Abstract
Niemann-Pick type C (NPC) disease is a lysosomal storage disorder resulting from mutations in either the NPC1 (95%) or NPC2 (5%) genes. NPC typically presents in childhood with visceral lipid accumulation and complex progressive neurodegeneration characterized by cerebellar ataxia, dysphagia, and dementia, resulting in a shortened lifespan. While cholesterol is widely acknowledged as the principal storage lipid in NPC, multiple species of sphingolipids accumulate as well. This accumulation of sphingolipids led to the initial assumption that NPC disease was caused by a deficiency in a sphingolipid catabolism enzyme, similar to sphingomyelinase deficiencies with which it shares a family name. It took about half a century to determine that NPC was in fact caused by a cholesterol traffcking defect, and still as we approach a century after the initial identification of the disease, the mechanisms by which sphingolipids accumulate remain poorly understood. Here we focus on the defects of sphingolipid catabolism in the endolysosomal compartment and how they contribute to the biology and pathology observed in NPC disease. This review highlights the need for further work on understanding and possibly developing treatments to correct the accumulation of sphingolipids in addition to cholesterol in this currently untreatable disease.
1. Introduction
There are roughly 50 lysosomal storage diseases characterized by the accumulation of various lipids and sugars in the endolysosomal compartment (Vitner et al., 2010). A subset of these diseases, often referred to as sphingolipidoses, are defined by their defective degradation and resulting accumulation of sphingolipids. The majority of sphingolipidoses, such as Fabry’s, Gaucher’s, Sandhoff’s, and Tay-Sachs’ diseases, arise from autosomal recessive mutations in the enzymes responsible for the degradation of individual sphingolipid species (Coant et al., 2017; Grassi et al., 2018). Also belonging to this group are the family of Niemann-Pick (NP) diseases. Named after the German physicians Albert Niemann and Ludwick Pick who first described the disorders in the early 20th century, the original classification was based on the pathological observation of hepatosplenomegaly with and without neurological defects. The accumulation of sphingomyelin in NP was first described in the 1930’s, and in 1966, Roscoe Brady was the first to demonstrate a sphingomyelinase defect (Brady et al., 1966). Surprisingly, in 1980 Vanier et al. discovered that while sphingomyelin storage is common to the family, only NP Type A (NPA) and NP Type B (NPB) are caused by defects in sphingomyelinase, whereas sphingomyelinase activity is normal in NP Type C (NPC) (Vanier et al., 1980). This finding distinguished NPC as a unique member of the NP family as it is not caused by a degradative enzyme deficiency, but further work was necessary to understand the root cause behind the accumulation of sphingomyelin (reviewed in (Vanier, 2015)).
The primary cellular lesion in NPC was first uncovered in studies of NPC patient fibroblasts and confirmed in a spontaneous mutant mouse model, which demonstrated the intracellular accumulation of low density lipoprotein (LDL)-derived, unesterified cholesterol (Butler et al., 1987; Pentchev et al., 1986a, 1986b; Sokol et al., 1988). It would be more than two decades before the seminal findings of Brown and Goldstein that elucidated the role the NPC proteins play in cholesterol transport and highlighted the tandem process by which NPC2, a soluble luminal protein, binds unesterified cholesterol and transfers it to the N-terminal domain of membrane-associated NPC1 that facilitates its transport out of the late endosome/lysosome (LE/L) compartment (Kwon et al., 2009; Wang et al., 2010; Xie et al., 2011). While structure-function studies have continued to shed light on the distinct functional domains of the NPC1 protein (Li et al., 2016a, 2016b), further work is still needed to determine the exact mechanism by which the NPC1 protein facilitates the transport of cholesterol across the glycocalyx and into the LE/L membrane.
2. Cholesterol is not the only lipid accumulated in NPC
NPC disease is caused predominantly by mutations in NPC1 (95% of cases) or NPC2 genes that decrease intracellular cholesterol trafficking resulting in accumulation of unesterified cholesterol (Carstea et al., 1997; Loftus et al., 1997; Neufeld et al., 1999). Important for this review, in addition to cholesterol, a diverse group of sphingolipid species also accumulate in NPC disease and have been implicated in a variety of NPC-related pathologies. Perhaps the most widely characterized are sphingomyelin and the glycosphingolipids (GSLs) that have been well documented in both animal models and in NPC patients (Abi-Mosleh et al., 2009; D’Arcangelo et al., 2016; Davidson et al., 2009; Heron et al., 2012; Newton et al., 2017; Ory et al., 2017; Park et al., 2003). The accumulation of GSLs in NPC and their role in pathology has led to the only disease-specific therapy currently approved for the treatment of NPC, the glucosylceramide synthase inhibitor Miglustat (Lachmann et al., 2004; Platt, 2014). While Miglustat has been shown to increase lifespan, delay the onset of neurological dysfunction, and reduce the magnitude of neurological symptoms in NPC animal models, it failed to address the pathogenic accumulation of cholesterol, in agreement with the original suggestion of a disconnect between cholesterol storage and the progression of neurodegeneration in NPC (Zervas et al., 2001). This is supported by work showing that genetic and pharmacological disruption of LDL uptake ameliorated the liver pathology in NPC mutant mice, but had little effect on the development of neurological defects (Erickson et al., 2000). Due to the compartmentalized nature of sphingolipid metabolism and potent bioactivity of the metabolites ceramide, sphingosine, and sphingosine-1-phosphate (S1P), it was suggested that examining these sphingolipids in the context of NPC may provide better understanding of the complex biology and heterogeneous nature of the disease (Lloyd-Evans et al., 2008; Lloyd-Evans and Platt, 2010). This review is focused on the defects of sphingolipid catabolism in the endolysosomal compartment and how those defects contribute to the biology and pathology observed in NPC disease.
