
The study presented focused on the specificity of methods for active PAI-1 in acidified plasma. For the evaluated methods for PAI-1 activity we showed that the majority was not specific according to the tests and criteria applied. Only 2 out of 15 methods were potentially specific. The three major problems causing these results were (a) the sensitivity of about half of the methods to interference by another inhibitory factor in plasma as evident from the results in PAI-1 depleted plasma, (b) the fact that some methods were not adapted to the use of acid or Stabilyte plasma, and (c) the sensitivity of many methods to endogenous t-PA as revealed by the addition of extra t-PA. In addition, most methods aimed to assess elevated PAI-1 and performed suboptimally at very low levels (cf 1).
The presence of an interfering plasma factor had been described previously (5,26); the identity of this interfering factor is still unknown. The sensitivity to this interference coincided with sensitivity to the addition of PAI-2. The effect of PAI-2 was debated in subsequent correspondence with some participants and at variance with their experience. This should be noted, but was not further evaluated.
The sensitivity to added t-PA was previously not considered a major aspect to circumvent since various methods aimed at studying elevated PAI-1 where endogenous t-PA is a minor component. Also the level of endogenous active t-PA was underestimated before the introduction of acidification of blood to inhibit in vitro interaction of t-PA and PAI-1. At present it is state-of-the art to use acidified plasma to avoid in vitro interactions and to attempt to measure active PAI-1 (see figure 1). This allows the use of methodology for all purposes independent of the t-PA status. It can be observed that methods using u-PA as target enzyme for PAI-1 were relatively insensitive to endogenous t-PA.
The two methods that fulfilled the criteria provided specificity for PAI-1 by the use of specific antibodies either to immobilise t-PA and detect only newly formed PAI-1-t-PA or to detect specifically the formation of the u-PA*PAI-1 complex. In method with u-PA the excess of u-PA used was apparently sufficient to avoid interference by endogenous t-PA. In later development also method 2 solved the problem of endogenous t-PA by the addition of PPACK (10). Later assay formats with the principle of methods 8 and 10 maintained a pH 6 during binding of the PAI-1, using acidified plasma.
The results of the exercise demonstrate the value of the criteria and test samples for the methods evaluated. It is possible that new criteria and test samples are necessary when other assay principles or standards are introduced. It should be noted that the exercise was not a complete evaluation; for instance matrix effects and analytical variability relative to the total variability were not assessed. The exercise was limited to the evaluation of a limited number of samples for each aspect of specificity and to one laboratory, for most methods and results should be confirmed in subsequent more elaborate and detailed experiments for definite conclusions.
The process in the subcommittee of fibrinolysis of the ISTH/SSC resulted in an increased awareness of the importance of specificity of methods and the experimental documentation, especially for standardisation and when a reference method is required. In addition it seemed adequately possible in all methods to use the NIBSC standards, though, this issue has not been formally investigated. It should be recognised that the evaluation did not focus on possible matrix interference. An inventory on this aspect in the present exercise was not possible because of the problems with the analyte added to buffer: the samples were excluded from evaluation. In future evaluations of these aspects require additional attention.
The ISTH/SSC can be considered a suitable expert forum for the definition of criteria and testing principles in haemostasis. The testing as executed for the method for PAI-1 activity was seen as a single exercise and in future the responsibility of parties who develop the methods and standards.
