Abstract
Alkyl gallates are anticipated for their use as anti-bacterial and anti-viral agents. Although their pharmacological activities depend on their alkyl chain length, no mechanism has yet been clarified. As described herein, we investigated the membrane binding properties of a series of alkyl gallates using fluorescence measurement to elucidate their different pharmacological activities. Membrane binding of the alkyl gallates increased concomitantly with increasing alkyl chain length, except for cetyl gallate and stearyl gallate. Dynamic light scattering revealed that alkyl gallates with a long alkyl chain are prone to self-association in the solution. Membrane binding abilities of the alkyl gallates are correlated with anti-bacterial and anti-virus activities, as described in previous reports. The partition constants of the alkyl gallates to lipid membranes depend on the membrane components and the membrane phase. Self-association and lipid binding of the alkyl gallates might be primary biophysical factors associated with their pharmacological activities.
Keywords: alkyl gallate, lipid membrane, partition constant, self-association, solubility
Gallate is a general term used to describe salts and esters of gallic acid with the galloyl group. Some gallate compounds, typically catechin and proanthocyanidin gallates, have been developed for pharmacological applications (1–7). Among them, alkyl gallates have recently been regarded as affecting microbial cell viability (8–18), virus activity (19–25) and human leukaemia cell proliferation (26). The alkyl gallates’ pharmacological activity increases concomitantly with their alkyl chain length. Therefore, their activity has been attributed to their surfactant-like effects, which can induce lipid membrane disruption and membrane protein inactivation (11–13). In fact, alkyl gallates have both a hydrophilic galloyl group and hydrophobic alkyl chain. More hydrophobic species with longer alkyl chains, e.g. dodecyl gallate (8, 9) and stearyl gallate (10, 11), do not function as anti-bacterial agents, suggesting an unknown factor that determines the cutoff point for activity (9).
Fluorescence spectra have been used often for analyses of interactions of various aromatic compounds with lipid membranes because fluorescence spectra depend greatly on the solution environment (27). For example, membrane binding of peptides and low-molecular weight compounds has been observed through changes in fluorescence intensity (28–34). Furthermore, fluorescence analyses present the advantage of being quantitative. For that reason, the thermodynamic parameters of the interactions, such as partition constants, can be determined. The membrane binding affinity for catechin derivatives has been measured to investigate their pharmacological activities (35).
Although partition constants of alkyl gallates with lipid membranes might be related to their pharmacological activity, partition constants have not been examined aside from qualitative observations (36). This study revealed that the partition constant depends on the alkyl chain length of gallate. The constant of the alkyl gallates increased concomitantly with increasing hydrophobicity, except for longer alkyl chain species such as cetyl and stearyl gallates. These two alkyl gallates are exceptional probably because of their self-association detected by dynamic light scattering measurements. Moreover, the membrane-binding of alkyl gallates was found to be correlated with the pharmacological activity of a series of alkyl gallates. Consequently, our findings are useful for understanding not only the physicochemical properties of alkyl gallates, but also for elucidating their pharmacological activity.
Materials and Methods
Chemicals
All alkyl gallates were obtained from Tokyo Kasei Kogyo Co. Ltd. (Tokyo, Japan). Dioleoylphosphatidylcholine (DOPC), dipalmitoylphosphatidylcholine (DPPC), dioleoylphosphatidylglycerol (DOPG) and cholesterol were obtained from NOF Corp. (Tokyo, Japan) along with sodium dihydrogen phosphate (Nacalai Tesque Inc., Kyoto, Japan) and ethanol (Kanto Chemical Co. Inc., Tokyo, Japan). All compounds were of the highest commercially available grade.
Solubility measurement of alkyl gallates
The respective solubilities of alkyl gallates in 10 mM phosphate buffer (pH 7.4) were measured as follows (37). An appropriate amount of alkyl gallate powder was transferred into a test tube, to which 1.5 ml of 10 mM phosphate buffer (pH 7.4) was added. The suspension was heated at 40°C for 1 h with frequent vortexing for complete dissolution of alkyl gallate powders. The solution was then incubated at 25°C for 3 days with frequent vortexing. Subsequently, the suspension was centrifuged at 25°C and 16,000g for 20 min to obtain a supernatant saturated with the alkyl gallates. After appropriate dilution of the supernatant with water, the supernatant absorbance was determined spectrophotometrically at 271 nm using a UV–VIS spectrophotometer (ND-1000; NanoDrop Technologies, Inc., Wilmington, DE, USA). The absorbance value was converted to the concentration based on the standard curve determined for methyl gallate. Solubility was determined in triplicate, from which the averages and standard errors were obtained.