3. Sphingolipid metabolism and endocytosis
All sphingolipids contain a sphingoid base consisting typically of an 18 carbon fatty amino alcohol known as sphingosine. De novo synthesis of sphingolipids occurs exclusively in the endoplasmic reticulum (ER), where the rate limiting enzyme, serine palmitoyl-transferase, catalyzes the condensation of palmitate and serine to form dihydrosphingosine. In subsequent reactions, dihydro-sphingosine is amino-acylated by fatty acylCoAs catalyzed by a family of six (dihydro)ceramide synthases to form dihydroceramide species with varying length (14–32 d) carbon chains. The final step of de novo synthesis occurs with the addition of a double bond between C4 and C5 in the sphingoid base by two isoforms of dihydroceramide desaturase (DES1/2) generating ceramide. In the ER, ceramide can be converted into relatively rare galactosphingolipids or actively transported to the Golgi by the soluble ceramide transfer protein or via vesicle transport where it is then utilized to form multiple complex sphingolipids, such as glucosylceramide and sphingomyelin by glucosylceramide synthase or sphingomyelin synthases (van Meer and Lisman, 2002). It should be noted that sphingosine, the namesake for the family of sphingolipids, is not generated during de novo synthesis and is only formed by the enzymatic breakdown of sphingolipids.
Similar to the compartmentalized nature of de novo synthesis, the catabolism and salvage pathways of sphingolipids occur in organelle-specific manners. Enzymes responsible for the metabolism of sphingolipids, such as the sphingomyelinases, galactosidase, and glucosidases, are predominantly lysosomal, whereas sphingomyelin synthase and the sphingosine kinases have been detected in the plasma membrane, mitochondria, and even the nucleus (Hannun and Obeid, 2018; Newton et al., 2017). In each instance, these enzymes are responsible for regulating and maintaining levels of bioactive sphingolipid metabolites with distinct cellular functions.
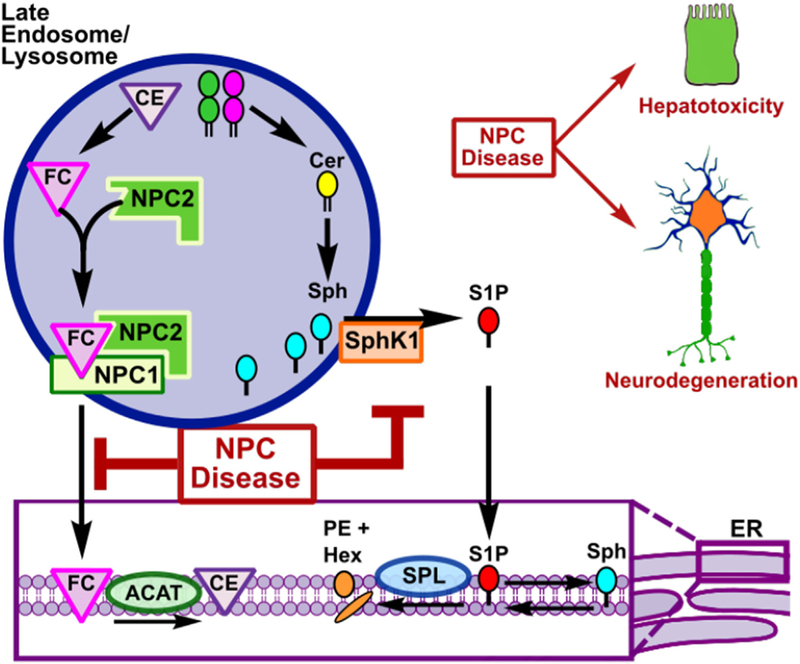
In the context of NPC disease, it has been suggested that perhaps the most important cellular process that contributes to the pathologic accumulation of sphingolipids is the endocytic pathway (Fig. 1). During the uptake of LDL and other endocytic events, cargo becomes wrapped in vesicles generated from the plasma membrane, which are highly enriched with sphingolipids that serve as both structural components and signaling modulators (Hannun and Obeid, 2018). Once taken into the cell, these sphingolipids are either recycled back to the plasma membrane or transported through the endosomal system to the lysosome, where the complex sphingolipids are enzymatically degraded in a series of reactions to produce free fatty acids and sphingoid bases. The sphingoid bases (sphingosine or dihydrosphingosine) released from ceramide carry a positive charge on the amine group, which dictates that they must exit the lysosome by a yet undiscovered active transport mechanism (Coant et al., 2017). After lysosomal egress, sphingosine can be phosphorylated by two isoforms of sphingosine kinase (SphK1/2) to generate S1P which moves to the ER where it can either be degraded by S1P phosphatases to sphingosine that is converted back to ceramide by ceramide synthases for reutilization in the salvage pathway (Geffken and Spiegel, 2018; Pyne et al., 2016) or irreversibly cleaved by S1P lyase (SPL), resulting in the formation of hexadecenal and ethanolamine phosphate and eventually, phosphatidylethanolamine (Aguilar and Saba, 2012). This compart-mentalization of sphingolipid degradation and the nature of the cellular biology of NPC disease led to the original notion that sphingolipid accumulation is a secondary effect in NPC. It is well established that there is a robust defect in the endocytic pathway at the late LE/L level (Lloyd-Evans et al., 2008). The accumulation of ganglioside GM1 in the early endosome of NPC cells that occurs upstream of a defective LE/L compartment is an often cited example of the “traffic jam” that occurs in NPC (Sugimoto et al., 2001). The fundamental requirement that all endocytosed membrane sphingolipids targeted for degradation are degraded to sphingosine in the LE/L does not contradict this theory, but multiple studies of the process by which sphingosine itself is transported out of the LE/L in NPC have provided evidence that sphingosine accumulation is at least partially independent of the LE/L fusion defect.
