Abstract
In our previous study, it was demonstrated that the Stathmin1 (STMN1) is overexpressed in gastric cancer (GC) and that its high expression level is associated with tumor invasion and metastasis. Epithelial-mesenchymal transition (EMT) has also been shown to be critically involved in GC invasion and metastasis. Certain studies have indicated that STMN1 may serve an important role in the EMT process. However, the association between STMN1 expression and EMT-associated markers, as well as clinicopathological characteristics of patients with GC, remains unclear. The aim of the present study was to investigate the clinicopathological significance and prognostic value of STMN1 and EMT-associated markers in GC. The expression of STMN1 and the EMT-associated proteins E-cadherin (E-Cad) and vimentin (VIM) were analyzed by immunohistochemistry in GC and adjacent non-tumorous tissues. Associations between the expression of these markers and clinicopathological parameters were analyzed. The association between STMN1 expression and EMT-associated markers was investigated in the GC cell lines BGC-803 and SGC-7901. The results revealed that STMN1 was expressed in 63.5% of the 167 GC tissues, which was significantly higher than the percentage observed in the adjacent non-tumorous tissues (P=0.003). The STMN1 expression was demonstrated to be positively associated with the VIM levels (P=0.001) and negatively associated with the E-Cad levels (P=0.022) in GC tissues. The STMN1 expression was associated with Lauren's Classification, invasion depth, lymph node metastasis and pathological Tumor-Node-Metastasis (pTNM) stage (P<0.05). In the univariate analyses, the high E-Cad expression was a positive prognostic indicator for overall survival, whereas the high STMN1 and VIM expression was a negative indicator. COX multiple regression analysis demonstrated that the pTNM stage [hazard ratio (HR) 1.912, 95% confidence interval (CI): 1.282–2.851, P=0.001] and E-Cad expression (HR 0.403, 95% CI: 0.249–0.650, P=0.000) were independent prognostic factors. It was also revealed that the expression level of E-Cad decreased, while the expression level of VIM increased by depleting STMN1 levels in GC cells. The present results suggest that the aberrant expression of STMN1 may promote tumor progression through EMT in GC.
Keywords: gastric cancer, Stathmin1, E-cadherin, vimentin, epithelial-mesenchymal transition, prognosis
Introduction
Gastric cancer (GC) is one of the most common types of malignant tumor and the secondary leading cause of cancer-associated mortality worldwide (1). Despite improved methods of diagnosis and treatment employed over the past several decades, the prognosis of patients with advanced GC remains poor, with the 5-year survival rates at <30% in the majority of countries (2). Surgical resection is the mainstay of treatment for GC. Even following curative resection, many GC patients die as a result of local recurrence and distant metastasis (3). Therefore, a better understanding of the molecular mechanisms of tumor invasion and metastasis is very important for tumor diagnosis and treatment.
Stathmin1 (STMN1), also called Oncoprotein-18/Op18 or metablastin, is a microtubule-destabilizing protein that has been found to play a critical role in the regulation of mitosis (4). Recently, STMN1 overexpression has been identified in a wide spectrum of human malignancies and may serve as a prognostic biomarker (5–7). Several studies have shown that an increased STMN1expression enhances proliferation, migration and invasion of cancer cells, while the downregulation of STMN1 expression produces the opposite effect (8–10). Our previous study also indicated that STMN1 is overexpressed in GC, with its high expression level being associated with tumor progression, and lymph node metastasis, and a predictive factor of poor survival (11). However, to date, the exact function of STMN1 in GC invasion and metastasis remains unclear.
Epithelial-mesenchymal transition (EMT) plays a crucial role in the invasion, migration and metastasis of cancer cells (12,13). During the process of EMT, tumor cells lose their epithelial characteristics and gain mesenchymal characteristics to become invasive (14). A major hallmark of EMT is the downregulation of the epithelial protein E-cadherin (E-Cad) and the upregulation of the mesenchymal protein vimentin (VIM) (15). E-Cad is a cell adhesion molecule that is essential to maintaining the integrity of cell-cell contact in epithelial cell layers. VIM is a cytoskeleton protein that may be associated with mitochondrial motility and localization, and VIM upregulation plays an important role in cell migration. Previous studies have established that EMT is associated with tumor progression and metastasis in many types of cancer (14,16,17). Furthermore, the expression of E-Cad and VIM has been associated with metastasis and OS in many types of human cancer, such as breast, lung, and colorectal cancer, as well as GC.
STMN1 is an important microtubule regulatory protein and previous studies have shown that microtubule dynamics could contribute to oncogenic EMT (18–20), suggesting that STMN1 may play an important role in the EMT. However, the association between STMN1 expression and EMT-related markers as well as clinicopathological characteristics of GC patients, remains unclear. Consequently, in the present study, the expression of STMN1, E-Cad and VIM was examined in patients with GC, and the correlation between these markers was analyzed. Furthermore, the association between their expression and clinicopathological parameters and prognosis was determined. The expression of STMN1 and EMT-related markers in GC cells was also examined. The present findings may uncover the clinical significance of STMN1 and EMT in GC.
Materials and methods
Patients and tissue specimens
The present study was approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). All patients provided written informed consent. Tumor samples were obtained from 167 GC patients who underwent radical surgical resection at the Tianjin Medical University Cancer Institute and Hospital (Tianjin, China) between 2008 and 2011. The inclusion criteria were as follows: i) Histologically confirmed adenocarcinoma; ii) not receiving neo-adjuvant therapy; iii) underwent curative resection with D2 lymph node dissection and retrieval of >15 lymph nodes; iv) no evidence for distant metastases; v) full clinical and pathological data were available; and vi) follow-ups had been completed and follow-up data were available for each patient. Matched normal gastric mucosa tissue samples were collected from 80 cases during surgery at a distance of 5 cm from the tumor margin, and used as the control. All patients included in the study were Chinese.