Observation of self-association of alkyl gallates
To verify the self-association of the alkyl gallates, dynamic light scattering (DLS) measurements of them at 3 µM were conducted at 25°C using a light-scattering spectrometer (DLS-7000; Ostuka Electronics Co., Ltd. Osaka, Japan) equipped with an argon ion laser at scattering angles of 90° (38). Samples that did not pass the instrument's internal quality criteria were omitted.
Preparation of lipid membranes
Large unilamellar vesicles (LUVs) composed of DOPC, DOPG, DPPC and cholesterol (Chol) were used as model lipid membranes, prepared using the extrusion method with 200 nm pore size polycarbonate membranes. The appropriate amounts of 5 mM lipids were mixed in chloroform. Subsequently, the solvent was removed completely in a vacuum desiccator connected to a rotary vacuum pump for 12 h. To this dry lipid film, 1 ml of 10 mM phosphate buffer (pH 7.4) was added; then the 1.5 mM lipid suspension was vortexed for several seconds above the phase-transition temperature (25°C for DOPC and DOPG, and 40°C for DPPC). Subsequently, the solutions were extruded through 200-nm pore-size polycarbonate membranes (Avanti Mini-Extruder; Avanti Polar Lipids, Inc., Alabaster, AL, USA) at a temperature higher than the phase-transition temperature.
Fluorescence analysis of the binding of alkyl gallates to phospholipid membranes
Fluorescence spectra of 3 µM alkyl gallate in 10 µM lipids with 10 mM phosphate buffer (pH 7.4) were recorded at 25°C. Only for C8–18 were they presolubilized in ethanol before mixing with the lipid solution to determine the alkyl gallate concentration. Thereby, the final component of the solution was 3 µM alkyl gallate, 10 µM lipids, 0.6% ethanol and 10 mM phosphate buffer (pH 7.4), where the residual ethanol did not affect their fluorescence spectra. Finally, the fluorescence intensity of each reference solution without the alkyl gallate was subtracted from the intensity of the sample solutions. The determined intensities were averages of triplicate experiments. Thereby, the average and standard error were obtained. These spectra were measured using a spectrofluorometer (FP6500; Jasco Corp., Tokyo, Japan). The emission fluorescence spectra were recorded for 200–500 nm, using the excitation wavelength of 271 nm.
Measurement of the partition constant of alkyl gallates to the membranes
To determine the partition constant of the alkyl gallates (C8–C16) to the membranes, the changes in fluorescence intensity of the alkyl gallate on the membrane binding were monitored using a spectrofluorometer. The prepared sample solutions contained various concentrations of the lipids comprising DOPC, DPPC/DOPC or DPPC/Chol, 60 nM alkyl gallates, 0.6% ethanol and 10 mM phosphate buffer (pH 7.4). The fluorescence intensity of each reference solution without the alkyl gallates was subtracted from the intensity of the sample solutions. The determined intensities were averages of triplicate experiments: the average and standard error were obtained.
Results
Solubility and hydrodynamic radius of alkyl gallates
The alkyl gallates used for this study are shown in Scheme 1, where they are termed C1–C18 according to the carbon number of the alkyl chain. The solubility of the alkyl gallates in 10 mM phosphate buffer solution was measured for physicochemical characterization of the alkyl gallates. As shown in Table I, the solubility of C2 is higher than that of C1, which is consistent with our previous study (37, 39). The solubility of C12 is lower than that of C16, which is not surprising when considering the dynamic light scattering measurements as follows. Results show that C16 and C18 tend to self-associate in the solution (Fig. 1). The solutions of C16 and C18 exhibit a unimodal size distribution with mean diameters of 84.6 ± 4.6 nm and 130.6 ± 17.6 nm, respectively. In contrast, C8 and C12 were not detected using the same apparatus. It can be concluded that the self-association of C16 and C18 increases their apparent solubilities.
Table I.
Solubility of the alkyl gallates in 10 mM phosphate buffer (pH 7.4) at 25°C.
| Gallate | Solubility (M) |
|---|---|
| Metyl (C1) | 6.46 ± 0.11 × 10−2 |
| Ethyl (C2) | 8.19 ± 0.05 × 10−2 |
| Propyl (C3) | 1.92 ± 0.01 × 10−2 |
| Butyl (C4) | 1.21 ± 0.02 × 10−2 |
| Octyl (C8) | 5.7 ± 0.6 × 10−4 |
| Dodecyl (C12) | 6.4 ± 0.8 × 10−5 |
| Cetyl (C16) | 9.1 ± 0.7 × 10−5 |
| Stearyl (C18) | 3.9 ± 0.7 × 10−5 |
Fig. 1.
