Abstract
After 20 years of successful targeting of pro-inflammatory cytokines for the treatment of IBD, an alternative therapeutic strategy has emerged, based on several decades of advances in understanding the pathogenesis of IBD. The targeting of molecules involved in leukocyte traffic has recently become a safe and effective alternative. With 2 currently approved drugs (ie, natalizumab, vedolizumab) and several others in phase 3 trials (eg, etrolizumab, ozanimod, anti-MAdCAM-1), the blockade of trafficking molecules has firmly emerged as a new therapeutic era for IBD. We discuss the targets that have been explored in clinical trials: chemokines and its receptors (eg, IP10, CCR9), integrins (eg, natalizumab, AJM300, vedolizumab, and etrolizumab), and its endothelial ligands (MAdCAM-1, ICAM-1). We also discuss a distinct strategy that interferes with lymphocyte recirculation by blocking lymphocyte egress from lymph nodes (small molecule sphingosine-phosphate receptor [S1PR] agonists: fingolimod, ozanimod, etrasimod, amiselimod). Strategies on the horizon include additional small molecules, allosteric inhibitors that specifically bind to the active integrin form and nanovectors that allow for the use of RNA interference in the quest to modulate pro-inflammatory leukocyte trafficking in IBD.
Keywords: α4β7 Integrin, MAdCAM-1, sphingosine-1-phosphate, gut homing, Crohn’s, colitis, ulcerative colitis
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) comprise the 2 main inflammatory bowel diseases (IBD), characterized by chronic persistent recruitment of inflammatory cells, as would happen during a chronic infection that fails to resolve (eg, intestinal tuberculosis). The current model for IBD pathogenesis considers microbial “dysbiosis” and impairment of intestinal epithelial barrier function as initiating events, triggering a dysregulated immune response in genetically predisposed individuals, with the contribution of poorly understood environmental factors. Microbial antigens are taken up by antigen-presenting cells (eg, dendritic cells), which migrate to secondary lymphoid tissues to present antigens to naïve T lymphocytes. This is followed by differentiation of the latter into effector T cells, with subsequent proliferation (clonal expansion), acquisition of a cytokine-producing program, and an array of surface molecules (eg, chemokine receptors, integrins) that allow them to recirculate back to the intestine.1,2 Subsequently, these antigen-primed T helper lymphocytes either retain their expression of lymphoid tissue homing receptors (eg, CCR7, central memory cells) and remain in the lymph node or they lose it, which allows them to traffic to the intestinal lamina propria. Here, they either become tissue-resident memory or recirculating cells.1 Once in the intestine, tissue-resident and trafficking effector cells are able to rapidly secrete pro-inflammatory cytokines in response to microbial antigens or inflammatory stimuli.2
Dysregulated leukocyte recruitment is considered a hallmark of IBD, as signified by accelerated recruitment or enhanced retention signals (likely chemoattractant chemokines) at sites of inflammation, particularly T cells.3, 4 Leukocytes migrate from blood into tissues across post-capillary venules by engaging specific molecules that are presented at the glycocalyx or expressed on the surface of specialized endothelial cells. These latter molecules, belonging to the immunoglobulin superfamily, serve as mechanical anchors and confer tissue specificity to the recruitment process. The molecules that mediate the sequential steps of the leukocyte adhesion cascade (ie, capture/tethering, rolling, activation, and firm adhesion) allow leukocytes to escape the circulation and migrate to sites of inflammation.3 The discovery of this sequence of steps was recognized by the Royal Swedish Academy of Sciences and the Crafoord Foundation with the Crafoord prize awarded to Eugene Butcher and Timothy Springer.
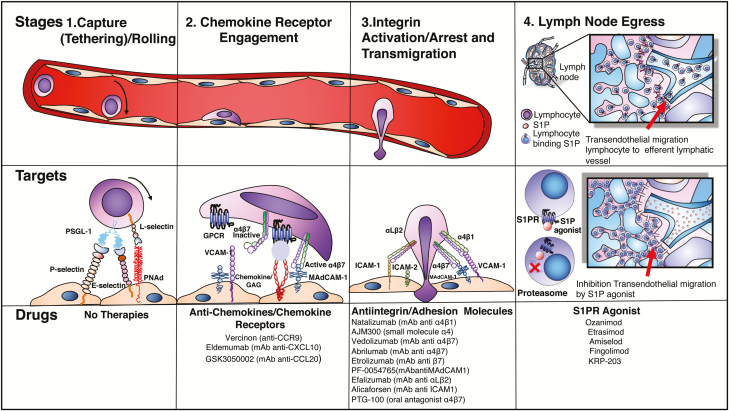
The success of interfering with each step in this sequence for therapeutic purposes in IBD has been variable. Some of the strategies failed at the preclinical stage (eg, targeting selectins/rolling), whereas others have become the latest safe and effective therapies in our armamentarium (ie, targeting integrins). In this review, we approach drug discovery based on our best understanding of IBD pathogenesis and the molecules and processes that allow leukocytes to escape the circulation to migrate to the intestine and induce or maintain IBD (Fig. 1).
FIGURE 1.
Lymphocyte trafficking: stages, molecular targets and drugs.
Capture Tethering/Rolling
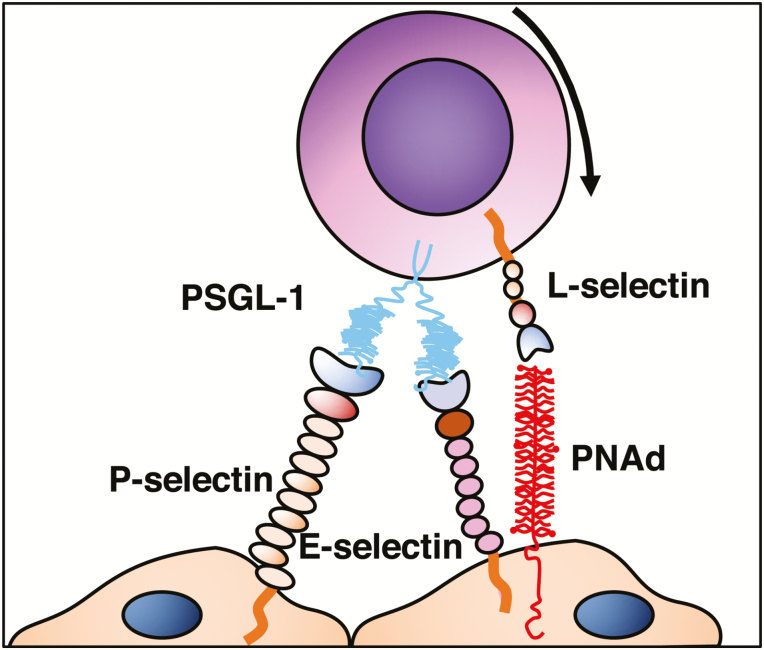
Leukocyte trafficking to the small and large intestine is tightly regulated to maintain intestinal immune homeostasis, mediate immune responses, and prevent overt inflammation.5 The first step in the recruitment process allows cells to slow down along endothelium, by engaging selectin ligands expressed on microvascular beds. This slowdown then allows leukocyte exposure to chemoattractant molecules. Selectins are carbohydrate-binding molecules that bind fucosylated and sialylated glycoprotein ligands, found on endothelial cell, leukocytes, and platelets. They are involved during homeostasis and during acute and chronic inflammatory processes.6
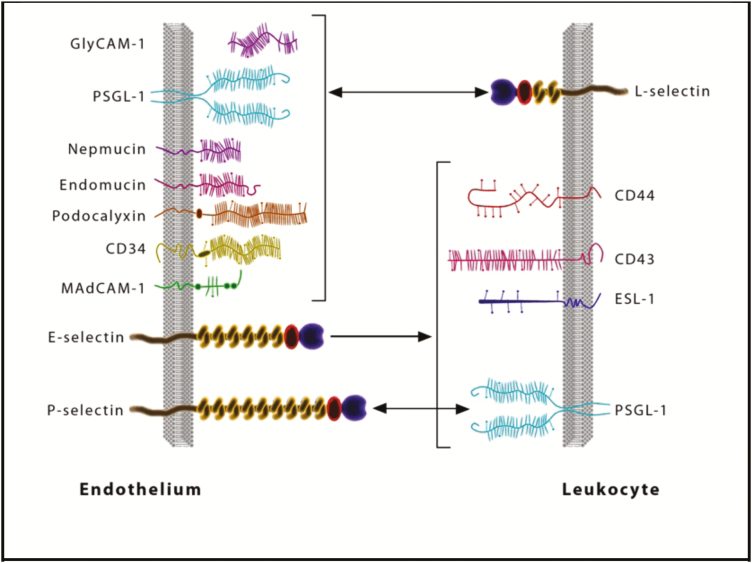
L-selectin is expressed on leukocytes, whereas P-selectin is stored in platelet alpha granules and in Weibel-Palade bodies of endothelial cells, translocating to the cell surface of activated endothelial cells and platelets. E-selectin is not expressed under baseline conditions—except in skin microvessels—but is rapidly induced on endothelial cells by inflammatory cytokines.7 The most important ligands for selectins include P-selectin glycoprotein ligand 1 (PSGL-1), E- selectin ligand-1 (ESL-1), and glycoproteins such as CD44, CD34, CD24, cellular adhesion molecule-1 (CAM-1), Glycosylation-dependent CAM-1 (GlyCAM-1), and mucosal addressin cell adhesion molecule-1 (MAdCAM-1). Of these ligands, the most extensively characterized at the molecular, cellular, and functional level is PSGL-1.6 The capture of T cells starts with the interaction between selectins and oligosaccharide moieties present in their ligands. The initial loose (carbohydrate-carbohydrate) interactions are mediated by PSLG-1 and L-selectin binding to endothelial E-selectin/P-selectin and PNAd, respectively.8
These loose initial contacts slow down the leukocyte to overcome the shear forces within the blood vessel and allow leukocytes to roll along the endothelium.8 By sequentially engaging their endothelial ligands, their velocity is slowed enough to allow exposure to chemoattractant/arrest chemokines that are held locally by the endothelial glycocalyx due to their basic properties (Figs. 2 and 3).