Fig. 1. Cholesterol and sphingosine accumulation in NPC disease.
During receptor-mediated endocytosis, plasma membrane sphingolipids are internalized. Those sphingolipids not targeted for recycling must be catabolized in the lysosome first to ceramide and then to sphingosine as they move through the endolysosomal pathway. Sphingosine is exported out of the late endosome/lysosome where it is phosphorylated by sphingosine kinases to S1P. In the endoplasmic reticulum, S1P can either be irreversible degraded by S1P lyase or dephosphorylated back to sphingosine and reused in the sphingolipid salvage pathway. In NPC, however, in addition to the hallmark cholesterol accumulation, there is accumulation of sphingosine that contributes to the cellular biology and pathology of NPC disease. Abbreviations: GSL, glycosphingolipid; LDL; low density lipo-protein; LDLR, low density lipoprotein receptor; SM, sphingomyelin; Cer, ceramide; Sph, sphingosine; SphK1, sphingosine kinase 1; S1P. sphin-gosine-1-phosphate; S1PRs, S1P receptors; CE; cholesterol esters; FC, free cholesterol; ACAT, cholesterol acyltransferase; SPL, sphingosine-1-phosphate lyase; PE, phosphatidylethanolamine; Hex, hexadecenal.
4. Sphingosine accumulation and NPC
There is a large body of work detailing the accumulation of complex sphingolipids in the brains and livers of multiple mouse models of sphingolipid storage disorders and in NPC (Fan et al., 2013; Goldin et al., 1992; Lee et al., 2014; Lloyd-Evans et al., 2008; Rodriguez-Lafrasse et al., 1994; te Vruchte et al., 2004). Furthermore, a spontaneously generated feline NPC model with a mutation in the NPC1 protein shows a similar pattern of sphingosine accumulation (Stein et al., 2012), and sphingosine and dihydrosphingosine are also increased in NPC patient livers and spleens, as well as in cultured primary NPC mutant fibroblasts (Rodriguez-Lafrasse et al., 1994). However, complex sphingolipids also accumulate in NPC, and some evidence exists that there are also alterations in the lysosomal enzymes responsible for degrading the complex sphingolipids to sphingosine, such as glucosylceramidase and acid sphingomyelinase (Salvioli et al., 2004). While animal models are informative in examinations of the pathogenic role that accumulation of sphingosine plays in NPC, determining the mechanism by which it accumulates requires examining the defect at the cellular level. In this context, while NPC1 or NPC2 mutations lead to storage of a diverse set of lipid species, only sphingosine induces a NPC phenotype when exogenously added at the levels seen in NPC mutant cells (Lloyd-Evans et al., 2008). Studies from the Platt lab using U18666A, a compound that binds to and directly inhibits the transport activity of NPC1 (Lu et al., 2015), demonstrated that sphingosine accumulates almost immediately, whereas cholesterol and other lipids increase hours later (Lloyd-Evans et al., 2008). This was the first suggestion that sphingosine accumulation could be a primary defect in NPC (Lloyd-Evans et al., 2008). Very recent studies confirmed that sphingosine indeed accumulates in the lysosomal compartment of cells after CRISPR/CAS9-mediated deletion of NPC1 (Tharkeshwar et al., 2017). Recently developed tools designed to elucidate the process by which sphingosine is transported in the LE/L compartment further strengthen this argument. Studies with a novel “caged” sphingosine that can be delivered directly to the LE/L compartment have shown that sphingosine transport out of this compartment is dramatically delayed in NPC patient fibroblasts, a phenotype replicated by siRNA mediated knockdown of NPC1 protein, or pharmacological inhibition of NPC1 by U18666A, and that the released sphingosine accumulates in luminal vesicles in the LE/L (Hoglinger et al., 2017). The development of more sophisticated technologies and techniques like CRISPR/CAS9 and “caged lipids” together with the ability to quantitatively measure sphingolipids by mass spectrometry, have strengthened our understanding of the nature of the sphingosine accumulation defect in NPC, although the mechanism underlying this defect has remained elusive.