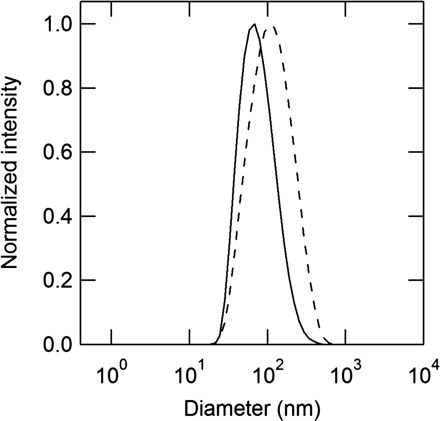
DLS measurements for 3 µM cetyl gallate (C16) (solid line) and stearyl gallate (C18) (broken line) in water.
Scheme 1.
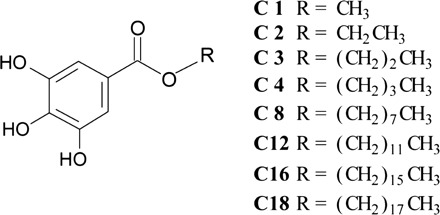
Chemical structures of the alkyl gallates.
Binding of alkyl gallates to phospholipid membranes
Binding of the alkyl gallates to the membranes was observed through the change of fluorescent intensities of their aromatic moiety. Figure 2 presents fluorescence spectra of C4 in the presence or absence of DOPG or DOPC. As expected, the fluorescence spectrum of C4 differed in the presence of positively charged DOPG or amphoteric DOPC. Accordingly, results suggest that the binding of C4 to the membranes is mainly attributable to the hydrophobic interaction between DOPC and the alkyl chains of the alkyl gallates. Similar data were obtained for C1, C2 and C3 (Supplementary Fig. S1). To support the suggestion presented above, we investigated the dependence of the alkyl chain length of the binding to DOPC. Ratios of the fluorescence intensity in the presence of DOPC to that in the absence of DOPC increased with elongation of the alkyl chain length (Fig. 3A). The ratios for C1–C12 increased with the alkyl chain length (Fig. 3, see Supplementary Fig. S2 for their spectral data). The changes in fluorescence intensities of their aromatic moiety are caused by the polarity of the solution environment (31). In contrast, the fluorescence intensity of C16, despite its longer alkyl chain, was weaker than that of C12. Additionally, the intensity of C18 did not change according to the presence of the lipid membrane.
Fig. 2.
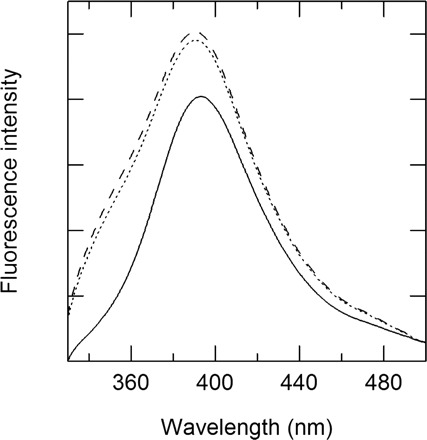
Fluorescence spectra of 3 µM butyl gallate (C4) in the presence and absence of 10 µM DOPC or DOPG: no lipid, solid line; DOPC, broken line; DOPG, dotted line. The excitation wavelength of the fluorescence spectra is 271 nm.
Fig. 3.
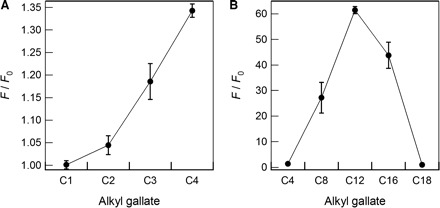
Ratios of the fluorescence intensity of the respective 3 µM alkyl gallates. (A) C1–C4 and (B) C4–C18 in the presence of 10 µM DOPC to those in the buffer solution.