FIGURE 2.
Tethering/rolling.
FIGURE 3.
Selectins and their ligands.75
Targeting selectins
The pharmacological blockade at this level of the leukocyte recruitment process was previously seen as promising strategy for therapeutic intervention in inflammatory disorders. Selectin-neutralizing monoclonal antibodies and small-molecule inhibitors have been tested in clinical trials on patients with trauma, cardiac indications, and asthma.9–11 Antiselectin antibodies have also been successfully used in preclinical models to deliver imaging contrast agents and therapeutics to the sites of inflammation.12 In patients with UC, a double-blind placebo control phase I-II with GI-270384X (an oral ICAM-1 and E-selectin inhibitor) was carried out. The study was concluded, yet the results were not published.13 Further improvements in the efficiency, availability, specificity, and pharmacokinetics of selectin inhibitors may hold promise for therapeutic indications.6
Chemokine Engagement
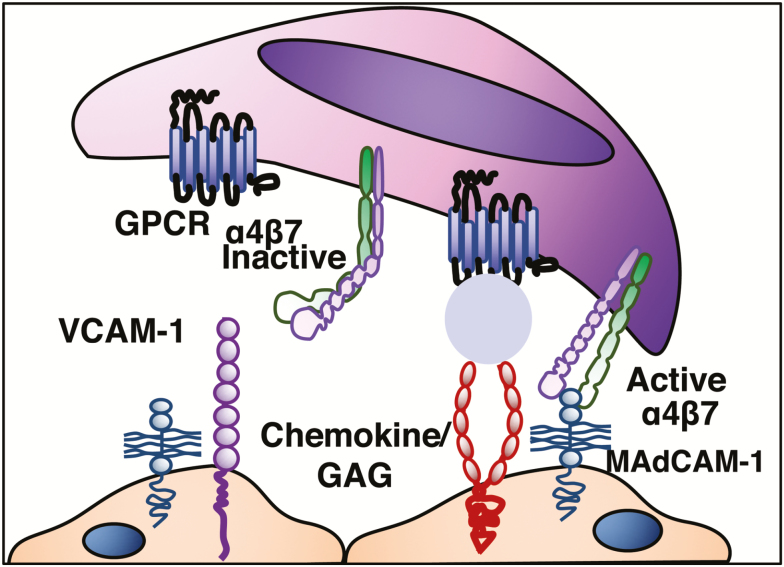
Chemokines (ie, chemo-tactic cyto-kines) are small secreted polypeptides that, among multiple other functions, direct the movement of circulating leukocytes to sites of inflammation (chemotaxis) (Table 1) and play a main role in integrin activation (arrest chemokines).14 During rolling, lymphocytes express specific chemokine receptors that bind chemokines displayed on glycosaminoglycans (GAGs) of endothelial cells. Chemokine receptors belong to a family of 7-transmembrane, G protein–coupled receptors (7-TM-GPCRs) (Fig. 4).
TABLE 1.
Major Human Chemokines and Chemokine Receptors Involved in Leukocyte Trafficking to the Small Intestine and Colon
| Chemokines | Receptor | Key/main inmune function |
|---|---|---|
| CXCL10 | CXCR3 | Leukocyte recruitment to inflamed intestine. |
| Th1 response. | ||
| CCL20 | CCR6 | Th17 responses.B cell and DC homing to gut-associated lymphoid tissues |
| CCL21 | CCR6, CCR7 | T cell and DC homing to LN. |
| CCL25 | CCR9 | T cell homing to gut:thymocyte migration. |
| CCL28 | CCR3, CCR10 | T cell and IgA plasma cell homing to mucosa. Homing to colon. |
Table is modified from reference (Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702.) Abbreviations: DC, dendritic cells;LN, lymph nodes, natural killer; Th, T helper cell
FIGURE 4.
Chemokine receptor engagement.
TARGETING CHEMOKINE AND CHEMOKINE RECEPTORS IN IBD.
Vercirnon (CCX282-B) is a small molecule antagonist against CCR9, which mediates homing of T and B lymphocytes and dendritic cells to the small intestine, through its interaction with CCL25, a chemokine expressed predominantly in small intestine and thymus under physiologic conditions. In a randomized, placebo-control phase 2 study in 436 patients with CD, this drug was not better than placebo.15, 16 In addition, the phase 3 trial did not demonstrate the efficacy of vercirnon as an induction therapy in patients with moderately to severely active Crohn’s disease. Its effect in maintenance therapy was not addressed.17 Basic pitfalls of the concept of CCR9/CCL25 axis targeting include: 1) extrapolation of CCR9 expression in mouse CD4+ cells, without regard to potential differences between the ligand/receptor expression in humans; 2) the fact that the trial included patients with Crohn’s colitis, a manifestation which could be fundamentally different from Crohn’s ileitis;18 3) and that CCR9/CCL25 might be predominantly homeostatic molecules with a limited role during inflammation.
Eldelumab is a fully human monoclonal antibody against Interferon-γ-inducible protein-10 (IP-10). IP-10 or CXCL10 is a chemokine that mediates trafficking of activated T cells, dendritic cells and monocytes to the inflamed colon. Its efficacy was evaluated in both UC (n = 252) and CD (n = 121). In both, eldelumab did not demonstrate efficacy over placebo.19, 20, 21
GSK3050002 (MorphotekTM) is a humanized IgG1κ antibody with high binding affinity to human CCL20. The chemokine CCL20 may be of particular interest in IBD, as it is upregulated in active ulcerative colitis and Crohn’s disease.22 CCL20 binds exclusively to CCR6 and is constitutively expressed by neutrophils, enterocytes, B cells and dendritic cells, and other cell types after stimulation with pro-inflammatory ligands.21 Its receptor, CCR6 is expressed by T regulatory (Treg), T helper type 17 (Th17), and immature dendritic and B cells. CCL20 has been shown to direct Treg, Th17, B cells and immature dendritic cell recruitment to the gut mucosa.22 Recently, GSK3050002 was administered to 48 healthy volunteers to evaluate safety, pharmacokinetics (PK), and pharmacodynamics (PD).23 An experimental skin suction blister model was employed to assess target engagement and the ability of the compound to inhibit recruitment of inflammatory CCR6-expressing cells. The results showed a relationship between PK, target engagement, and PD, suggesting selective inhibition of recruitment of CCR6+ cells by GSK3050002 and supporting further development.24
Integrin Activation, Arrest, and Transmigration
Integrins are cell-adhesion receptors, expressed on leukocytes as heterodimeric transmembrane proteins. They interact with molecular components of the extracellular matrix but also with ligands displayed by endothelial and other cells. Chemokine binding triggers conformational changes, resulting in their activation.8 This process, referred to as inside-out signaling, is initiated by adaptor molecules that affect the position of the integrin α and β cytoplasmic tails relative to each other and to the plasma membrane. The best known positive regulators of integrin activation are the adaptor molecules talin-1 and the kindlins.25, 26 Integrins on resting cells are maintained in an inactive state, in which the headpiece is folded back to the leg pieces, thereby exhibiting a bent conformation. In this inactive conformation, the ligand-binding domain is in a low affinity configuration, oriented unfavorably for interacting with ligand. Upon integrin activation, the interface between headpiece and tailpiece is opened in a switchblade-like movement, thereby exhibiting an extended conformation, in which the ligand-binding headpiece is oriented favorably toward ligand on endothelial cells. The conversion from the inactive bent conformation to the active extended conformation with open headpiece is triggered by the separation of the α/β cytoplasmic domains and is linked to structural rearrangements in the ligand-binding domain, leading to the high-affinity configuration.26, 27
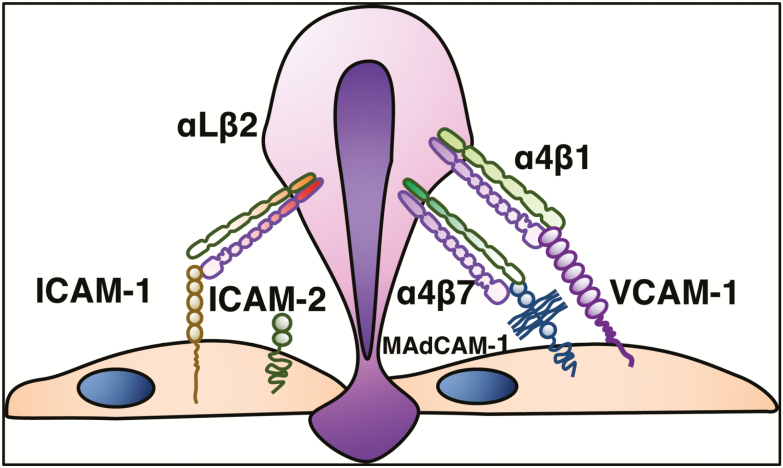
After activation, integrins demonstrate strong and stable protein-protein binding to ligand, resulting in arrest of leukocytes on the vessel wall. These firm, adhesive bonds allow for arrest of lymphocytes to sites of inflammation, guided by chemoattract gradients, which further localizes them within tissues. The endothelial ligands for integrins are members of the immunoglobulin superfamily (ie, ICAM-1, ICAM-2, VCAM-1, MAdCAM-1).