Acknowledgements
Dr. P.J. Declerck (Immunodepleted plasma, rPAI-1), Dr. B.R. Binder (PAI-3), Dr D.J. Ballance (rPAI-2) and Dr P.J. Gaffney (NIBSC standards) are gratefully acknowledged for supply of materials and samples. Ing. P. Meijer is gratefully acknowledged for sample preparation and distribution The following groups are acknowledged for their voluntary participation in the analysis: Dr S. Thorsen, M. Philips and B. Lillethorup, Rigshospitalet, Section for Hemostasis & Thrombosis, Dept of Clinical Biochemistry, Copenhagen, Denmark Dr. P.J. Declerck and I. Knockaert, Laboratory of pharmaceutical biology and phytopharmacology, Institute for Pharmaceutical Sciences, Leuven, Belgium Dr. W. Nieuwenhuizen and R. Laterveer, Dept of Lipids and Endothelium, Gaubius Laboratory, IVVO-TNO, Leiden, The Netherlands Dr. N. Booth and A.M. Croll. Dept of Molecular and Cell biology, University of Aberdeen, Marischal College, Aberdeen, Scotland Dr C.Kluft and P. Meijer. Gaubius Laboratory, IVVO-TNO, Leiden, the Netherlands Dr S. Rosen, E. Ersdal-Badju and L Wejkum. Chromogenix AB, Molndal, Sweden Dr A. Takada and T. Urano.Dept of physiology, Hamamatsu University School of Medicine, Hamamatsu, Shizuoka, Japan. Dr I. Juhan-Vague and J. Ansaldi. Laboratoire d’hematologie, Hôpital d’adultes Timone et Universitaire de Marseille, Marseille, France. Dr K. Okada and O. Matsuo. Dept of Physiology, Kinki University School of Medicine, Osakasayama City, Japan. Dr. E. Angles-Cano and S. Loyau. INSERM U.143, Institut de pathologie cellulaire, Hôpital de Bicetre, Paris, France. Dr. B.R. Binder, R. Beckmann and V. Carroll. Dept of Medical Physiology, Lab. for Clinical Experimental physiology, University of Vienna, Vienna, Austria. Dr E. Eriksson, P. Falk and A. Hansevi. Fibrinolyslab, Dept of Surgery, East Hospital, Gothenburg, Sweden. Dr G. Contant, F. Nicham and N. Barat. Serbio Laboratory, Gennevilliers, France. Dr H. Keuper and P. Lenz. Dept of Coagulation and Fibrinolysis diagnostics, Behringwerke AG, Marburg, W-Germany.
References
- 1).Gram J, Declerck PJ, Sidelmann J, Jespersen J, Kluft C. Multicentre evaluation of commercial kit methods: plasminogen activator inhibitor activity. Thromb Haemostas 1993:70:852?7. [PubMed] [Google Scholar]
- 2).Declerck PJ, Moreau H, Jespersen J, Gram J, Kluft C. Multicenter evaluation of commercially available methods for the immunological determination of plasminogen activator inhibitor?1 (PAI?1). Thromb Haemostas 1993;70:858?63. [PubMed] [Google Scholar]
- 3).Declerck PJ, De Mol M, Alessi M-C, Baudner S, Paques GP, Preissner KT, Muller-Berghaus G., Collen D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. J Biol Chem 1988;263:15454-15461. [PubMed] [Google Scholar]
- 4).Alessi M-C, Declerck PJ, De Mol M, Nelles L, Collen D. Purification and characterization of natural and recombinant plasminogen activator inhibitor 1(PAI-1). Eur J Biochem 1988;175:531-540. [DOI] [PubMed] [Google Scholar]
- 5).Kluft C, Jie AF, Sprengers ED, Verheijen JH. Identification of a reversible inhibitor of plasminogen activators in blood plasma. FEBS Lett. 1985;190:315?8. [DOI] [PubMed] [Google Scholar]
- 6).Ranby M, Sundell IB, Nilsson TK. Blood collection in strong acidic citrate anticoagulant used in a study of dietary influence on basal tPA activity. Thromb Haemost 1989;62:917?22. [PubMed] [Google Scholar]
- 7).Kluft C, van Wezel AL, van der Velden CAM, Emeis JJ, Verheijen JH, Wijngaards G. Large scale production of extrinsic (tissue-type) plasminogen activator from human melanoma cells. Mizrahe A, van Wezel AL, eds. Advances in biotechnological processes, 2nd ed. New York: AR Liss, 1983:97-110. [Google Scholar]
- 8).Gaffney PJ, Edgell TA. The international standard for plasminogen activator inhibitor?1 (PAI?1) activity. Thromb Haemost 1996;76:80? 3. [PubMed] [Google Scholar]