5. Sphingosine, S1P, and the cellular pathology of NPC
A possible explanation for sphingosine accumulation that has been suggested is a deficiency in the conversion of sphingosine to S1P. Early studies of a spontaneous mutant mouse model of NPC reported that sphingosine and dihydrosphingosine accumulate in the livers of these mice, and assays with both crude liver homogenates as well as with purified SphK implicated a defect or inhibition of the enzyme (Goldin et al., 1992). More recently, work by Lee et al. demonstrated a defect in VEGF-mediated SphK1 activation in patient mutant NPC1 fibroblasts as well as in primary neurons from Balb/c Npc1nih mice, a defect that could be corrected by the addition of VEGF (Lee et al., 2014). This provided hints that the defect in activation of SphK1 leading to accumulation of sphingosine and decreased S1P may contribute to cellular processes that are aberrant in NPC disease.
Recent work by our lab and others has highlighted the importance of the conversion of sphingosine to S1P by SphK1 in endocytic processing (Lima et al., 2017; Shen et al., 2014; Young et al., 2016). SphK1 is recruited to nascent endosomes and knockdown of SphKs results in endocytic recycling defects (Shen et al., 2014). This was the first suggestion of a role for sphingosine phosphorylation in endocytic membrane trafficking beyond the established function of S1P as a ligand for specific G protein-coupled receptors (Shen et al., 2014). Similarly, treating cells with sphingosine or sphingosine analogs in the presence of an inhibitor of SphK1 resulted in delayed endocytic maturation and the accumulation of dilated dysfunctional LEs that impair autophagy (Lima et al., 2017; Young et al., 2016). This phenotype is remarkably similar to that observed in NPC mutant fibroblasts that accumulate LEs enriched in sphingolipids and cholesterol accompanied by stalled autophagy (Sarkar et al., 2013, 2014). Because SphK activity is defective in NPC (Lee et al., 2014), it is possible that a failure to recruit SphK to endosomal membranes may cause sphingosine accumulation that is involved in the pathogenesis of NPC. Interestingly, embryonic fibroblasts generated from mice lacking the S1P lyase gene (Sgpl1), which are unable to terminally degrade S1P (and thus all sphingolipids), accumulate both S1P and sphingosine and display a phe-notype strikingly similar to that of NPC1 mutant cells, including increased cholesterol accumulation and impaired autophagic flux (Vienken et al., 2017).
6. Sphingosine and lysosomal calcium signaling
Defective calcium signaling is a common characteristic of many lysosomal storage disorders, including NPC (Lloyd-Evans et al., 2010; Vitner et al., 2010). Calcium plays a key role in the endolysosomal pathway where it both regulates vesicular fusion events responsible for the trafficking and recycling of endocytosed substrates through activation of SNARE complexes and controls autophagy (Lloyd-Evans et al., 2010; Medina and Ballabio, 2015). Attenuated elevation of cytoplasmic calcium was the first clue that calcium homeostasis is altered in NPC disease (Yamamoto et al., 1994) Later studies showed that calcium was increased, at least in part, by the dysregulation of lysosomal calcium refilling and that this phenomenon may be directly related to the accumulation of intracellular sphingosine (Lloyd-Evans et al., 2008). While the identity of the calcium channel involved in the NPC related signaling defect was initially controversial (Kiselyov et al., 2010; Lloyd-Evans et al., 2010), recent studies utilizing caged sphingosine to spontaneously increase intracellular levels of sphingosine resulted in a dynamic release of calcium from the endolysosomal compartment that was independent of S1P and extracellular and ER calcium levels, but dependent on the two-pore calcium channel 1 (TPC1) that resides on endosomes and lysosomes (Hoglinger et al., 2015). This is an important observation because calcium changes in acidic compartments have been shown to regulate vesicle fusion and trafficking as well as autophagy and lysosomal biogenesis (Medina et al., 2015; Patel and Docampo, 2010). Indeed, sphingosine-induced lysosomal calcium release induced translocation of transcription factor EB (TFEB) to the nucleus (Hoglinger et al., 2015), where it acts as a central regulator for lysosomal biogenesis and autophagy, suggesting that not only are the calcium defects responsible for the observed dysfunction in SNARE activity in NPC but also have a role in the autophagic defects as well (Fraldi et al., 2010; Medina et al., 2015; Sarkar et al., 2014; Tong and Song, 2015). In this regard, during the clearance of vacuoles whose formation was induced by a specific SphK1 inhibitor (SK1-I), TFEB is translocated to the nucleus (Young et al., 2016). This in turn restored autophagic flux and allowed for vacuole clearance by an undetermined mechanism.