The following clinical data were collected for all patients: Sex, age, tumor size, and location, histological differentiation, serosal invasion, lymph node metastasis and pathological Tumor-Node-Metastasis (pTNM) stage. The TNM stage of each patient was confirmed by pathologists according to the 7th edition of the American Joint Committee on Cancer TNM classification of gastric cancer (21). All patients were followed up until either death or the end of the follow-up period (December 30, 2016).
Immunohistochemistry
All freshly excised tissues were paraffin fixed and serially sectioned at 4-µm thickness. The paraffin sections were baked at 65°C for 4 h, deparaffinized with xylene, and dehydrated through graded ethanol. Antigen retrieval treatment was done at 95°C for 20 min in 0.01 mol/l sodium citrate buffer (pH 6.0), and endogenous peroxidases were blocked using 3% H2O2 for 30 min. Sections were then blocked with 10% goat serum albumin (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China) for 30 min. Next, the slides were incubated overnight at 4°C in a humidified chamber with the following primary antibodies: Anti-STMN1 (cat. no. ab52630; dilution 1:200; Abcam, Cambridge, UK), anti-E-Cad (cat. no. ab40772; dilution 1:200; Abcam), and anti-VIM (cat. no. ab92547; dilution 1:200; Abcam). Slides were then washed three times with PBS and incubated with horseradish peroxidase-conjugated secondary antibody (OriGene Technologies, Inc., Beijing, China) for 1 h at 37°C. Finally, staining was visualized using 3,3′-diaminobenzidine and counterstained with hematoxylin. The slides were dehydrated, cleared and permanently mounted with resinous mounting medium. Known positive controls were included in every staining procedure. PBS was used to replace primary antibodies in the negative control.
Immunohistochemical staining was evaluated by two independent pathologists who were blinded to the clinical data. In each case, five representative fields were selected and at least 200 tumor cells were observed at a magnification of ×400. The staining score was based on the percentage of positive cells and staining intensity (22). The percentage of cells stained was counted and scored as follows: 0, <5; 1, 5–25; 2, 26–50; 3, 51–75; and 4, 76–100%. The staining intensity was scored as follows: 0, no staining; 1, buff; 2, buffy; and 3, puce. The total score ranged from 0 to 12. When the multiplication product of the two scores was >3, the samples were considered positively stained.
Cell culture and siRNA transfection
The human GC cell lines BGC-803 (mucinous GC, poorly differentiated) and SGC-7901 (adenocarcinoma, moderately differentiated) used in this study were purchased from the Cell Resource Center of the Institutes for Biological Science (Shanghai, China). All cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 mg/ml streptomycin (Merck KGaA, Darmstadt, Germany). The cells were grown at 37°C in a humidified atmosphere containing 5% CO2.
The small interfering RNA (siRNA) targeting STMN1 gene was purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA; cat. no. sc-36127). The siRNA transfection was performed using Lipofectamine 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. The culture medium was replaced following a 6-h transfection. After 48 h, the cells were harvested for subsequent experiments. A sequence non-specific to any known gene was used as the negative control siRNA (sc-37007; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Western blot analysis
Cells were collected and washed with PBS, and protein was extracted using lysis buffer (PMSF: RIPA=1:100, Beyotime Institute of Biotechnology, Haimen, China). Protein concentrations were measured by BCA Protein Assay Reagent (Pierce; Thermo Fisher Scientific, Inc.). Total proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, electro-transferred onto polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA, USA), and blocked with 5% non-fat milk in PBS with 0.05% Tween-20 for 2 h. The membranes were incubated overnight at 4°C with the following antibodies: Anti-STMN1 (cat. no. ab52630; dilution 1:500; Abcam), anti-E-Cad (cat. no. ab40772; dilution 1:500; Abcam), anti-VIM (cat. no. ab92547; dilution 1:500; Abcam), and anti-β-actin (cat. no. ab8227; dilution 1:2,000; Abcam). The membrane was incubated with secondary antibodies for 1 h at room temperature. The immunocomplexes were visualized by enhanced chemiluminescence.
Follow-up and statistical analysis
All statistical analysis was performed using SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA). The follow-up assessments were conducted by mail, telephone, and personal interviews. All patients were followed up for a minimum of 5 years or until death. The association between the patients' clinicopathological features and the expression of STMN1, E-Cad and VIM was estimated using the χ2 test or Fisher's exact test. The correlations between STMN1, E-Cad and VIM were assessed using Spearman's rho. OS was defined as the interval between the date of surgery and the date of death or final follow-up. Kaplan-Meier survival analysis (log-rank test) was used to evaluate the association between markers and survival. Factors with potential importance on univariate analysis were included in multivariate analysis. Multivariate analysis of the OS was performed using the Cox proportional hazards model with forward step procedures. P<0.05 were considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 167 cases of primary GC were analyzed, including 120 (71.9%) men and 47 (28.1%) women. The age of patients ranged from 26 to 79 years, with a mean age of 58.8 years. The follow-up period ranged from 2 to 95 months (median, 34.2 months). Primary tumor size ranged from 0.5 to 16 cm with a mean size of 5.17±2.58 cm. From the 167 patients, 145 (86.8%) had serosal invasion and 126 (75.4%) had lymph node metastasis. The number of patients with stage I/II and III GC were 48 and 119, respectively. The main clinical characteristics of patients are detailed in Table I.