- 9).Declerck PJ, Verstreken M, Collen D. An immunofunctional assay for active plasminogen activator inhibitor-1 (PAI-1). Fibrinolysis. 1988;2 suppl. 2:77-78. [Google Scholar]
- 10).Nieuwenhuizen W, Laterveer R, Hoegee B. An enzyme immunoassay for the simultaneous determination of active type-1 plasminogen activator inhibitor (PAI-1), and t-PA/PAI-1 complexes. Blood Coagul Fibrinolysis 1995;6:520-526. [DOI] [PubMed] [Google Scholar]
- 11).Booth NA, Croll A, Bennett B. The activity of plasminogen activator inhibitor-1 (PAI-1) of human platelet. Fibrinolysis. 1990;4 supp 2:138-140. [Google Scholar]
- 12).Verheijen JH, Chang GT, Kluft C. Evidence for the occurrence of a fast?acting inhibitor for tissue?type plasminogen activator in human plasma. Thromb Haemost. 1984;51:392?5. [PubMed] [Google Scholar]
- 13).Wejkum L, Chmielewska J. A new adaptation of Coatest PAI for measurement of low inhibitor concentrations in plasma. Fibrinolysis 1990;4:130. [Google Scholar]
- 14).Chmielewska J, Ranby M, Wiman B. Evidence for a rapid inhibitor to tissue plasminogen activator in plasma. Thromb Res 1983;31:427-436. [DOI] [PubMed] [Google Scholar]
- 15).Chmielewska J, Wiman B. Determination of tissue plasminogen activator and its fast inhibitor in plasma. Clin Chem 1986;32:482-485. [PubMed] [Google Scholar]
- 16).Eriksson E, Ranby M, Gyzander E, Risberg B. Determination of plasminogen activator inhibitor in plasma using t-PA and a chromogenic single-point poly-D-lysine stimulated assay. Thromb Res 1998;50:910101. [DOI] [PubMed] [Google Scholar]
- 17).Angles-Cano E, Masson-Lunven C, Gaussem P. Development of an internal standard for plasminogen activator inhibitor-1 PAI-1 and its use in a simplified assay for measuring PAI-1 activity in human plasma. Fibrinolysis. 1990;4 supp 2: 127-129. [Google Scholar]
- 18).Korninger C, Wagner O, Binder BR. Tissue plasminogen activator inhibitor in human plasma: development of a functional assay system and demonstration of a correlating Mr=50.000 antiactivator. J Lab Clin Med 1985;105:718-724. [PubMed] [Google Scholar]
- 19).Thorsen S, Philips M, Selmer J, Lecander I, Astedt B. Kinetics of inhibition of tissue-type and urokinase-type plasminogen activator by plasminogen-activator inhibitor type 1 and type 2. Eur J Biochem 1988;175:33-39. [DOI] [PubMed] [Google Scholar]
- 20).Philips M, Juul A-G, Selmer J, Lind B, Thorsen S. A specific immunological assay for functional plasminogen activator inhibitor 1 in plasma. Standardized measurements of the inhibitor and related parameters in patients with venous thromboembolic disease. Thromb Haemost 1992;68:486-494. [PubMed] [Google Scholar]
- 21).Contant G, Nicham F, Martinoli JL. Determination of plasminogen activator inhibitor (PAI) by a new venom based assay. Fibrinolysis. 1992; 6 supp 3:85-86. [Google Scholar]
- 22).Stief TW, Lenz P, Becker U, Heimburger N. Determination of plasminogen activator inhibitor (PAI) capacity of human plasma in presence of oxidants: a novel principle. Thromb Res 1988;50:559-573. [DOI] [PubMed] [Google Scholar]
- 23).Stief TW, Lenz P, Becker U, Heimburger N. Functional determination of plasminogen activator inhibitor (PAI) based on oxidative inactivation of alpha-2-antiplasmin: no influence of sample heparin and fibrinogen split products (FSP). Fibrinolysis. 1988;2 supp 2:73-74. [Google Scholar]
- 24).Kluft C, Verheijen JH. Fibrinolysis Working Party: Blood collection and handling procedures for assessment of tissue-type plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1). Fibrinolysis. 1990;4 supp 2:155-161. [Google Scholar]
- 25).Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci 1989;27:409-437. [DOI] [PubMed] [Google Scholar]
- 26).Sprengers ED, Kluft C. Plasminogen activator inhibitors. Blood 1987;69:381?7. [PubMed] [Google Scholar]