Integrins and their ligands play pivotal roles in IBD, as they are major mediators of the dysregulated traffic of lymphocytes and other immune cells to the inflamed intestine. In addition, they may participate in the pathogenesis of extraintestinal inflammatory manifestations of IBD.3 Among the several members of the immunoglobulin superfamily, the following adhesion molecules have established roles in IBD: intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and MAdCAM-1.3, 8
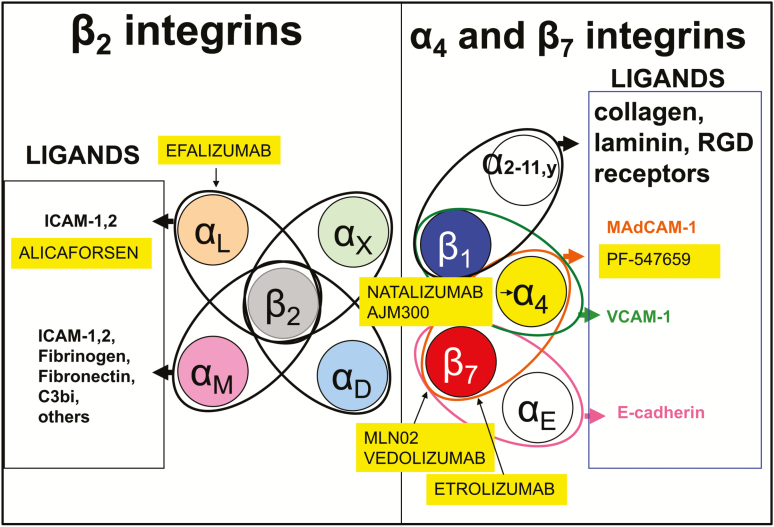
The integrins are formed by the noncovalent association of an α and β subunit. Both α subunits determine the specificity of the integrin ligand (α4β1/VCAM-1, α4β7/MAdCAM-1), whereas the β subunit also regulates binding of ligands.3 The β7 subunit pairs with α4 (α4β7), which binds to MAdCAM-1 expressed on gut endothelial and MLN cells. The α4β7 can also bind to VCAM-1 and the extracellular matrix protein fibronectin, when on a highly activated state.3, 28 Integrin α4β7 is expressed on lymphocytes that migrate to the gut and gut-associated lymphoid tissues (GALT). The β7 subunit can also dimerize with αE to form αEβ7 heterodimer. The only known ligand for αEβ7 is E-cadherin, an adhesion molecule present on epithelial cells and absent on endothelial cells.27 Integrin αEβ7 is expressed by intraepithelial T lymphocytes in the spleen, thymus, skeletal muscle, heart, liver, kidney, brain, gastrointestinal tract, urogenital tract and lungs, binding selectively to E-cadherin on epithelial cells.5, 29
The α4 subunit pairs with β1 to form α4β1 (very late activation-4, VLA-4), the primary receptor recognizing VCAM-1, being mainly responsible for lymphocyte and monocyte adhesion to vascular endothelium. The steady state-expression of VCAM-1 on endothelium is very low or undetectable. Under inflammatory or other stimulatory conditions, VCAM-1 expression is upregulated.3 VCAM-1 is not exclusively expressed by endothelial cells, being also detected on epithelial cells, dendritic cells (DCs), Kupffer cells, and smooth muscle cells within atherosclerotic lesions.
Another group of integrins involved in the trafficking of leukocytes is the CD18 integrins. The αLβ2 (CD11a/CD18), also known as leukocyte functional antigen-1 (LFA-1), is expressed on lymphocytes and neutrophils and binds to ICAM-1 and ICAM-2 expressed on endothelial cells. It mediates migration, antigen presentation, and cell proliferation. ICAM-2 is expressed constitutively on all endothelial cells, whereas ICAM-1 is expressed at low levels on endothelial cell membrane and on macrophages and lymphocytes in the absence of inflammation. Its expression is induced by inflammatory cytokines. LFA-1 enables migration of both naïve and effector T cells to sites of inflammation.3 (Fig. 5).
FIGURE 5.
Integrin activation, firm adhesion/transmigration.
ANTI-INTEGRIN THERAPIES IN IBD
Natalizumab is a recombinant humanized IgG4 monoclonal antibody that binds to α4 integrin. It was approved by US Food and Drug Administration for the treatment of multiple sclerosis (MS) and CD in 1998, therefore being the prototype anti-integrin strategy. By targeting the shared α4 subunit of 2 distinct integrin heterodimers, natalizumab blocks both α4β1 and α4β7 integrin interactions with fibronectin, VCAM-1, and MAdCAM-1. Its use in IBD has been limited by its association with progressive multifocal leukoencephalopathy (PML).30 The pathogenesis of PML in patients receiving natalizumab is unknown; however, it is primarily associated with the blockade of α4β1 integrin/VCAM-1 interactions by natalizumab in the central nervous system. Thus, natalizumab confers a risk of PML on those that are JCV seropositive, which is otherwise seen only in severely immunocompromised patients.30 Risk factors to PML include a longer than 2-year treatment duration, prior immunosuppressant use, and positive anti-JC virus serology. Patients with all 3 risk factors have a 1 in 100 chances of developing PML.31, 32
Efalizumab (Raptiva) targets the αL subunit of LFA-1. An open-label study evaluated the efficacy and safety of efalizumab in 15 subjects with moderate to severe CD. At 8 weeks, 10 (67%) subjects had clinical response, and 6 (40%) were in remission. No serious adverse events (AEs) occurred.33 Nevertheless, similar to natalizumab, its use was associated with PML and subsequently withdrawn from the market.4, 34
Vedolizumab, a humanized monoclonal antibody that specifically blocks α4β7-MAdCAM-1 interactions, is approved for the treatment of adults with UC and CD. The efficacy of vedolizumab in patients with UC and CD was demonstrated in the GEMINI 1 and GEMINI 2 trials, achieving up to 44% and 39% of clinical remission at week 52, respectively.35, 36 Its efficacy in both UC and CD is greater in patients naïve to anti-TNF therapy.37, 38
Vedolizumab has a favorable safety profile. No cases of progressive PML have been reported, nor has it been associated with increased risk of serious or opportunistic infections. Furthermore, the rate of malignancy is consistent with baseline rates observed in patients with IBD.39 In comparison with anti-TNF antibodies, vedolizumab needs a longer treatment time to exert its full effect. This could be due to the dynamics of T cell recirculation and our limited understanding of the role of tissue resident T cells during IBD. Blockade of de novo T-cell recruitment may be only apparent after a significant portion of the existing lamina propria T-effector cells undergo apoptosis and their replenishment is impeded.1, 35, 36 This seems to be a class effect for all antitrafficking therapies.
Treatment with vedolizumab decreases gut mucosal immune responses to oral cholera vaccine, while preserving the normal immune response to systemic immunization with hepatitis B surface antibody.40 The accumulated evidence suggests that the risk of systemic infections and PML is lower with drugs that selectively target leukocyte trafficking to the gut.39 Questions remain as to whether patients treated with such agents will be more susceptible to enteric pathogens such as cytomegalovirus, giardiasis, or Clostridium difficile.32, 40
An ongoing study will determine the effect of combination therapy (vedolizumab, adalimumab, and methotrexate) on endoscopic remission in newly diagnosed patients with Crohn’s disease, who are stratified at higher risk for complications.41 Furthermore there are 2 ongoing phase 4 studies evaluating its effectiveness for treatment of fistulizing disease42 and pouchitis.43
Abrilumab (AMG 181, Amgen), a completely human subcutaneously administered antibody against α4β7 integrin, has recently completed phase 2b studies.44, 45 In the CD study (n = 249), the primary endpoint (clinical remission at week 8, defined as a Crohn’s disease activity index [CDAI] score <150) was not met.45 However, in the UC study (n = 354), a significantly higher proportion of patients experienced clinical remission (defined as a total Mayo Clinic score <3 with no subscore >1) at week 8 in the highest abrilumab dose groups as compared with placebo.44
Etrolizumab, a humanized monoclonal antibody that selectively binds to the β7 subunit of both α4β7 and αEβ7 integrin heterodimers, antagonizes α4β7-MAdCAM interactions and αEβ7-E-cadherin interactions (believed to be involved in retention of αEβ7 cells within the intraepithelial compartment and migration of DC subsets to mesenteric lymph nodes).46 Etrolizumab efficacy was assessed in a double-blind, placebo-controlled, randomized, phase 2 study in patients (n = 119) with moderately to severely active UC who had not responded to conventional therapy. Clinical remission at week 10 was achieved in none of the subjects in the placebo group compared with 21% in the 100-mg group and 10% in the 300-mg plus loading dose group. Safety and pharmacokinetics were examined at various time points. Etrolizumab was well tolerated with the most common side effect being exacerbation of colitis, followed by nasopharyngitis (also observed with vedolizumab). No serious opportunistic infections were reported. Interestingly, the higher dose was not superior to placebo, a feature that was shared with higher doses of the anti-MAdCAM-1 antibody in the UC trial.46 Although this could be an artifact, we may speculate that higher drug tissue levels might potentially interfere with CD103:E-cadherin interactions or recruitment of T-regs. A phase 3 study was designed to evaluate the safety and efficacy of etrolizumab in patients with moderate to severe CD. In this study, 300 patients (73% anti TNF-experienced) with moderate to severe CD received 105 mg of etrolizumab (n = 120), 210 mg of etrolizumab (n = 121), or placebo (n = 59). Symptomatic remission was observed in a greater proportion of patients treated with 105 mg and 210 mg of etrolizumab compared with placebo at weeks 6, 10, and 14. A greater proportion of patients achieved endoscopic improvement with 105 mg and 210 mg of etrolizumab compared with placebo at week 14. CDAI remission was achieved at week 14 in 23.3% of patients receiving 105 mg of etrolizumab, 28.9% of those receiving 210 mg, and 16.9% of those receiving placebo. In this induction cohort, treatment with etrolizumab was well tolerated and resulted in clinically meaningful endoscopic improvement, with rapid symptomatic remission as early as week 6 and sustained through week 14. These early results are indicative of the efficacy of etrolizumab in treating CD.47 Currently, phase 3 clinical trials are ongoing in CD and UC, evaluating etrolizumab safety and efficacy. An ongoing clinical trial in UC will compare the effectiveness of etrolizumab with anti-TNF.48, 49, 50, 51
AJM300 (AjinomotoTM, EA PharmaTM) is an orally administered small molecule that, like natalizumab, blocks binding of the shared α4 integrin subunit. Its efficacy and safety were evaluated in a double-blind, placebo-controlled, phase 2a study in UC (n = 102). Clinical response rates were 62.7% and 25.5% at week 8 in the AJM300 group and placebo group, respectively. Rates of clinical remission (Mayo Clinic score ≤2 and no subscore >1) were 23.5% and 3.9% in the AJM300 group and placebo groups, and rates of mucosal healing (endoscopic subscores of 0 or 1) were 58.8% and 29.4%. No serious adverse events, including PML, were observed in this short-term trial.52 However concerns remain regarding the risk of PML during long-term treatment that block the shared α4 integrin subunit.