Table I.
Associations between STMN1, E-Cad and VIM expression and clinicopathological characteristics of GC.
| STMN1 | E-Cad | VIM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Cases (n) | Positive | Negative | P-value | Positive | Negative | P-value | Positive | Negative | P-value |
| Sex | 167 | 106 | 61 | 0.311 | 48 | 119 | 0.184 | 62 | 105 | 0.383 |
| Male | 120 | 79 | 41 | 31 | 89 | 47 | 73 | |||
| Female | 47 | 27 | 20 | 17 | 30 | 15 | 32 | |||
| Age (years) | 0.406 | 0.557 | 0.731 | |||||||
| <60 | 86 | 52 | 34 | 23 | 63 | 33 | 53 | |||
| ≥60 | 81 | 54 | 27 | 25 | 56 | 29 | 52 | |||
| Tumor size (cm) | 0.694 | 0.271 | 0.653 | |||||||
| <5 | 69 | 45 | 24 | 23 | 46 | 27 | 42 | |||
| ≥5 | 98 | 61 | 37 | 25 | 73 | 35 | 63 | |||
| Histological differentiation | 0.061 | 0.014a | 0.408 | |||||||
| Well/moderate | 41 | 21 | 20 | 18 | 23 | 13 | 28 | |||
| Poor/undifferentiation | 126 | 85 | 41 | 30 | 96 | 49 | 77 | |||
| Lauren's classification | 0.037a | 0.017 | 0.581 | |||||||
| Intestinal type | 47 | 24 | 23 | 18 | 29 | 19 | 28 | |||
| Diffuse type | 120 | 82 | 38 | 30 | 80 | 43 | 77 | |||
| Serosal invasion | 0.019a | 0.007b | 0.534 | |||||||
| Negative | 31 | 14 | 17 | 15 | 16 | 10 | 21 | |||
| Positive | 136 | 92 | 44 | 33 | 103 | 52 | 84 | |||
| Lymph node metastasis | 0.005b | 0.0001c | 0.0001c | |||||||
| N0 | 42 | 19 | 23 | 21 | 21 | 16 | 73 | |||
| N (+) | 125 | 87 | 38 | 27 | 98 | 46 | 32 | |||
| TNM stage | 0.008b | 0.0001c | 0.039a | |||||||
| I+II | 48 | 23 | 25 | 24 | 24 | 12 | 36 | |||
| III | 119 | 83 | 36 | 24 | 95 | 50 | 69 | |||
P<0.05
P<0.01
P<0.0001. STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin; GC, gastric cancer; TNM, tumor-node-metastasis.
Protein expression of STMN1, E-Cad and VIM in GC tissues
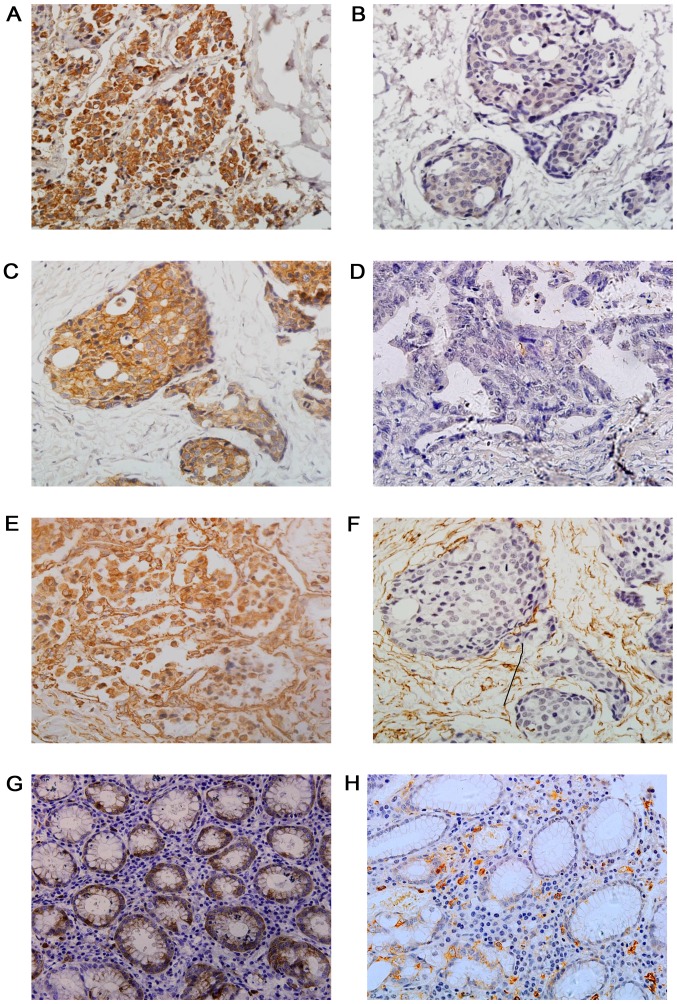
The results of immunohistochemistry are presented in Fig. 1. Positive expression of STMN1 was observed in the cytoplasm of the GC cells, with a pattern of buffy granules (Fig. 1A). From the 167 patients, 106 (63.5%) exhibited a positive expression of STMN1. In the adjacent normal gastric mucosa, STMN1 was present at a low level (43.5%; 35/80), indicating significant differences between the two groups (Table II).