Estimation of the partition constant of alkyl gallates to phospholipid membranes
Partition constants of the alkyl gallates to the membranes were estimated from the binding isotherms as follows. Figure 4A shows representative data for the peak intensity of the fluorescence spectra of C8 in various concentrations of DOPC, as normalized by the maximum value. The normalized intensity in Fig. 4A increased by the addition of DOPC and subsequently reached a plateau at which all C8 molecules bind to the membrane. Here, a two-state transition was presumed: binding or non-binding states of the alkyl gallates to the membrane. Additionally, it was assumed that the alkyl gallates bind to both the internal and external monolayer. Consequently, the equilibrium concentration of the alkyl gallate in the bulk solutions, as denoted by Ceq, was obtained as (28)
| (1) |
where CT denotes the total concentration of the alkyl gallate in solution and CM denotes its concentration in the lipid membranes. Therein, FN denotes the normalized fluorescence intensity corresponding to the vertical axis in Fig. 4A. The molar ratio of the alkyl gallate molecules in the membranes to total lipid molecules, denoted by Xb, is given as
| (2) |
where CL is the lipid concentration in solution. Finally, because the ratio of the concentration of the alkyl gallate to that of the lipid membrane in the solution is low, Xb can be expressed as (29)
| (3) |
where Kint is the intrinsic partition constant of the alkyl gallate to the lipid membrane. According to these analyses, Xb and Ceq are actually mutually proportional (Fig. 4B). These calculations are applicable to the other alkyl gallates, except for C1–C4 and C18: because of their weak binding affinity to the membrane, their binding curves were not obtained. The partition constants for C8, C12 and C16 are presented in Table II; the partition constants for C12 and C16 were of the same order and an order of magnitude higher than that for C8. These results reflect that the partition constant for C16 might be affected by self-association of C16, as described above (Fig. 1).
Fig. 4.
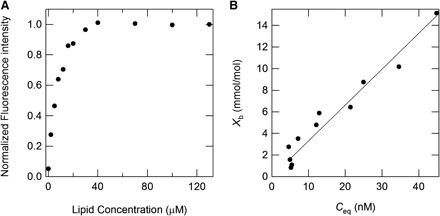
Partition constant of alkyl gallates to the membranes. (A) Normalized fluorescent intensity of 60 nM octyl gallate (C8) at 350 nm in various concentrations of DOPC, normalized by the maximum value. (B) Binding isotherms of octyl gallate (C8) to the lipid membrane of DOPC at 25°C.
Table II.
Intrinsic partition constant (Kint) of octyl (C8), dodecyl (C12) and cetyl (C16) gallates to the lipid membrane of DOPC.
| Gallate | K int (1/M) |
|---|---|
| Octyl (C8) | 1.5 ± 0.1 × 105 |
| Dodecyl (C12) | 8.2 ± 0.7 × 105 |
| Cetyl (C16) | 8.4 ± 0.6 × 105 |
Effect of membrane components on the binding of alkyl gallate to phospholipid membranes
The membrane liquid–solid phase differs according to its components (40). They can be expected to affect the binding affinity of the alkyl gallates. Figure 5A exhibits the partition constants for C8, C12 and C16 to the lipid membranes composed of various ratios of DPPC/DOPC. The partition constants for these alkyl gallates increased concomitantly with the increasing ratio of DOPC to DPPC; the value for DOPC was twice that for DPPC. Figure 5B shows alteration of the partition constants for these alkyl gallates to the membranes containing cholesterol. The partition constants for C8 and C12 decreased concomitantly with increasing concentration of cholesterol, although that for C16 was maintained.
Fig. 5.
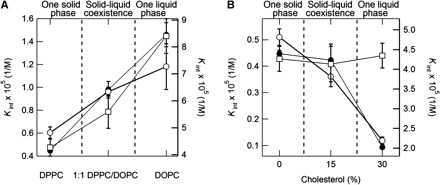
Dependence of the partition constant of alkyl gallates on the components of the membranes. Partition constants (Kint) of 60 nM octyl gallate (C8) (closed circles, left axes), dodecyl gallate (C12) (open circles, right axes) and cetyl gallate (C16) (open squares, right axes) in the presence of DPPC/DOPC (A) and DPPC/Chol (B).