PTG-100 (Protagonist) is a novel oral α4β7 antagonist peptide with minimal systemic absorption and distribution largely restricted to the gut. PTG-100 was evaluated in patients with moderate to severe ulcerative colitis in the phase 2b PROPEL study and was discontinued because it did not meet its primary endpoint of clinical remission.53, 54
ET3764 (Encycle TherapeuticsTM) is an oral inhibitor of integrin α4β7 that is being developed as a candidate drug to treat IBD and other inflammatory disorders.55
TARGETING THE IMMUNOGLOBULIN SUPERFAMILY OF MOLECULES
ICAM-1
Alicaforsen is an antisense oligonucleotide against ICAM-1, which acts via downregulation of ICAM-1 mRNA. In CD, parenterally administered alicaforsen was not more effective than placebo in phase 2 and 3 trials.56, 57 In UC, a placebo-controlled, double-blind, multicenter study randomized 112 patients with UC to receive 1 of 4 alicaforsen enema regimens or placebo daily for 6 weeks. The primary endpoint was Disease Activity Index (DAI) at week 6; however, no significant differences were observed between the treatment arms and placebo. However, a post hoc analysis was performed on subjects whose extent of bowel involvement ranged from 15 to 40 cm at baseline (n = 70) showed a difference in response to the 240-mg daily alicaforsen enema treatment as early as week 3, with a 41% reduction in mean DAI vs 14% in placebo (P = 0.01); this extended to week 30 with a 57% vs 10% reduction of the same (P = 0.04).58 Another randomized, double-blind, active controlled trial compared the effects of alicaforsen enema with mesalazine enema in subjects with mild to moderate, active, left-sided ulcerative colitis. Subjects received a nightly enema of 120 mg of alicaforsen (n = 55), 240 mg of alicaforsen (n = 50), or 4 g of mesalazine (n = 54) for 6 weeks, followed by a 24-week monitoring period. There was no difference compared with mesalamine enemas, although alicaforsen seemed to have a more durable effect in comparison with mesalazine.59 In pouchitis, an open-label trial showed encouraging results, and currently, a phase 3, multicenter, double-blind, randomized, controlled trial in subjects with chronic antibiotic refractory pouchitis is underway.56, 60
MAdCAM-1 Blockade (PF-00547659/SHP647) is a humanized monoclonal antibody against MAdCAM-1 (the endothelial ligand for integrin α4β7). Recently, the results of the TURANDOT—a phase 2, randomized, double-blind, placebo-controlled trial in patients with UC—have been reported. Patients (n = 357) were treated with subcutaneous injections of 1 of 4 doses (7.5, 22.5, 75, or 225 mg) of PF-00547659 or placebo. The primary endpoint was remission at week 12. This was met in 3 of the 4 groups (7.5, 22.5, or 75 mg); the highest difference in efficacy compared with placebo was observed in the 22.5-mg group.61 In this study, higher doses were less effective (hormesis), with the maximum effect observed below the highest dose studied. The authors propose that decreased efficacy at increased doses is related to overdepletion of regulatory T cell and known to express α4β7 integrin, like intraepithelial lymphocytes, mucosal-associated invariant T cells, and eosinophils. This effect is potentially undesirable and could explain the weaker responses seen at higher doses.62, 63, 64 The authors conclude that the gut effector cell population is more susceptible than the regulatory T-cell population to MAdCAM-1 blockade, resulting in a net immunoregulatory phenotype in the intestine at lower efficacious doses.61
In contrast, 265 patients with active moderate to severe CD (CDAI 220–450), history of failure or intolerance to anti-TNF and/or immunosuppressive agents, high-sensitivity C reactive protein >3.0 mg/L, and ulcers on colonoscopy were randomized to PF-00547659—22.5 mg, 75 mg, 225 mg, or placebo. Patients receiving anti-MAdCAM-1 therapy in the OPERA study had no greater efficacy than placebo in any of the studied doses.65 The primary endpoint (70-point decrease CDAI from baseline at week 8 or 12) was not met, in part due to high placebo response rate. However, in patients with evidence of inflammation by high-sensitivity C reactive protein or Simple Endoscopic Activity Score, post hoc analyses suggested the presence of a drug effect.65 The Opera II was a 72-week, phase 2 extension study designed to assess the long safety and efficacy of SHP647 (Shire designation). A total of 268 patients were enrolled, and 149 completed the study. The antibody was well tolerated, with a safety profile similar to that observed in previous trials and no PML cases reported; the clinical response and remission rates were sustained, suggesting efficacy over 72 weeks.66 Future clinical trials are needed to determine the role of anti-MAdCAM therapy for the treatment of CD (Fig. 6).67
FIGURE 6.
Integrin targeting.75
Lymph Node Egress
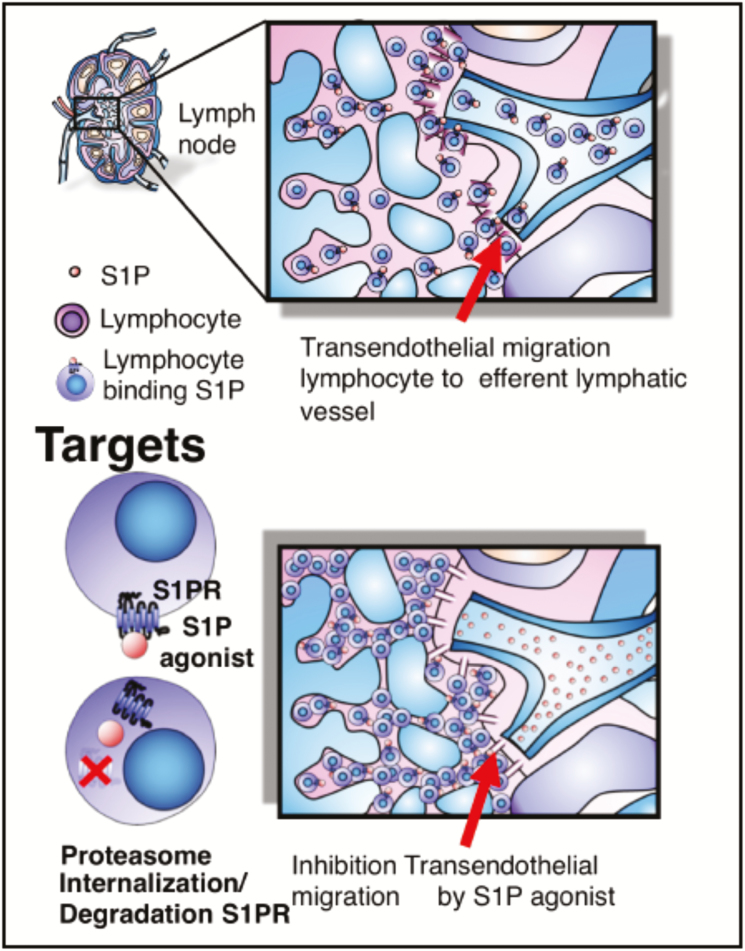
The egress of lymphocytes from lymph nodes is an early event that allows lymphocytes to traffic to circulation and back to effector sites for immune surveillance (Fig. 7). T cell exit from the lymph nodes is dependent on sphingosine 1-phosphate (S1P), a membrane-derived lysophospholipid signaling molecule, which binds to 7 membrane–spanning, G protein–coupled receptors on T cells and other cell types.68
FIGURE 7.
Lymph node egress and targets.
T-cell exit from the lymph nodes is dependent on sphingosine 1-phosphate (S1P), a membrane-derived lysophospholipid-signaling molecule, which binds to 7 membrane–spanning, G protein–coupled receptors on T cells and other cell types.68 Sphingolipids are essential constituents of cellular membranes, and S1P is a sphingolipid metabolite derived from sphingosine by sphingosine kinases 1, 2 actions and can be reversibly dephosphorylated to sphingosine by sphingosine phosphatases 1, 2 and irreversibly degraded by S1P lyase. This enzymatic pathway is responsible for the regulation of S1P levels.69 S1P receptors (S1PR) show overlapping or distinct expression patterns in various cells and tissues and regulate diverse cellular functions by signaling through its receptor subtypes (S1PR 1–5). S1PR1 plays a predominant role in controlling lymphocyte egress from secondary lymphoid organs, thymus, and bone marrow.70–73
The S1P gradient between tissues and systemic circulation is essential for lymphocytes to egress from the thymus and lymph nodes. S1P is present at higher concentrations in the blood and lymph than in tissues. This concentration gradient is maintained by the S1P degrading enzyme S1P lyase, which is present in tissues but not in blood.74 S1P/S1PR1 interactions are important for the trafficking of both naïve and activated T cells. The signals generated by S1P binding to S1PR1 on naïve T cells stimulate directed movement of the cells along the S1P concentration gradient out of the lymph node parenchyma. Circulating naïve T cells have very little surface S1PR1 because the high blood concentration of S1P induces internalization of the receptor. After naïve T cells enter the lymph node, where S1P concentrations are low, S1PR1 is re-expressed on the cell surface over a period of several hours. This time lag allows a naïve T cell to interact with antigen-presenting cells. Once S1PR1 receptor is re-expressed, T cells leave the lymph node by sensing the S1P concentration gradient into the efferent lymphatics (Fig. 7).74 Activated T cells in lymphoid organs downregulate the surface expression of the S1P receptor, S1PR1, for several days. During this period, T cells cannot respond to the S1P gradient and do not exit the lymphoid organs. After several days of proliferation, as T-cell activation wanes, S1PR1 is re-expressed, allowing them to migrate out of the lymphoid tissue in response to the S1P gradient.74 S1PR1 agonists binding to S1P1 lead to the internalization of the receptor on naïve and central memory T and B cells, rendering them unable to respond to the S1P gradient and impeding lymph node exit.74 Receptor internalization, induced by continuous exposure to effective concentrations of S1PR agonists, leads to receptor ubiquitination and proteasome degradation in lymphocytes, causing long-lasting receptor downregulation and lymphocyte sequestration in lymphoid tissues, potentially preventing them from reaching sites of inflammation.2 (Fig. 7).