Figure 1.
Immunohistochemical staining of STMN1, E-Cad and VIM in GC and adjacent normal tissues. Representative images displaying (A) positive and (B) negative STMN1 expression, (C) positive and (D) negative E-cad expression, and (E) positive and (F) negative VIM expression in GC tissues, as well as the (G) positive and (H) negative STMN1 expression in adjacent normal tissues. Magnification, ×400. STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin; GC, gastric cancer.
Table II.
Protein expression of STMN1, E-Cad and VIM in GC and adjacent normal tissues.
| Group | Cancer tissue, n (%) | Normal tissue, n (%) | χ2 | P-value |
|---|---|---|---|---|
| STMN1 | 8.589 | 0.003a | ||
| Positive | 106 (63.5) | 35 (43.5) | ||
| Negative | 61 (36.5) | 45 (56.5) | ||
| E-Cad | 62.896 | 0.0001b | ||
| Positive | 48 (28.7) | 66 (82.5) | ||
| Negative | 119 (71.3) | 14 (17.5) | ||
| VIM | 14.196 | 0.0001b | ||
| Positive | 62 (37.1) | 11 (13.8) | ||
| Negative | 105 (62.9) | 69 (86.2) |
P<0.01
P<0.0001. STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin; GC, gastric cancer.
E-Cad protein expression was observed at the intercellular junctions (Fig. 1C). Adjacent non-tumorous gastric mucosa exhibited a high E-Cad expression (82.5%, 66/80). The present results showed 28.7% (48/167) of GC cases to be positive for E-Cad, a markedly lower percentage than that in adjacent normal gastric tissues.
VIM expression was mainly identified on the stromal structures of normal or neoplastic tissues, as well as the cytoplasm of neoplastic tissues (Fig. 1E). In the present study, 31.7% (62/167) of GC tissues were positive for VIM, which was significantly higher than the positive expression in adjacent normal tissues (13.8%; 11/80).
Association between clinicopathological variables and the expression levels of STMN1, E-Cad and VIM
As shown in Table I, it was found that the STMN1 expression was significantly correlated with Lauren's Classification, invasion depth, lymph node metastasis and pTNM stage (P<0.05), but not with sex, age, tumor size or histological differentiation (P>0.05). Positive STMN1 expression was closely associated with diffuse-type cancer, greater invasion depth, advanced pTNM stage and higher percentages of lymph node metastasis. The loss of E-Cad expression was closely associated with poorly differentiated tumor grade, greater invasion depth, advanced pTNM stage and lymph node metastasis (P<0.05). In addition, it was found that the positive VIM expression group had a higher percentage of patients with lymph node metastasis or pTNM stage III cancer than did the negative VIM expression group (P<0.05).
Correlation among the expression of STMN1, E-Cad and VIM
As shown in Table III, there was a negative correlation between the expression of STMN1 and E-Cad (P=0.022), and a positive correlation between the expression of STMN1 and VIM (P=0.001).
Table III.
Association among the expression of STMN1, and E-Cad and VIM expression in gastric cancer.
| STMN1 expression | |||
|---|---|---|---|
| Variable | Positive | Negative | P-value |
| E-Cad | 0.022 | ||
| Positive | 24 | 24 | |
| Negative | 82 | 37 | |
| VIM | 0.001 | ||
| Positive | 49 | 13 | |
| Negative | 57 | 48 | |
STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin.
Univariate and multivariate analysis of prognostic factors
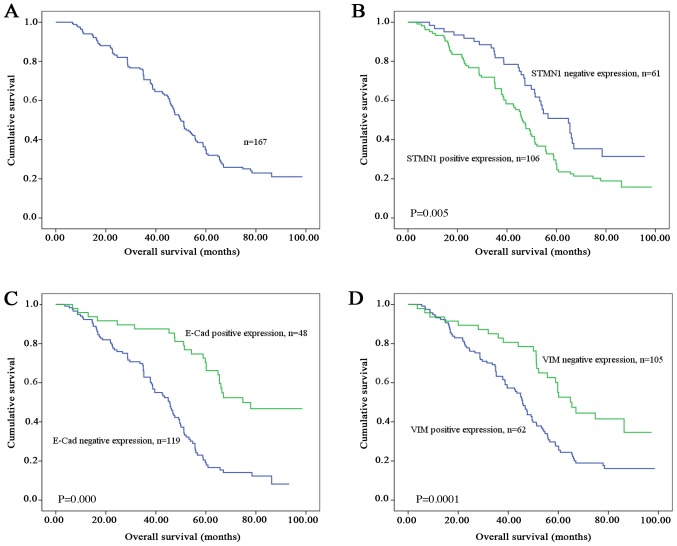
Of the 167 GC patients, the median survival time was 50.0 months (range, 3–98 months), and the 5-year survival rate was 32.7%. The OS curve is shown in Fig. 2A. In order to identify prognostic factors for survival, univariate and multivariate Cox's regression test was performed. According to the univariate analysis results, the OS was significantly correlated with tumor size, serosal invasion, lymph node metastasis, pTNM stage, and STMN1, E-Cad, and VIM expressions (P<0.05); by contrast, no significant relationships were detected between OS and sex, age, histological differentiation and Lauren's classification (P>0.05; Table IV). Patients with tumors that highly expressed STMN1 and VIM had a significantly lower 5-year survival rate than those with tumors expressing STMN1 (26.8 vs. 48.4%; Fig. 2B) and VIM (27.6 vs. 52.6%; Fig. 2D) at a low level. The group of patients with a positive or high E-Cad expression in their tumors had a better prognosis than those with a low E-Cad expression (5-year survival rate, 46.7 vs. 19.2%; Fig. 2C).