Discussion
Alkyl gallates are anticipated for use as anti-bacterial and anti-viral agents because various studies have already shown their pharmacological activity in vitro (8–25). Therefore, their biophysical properties should be clarified in advance of their application. Nevertheless, quantitative studies such as thermodynamic studies of the binding interaction of the alkyl gallates to lipid membranes have not been reported. Additionally, it has not been established why highly hydrophobic alkyl gallates with a longer alkyl chain did not function as anti-bacterial agents (8–11). Regarding the binding interaction of the alkyl gallates to lipid membranes, only qualitative observations have been performed (36). In this study, we quantitatively examined the binding ability of alkyl gallates with different alkyl chain lengths to various lipid membranes. It is worth mentioning that this is the first report of a partition constant between the alkyl gallates and phospholipid membrane. Ratios of fluorescence intensity of C1–C12 have increased concomitantly with increasing alkyl chain length (Fig. 3). The ratios for C1–C12 increased with the logarithm of the octanol–water partition coefficient (log P) of the alkyl gallates. Therefore, it was suggested that the membrane-binding ability of C1–C12 depends on their hydrophobicity derived from the alkyl chain. However, for more hydrophobic species with a longer alkyl chain (C16, C18), the intensity decreased. Here, recall that DLS measurements for C8–C18 indicated self-association of C16 and C18, even at concentrations below solubility (Fig. 1, Table I). Assuming that the self-associated forms have no ability to interact with the lipid membrane, the observed reduction of the membrane-binding ability for C16 and C18 can be ascribed to the promotion of self-association with increasing alkyl chain length. The result, that C16 but not C18 interacted with the membranes (Fig. 3B), presents the possibility that the self-association state of C16 is an equilibrium, with the soluble monomeric state binding to the lipid membranes.
Reportedly, anti-bacterial and anti-viral activities of the alkyl gallates decrease as their alkyl chain length increases beyond a certain length (8–11), indicating a cutoff point for the activity of the alkyl gallates (9). Reduction of the activity will be reasonable according to our observations. The reduction can be attributed to the promotion of the self-association with increasing alkyl chain length. In other words, such self-associated species can not interact with the bacterial cell membranes or envelopes of viruses. Therefore, their activities would be reduced. Consequently, the pharmacological activity of the alkyl gallates can be expected to be associated closely with their physicochemical properties such as hydrophobicity and the monomeric solubility. To assess the relation between the physicochemical properties and the pharmacological activity, we investigated the correlation between the membrane binding ability and pharmacological activity (Table III), where the binding ability was evaluated using the ratios of the fluorescence intensity of the alkyl gallates (F/F0). Most correlation coefficients were positive and greater than 0.7. Therefore, the membrane-binding ability of the alkyl gallates will probably account for the results obtained for the pharmacological activity. It is noteworthy that the correlation coefficients for Trichophyton rubrum and Microsporum gypseum were lower than the others. Consequently, the pharmacological activity of the alkyl gallates might be partly accounted for by other functions including disruption of lipid membranes, inactivation of membrane proteins and their downstream activities.
Table III.
Calculated correlation coefficients between ratios of the fluorescence intensity (F/F0) and the pharmacological activity of the alkyl gallates against bacteria and viruses.
| Targets | r a | Ref. |
|---|---|---|
| Propionibacterium acnes | 0.949 | (13) |
| Brevibacterium ammoniagenes | 0.892 | (13) |
| Staphylococcus aureus (MRSA) | 0.892 | (13) |
| Micrococcus luteus | 0.824 | (13) |
| Bacillus subtilis | 0.824 | (13) |
| Streptococcus mutans | 0.593 | (13) |
| Trichophyton mentagrophytes | 0.409 | (16) |
| Trichophyton rubrum | 0.135 | (16) |
| Microsporum gypseum | 0.129 | (16) |
|
| ||
| Targets | r b | Ref |
|
| ||
| Herpes simplex virus type-1 | 0.745 | (19) |
| Lenzites betulina | 0.785 | (14) |
| Gloeophyllum trabeum | 0.729 | (14) |
| Chaetomium globosum | 0.716 | (14) |
| Trametes versicolor | 0.683 | (14) |
The alkyl gallates’ interaction with cholesterol and with phospholipids also constitutes important information. Lipid membranes composed of DPPC, DOPC and cholesterol are often used as cell membrane models with phase changes depending on the composition ratio (40, 42–46). The results presented in Fig. 5 indicate that the alkyl gallates are more stable in the liquid phase of DPPC/DOPC than in the solid phase, although they are less stable in the liquid phase of DPPC/Chol than in the solid phase. Consequently, the stability of alkyl gallates in lipid membranes depends substantially on the membrane components rather than the membrane phase. The direct interaction of the alkyl gallates with the lipid components might be related with various pharmacological effects toward bacterial cells (12, 14) and viruses (19, 21).