S1P may also act through a completely different mechanism. S1PR1 is strongly expressed by lymphatic endothelium. S1PR1 agonists tighten the lymphatic endothelial barrier, inhibiting transendothelial migration and blocking lymphocyte egress from the lymph node. Consequently, lymphocytes are trapped, decreasing circulating lymphocyte counts (Fig. 7). Upon withdrawal of the agent, the permeability is restored and lymphocyte trafficking resumes.76–78
Further investigation revealed that traffic of specific subsets of immune cells is compromised by S1PR agonists; thus, immune surveillance and the innate immune system against cancer and virally infected cells are maintained, at least in the short term. In particular, central memory and naïve T cells and B cells are decreased, with the effect on CD4+T cells being more pronounced as compared with cytotoxic CD8+T cells. No effect was observed on effector memory T cells.79, 80 Research on the traffic of lymphocytes has come to the forefront by exploring the possible roles of S1PR agonists in IBD and various immune-mediated disorders. Its promising profile is based on receptor specificity of novel agents, a short half-life that allows a faster recovery of lymphocyte counts in case of complications, convenient oral route of administration, absence of immunogenicity, selective effects on memory and naïve cells, with limited effects on effector T-cell pool, maintenance of immune surveillance, and potentially lower cost of manufacturing and administration as compared with monoclonal antibodies. Further understanding of the relevant receptor and cellular targets may allow for improved efficacy and safety profiles.81
THERAPEUTIC TARGETING OF THE SPHINGOSINE 1-PHOSPHATE PATHWAY IN IBD
Given the potential role for S1PR in the pathogenesis of immune-mediated diseases, the S1P/R axis could be potentially targeted to treat MS, psoriasis, rheumatoid arthritis (RA), and IBD.68, 82, 83 Several potential drug candidates that modulate S1P receptors have been or are being tested for the treatment of IBD.
Fingolimod/FTY720 (Gilenya) is a S1P-analogue, acting as nonselective potent agonist of S1PR1,3,4,5. FTY720 was the first S1PR agonist approved for clinical use for relapsing MS.2, 68 Numerous preclinical studies have shown its efficacy at ameliorating intestinal inflammation.78, 84–87 The safety profile of FTY720 therapy has been carefully examined, being generally well tolerated, with most adverse events being mild to moderate in severity. The most common AEs are cardiovascular events, including bradycardia and first‐degree or second‐degree atrioventricular block. Cases of PML during FTY720 treatment have been identified; however in most cases, it was difficult to determine whether PML was related to FTY720 or a “carry‐over” effect of previously natalizumab use.88 Finally, more S1PR agonists have been developed to improve the drug safety profile.68
KRP-203 (NovartisTM) is a S1PR1,4,5 agonist and partial agonist of S1PR3. Its efficacy, safety, and tolerability were evaluated in patients with moderately active refractory UC in a multicenter, double-blind, placebo-controlled, parallel group study. KRP203 was safe and well tolerated, and there were no reported cardiac adverse events. However, KRP203 was inferior to the minimal clinically relevant threshold, but remission was observed in 2 of 14 patients (14%) in the KRP203 group and 0 of 8 (0%) in the placebo group.82 Nevertheless, this small study was terminated.89 Ozanimod/RPC1063 (Celgene) is an oral S1P1 receptor agonist, with enhanced selectivity for S1PR1 and S1PR5. Ozanimod has a half-life of 19 hours; thus upon drug discontinuation, lymphocyte counts rebound to the normal range within 3 days.90 A multicenter, phase 2 human study examined the safety and efficacy of 0.5 and 1 mg of ozanimod daily compared with placebo in patients (n = 199) with moderate to severe UC. The primary outcomes were that clinical remission at week 8 was achieved by 13.8% and 16.4% in the 0.5-mg and 1-mg ozanimod groups, compared with 6.2% in placebo. Clinical response rates at 8 weeks occurred in 57%, 54%, and 37% for 1 mg, 0.5 mg, and placebo, respectively. Ozanimod also induced mucosal improvement or healing (34%, 28%, and 12%, respectively) at week 8. However, histological remission rates at week 8 were nonsignificant for both high- and low-dose ozanimod. At week 32, the rate of clinical remission was 21%, 26%, and 6% for 1 mg, 0.5 mg, and placebo, respectively. Clinical response rates were 51%, 35%, and 20%, and mucosal improvement or healing was 33%, 32%, and 12%, respectively. Histological remission rates at week 32 were 31% and 23% for the active drug arm and 8% for the placebo arm. At week 8, absolute lymphocyte counts declined 49% from baseline in the group that received 1 mg and 32% in the group that received 0.5 mg. The investigators concluded that the 1-mg dose was optimal for future studies. The adverse-event profile was similar across groups. One patient who had evidence of preexisting bradycardia had mild asymptomatic and transient bradycardia and first-degree AV block that resolved without intervention.91
The results of the phase 2, open-label, nonplacebo controlled study with oral ozanimod in moderate to severe active Crohn’s disease were reported, showing meaningful clinical improvement at week 4 and endoscopic improvement at week 12.92 The role of ozanimod continues to be investigated in phase 3 studies in IBD.81 The shorter half-life and rapid peripheral lymphocyte recovery of ozanimod may provide safety advantages over fingolimod, should opportunistic infections or other treatment-related complications arise during the perioperative period. The drug should be avoided during pregnancy due to known teratogenicity.90
Etrasimod/APD334 (Arena) is a S1PR1,4,5 selective agonist.93 Two randomized, double-blind studies in healthy subjects (n = 100) evaluated its safety and impact on lymphocyte subpopulations. Etrasimod was administered in single-ascending doses or multiple-ascending doses for up to 21 days. The drug rapidly decreased circulating T lymphocytes in healthy subjects with a fast recovery after therapy cessation. The primary effect was on T-helper and naïve cells and seemed safe and well tolerated in healthy volunteers when administered at the target therapeutic dose of 2.0 mg daily.94 Recently, the preliminary phase 2 results from the OASIS trial in moderate to severe UC were reported.93, 95 The primary endpoint, defined as an improvement in the 3-component (stool frequency, rectal bleeding, and findings on endoscopy) Mayo Clinic Score with 2 mg at week 12, was met. In addition, significantly more patients in the 2-mg group achieved endoscopic improvement compared with placebo (41.8% vs17.8%, P = 0.003). The proportion of patients achieving clinical remission, defined by the 3-component Mayo Clinic Score, was 33.0% in the 2-mg group compared with 8.1% in the placebo group (P < 0.001). Remission, as defined by the 4-component Total Mayo Score, was 24.5% and 6.0% for the 2-mg and placebo groups, respectively (P = 0.004). Etrasimod was well tolerated, and there were fewer patients with serious adverse events (SAEs) compared with placebo (0% in 2 mg, 5.8% in 1 mg, and 11.1% in placebo). Arena intends to initiate a phase 3 program in ulcerative colitis.96
Amiselimod/MT-1303 (Biogen) is an oral selective S1PR1,5 receptor developed for the treatment of autoimmune diseases. Amiselimod, unlike to Fingolimod, has little agonistic activity at human S1PR3 receptors, resulting in a better cardiac safety profile in preclinical, phase 1 and 2 studies.97 Amiselimod was under investigation for moderate to severe, active Crohn’s disease in a 14-week, randomized, placebo-controlled, phase 2 trial, with an open-label extension of ≥22 weeks; nevertheless, the results of this trial have not been reported. Amiselimod was also studied for ulcerative colitis, MS, and other autoimmune conditions; however, Biogen stopped its development.68
Future Directions for the S1P Pathway
Other S1PR agonists (eg, AUY954, SEW2871, AUY954, W061, CS-0777, Ponesimod, Ceralifimod, GSK2018682, and Siponimod) are being studied in other inflammatory diseases.98 In the search for new therapies within the S1P pathway, there are several potential drug candidates, targeting S1P synthesis, transport, and degradation. Some aspects in the mechanism of action of these potential drugs that needs to be investigated comprehensively are the effects beyond T cells on innate immunity, dendritic cells, and vascular barrier.