Figure 2.
Kaplan-Meier survival curves of 167 patients with GC. (A) Overall survival curve. Survival of patients with GC stratified by the expression of (B) STMN1, (C) E-Cad and (D) VIM. GC, gastric cancer; STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin.
Table IV.
Univariate and multivariate Cox's regression test of OS in patients with GC.
| Univariate analyses | Multivariate analyses | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Sex (male vs. female) | 1.281 | 0.883–1.858 | 0.193 | |||
| Age (<60 vs. ≥60) | 1.202 | 0.836–1.728 | 0.320 | |||
| Tumor size (<5 vs. ≥5) | 2.202 | 1.368–3.543 | 0.001b | |||
| Histological differentiation: Well/moderate vs. poor/undifferentiation | 1.473 | 0.972–2.010 | 0.084 | |||
| Lauren's classification: Intestinal vs. diffuse | 1.347 | 0.929–1.952 | 0.117 | |||
| Serosal invasion: Negative vs. positive | 3.850 | 1.565–9.468 | 0.003a | |||
| Lymph node metastasis: N(−) vs. N(+) | 2.066 | 1.430–2.985 | 0.0001c | |||
| TNM stage: I+II vs. III | 3.877 | 2.399–6.268 | 0.0001c | 1.912 | 1.282–2.851 | 0.001b |
| STMN1 expression: Negative vs. positive | 1.755 | 1.177–2.616 | 0.005a | |||
| VIM expression: Negative vs. positive | 2.110 | 1.360–3.274 | 0.001b | |||
| E-Cad expression: Negative vs. positive | 0.297 | 0.187–0.472 | 0.0001c | 0.403 | 0.249–0.650 | 0.0001c |
P<0.01
P<0.001
P<0.0001. STMN1, Stathmin1; E-Cad, E-cadherin; VIM, vimentin; GC, gastric cancer; OS, overall survival; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis.
In the multivariate analysis, the E-Cad expression level [hazard ratio (HR)=0.403, 95% confidence interval (CI): 0.249–0.650, P=0.000] and pTNM stage (HR=1.912, 95% CI: 1.282–2.851, P=0.001) were independent prognostic factors for OS.
STMN1-siRNA induces a change in EMT-related protein expression in GC cells
Western blotting was performed to evaluate the association between STMN1 and EMT-related proteins in GC cells. The results indicated that STMN1 expression was successfully inhibited by STMN RNAi in BGC-803 and SGC-7901 cells. As compared with the negative or blank control groups, the STMN1 RNAi clearly downregulated VIM in the RNAi group, but E-Cad was upregulated (Fig. 3).
Figure 3.
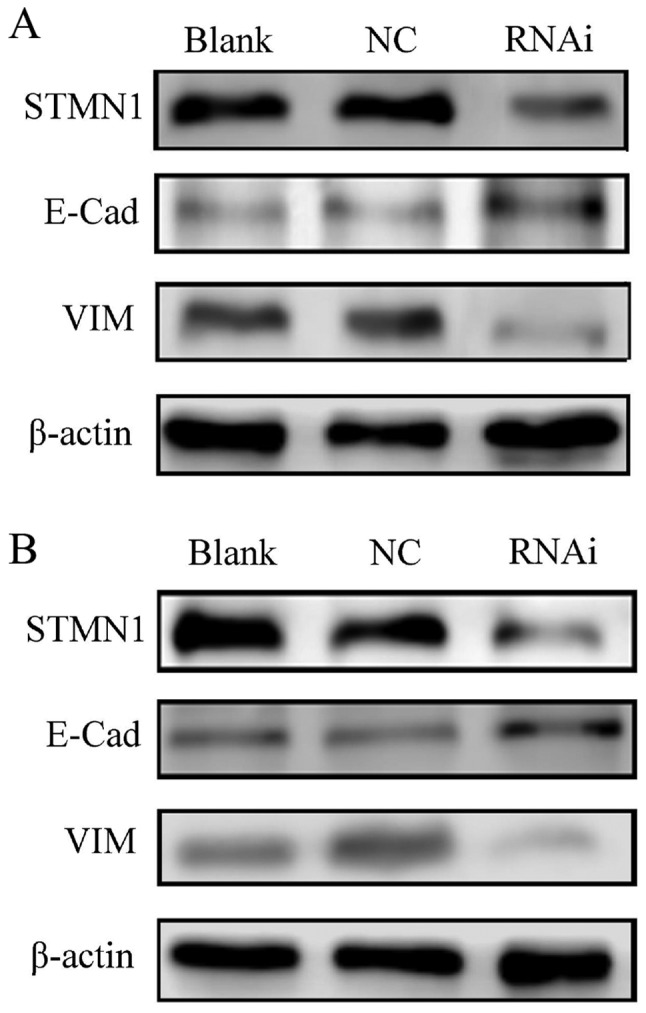
STMN1 and EMT-associated proteins detected by western blotting. The protein expression level of STMN1, E-Cad and VIM in (A) BGS-803 and (B) SGC-7901 GC cell lines was determined using western blotting. NC, negative control; STMN1, Stathmin1; EMT, epithelial-mesenchymal transition; E-Cad, E-cadherin; VIM, vimentin; GC, gastric cancer; RNAi, RNA interference.