While evidence strongly supports a relationship between sphingosine accumulation and calcium defects in the endolysosomal compartment in NPC disease, there is some evidence that accumulation of intracellular sphingosine does not always induce decreases in calcium levels in acidic compartments. For example, in Sgpl1 knockout fibroblasts, which also accumulate sphingosine, the opposite effect on calcium was found (Claas et al., 2010; Vienken et al., 2017). There are some key differences in these cases, however, as accumulation of other sphingolipids found in NPC, such as sphingomyelin and glycosphingolipids, does not occur in Sgpl1 mutant cells. Moreover, while NPC cells have reduced S1P levels, Sgpl1 deleted cells in contrast have marked increases in the levels of this potent signaling molecule (Vienken et al., 2017), which might explain these discrepancies.
7. Sphingolipid biomarkers of NPC
Recent advances in mass spectrometry detection methods have spurred the exploration of sphingolipids as biomarkers for NPC disease. Diagnosis of NPC disease can be problematic due to heterogeneity in age of onset, complexity of the current diagnostic criteria that includes cell based filipin staining for cholesterol accumulation and genetic testing, and the rarity of these disorders, resulting in a general knowledge deficit by physicians (Vanier et al., 2016). Attempts have been made to find new methods to confirm diagnoses, such as measurements of the relative acidic compartment volume in circulating B cells using commercially available probes and flow cytometry (te Vruchte et al., 2014), but these cell-based methods also require the collection of live cells and biochemical methods that are not standard in most clinical labs. Unlike the cellular based approaches, sphingolipid analysis by mass spectrometry can be applied to samples that do not require immediate testing, such as dried blood spots, plasma, or fecal matter (Jiang et al., 2016; Rusconi et al., 2018). Furthermore, such samples can be obtained from neonates before the development of severe disease symptoms and have already been shown to have diagnostic benefits in NPC (Jiang et al., 2011, 2016).
Initial biomarker analysis in NPC focused on the circulating oxysterol species, cholestane-3β,5α,6β-triol (c-trio) and 7-ketocho-lesterol (7-KC), which were found to be elevated in plasma from both NPC mutant mice and NPC patients (Porter et al., 2010) and in dried blood spots (Jiang et al., 2016). However, further work indicated that increased levels of these cholesterol metabolites do not distinguish between NPC and a variety of other disorders, including NPA and NPB (Bjorkhem et al., 2014; Klinke et al., 2015; Reunert et al., 2016). In addition to c-trio and 7-KC, the cholesterol metabolite 24(S)-hydroxycholesterol (24(S)-HC) was also investigated as a possible biomarker of NPC in a NIH clinical trial (Ory et al., 2017; Sidhu et al., 2015). However, levels of 24(S)-HC were also altered in other neurological disorders including Parkinson’s (Di Natale et al., 2018), multiple sclerosis (Mukhopadhyay et al., 2017) and Alzheimer’s disease (Benussi et al., 2017). This promiscuity indicates that 24(S)-HC may be a more general marker of brain health, rather than a specific marker of NPC. Despite this, the potential of the use of oxysterol biomarkers to monitor NPC patient responses to therapies is promising.
Sphingolipid quantification by mass spectrometry has recently become a hot topic in the field of other lysosomal storage disorders, such as Krabbe’s, Gaucher’s and Farber’s disease, suggesting that this approach may be a powerful tool for diagnosis of NPC as well (Cozma et al., 2017). While it is known that many sphingolipids accumulate in the brains and visceral tissues of NPC patients, these accumulations do not always translate into observable increases in their circulating levels. An initial lipidomic evaluation of plasma from NPC patients reported increases in monohexosylceramides and ceramide, yet unlike results from patient tissues, sphingosine, S1P, dihydrosphingosine, and dihydro-S1P were all markedly decreased compared to normal controls (Fan et al., 2013). In this same report, after Miglustat treatment, total plasma levels of ceramide and gangliosides (namely GM1 and GM3) decreased, and the cerebrospinal fluid levels of monohexosylceramides were significantly increased. Measurements of plasma lysosphingomyelin levels also have been reported to be potentially useful in NPC disease (Welford et al., 2014). Lysosphingolipids, N-deacylated versions of their precursor sphingolipids, appear to be elevated in multiple disorders due to deficiencies of enzymes responsible for the breakdown of complex sphingolipids (Piraud et al., 2018). Moreover, lysosphingolipid measurements have attracted attention since compared to their acylated precursors, their accumulation is often up to an order of magnitude higher in these disease states, suggesting that they could be more reliable biomarkers than the other previously discussed sphingolipid metabolites (Welford et al., 2014). An analog of sphingosylphosphorylcholine known as lysosphingomyelin 509 has been suggested to be of particular interest in regard to NPC disease (Kuchar et al., 2017). Measurements of plasma samples from patients presenting with all three NPC diseases suggested that in conjunction with sphingosylphosphorylcholine, lysosphingomyelin 509 can be used to differentially screen for NPC, as both are increased in NPA and NPB samples, but only lysosphingomyelin 509 is elevated in NPC (Kuchar et al., 2017). These observations if confirmed could potentially pave the way for the use of these non-cell based assays to discriminate between NPC and NPA/B.