LESSONS FROM THE CLINICS, CONCLUSIONS, AND FUTURE DIRECTIONS
The translation of basic science concepts to the clinics has now allowed for real-life lessons from the clinical application of these concepts to illuminate gaps in our understanding of basic concepts, only superficially understood from in vitro or animal studies. Indeed, a more precise understanding of the mechanisms of action of anti-integrin therapies is still needed. A recent study suggested that effects of α4β7 antibodies extend beyond T cells to innate immunity, including changes in macrophage populations (eg, switch from an M1 to an M2 macrophage profile in patients who achieved remission specifically with vedolizumab) and pronounced alterations in the expression of molecules involved in microbial sensing, chemoattraction, and regulation of innate effector response.99 Although this data seems to indicate that α4β7 plays a limited role in the regulation of intestinal T-cell trafficking in humans, it is important to highlight that their longest time point from the onset of vedolizumab was 14 weeks. Given that lymphocytes are long-lived cells and that the clinical data seems to show added benefit with longer duration of therapy, longer time points should have been assessed.99 This might be particularly relevant due to the description of long-lived, tissue-resident memory cells, which may not depend on trafficking molecules.100 Another relevant question in this context is whether current anti-adhesion therapy with vedolizumab and potential future therapies with etrolizumab or anti-MAdCAM could differ in their potential to inhibit α4β7-MAdCAM-1 interactions or in their overall efficacy.101
Similarly, for S1PR agonists, its mechanism of action has been focused on the retention of lymphocytes on lymph nodes, but given the expression of S1PR1 on dendritic cells and lymphatics, this simple explanation is likely another oversimplification.78
Pharmacokinetic-pharmacodynamic data from a prospective study of real-life patients with IBD showed that vedolizumab drug levels were associated with remission, and this study also showed that integrin α4β7 was blocked in almost all T cells, regardless of serum drug levels or response to treatment.102 This is fundamentally different from what is known of anti-TNF levels and how they are used to guide anti-TNF dosing in the clinics.103 Thus, our understanding of the pharmacokinetic-pharmacodynamics of these drugs is superficial, representing another important knowledge gap that needs to be addressed. There could additionally be an immunological mechanism of escape. In mice and humans, there is evidence to suggest that chronic inflammation may allow pathogenic cell subsets to escape their dependence on α4β7-MAdCAM-1 interactions, perhaps by utilizing α4β1 -VCAM-1 to traffic to the chronically inflamed bowel.104, 105
The success of TNF inhibitors during the late 1990s revolutionized the treatment of IBD; however, only around two thirds of patients respond to this therapy, and around 50% lose response during maintenance. TNF inhibitors are associated with important toxicities, making it necessary to search for novel strategies with distinct mechanisms of action. Understanding the molecules involved in cell trafficking to the intestine and elsewhere has led to the development of a novel, effective, and well-tolerated strategy for the treatment of IBD. Small molecules (eg, S1PR agonists) could be a good alternative to biologics due to inherent advantages such as low manufacturing cost, absence of immunogenicity, short half-life, and oral administration; however, more studies are necessary to evaluate their position among IBD therapies.
Strategies utilizing nanoparticles as vectors of small interfering RNA (siRNA), microRNA (miRNA) or antisense oligonucleotides (ASO) modifying the expression of adhesion molecules, integrins, and other adhesion molecules could potentially have a place within the IBD treatment algorithms in the future.106–108 Competitive antagonists and allosteric integrin inhibitors that selectively bind to the active integrin conformation might also have important clinical applications.26, 27, 109 The design of nano-delivery systems has significantly advanced the future for IBD therapy by improving the selective targeting, increasing the drug availability in disease tissue with fewer systemic adverse effects.110 Also, siRNA directed against integrins or adhesion molecule mRNAs might be useful novel strategies for IBD. However, low penetration of siRNA across cell membranes is a major obstacle for siRNA delivery. This could be circumvented by a stable nanoparticle delivery system.107 The intestine is an attractive site for nanomaterial delivery, which enables safe and efficient delivery of peptides to the intestinal mucosa.106 ASO are oligomers designed to hybridize to mRNAs coding for a targeted protein. Antisense oligonucleotides can reduce the abundance of specific RNAs through multiple mechanisms such as the RNase H (an intracellular endoribonuclease that recognizes DNA:RNA heteroduplexes for selective hydrolysis), mediated degradation of target RNA, translational arrest, and altered RNA splicing.107 Alicaforsen, an ASO therapy, is a good example of this technology, and a nanoparticle delivery system could improve its efficacy.60 Inhibition of MAdCAM-1 expression by ASO in TBNS-induced mice colitis significantly suppressed the development of colitis compared with controls.111 However, the clinical use of these nanoparticles will require that several issues are addressed such as safety, stability in the human GI tract, best formulation, and doses for human administration.106, 112
Conflicts of interest: TPJ, CJT, JDB, TK, GB, DP, and JRN have no disclosures or potential conflicts of interest. PSD has received research support, travel support, and honorarium from Takeda, research support from Pfizer, and support from a training grant through the National Institute of Diabetes and Digestive and Kidney Diseases (5T32DK007202). BSB has received research support from Takeda, and support from CCFA career development award, and UCSD KL2 (1KL2TR001444). WJS has received personal fees from Kyowa Hakko Kirin, Millennium Pharmaceuticals, Celgene Cellular Therapeutics, Santarus, Salix Pharmaceuticals, Catabasis Pharmaceuticals, Vertex Pharmaceuticals, Warner Chilcott, Cosmo Pharmaceuticals, Ferring Pharmaceuticals, Sigmoid Biotechnologies, Tillotts Pharma, Am Pharma BV, Dr. August Wolff, Avaxia Biologics, Zyngenia, Ironwood Pharmaceuticals, Index Pharmaceuticals, Nestle, Lexicon Pharmaceuticals, UCB Pharma, Orexigen, Luitpold Pharmaceuticals, Baxter Healthcare, Ferring Research Institute, Novo Nordisk, Mesoblast Inc., Shire, Ardelyx Inc., Actavis, Seattle Genetics, MedImmune (AstraZeneca), Actogenix NV, Lipid Therapeutics Gmbh, Eisai, Qu Biologics, Toray Industries Inc., Teva Pharmaceuticals, Eli Lilly, Chiasma, TiGenix, Adherion Therapeutics, Immune Pharmaceuticals, Celgene, Arena Pharmaceuticals, personal fees from Ambrx Inc., Akros Pharma, Vascular Biogenics, Theradiag, Forward Pharma, Regeneron, Galapagos, Seres Health, Ritter Pharmaceuticals, Theravance, Palatin, Biogen, University of Western Ontario (owner of Robarts Clinical Trials), grants and personal fees from Prometheus Laboratories, AbbVie, Gilead Sciences, Boehringer Ingelheim, Amgen, Takeda, Atlantic Pharmaceuticals, Bristol-Myers Squibb Genentech, GlaxoSmithKline, Pfizer, Nutrition Science Partners, Receptos, Amgen, grants, personal fees, and nonfinancial support from Janssen, grants from Broad Foundation, American College of Gastroenterology, and Exact Sciences. DRP has been a speaker bureau ABBVIE.
Supported by: This work is funded by grants from the National Institutes of Health (DK108670) and VA Merit grant BLRD-I01 BX003436.
REFERENCES
- 1. Zundler S, Becker E, Weidinger C, et al. Anti-adhesion therapies in inflammatory bowel disease-molecular and clinical aspects. Front Immunol. 2017;8:891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Currò D, Pugliese D, Armuzzi A. Frontiers in drug research and development for inflammatory bowel disease. Front Pharmacol. 2017;8:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bamias G, Clark D, Rivera-Nieves J. Leukocyte traffic blockade as a therapeutic strategy in inflammatory bowel disease. Curr Drug Targets. 2013;14:1490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas S, Baumgart DC. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology. 2012;20:1–18. [DOI] [PubMed] [Google Scholar]
- 5. Lobaton T, Vermeire S, Van Assche G, et al. Review article: anti-adhesion therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:579–94. [DOI] [PubMed] [Google Scholar]
- 6. Impellizzeri D, Cuzzocrea S. Targeting selectins for the treatment of inflammatory diseases. Expert Opin Ther Targets. 2014;18:55–67. [DOI] [PubMed] [Google Scholar]
- 7. Ley K. The role of selectins in inflammation and disease. Trends Mol Med. 2003;9:263–268. [DOI] [PubMed] [Google Scholar]
- 8. Marsal J, Agace W. Targeting T-cell migration in inflammatory bowel disease. J Intern Med. 2012;272:411–429. [DOI] [PubMed] [Google Scholar]
- 9. Seekamp A, van Griensven M, Dhondt E, et al. The effect of anti-L-selectin (aselizumab) in multiple traumatized patients - Results of a phase II clinical trial. Crit Care Med. 2004;32:2021–2028. [DOI] [PubMed] [Google Scholar]
- 10. Stähli BE, Gebhard C, Duchatelle V, et al. Effects of the P-Selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention according to timing of infusion: insights from the SELECT-ACS trial. J Am Heart Assoc. 2016;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beeh KM, Beier J, Meyer M, et al. Bimosiamose, an inhaled small-molecule pan- selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther. 2006;19:233–241. [DOI] [PubMed] [Google Scholar]
- 12. Bhatti M, Chapman P, Peters M,et al. Visualising E-selectin in the detection and evaluation of inflammatory bowel disease. Gut. 1998;43:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ClinicalTrial.gov. NCT00457171 GI-270384 Study In Patients With Mild To Moderate Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/NCT00457171? term=selectin&cond=ulcerative+colitis&rank=1. Accessed May 10, 2018. [Google Scholar]