Discussion
GC is a highly heterogeneous disease, and even in patients with similar clinical and pathological features, the outcome can be considerably different. Tumor progression and metastasis are the main factors influencing the prognosis. Several mechanisms have been identified as key factors in tumor progression and metastasis, such as EMT (12), signaling pathway activation (23) and cancer stem cell theory (24).
Recent studies have shown that EMT plays an important role in tumor invasion and metastasis in GC (16,25) and our previous study also suggested that STMN1 may also influence GC tumor progression (11). Although certain studies revealed a critical role of STMN1 expression in EMT (26), the interaction between the expression levels of STMN1 and EMT-related markers in GC remains unclear. In the present study, the expression of STMN1 and EMT-related makers was semi-quantitatively examined in postoperative patients with GC, and the association between the expressions of these markers and the clinicopathological variables and patient OS was further analyzed. It was demonstrated that STMN1 and EMT-related markers are differentially expressed between GC and adjacent non-tumorous tissues. In addition, the STMN1 expression in GC tissues was found to be associated with the EMT-related markers E-Cad and VIM, proteins closely linked with cancer progression and survival.
STMN1 has been reported as an important cytosolic phosphoprotein that can regulate microtubule dynamics by participating in microtubule catastrophe and/or in the sequestering of α/β-tubulin heterodimers. As an important microtubule-destabilizing protein, STMN1 has already been found to be involved in a variety of other biological processes, including cell differentiation, cell proliferation, cell migration, metastasis and drug resistance (4,8). In our previous study, STMN1 was found to be overexpressed in GC tissues, and the high STMN1 expression level was associated with poor prognosis in GC (11). Jeon et al (27) suggested that the knockdown of STMN1 in the GC cells resulted in decreased proliferation, migration and invasion in vitro and in vivo. In agreement with these reports, it was found that the STMN1 expression was higher in GC than in adjacent non-tumorous tissues, and its high expression was correlated with invasion depth, lymph node metastasis and pTNM stage. These results suggested that the abnormal expression of STMN1 is closely associated with the malignant features of GC. Survival analysis indicated that patients who exhibited a high STMN1 expression had a shorter 5-year OS than patients with a low STMN1 expression. However, multivariate analysis showed that STMN1 expression was not an independent predictor of poor prognosis. These results are consistent with our previous study, as well as others (11,27,28), indicating that STMN1 overexpression is associated with tumor progression and poor prognosis.
EMT is an important process in tumor invasion and metastasis, including in GC (16,25). It is defined by the loss of epithelial morphology and the acquisition of a mesenchymal phenotype (15). E-Cad is a calcium-dependent transmembrane glycoprotein and plays a crucial role in maintaining tight junctions and structures between different epithelia. By contrast, VIM is a cytoskeletal protein, usually associated with cells of mesenchymal origin. Interacting with other cytoskeletal proteins, VIM acts on cell morphology and participates in cell motility and migration. The diverse roles of these two proteins lead to their different functions in the EMT process of cancer (29). It is generally accepted that a reduced E-Cad expression coupled with an increased VIM expression is characteristic of EMT. In the present study, the expression of EMT-related molecular markers, E-Cad and VIM, was assessed by IHC. The results demonstrated that the expression level of epithelial marker E-Cad was clearly lower in GC tumor tissues than in adjacent non-tumorous tissues, while the expression level of mesenchymal marker VIM was significantly higher in tumor tissues. Previous studies (30–33) have reported that the E-Cad expression ranged from 30.0 to 42.9% in GC. In the present study, the E-Cad expression level was 28.7%, which was similar to the levels observed in previous studies. Consistent with the literature, the results of the present study indicated that the decreased E-Cad expression was positively correlated with invasion depth, lymph node metastasis and advanced pTNM stage. Multivariate analysis showed that the E-Cad expression was independently associated with the outcome of GC. Several studies have reported that the rate of positive VIM expression in surgically resected GC ranged from 12.2 to 60.4% (32,34,35). In agreement with these results, a positive VIM expression was observed in 31.7% of the cases in the present study. The VIM expression was correlated with adverse clinical features, such as lymph node metastasis and advanced pTNM stage. However, the prognostic value of VIM for GC remains under debate. Survival analysis showed that the increased VIM expression was correlated with a poor prognosis. These findings suggested that EMT is associated with the progressive phenotypes and poor outcome in GC.
Certain previous studies may shed light on the role of STMN1 in EMT. Li et al (18) reported that STMN1 might promote the EMT process through regulating microtubule dynamics in cancer. Williams et al (19) found that the inactivation of STMN1 is key to promoting oncogenesis and EMT. Loss of STMN1 gene compromises cell-cell adhesion, which is followed by EMT. These findings indicated that SMTN1 may regulate EMT in human cancer. However, whether STMN1 is associated with EMT in GC remains unclear. The present results demonstrated that the proteins STMN1 and VIM are upregulated in GC tissues, whereas E-Cad is downregulated, which indicated that SMTN1 and EMT should be involved in tumor development and progression. Further tests showed that a high STMN1 expression is significantly and positively correlated with VIM expression levels and negatively correlated with those of E-Cad. Our investigations also suggested that reducing the expression of STMN1 would result in the increased the expression of epithelial marker E-cad, and decreased the expression of mesenchymal marker VIM in GC cells. These findings indicated that overexpressed STMN1 may enhance metastasis and aggressiveness in GC, partly via facilitating the EMT process. However, the exact relationship between STMN1 and EMT remains to be determined, and further studies are required to uncover the potential mechanisms of STMN1 and EMT.