Finally, the stability of the alkyl gallate in aqueous solution depends on the co-existing solutes, as shown in our previous study (37, 39). Alteration of the stability in the solution by the solutes can affect the partition constants and thereby affect their activity toward bacterial cells and viruses. Such effects of solutes are potentially applicable to prevent self-association and to enhance their bioavailability as drugs and food additives. We are carrying out systematic investigations of the solute effects to elucidate their potential benefits.
Supplementary Material
Acknowledgements
We thank Prof. Yukio Nagasaki for use of the light-scattering spectrometer. We thank Dr D. Ejima for valuable discussion.
Conflict of Interest
None declared.
Glossary
Abbreviations
- C1
methyl gallate
- C2
ethyl gallate
- C3
propyl gallate
- C4
butyl gallate
- C8
octyl gallate
- C12
dodecyl gallate
- C16
cetyl gallate
- C18
stearyl gallate
- Chol
cholesterol
- DLS
dynamic light scattering
- DOPC
dioleoylphosphatidylcholine
- DOPG
dioleoylphosphatidylglycerol
- DPPC
dipalmitoylphosphatidylcholine
References
- 1. Kuo PL, Hsu YL, Lin TC, Lin CC. 2005. The antiproliferative activity of prodelphinidin B-2 3'-O-gallate from green tea leaf is through cell cycle arrest and Fas-mediated apoptotic pathway in A549 cells. Food Chem. Toxicol. 43, 323–323 [DOI] [PubMed] [Google Scholar]
- 2. Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. 1997. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J. Natl Cancer Inst. 89, 1886–1886 [DOI] [PubMed] [Google Scholar]
- 3. Tsukiyama F, Nakai Y, Yoshida M, Tokuhara T, Hirota K, Sakai A, Hayashi H, Katsumata T. 2006. Gallate, the component of HIF-inducing catechins, inhibits HIF prolyl hydroxylase. Biochem. Biophys. Res. Commun. 351, 239–239 [DOI] [PubMed] [Google Scholar]
- 4. Fang MZ, Wang YM, Ai N, Hou Z, Sun Y, Lu H, Welsh W, Yang CS. 2003. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 63, 7570–7570 [PubMed] [Google Scholar]
- 5. Lin YL, Lin JK. 1997. (-)-epigallocatechin-3-gallate blocks the induction of nitric oxide synthase by down-regulating lipopolysaccharide-induced activity of transcription factor nuclear factor-kappa B. Mol. Pharmacol. 52, 472–472 [PubMed] [Google Scholar]
- 6. Yokoyama M, Noguchi M, Nakao Y, Pater A, Iwasaka T. 2004. The tea polyphenol, (-)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol. Oncol. 92, 204–204 [DOI] [PubMed] [Google Scholar]
- 7. Kubo I, Xiao P, Nihei K, Fujita K, Yamagiwa Y, Kamikawa T. 2002. Molecular design of antifungal agents. J. Agric. Food Chem. 50, 3998–3998 [DOI] [PubMed] [Google Scholar]
- 8. Fujita K, Kubo I. 2002. Antifungal activity of octyl gallate. Int. J. Food Microbiol. 79, 201–201 [DOI] [PubMed] [Google Scholar]
- 9. Fujita K, Kubo I. 2002. Plasma membrane injury induced by nonyl gallate in Saccharomyces cerevisiae. J. Appl. Microbiol. 92, 1042–1042 [DOI] [PubMed] [Google Scholar]
- 10. Leal PC, Mascarello A, Derita M, Zuljan F, Nunes RJ, Zacchino S, Yunes RA. 2009. Relation between lipophilicity of alkyl gallates and antifungal activity against yeasts and filamentous fungi. Bioorg. Med. Chem. Lett. 19, 1796–1796 [DOI] [PubMed] [Google Scholar]
- 11. Hsu FL, Chen PS, Chang HT, Chang ST. 2009. Effects of alkyl chain length of gallates on their antifungal property and potency as an environmentally benign preservative against wood-decay fungi. Int. Biodeter. Biodegrad. 63, 547–547 [Google Scholar]
- 12. Kubo I, Xiao P, Fujita K. 2001. Antifungal activity of octyl gallate, Structural criteria and mode of action. Bioorg. Med. Chem. Lett. 11, 350–350 [DOI] [PubMed] [Google Scholar]