- 14. Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. [DOI] [PubMed] [Google Scholar]
- 15. Keshav S, Vaňásek T, Niv Y, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS ONE. 2013;8:e60094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wendt E, Keshav S. CCR9 antagonism: potential in the treatment of inflammatory bowel disease. Clin Exp Gastroenterol. 2015;8:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feagan BG, Sandborn WJ, D’Haens G, et al. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn’s disease. Aliment Pharmacol Ther. 2015;42:1170–81. [DOI] [PubMed] [Google Scholar]
- 18. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 19. Mayer L, Sandborn WJ, Stepanov Y, et al. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2014;63:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandborn WJ, Rutgeerts P, Colombel JF, et al. Induction therapy for active Crohn’s disease: a randomised, double-blind, placebo-controlled phase iia study. J Crohns Colitis. 2017;11:811–819. [DOI] [PubMed] [Google Scholar]
- 21. Sandborn WJ, Colombel JF, Ghosh S, et al. Eldelumab [Anti-IP-10] induction therapy for ulcerative colitis: a randomised, placebo- controlled, phase 2b study. J Crohns Colitis. 2016;10:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skovdahl HK, Granlund AV, Østvik AE, et al. Expression of CCL20 and its corresponding receptor CCR6 is enhanced in active inflammatory bowel disease, and TLR3 mediates CCL20 expression in colonic epithelial cells. PLoS One. 2015;10:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrial.gov. NCT01984047. Single Dose Escalation Trial to Evaluate the Safety, Tolerability Pharmacokinetics and Pharmacodynamics of GSK3050002. https://clinicaltrials.gov/ct2/show/NCT01984047 (1 January 2018, date last accessed). [Google Scholar]
- 24. Bouma G, Zamuner S, Hicks K, et al. CCL20 neutralization by a monoclonal antibody in healthy subjects selectively inhibits recruitment of CCR6+ cells in an experimental suction blister. Br J Clin Pharmacol. 2017;83:1976–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ley K, Rivera-Nieves J, Sandborn WJ. et al. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park EJ, Yuki Y, Kiyono H, et al. Structural basis of blocking integrin activation and deactivation for anti-inflammation. J Biomed Sci. 2015;22:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shimaoka M, Springer TA. Therapeutic antagonists and conformational regulation of integrin function. Nat Rev Drug Discov. 2003;2:703–716. [DOI] [PubMed] [Google Scholar]
- 28. Berlin C, Berg EL, Briskin MJ, et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–95. [DOI] [PubMed] [Google Scholar]
- 29. Smids C, Horjus Talabur Horje CS, van Wijk F, et al. The complexity of alpha E beta 7 blockade in inflammatory bowel diseases. J Crohns Colitis. 2017;11:500–508. [DOI] [PubMed] [Google Scholar]
- 30. Bellaguarda E, Keyashian K, Pekow J. Prevalence of antibodies against JC virus in patients with refractory Crohn’s disease and effects of Natalizumab therapy. Clin Gastroenterol Hepatol. 2015;13:1919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rivera-Nieves J. Strategies that target leukocyte traffic in IBD: recent developments. Curr Opin Gastroenterol. 2015;31:441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sands BE. Leukocyte anti-trafficking strategies: current status and future directions. Dig Dis. 2017;35:13–20. [DOI] [PubMed] [Google Scholar]
- 33. James DG, Seo DH, Chen J, et al. Efalizumab, a human monoclonal anti-CD11a antibody, in the treatment of moderate to severe Crohn’s disease: an open-label pilot study. Dig Dis Sci. 2011;56:1806–1810. [DOI] [PubMed] [Google Scholar]
- 34. Kothary N, Diak IL, Brinker A, et al. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol. 2011;65:546–551. [DOI] [PubMed] [Google Scholar]
- 35. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 36. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 37. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627. [DOI] [PubMed] [Google Scholar]
- 38. Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients with ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15:229–239. [DOI] [PubMed] [Google Scholar]
- 39. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wyant T, Leach T, Sankoh S, et al. Vedolizumab affects antibody responses to immunisation selectively in the gastrointestinal tract: randomised controlled trial results. Gut. 2015;64:77–83. [DOI] [PubMed] [Google Scholar]
- 41. ClinicalTrial.gov. NCT02764762. Triple Combination Therapy in High Risk Crohn’s Disease. https://clinicaltrials.gov/ct2/show/NCT02764762. Accessed May 10, 2018. [Google Scholar]
- 42. ClinicalTrial.gov. NCT02630966. Vedolizumab IV 300 mg in the Treatment of Fistulizing Crohn’s Disease (ENTERPRISE). https://clinicaltrials.gov/ct2/show/NCT02630966. Accessed May 10, 2018. [Google Scholar]
- 43. ClinicalTrial.gov. NCT02790138. Phase 4 Study to Evaluate the Efficacy and Safety of Vedolizumab in the Treatment of Chronic Pouchitis (EARNEST). https://clinicaltrials.gov/ct2/show/NCT02790138. Accessed May 10, 2018. [Google Scholar]
- 44. Sandborn WJ, Cyrille M, Hansen MB, et al. Efficacy and safety of abrilumab in subjects with moderate to severe ulcerative colitis: results of a phase 2b, randomised, double-blind, multiple-dose, placebo-controlled study. Eur Crohn`s Colitis Organ. 2017;11:S21–S22. [Google Scholar]
- 45. Sandborn WJ, Cyrille M, Hansen MB, et al. Efficacy and safety of abrilumab (AMG 181 / MEDI 7183) therapy for moderate to severe Crohn’ s disease. J Crohns Colitis. 2017;11:S22–S23. [Google Scholar]
- 46. Vermeire S, O’Byrne S, Keir M, et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384:309–18. [DOI] [PubMed] [Google Scholar]
- 47. Sandborn WJ, et al. Etrolizumab as induction therapy in moderate to severe Crohn’s Disease:Results from Bergamot cohort 1. https://www.ueg.eu/education/document/etrolizumab-as-induction-therapy-in-moderate-to-severe-crohn-s-disease-results-from-bergamot-cohort-1/151599/. Accessed May 20, 2018. [Google Scholar]
- 48. ClinicalTrial.gov. NCT02394028. A Study to Assess Whether Etrolizumab is a Safe and Effective Treatment for Participants With Moderately to Severely Active Crohn’s Disease (CD). https://clinicaltrials.gov/ct2/results?cond=inflammatory+bowel+diseases&term=etrolizumab&cntry=&state=&city=&dist=. Accessed May 10, 2018. [Google Scholar]
- 49. ClinicalTrial.gov. NCT02403323. Open-Label Extension and Safety Study for Patients With Crohn’s Disease Previously Enrolled in the Etrolizumab Phase III Study GA29144. https://clinicaltrials.gov/ct2/results?cond=inflammatory+bowel+diseases&term=etrolizumab&cntry=&state=&city=&dist=. Accessed May 10, 2018. [Google Scholar]
- 50. ClinicalTrial.gov. NCT02165215 A Study of the Efficacy and Safety of Etrolizumab Treatment in Maintenance of Disease Remission in Ulcerative Colitis (UC) Participants Who Are Naive to Tumor Necrosis Factor (TNF) Inhibitors. https://clinicaltrials.gov/ct2/results?cond=inflammatory+bowel+diseases&term=etrolizumab&cntry=&state=&city=&dist=. Accessed May 10, 2018. [Google Scholar]
- 51. ClinicalTrial.gov. NCT02171429. A Study Comparing the Efficacy and Safety of Etrolizumab With Adalimumab and Placebo in Participants With Moderate to Severe Ulcerative Colitis (UC) in Participants Naive to Tumor Necrosis Factor (TNF) Inhibitors (Study #2). https://clinicaltrials.gov/ct2/results?cond=inflammatory+bowel+diseases&term=etrolizumab&cntry=&state=&city=&dist=. Accessed May 10, 2018. [Google Scholar]
- 52. Yoshimura N, Watanabe M, Motoya S, et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology. 2015;149:1775–1783. [DOI] [PubMed] [Google Scholar]
- 53. ClinicalTrial.gov. NCT02895100. Safety and Efficacy Study of PTG-100 in the Treatment of Moderate to Severe Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/NCT02895100?term=PTG-100&cond=IBD&rank=1. Accessed May 10, 2018. [Google Scholar]
- 54. Protagonist’s ‘futile’ Phase IIb ulcerative colitis trial halted. https://www.pharmaceutical-technology.com/news/protagonistsphase-iib-ulcerative-colitis/. Accessed April 4, 2018. [Google Scholar]
- 55. ET3764. http://encycletherapeutics.com. Accessed May 20, 2018. [Google Scholar]
- 56. Jairath V, Khanna R, Feagan BG. Alicaforsen for the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 2017;26:991–997. [DOI] [PubMed] [Google Scholar]
- 57. Yacyshyn B, Chey WY, Wedel MK, et al. A randomized, double-masked, placebo-controlled study of alicaforsen, an antisense inhibitor of intercellular adhesion molecule 1, for the treatment of subjects with active Crohn’s disease. Clin Gastroenterol Hepatol. 2007;5:215–220. [DOI] [PubMed] [Google Scholar]
- 58. van Deventer SJ, Wedel MK, Baker BF, et al. A phase II dose ranging, double- blind, placebo-controlled study of alicaforsen enema in subjects with acute exacerbation of mild to moderate left-sided ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1415–1425. [DOI] [PubMed] [Google Scholar]
- 59. Miner PB, Wedel MK, Xia S, et al. Safety and efficacy of two dose formulations of alicaforsen enema compared with mesalazine enema for treatment of mild to moderate left-sided ulcerative colitis: a randomized, double-blind, active-controlled trial. Aliment Pharmacol Ther. 2006;23:1403–1413. [DOI] [PubMed] [Google Scholar]
- 60. Miner P, Wedel M, Bane B, et al. An enema formulation of alicaforsen, an antisense inhibitor of intercellular adhesion molecule-1, in the treatment of chronic, unremitting pouchitis. Aliment Pharmacol Ther. 2004;19:281–6. [DOI] [PubMed] [Google Scholar]
- 61. Vermeire S, Sandborn WJ, Danese S, et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:135–144. [DOI] [PubMed] [Google Scholar]