Some studies have shown that STMN1 expression and EMT are associated with the prognosis of patients with GC. The present results showed that patients with a high expression of STMN1 and VIM in their tumors had a poorer OS than those with a low expression. Meanwhile, patients with tumors that highly expressed E-Cad had a better prognosis than patients with tumors with a low E-Cad expression. Multivariate analysis results indicated that only the E-Cad expression was an independent factor influencing the prognosis of GC patients. This result suggested that E-Cad may function as a clinically relevant tumor marker in conjunction with other clinicopathological parameters to affect prognoses in GC patients following surgery. However, the prognostic value of E-Cad for GC remains under debate. Some studies have reported that E-Cad expression is linked to improved patient survival; this has been supported by in vitro studies reporting that the EMT is associated with tumor progression and metastasis (36). Other studies have reported that E-Cad expression does not predict prognosis in GC (37). The discrepancies between these studies might be due to difference in sample size and population.
Despite the suggestive nature and replicability of the findings, the study has certain limitations: Only one technique, immunochemistry, was used, and the sample size was limited. Further studies with larger sample sizes and different techniques used are required to confirm the present results.
In conclusion, the present study demonstrated that the expression level of STMN1 and EMT-related markers E-Cad and VIM were associated with tumor progression and prognosis in GC. STMN1 expression levels were found to be associated with those of E-Cad and VIM. STMN1 may promote tumor progression through EMT in GC. However, the relationship between STMN1 and EMT in the field of GC remains to be elucidated.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- GC
gastric cancer
- EMT
epithelial-mesenchymal transition
- STMN1
Stathmin1
- E-Cad
E-cadherin
- VIM
vimentin
- TNM
tumor-node-metastasis classification
- OS
overall survival
- CI
confidence interval
- HR
hazard ratio
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81401952) and the Science and Technology Research Projects of Tianjin Municipal Health Bureau (grant no. 2014KZ082).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All of the authors have read and approved the final version of this manuscript. BK and HL designed the experiments. BK, XFG and LLW performed the experiments. BK and BL analyzed and interpreted data. NL and RPZ contributed to the analysis and interpretation of data. All authors participated in the writing of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital (Tianjin, China). All patients in the present study signed written informed consent forms prior to study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM. Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J Am Coll Surg. 2014;219:664–675. doi: 10.1016/j.jamcollsurg.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 4.Belletti B, Baldassarre G. Stathmin: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15:1249–1266. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- 5.Hemdan T, Linden M, Lind SB, Namuduri AV, Sjöstedt E, de Ståhl TD, Asplund A, Malmström PU, Segersten U. The prognostic value and therapeutic target role of stathmin-1 in urinary bladder cancer. Br J Cancer. 2014;111:1180–1187. doi: 10.1038/bjc.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu K, Ohtaki Y, Altan B, Yokobori T, Nagashima T, Arai M, Mogi A, Kuwano H. Prognostic impact of stathmin 1 expression in patients with lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;154:1406–1417.e3. doi: 10.1016/j.jtcvs.2017.03.125. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki S, Yokobori T, Altan B, Hara K, Ozawa D, Tanaka N, Sakai M, Sano A, Sohda M, Bao H, et al. High stathmin 1 expression is associated with poor prognosis and chemoradiation resistance in esophageal squamous cell carcinoma. Int J Oncol. 2017 Mar 7; doi: 10.3892/ijo.2017.3899. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Tian FJ, Qin CM, Li XC, Wu F, Liu XR, Xu WM, Lin Y. Decreased stathmin-1 expression inhibits trophoblast proliferation and invasion and is associated with recurrent miscarriage. Am J Pathol. 2015;185:2709–2721. doi: 10.1016/j.ajpath.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Tseng YH, Huang YH, Lin TK, Wu SM, Chi HC, Tsai CY, Tsai MM, Lin YH, Chang WC, Chang YT, et al. Thyroid hormone suppresses expression of stathmin and associated tumor growth in hepatocellular carcinoma. Sci Rep. 2016;6:38756. doi: 10.1038/srep38756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Yao Y, Ming Y, Shen S, Wu N, Liu J, Liu H, Suo T, Pan H, Zhang D, et al. Downregulation of stathmin 1 in human gallbladder carcinoma inhibits tumor growth in vitro and in vivo. Sci Rep. 2016;6:28833. doi: 10.1038/srep28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke B, Wu LL, Liu N, Zhang RP, Wang CL, Liang H. Overexpression of stathmin 1 is associated with poor prognosis of patients with gastric cancer. Tumour Biol. 2013;34:3137–3145. doi: 10.1007/s13277-013-0882-0. [DOI] [PubMed] [Google Scholar]