- 13. Kubo I, Fujita K, Nihei K. 2003. Molecular design of multifunctional antibacterial agents against methicillin resistant Staphylococcus aureus (MRSA). Bioorg. Med. Chem. 11, 4262–4262 [DOI] [PubMed] [Google Scholar]
- 14. Strippoli V, D'Auria FD, Tecca M, Callari A, Simonetti G. 2000. Propyl gallate increases in vitro antifungal imidazole activity against Candida albicans. Int. J. Antimicrob. Agents 16, 76–76 [DOI] [PubMed] [Google Scholar]
- 15. Nihei K, Nihei A, Kubo I. 2003. Rational design of antimicrobial agents, antifungal activity of alk(en)yl dihydroxybenzoates and dihydroxyphenyl alkanoates. Bioorg. Med. Chem. Lett. 13, 3996–3996 [DOI] [PubMed] [Google Scholar]
- 16. Hsu FL, Chang HT, Chang ST. 2007. Evaluation of antifungal properties of octyl gallate and its synergy with cinnamaldehyde. Bioresour. Technol. 98, 738–738 [DOI] [PubMed] [Google Scholar]
- 17. Kubo I, Fujita K, Nihei K, Nihei A. 2004. Antibacterial activity of akyl gallates against Bacillus subtilis. J. Agric. Food. Chem. 52, 1076–1076 [DOI] [PubMed] [Google Scholar]
- 18. Sierra-Campos E, Valdez-Solana MA, Matuz-Mares D, Velazquez I, Pardo JP. 2009. Induction of morphological changes in Ustilago maydis cells by octyl gallate. Microbiology 155, 611–611 [DOI] [PubMed] [Google Scholar]
- 19. Uozaki M, Yamasaki H, Katsuyama Y, Higuchi M, Higuti T, Koyama AH. 2007. Antiviral effect of octyl gallate against DNA and RNA viruses. Antiviral Res. 73, 91–91 [DOI] [PubMed] [Google Scholar]
- 20. Hurtado C, Bustos MJ, Sabina P, Nogal ML, Granja AG, Gonzalez ME, Gonzalez-Porque P, Revilla Y, Carracosa AL. 2008. Antiviral activity of lauryl gallate against animal viruses. Antiviral Ther. 13, 917–917 [PubMed] [Google Scholar]
- 21. Yamasaki H, Uozaki M, Katsuyama Y, Utsunomiya H, Arakawa T, Higuchi M, Higuti T, Koyama AH. 2007. Antiviral effect of octyl gallate against influenza and other RNA viruses. Int. J. Mol. Med. 19, 688–688 [PubMed] [Google Scholar]
- 22. Kane CJM, Menna JH, Sung C-C, Yeh Y-C. 1988. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 8, 1. [DOI] [PubMed] [Google Scholar]
- 23. Kratz JM, Andrighetti-Frohner CR, Kolling DJ, Leal PC, Cirne-Santos CC, Yunes RA, Nunes RJ, Trybala E, Bergstrom T, Frugulhetti IC, Barardi CR, Simoes CM. 2008. Anti-HSV-1 and anti-HIV-1 activity of gallic acid and pentyl gallate. Mem. Inst. Oswaldo. Cruz. 103, 442–442 [DOI] [PubMed] [Google Scholar]
- 24. Kratz JM, Andrighetti-Fröhner CR, Leal PC, Nunes RJ, Yunes RA, Trybala E, Bergström T, Barardi CRM, Simões CMO. 2008. Evaluation of anti-HSV-2 activity of gallic acid and pentyl gallate. Biol. Pharm. Bull. 31, 907–907 [DOI] [PubMed] [Google Scholar]
- 25. Chavez JH, Leal PC, Yunes RA, Nunes RJ, Barardi CR, Pinto AR, Simoes CM, Zanetti CR. 2006. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 116, 59–59 [DOI] [PubMed] [Google Scholar]
- 26. Dodo K, Minato T, Noguchi-Yachide T, Suganuma M, Hashimoto Y. 2008. Antiproliferative and apoptosis-inducing activities of alkyl gallate and gallamide derivatives related to (-)-epigallocatechin gallate. Bioorg. Med. Chem. 16, 7982–7982 [DOI] [PubMed] [Google Scholar]
- 27. Cowgill RW. 1967. Fluorescence and protein structure, X. Reappraisal of solvent and structural effects. Biochim. Biophys. Acta 133, 18–18 [DOI] [PubMed] [Google Scholar]
- 28. Tamba Y, Yamazaki M. 2009. Magainin 2-induced pore formation in the lipid membranes depends on its concentration in the membrane interface. J. Phys. Chem. B. 113, 4852–4852 [DOI] [PubMed] [Google Scholar]
- 29. Wieprecht T, Apostolov O, Beyermann M, Seelig J. 2000. Membrane binding and pore formation of the antibacterial peptide PGLa, thermodynamic and mechanistic aspects. Biochemistry 39, 452–452 [DOI] [PubMed] [Google Scholar]