In summary, while NPC disease is accurately classified as a sphingolipidosis, the genetic defects leading to accumulation of sphingolipids are unique amongst the group. The primary defect in NPC is a result of mutations of the cholesterol transport proteins NPC1 and NPC2 and accumulation of sphingolipids in the disease has been strongly implicated in the pathology of this disease variant. Similar to the yet undescribed mechanism by which NPC1 transports cholesterol through the glycocalyx and out of the lysosome, the mechanism by which sphingolipids accumulate in NPC is equally elusive at this point. On a cellular level, it is apparent that the accumulation of sphingolipids, and sphingosine in particular, results in a wide disruption of signaling events mediated by S1P and calcium. Importantly, these disruptions affect vital cellular processes, including vesicular fusion events, autophagy, and cellular survival. Beyond the role that sphingolipid accumulation plays in the complex biology of NPC disease, mass spectrometry evaluation of the disruption of sphingolipid metabolism offers some potential diagnostic value that may eventually lead to increased diagnostic accuracy and perhaps earlier intervention.
Acknowledgements
This work was supported by NIH grants K99HD096117 (J. Newton) and 2R01GM043880 (S. Spiegel).
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
References
- Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS, 2009. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. U. S. A 106 (46), 19316–19321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar A, Saba JD, 2012. Truth and consequences of sphingosine-1-phosphate lyase. Adv. Biol. Regul 52 (1), 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benussi L, Ghidoni R, Dal Piaz F, Binetti G, Di Iorio G, Abrescia P, 2017. The level of 24-hydroxycholesteryl esters is an early marker of Alzheimer’s disease. J Alzheimers Dis 56 (2), 825–833. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Diczfalusy U, Lovgren-Sandblom A, Starck L, Jonsson M, Tallman K, Schirmer H, Ousager LB, Crick PJ, Wang Y, Griffiths WJ, Guengerich FP, 2014. On the formation of 7-ketocholesterol from 7-dehydrocholesterol in patients with CTX and SLO. J. Lipid Res 55 (6), 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Mock MB, Fredrickson DS, 1966. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc. Natl. Acad. Sci. U. S. A 55 (2), 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JD, Comly ME, Kruth HS, Vanier M, Filling-Katz M, Fink J, Barton N, Weintroub H, Quirk JM, Tokoro T, et al. , 1987Niemann-pick variant disorders: comparison of errors of cellular cholesterol homeostasis in group D and group C fibroblasts. Proc. Natl. Acad. Sci. U. S. A 84 (2), 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA, 1997. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277 (5323), 228–231. [DOI] [PubMed] [Google Scholar]
- Claas RF, ter Braak M, Hegen B, Hardel V, Angioni C, Schmidt H, Jakobs KH, Van Veldhoven PP, zu Heringdorf DM, 2010. Enhanced Ca2+ storage in sphingosine-1-phosphate lyase-deficient fibroblasts. Cell. Signal 22 (3), 476–483. [DOI] [PubMed] [Google Scholar]
- Coant N, Sakamoto W, Mao C, Hannun YA, 2017. Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 63, 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozma C, Iurascu MI, Eichler S, Hovakimyan M, Brandau O, Zielke S, Bottcher T, Giese AK, Lukas J, Rolfs A, 2017. C26-Ceramide as highly sensitive biomarker for the diagnosis of Farber Disease. Sci. Rep 7 (1), 6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Arcangelo G, Grossi D, Racaniello M, Cardinale A, Zaratti A, Rufini S, Cutarelli A, Tancredi V, Merlo D, Frank C, 2016. Miglustat reverts the impairment of synaptic plasticity in a mouse model of NPC disease. Neural Plast 2016, 3830424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU, 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One 4 (9) e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Natale C, Monaco A, Pedone C, Tessitore A, De Mase A, Tedeschi G, Netti PA, Abrescia P, 2018. The level of 24-hydroxycholesteryl esters decreases in plasma of patients with Parkinson’s disease. Neurosci. Lett 672, 108–112. [DOI] [PubMed] [Google Scholar]
- Erickson RP, Garver WS, Camargo F, Hossain GS, Heidenreich RA, 2000. Pharmacological and genetic modifications of somatic cholesterol do not sub-stantially alter the course of CNS disease in Niemann-Pick C mice. J. Inherit. Metab. Dis 23 (1), 54–62. [DOI] [PubMed] [Google Scholar]