- 12.Micalizzi DS, Farabaugh SM, Ford HL. Epithelial-mesenchymal transition in cancer: Parallels between normal development and tumor progression. J Mammary Gland Biol Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 15.Kang Y, Massagué J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, Wu RL, Xu AM. Epithelial-mesenchymal transition in gastric cancer. Am J Transl Res. 2015;7:2141–2158. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Liu J, Yue D, Gao L, Wang D, Zhang H, Wang C. Clinical significance of E-cadherin, β-catenin, vimentin and S100A4 expression in completely resected squamous cell lung carcinoma. J Clin Pathol. 2013;66:937–945. doi: 10.1136/jclinpath-2013-201467. [DOI] [PubMed] [Google Scholar]
- 18.Li N, Jiang P, Du W, Wu Z, Li C, Qiao M, Yang X, Wu M. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules. Proc Natl Acad Sci USA. 2011;108:12851–12856. doi: 10.1073/pnas.1017372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams K, Ghosh R, Giridhar PV, Gu G, Case T, Belcher SM, Kasper S. Inhibition of stathmin1 accelerates the metastatic process. Cancer Res. 2012;72:5407–5417. doi: 10.1158/0008-5472.CAN-12-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Cao J, Zhao X. miR-221 facilitates the TGFbeta1-induced epithelial-mesenchymal transition in human bladder cancer cells by targeting STMN1. BMC Urol. 2015;15:36. doi: 10.1186/s12894-015-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobin LH, Gospodarowicz MK, Wittekind C. 7th. Wiley-Blackwell; Oxford: 2010. TNM Classification of Malignant Tumors. [Google Scholar]
- 22.Gao Y, Niu Y, Wang X, Wei L, Zhang R, Lv S, Yu Q, Yang X. Chromosome aberrations associated with centrosome defects: A study of comparative genomic hybridization in breast cancer. Human Pathol. 2011;42:1693–1701. doi: 10.1016/j.humpath.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Bi T, Dai W, Wang G, Qian L, Shen G, Gao Q. Lupeol induces apoptosis and cell cycle arrest of human osteosarcoma cells through PI3K/AKT/mTOR pathway. Technol Cancer Res Treat. 2016;15:NP16–NP24. doi: 10.1177/1533034615609014. [DOI] [PubMed] [Google Scholar]
- 24.Xia P, Xu XY. Epithelial-mesenchymal transition and gastric cancer stem cell. Tumour Biol. 2017;39:1010428317698373. doi: 10.1177/1010428317698373. [DOI] [PubMed] [Google Scholar]
- 25.Peng Z, Wang CX, Fang EH, Wang GB, Tong Q. Role of epithelial-mesenchymal transition in gastric cancer initiation and progression. World J Gastroenterol. 2014;20:5403–5410. doi: 10.3748/wjg.v20.i18.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y, Liu C, Xu YF, Cheng H, Shi S, Wu CT, Yu XJ. Stathmin destabilizing microtubule dynamics promotes malignant potential in cancer cells by epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int. 2014;13:386–394. doi: 10.1016/S1499-3872(14)60038-2. [DOI] [PubMed] [Google Scholar]
- 27.Jeon TY, Han ME, Lee YW, Lee YS, Kim GH, Song GA, Hur GY, Kim JY, Kim HJ, Yoon S, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–718. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang W, Tong JH, Chan AW, Lung RW, Chau SL, Wong QW, Wong N, Yu J, Cheng AS, To KF. Stathmin1 plays oncogenic role and is a target of microRNA-223 in gastric cancer. PLoS One. 2012;7:e33919. doi: 10.1371/journal.pone.0033919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Wever O, Pauwels P, De Craene B, Sabbah M, Emami S, Redeuilh G, Gespach C, Bracke M, Berx G. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–494. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Chen XY, Huang KJ, Wu WD, Jiang T, Cao J, Zhou LS, Qiu ZJ, Huang C. Expression of FoxM1 and the EMT-associated protein E-cadherin in gastric cancer and its clinical significance. Oncol Lett. 2016;12:2445–2450. doi: 10.3892/ol.2016.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Bartolomeo M, Pietrantonio F, Pellegrinelli A, Martinetti A, Mariani L, Daidone MG, Bajetta E, Pelosi G, de Braud F, Floriani I, Miceli R. Osteopontin, E-cadherin, and β-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer. 2016;19:412–420. doi: 10.1007/s10120-015-0495-y. [DOI] [PubMed] [Google Scholar]
- 32.Liu WF, Ji SR, Sun JJ, Zhang Y, Liu ZY, Liang AB, Zeng HZ. CD146 expression correlates with epithelial-mesenchymal transition markers and a poor prognosis in gastric cancer. Int J Mol Sci. 2012;13:6399–6406. doi: 10.3390/ijms13056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong XY, Zhang LH, Jia SQ, Shi T, Niu ZJ, Du H, Zhang GG, Hu Y, Lu AP, Li JY, Ji JF. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology. 2008;52:560–568. doi: 10.1111/j.1365-2559.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Yamada S, Izumi H, Li Z, Shimajiri S, Wang KY, Liu YP, Kohno K, Sasaguri Y. Strong YB-1 expression is associated with liver metastasis progression and predicts shorter disease-free survival in advanced gastric cancer. J Surg Oncol. 2012;105:724–730. doi: 10.1002/jso.23030. [DOI] [PubMed] [Google Scholar]
- 35.Kim MA, Lee HS, Lee HE, Kim JH, Yang HK, Kim WH. Prognostic importance of epithelial-mesenchymal transition-related protein expression in gastric carcinoma. Histopathology. 2009;54:442–451. doi: 10.1111/j.1365-2559.2009.03247.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang ZS, Shen Y, Li X, Zhou CZ, Wen YG, Jin YB, Li JK. Significance and prognostic value of Gli-1 and Snail/E-cadherin expression in progressive gastric cancer. Tumour Biol. 2014;35:1357–1363. doi: 10.1007/s13277-013-1185-1. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Zhang J, Yan Y, Cai H, Li M, Sun K, Wang J, Liu X, Wang J, Duan X. Low expression of Rap1GAP is associated with epithelial-mesenchymal transition (EMT) and poor prognosis in gastric cancer. Oncotarget. 2017;8:8057–8068. doi: 10.18632/oncotarget.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.