- 30. Lampio A, Kilpelainen I, Pesonen S, Karhi K, Auvinen P, Somerharju P, Kaariainen L. 2000. Membrane binding mechanism of an RNA virus-capping enzyme. J. Biol. Chem. 275, 37859–37859 [DOI] [PubMed] [Google Scholar]
- 31. Kraft CA, Garrido JL, Leiva-Vega L, Romero G. 2009. Quantitative analysis of protein-lipid interactions using tryptophan fluorescence. Sci. Signal 2, pl4. [DOI] [PubMed] [Google Scholar]
- 32. Surewicz WK, Epand RM. 1984. Role of peptide structure in lipid-peptide interactions, a fluorescence study of the binding of pentagastrin-related pentapeptides to phospholipid vesicles. Biochemistry 23, 6077–6077 [DOI] [PubMed] [Google Scholar]
- 33. Chaudhuri S, Pahari B, Sengupta PK. 2009. Ground and excited state proton transfer and antioxidant activity of 7-hydroxyflavone in model membranes, absorption and fluorescence spectroscopic studies. Biophys. Chem. 139, 26–26 [DOI] [PubMed] [Google Scholar]
- 34. Klymchenko AS, Duportail G, Ozturk T, Pivovarenko VG, Mely Y, Demchenko AP. 2002. Novel two-band ratiometric fluorescence probes with different location and orientation in phospholipid membranes. Chem. Biol. 9, 1208–1208 [DOI] [PubMed] [Google Scholar]
- 35. Kajiya K, Hojo H, Suzuki M, Nanjo F, Kumazawa S, Nakayama T. 2004. Relationship between antibacterial activity of (+)-catechin derivatives and their interaction with a model membrane. J. Agric. Food. Chem. 52, 1519–1519 [DOI] [PubMed] [Google Scholar]
- 36. Shibata H, Nakano T, Parvez MA, Furukawa Y, Tomoishi A, Niimi S, Arakaki N, Higuti T. 2009. Triple combinations of lower and longer alkyl gallates and oxacillin improve antibiotic synergy against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53, 2220–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ariki R, Hirano A, Arakawa T, Shiraki K. 2011. Arginine increases the solubility of alkyl gallates through interaction with the aromatic ring. J. Biochem. 149, 394–394 [DOI] [PubMed] [Google Scholar]
- 38. Georgalis Y, Kierzek AM, Saenger W. 2000. Cluster formation in aqueous electrolyte solutions observed by dynamic light scattering. J. Phys. Chem. B. 104, 3406–3406 [Google Scholar]
- 39. Hirano A, Kameda T, Arakawa T, Shiraki K. 2010. Arginine-assisted solubilization system for drug substances, solubility experiment and simulation. J. Phys. Chem. B. 114, 13462–13462 [DOI] [PubMed] [Google Scholar]
- 40. Veatch SL, Keller SL. 2003. Separation of liquid phases in giant vesicles of ternary mixtures of phospholipids and cholesterol. Biophys. J. 85, 3083–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zou KH, Tuncali K, Silverman SG. 2003. Correlation and simple linear regression. Radiology 227, 622–622 [DOI] [PubMed] [Google Scholar]
- 42. Veatch SL, Keller SL. 2002. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89, 268101. [DOI] [PubMed] [Google Scholar]
- 43. Veatch SL, Keller SL. 2005. Seeing spots, complex phase behavior in simple membranes. Biochim. Biophys. Acta 1746, 185–185 [DOI] [PubMed] [Google Scholar]
- 44. Veatch SL, Keller SL. 2005. Miscibility phase diagrams of giant vesicles containing sphingomyelin. Phys. Rev. Lett. 94, 148101. [DOI] [PubMed] [Google Scholar]
- 45. Cicuta P, Keller SL, Veatch SL. 2007. Diffusion of liquid domains in lipid bilayer membranes. J. Phys. Chem. B. 111, 3331–3331 [DOI] [PubMed] [Google Scholar]
- 46. Veatch SL, Soubias O, Keller SL, Gawrisch K. 2007. Critical fluctuations in domain-forming lipid mixtures. Proc. Natl Acad. Sci. USA 104, 17655–17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