- 62. Sheridan BS, Lefrançois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep. 2010;12:513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Serriari NE, Eoche M, Lamotte L, et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol. 2014;176:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jung Y, Rothenberg ME. Roles and regulation of gastrointestinal eosinophils in immunity and disease. J Immunol. 2014;193:999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sandborn WJ, Lee SD, Tarabar D, et al. Phase II evaluation of anti-MAdCAM antibody PF-00547659 in the treatment of Crohn’s disease: report of the OPERA study. Gut. 2017;0:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. D’Haens GR. OP024 Long-term safety and efficacy of the anti-MAdCAM monoclonal antibody SHP647 for the treatment of Crohn’s disease: the OPERA II study. https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2018/item/op024-long-term-safety-and-efficacy-of-the-anti-madcam-monoclonal-antibody-shp647-for-the-treatment-of-crohn-x2019-s-disease-the-opera-ii-study.html. Accessed May 10, 2018. [Google Scholar]
- 67. ClinicalTrial.gov. NCT03283085. A Phase 3 Long-term Safety Extension Study of SHP647 in Subjects with Moderate to Severe Ulcerative Colitis or Crohn’s Disease (AIDA). https://clinicaltrials.gov/ct2/show/NCT03283085. Accessed June 5, 2018. [Google Scholar]
- 68. Peyrin-Biroulet L, Christopher R., Behan D, et al. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. 2017;16:495–503. [DOI] [PubMed] [Google Scholar]
- 69. Le Stunff H, Milstien S, Spiegel S. Generation and metabolism of bioactive sphingosine-1-phosphate. J Cell Biochem. 2004;92:882–899. [DOI] [PubMed] [Google Scholar]
- 70. Schwab SR, Pereira JP, Matloubian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. [DOI] [PubMed] [Google Scholar]
- 71. Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. [DOI] [PubMed] [Google Scholar]
- 72. Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. [DOI] [PubMed] [Google Scholar]
- 73. Maeda Y, Seki N, Sato N, et al. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol. 2010;22:515–525. [DOI] [PubMed] [Google Scholar]
- 74. Abbas AK, Lichtman AH, Pillai S.. Cellular and Molecular Immunology. 8th ed Philadelphia, PA: Elsevier/Saunders; 2015:45–48. [Google Scholar]
- 75. Rivera-Nieves J, Gorfu G, Ley K. Leukocyte adhesion molecules in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1715–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rivera-Nieves J. Strategies that target leukocyte traffic in IBD: recent developments. Curr Opin Gastroenterol. 2015;39:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Horga A, Montalban X. FTY720 (fingolimod) for relapsing multiple sclerosis. Expert Rev Neurother. 2008;8:699–714. [DOI] [PubMed] [Google Scholar]
- 78. Karuppuchamy T, Behrens EH, González-Cabrera P, et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 2016;1:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Juif PE, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety aspects of sphingosine-1-phosphate receptor modulators. Expert Opin Drug Metab Toxicol. 2016;12:879–895. [DOI] [PubMed] [Google Scholar]
- 80. Mehling M, Lindberg R, Raulf F. TH17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology. 2010;75:403–410. [DOI] [PubMed] [Google Scholar]
- 81. Nielsen OH, Li Y, Johansson-Lindbom B, et al. Sphingosine-1-phosphate signaling in inflammatory bowel disease. Trends Mol Med. 2017;23:362–374. [DOI] [PubMed] [Google Scholar]
- 82. Radeke HH, Stein J, Kruis W. P372. A multicentre, double-blind, placebo-controlled, parallel group, proof of concept study to evaluate the efficacy, safety and tolerability of the S1P receptor modulator KRP203 in subjects with moderately active refractory ulcerative colitis. J Crohns Colitis. 2016;10:S285–S286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Donoviel MS, Hait NC, Ramachandran S, et al. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 2015;29:5018–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Deguchi Y, Andoh A, Yagi Y, et al. The S1P receptor modulator FTY720 prevents the development of experimental colitis in mice. Oncol Rep. 2006;16:699–703. [PubMed] [Google Scholar]
- 85. Daniel C, Sartory N, Zahn N, et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J Immunol. 2007;178:2458–2468. [DOI] [PubMed] [Google Scholar]
- 86. Radi ZA, Heuvelman DM, Masferrer JL, et al. Pharmacologic evaluation of sulfasalazine, FTY720, and anti-IL-12/23p40 in a TNBS-induced Crohn’s disease model. Dig Dis Sci. 2011;56:2283–2291. [DOI] [PubMed] [Google Scholar]
- 87. Tomita T, Kanai T, Nemoto Y, et al. Colitogenic CD4+ effector-memory T cells actively recirculate in chronic colitic mice. Inflamm Bowel Dis. 2008;14:1630–1640. [DOI] [PubMed] [Google Scholar]
- 88. Yoshii F, Moriya Y, Ohnuki T, et al. Neurological safety of fingolimod: an updated review. Clin Exp Neuroimmunol. 2017;8:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. ClinicalTrials.gov. NCT01375179. A Multi-centre, Double-blind, Placebo Controlled, Parallel Group, Proof of Concept Study to Evaluate the Efficacy, Safety and Tolerability of KRP203 in Subjects With Moderately Active Refractory Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/NCT01375179?term =Krp203&cond=Inflammatory+Bowel+Diseases&rank=1. Accessed 10 2018. [Google Scholar]
- 90. Scott FL, Clemons B, Brooks J, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P1) and receptor-5 (S1P5) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sandborn WJ, Feagan BG, Wolf DC, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–1762. [DOI] [PubMed] [Google Scholar]
- 92. Feagan BG, et al. P1272 - Endoscopic and Clinical Efficacy Demonstrated With Oral Ozanimod in Moderately to Severely Active Crohn’s Disease. WCG at ACG (2017). http://ir.celgene.com/releasedetail.cfm?releaseid=1044007. Accessed May 10, 2018. [Google Scholar]
- 93. ClinicalTrials.gov. NCT02447302 Safety and Efficacy of Etrasimod (APD334) in Patients With Ulcerative Colitis. ClinicalTrials.gov; NCT02447302. https://clinicaltrials.gov/ct2/show/NCT02447302?term=APD334-003&draw=2&rank=2. Accessed December 2, 2018. [Google Scholar]
- 94. Adams J, et al. P573 Safety and immune modulatory properties of etrasimod (APD334), a next-generation oral, selective sphingosine 1-phosphate receptor (S1PR) modulator, in healthy volunteers. https://www.ecco-ibd.eu/publications/congress-abstract-s/abstracts-2018/item/p573-safety-and-immune-modulatory-properties-of-etrasimod-apd334-a-next-generation-oral-selective-sphingosine-1-phosphate-receptor-s1pr-modulator-in-healthy-volunteers.html. Accessed May 13, 2018. [Google Scholar]
- 95. ClinicalTrials.gov NCT02536404 Extension Study of APD334-003 in Patients With Moderately to Severely Active Ulcerative Colitis. ClinicalTrials.gov; NCT02536404. https://clinicaltrials.gov/ct2/show/NCT02536404?term=APD334-003&rank=1. Accessed December 2, 2017. [Google Scholar]
- 96. Arena Pharmaceuticals Reports Positive Phase 2 Results from the OASIS Trial for Etrasimod in Patients with Ulcerative Colitis. https://www.reuters.com/finance/stocks/ARNA.O/key-developments/article/3788737. Accessed 10 2018. [Google Scholar]
- 97. Harada T, Wilbraham D, de La Borderie G, et al. Cardiac effects of amiselimod compared with fingolimod and placebo: results of a randomised, parallel-group, phase I study in healthy subjects. Br J Clin Pharmacol. 2017;83:1011–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Park SJ, Im DS. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol Ther. (Seoul). 2017;25:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zeissig S, Rosati E, Dowds CM, et al. Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut Published Online First: 05 May 2018. doi:10.1136/gutjnl-2018-316023. https://gut.bmj.com/content/gutjnl/early/2018/05/05/gutjnl-2018-316023.full.pdf. [DOI] [PubMed] [Google Scholar]
- 100. Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol. 2018;9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Binder MT, Becker E, Wiendl M, et al. Similar inhibition of dynamic adhesion of lymphocytes from IBD patients to MAdCAM-1 by vedolizumab and etrolizumab-s. Inflamm Bowel Dis. 2018;24:1237–1250. [DOI] [PubMed] [Google Scholar]
- 102. Ungar B, Kopylov U, Yavzori M, et al. Association of vedolizumab level, anti-drug antibodies, and α4β7 occupancy with response in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16:697–705. [DOI] [PubMed] [Google Scholar]
- 103. Van de Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;7:1320–9. [DOI] [PubMed] [Google Scholar]
- 104. Rivera-Nieves J, Olson T, Bamias G, et al. L-Selectin, α4β1, and α4β7 integrins participate in CD4 T cell recruitment to chronically inflamed small intestine. J Immunol. 2005;174:2343–2352. [DOI] [PubMed] [Google Scholar]
- 105. Zundler S, Fischer A, Schillinger D, et al. The α4β1 homing pathway is essential for ileal homing of Crohn’s disease effector T cells in vivo. Inflamm Bowel Dis. 2017;23:379–391. [DOI] [PubMed] [Google Scholar]
- 106. Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117–1132. [DOI] [PubMed] [Google Scholar]
- 107. Takedatsu H, Mitsuyama K, Torimura T. Nanomedicine and drug delivery strategies for treatment of inflammatory bowel disease. World J Gastroenterol. 2015;21:11343–11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kedmi R, Veiga N, Ramishetti S, et al. A modular platform for targeted RNAi therapeutics. Nat Nanotechnol. 2018;13:214–219. [DOI] [PubMed] [Google Scholar]
- 109. Rantala JK, Pouwels J, Pellinen T, et al. SHARPIN is an endogenous inhibitor of β1-integrin activation. Nat Cell Biol. 2011;13:1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mittal R, Patel AP, Jhaveri VM, et al. Recent advancements in nanoparticle-based drug delivery for gastrointestinal disorders. Expert Opin Drug Deliv. 2018;15:1–18. [DOI] [PubMed] [Google Scholar]
- 111. Goto A, Arimura Y, Shinomura Y, et al. Antisense therapy of MAdCAM-1 for trinitrobenzenesulfonic acid-induced murine colitis. Inflamm Bowel Dis. 2006;12:758–765. [DOI] [PubMed] [Google Scholar]
- 112. Jairath V, Khanna R, Feagan BG. Expert opinion on investigational drugs Alicaforsen for the treatment of inflammatory bowel disease Alicaforsen for the treatment of inflammatory bowel disease. Expert Opin Investig Drugs. 2017;26:991–997. [DOI] [PubMed] [Google Scholar]