- Fan M, Sidhu R, Fujiwara H, Tortelli B, Zhang J, Davidson C, Walkley SU, Bagel JH, Vite C, Yanjanin NM, Porter FD, Schaffer JE, Ory DS, 2013. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J. Lipid Res 54 (10), 2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A, Annunziata F, Lombardi A, Kaiser HJ, Medina DL, Spampanato C, Fedele AO, Polishchuk R, Sorrentino NC, Simons K, Ballabio A, 2010. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J 29 (21), 3607–3620. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Geffken K, Spiegel S, 2018. Sphingosine kinase 1 in breast cancer. Adv Biol Regul 67, 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin E, Roff CF, Miller SP, Rodriguez-Lafrasse C, Vanier MT, Brady RO, Pentchev PG, 1992. Type C Niemann-Pick disease: a murine model of the lysosomal cholesterol lipidosis accumulates sphingosine and sphinganine in liver. Biochim. Biophys. Acta 1127 (3), 303–311. [DOI] [PubMed] [Google Scholar]
- Grassi S, Chiricozzi E, Mauri L, Sonnino S, Prinetti A, 2018. Sphingolipids and neuronal degeneration in lysosomal storage disorders. J. Neurochem 10.1111/jnc.14540. [DOI] [PubMed]
- Hannun YA, Obeid LM, 2018. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol 19 (3), 175–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron B, Valayannopoulos V, Baruteau J, Chabrol B, Ogier H, Latour P, Dobbelaere D, Eyer D, Labarthe F, Maurey H, Cuisset JM, de Villemeur TB, Sedel F, Vanier MT, 2012. Miglustat therapy in the French cohort of paediatric patients with Niemann-Pick disease type C. Orphanet J. Rare Dis 7, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Haberkant P, Aguilera-Romero A, Riezman H, Porter FD, Platt FM, Galione A, Schultz C, 2015. Intracellular sphingosine releases calcium from lysosomes. Elife 4, e10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Nadler A, Haberkant P, Kirkpatrick J, Schifferer M, Stein F, Hauke S, Porter FD, Schultz C, 2017. Trifunctional lipid probes for comprehensive studies of single lipid species in living cells. Proc. Natl. Acad. Sci. U. S. A 114 (7), 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Sidhu R, Mydock-McGrane L, Hsu FF, Covey DF, Scherrer DE, Earley B, Gale SE, Farhat NY, Porter FD, Dietzen DJ, Orsini JJ, Berry-Kravis E, Zhang X, Reunert J, Marquardt T, Runz H, Giugliani R, Schaffer JE, Ory DS, 2016. Development of a bile acid-based newborn screen for Niemann-Pick disease type C. Sci. Transl. Med 8 (337) 337ra363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Sidhu R, Porter FD, Yanjanin NM, Speak AO, te Vruchte DT, Platt FM, Fujiwara H, Scherrer DE, Zhang J, Dietzen DJ, Schaffer JE, Ory DS, 2011. A sensitive and specific LC-MS/MS method for rapid diagnosis of Niemann-Pick C1 disease from human plasma. J. Lipid Res 52 (7), 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Yamaguchi S, Lyons CW, Muallem S, 2010. Aberrant Ca2+ handling in lysosomal storage disorders. Cell Calcium 47 (2), 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke G, Rohrbach M, Giugliani R, Burda P, Baumgartner MR, Tran C, Gautschi M, Mathis D, Hersberger M, 2015. LC-MS/MS based assay and reference intervals in children and adolescents for oxysterols elevated in Niemann-Pick diseases. Clin. Biochem 48 (9), 596–602. [DOI] [PubMed] [Google Scholar]
- Kuchar L, Sikora J, Gulinello ME, Poupetova H, Lugowska A, Malinova V, Jahnova H, Asfaw B, Ledvinova J, 2017. Quantitation of plasmatic lyso-sphingomyelin and lysosphingomyelin-509 for differential screening of Niemann-Pick A/B and C diseases. Anal. Biochem 525, 73–77. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE, 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell 137 (7), 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann RH, te Vruchte D, Lloyd-Evans E, Reinkensmeier G, Sillence DJ, Fernandez-Guillen L, Dwek RA, Butters TD, Cox TM, Platt FM, 2004. Treatment with miglustat reverses the lipid-trafficking defect in Niemann-Pick disease type C. Neurobiol. Dis 16 (3), 654–658. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee JK, Park MH, Hong YR, Marti HH, Kim H, Okada Y, Otsu M, Seo EJ, Park JH, Bae JH, Okino N, He X, Schuchman EH, Bae JS, Jin HK, 2014. Pathological roles of the VEGF/SphK pathway in Niemann-Pick type C neurons. Nat. Commun 5, 5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Saha P, Li J, Blobel G, Pfeffer SR, 2016a. Clues to the mechanism of cholesterol transfer from the structure of NPC1 middle lumenal domain bound to NPC2. Proc. Natl. Acad. Sci. U. S. A 113 (36), 10079–10084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang J, Coutavas E, Shi H, Hao Q, Blobel G, 2016b. Structure of human Niemann-Pick C1 protein. Proc. Natl. Acad. Sci. U. S. A 113 (29), 8212–8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima S, Milstien S, Spiegel S, 2017. Sphingosine and sphingosine kinase 1 involvement in endocytic membrane trafficking. J. Biol. Chem 292 (8), 3074–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM, 2008. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med 14 (11), 1247–1255. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM, 2010. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic 11 (4), 419–428. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Waller-Evans H, Peterneva K, Platt FM, 2010. Endolysosomal calcium regulation and disease. Biochem. Soc. Trans 38 (6), 1458–1464. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, Pavan WJ, 1997. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science 277 (5323), 232–235. [DOI] [PubMed] [Google Scholar]
- Lu F, Liang Q, Abi-Mosleh L, Das A, De Brabander JK, Goldstein JL, Brown MS, 2015. Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife 4, e12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Ballabio A, 2015. Lysosomal calcium regulates autophagy. Autophagy 11 (6), 970–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A, 2015. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol 17 (3), 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, Wastney M, Patel S, Suresh S, Cooney AM, Dwyer NK, Roff CF, Ohno K, Morris JA, Carstea ED, Incardona JP, Strauss JF 3rd, Vanier MT, Patterson MC, Brady RO, Pentchev PG, Blanchette-Mackie EJ, 1999. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem 274 (14), 9627–9635. [DOI] [PubMed] [Google Scholar]
- Newton J, Hait NC, Maceyka M, Colaco A, Maczis M, Wassif CA, Cougnoux A, Porter FD, Milstien S, Platt N, Platt FM, Spiegel S, 2017. FTY720/fingolimod increases NPC1 and NPC2 expression and reduces cholesterol and sphingolipid accumulation in Niemann-Pick type C mutant fibroblasts. Faseb. J 31 (4), 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory DS, Ottinger EA, Farhat NY, King KA, Jiang X, Weissfeld L, Berry-Kravis E, Davidson CD, Bianconi S, Keener LA, Rao R, Soldatos A, Sidhu R, Walters KA, Xu X, Thurm A, Solomon B, Pavan WJ, Machielse BN, Kao M, Silber SA, McKew JC, Brewer CC, Vite CH, Walkley SU, Austin CP, Porter FD, 2017. Intrathecal 2-hydroxypropyl-beta-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: a non-randomised, open-label, phase 1–2 trial. Lancet 1758–1768. [DOI] [PMC free article] [PubMed]
- Park WD, O’Brien JF, Lundquist PA, Kraft DL, Vockley CW, Karnes PS, Patterson MC, Snow K, 2003. Identification of 58 novel mutations in Niemann-Pick disease type C: correlation with biochemical phenotype and importance of PTC1-like domains in NPC1. Hum. Mutat 22 (4), 313–325. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R, 2010. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol 20 (5), 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentchev PG, Comly ME, Kruth HS, Patel S, Proestel M, Weintroub H, 1986a. The cholesterol storage disorder of the mutant BALB/c mouse. A primary genetic lesion closely linked to defective esterification of exogenously derived cholesterol and its relationship to human type C Niemann-Pick disease. J. Biol. Chem 261 (6), 2772–2777. [PubMed] [Google Scholar]
- Pentchev PG, Kruth HS, Comly ME, Butler JD, Vanier MT, Wenger DA, Patel S, 1986b. Type C Niemann-Pick disease. A parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J. Biol. Chem 261 (35), 16775–16780. [PubMed] [Google Scholar]
- Piraud M, Pettazzoni M, Lavoie P, Ruet S, Pagan C, Cheillan D, Latour P, Vianey-Saban C, Auray-Blais C, Froissart R, 2018. Contribution of tandem mass spectrometry to the diagnosis of lysosomal storage disorders. J. Inherit. Metab. Dis 41 (3), 457–477. [DOI] [PubMed] [Google Scholar]
- Platt FM, 2014. Sphingolipid lysosomal storage disorders. Nature 510 (7503), 68–75. [DOI] [PubMed] [Google Scholar]
- Porter FD, Scherrer DE, Lanier MH, Langmade SJ, Molugu V, Gale SE, Olzeski D, Sidhu R, Dietzen DJ, Fu R, Wassif CA, Yanjanin NM, Marso SP, House J, Vite C, Schaffer JE, Ory DS, 2010. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci. Transl. Med 2 (56), 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, McNaughton M, Boomkamp S, MacRitchie N, Evangelisti C, Martelli AM, Jiang HR, Ubhi S, Pyne S, 2016. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul 60, 151–159. [DOI] [PubMed] [Google Scholar]
- Reunert J, Fobker M, Kannenberg F, Du Chesne I, Plate M, Wellhausen J, Rust S, Marquardt T, 2016. Rapid diagnosis of 83 patients with Niemann pick type C disease and related cholesterol transport disorders by cholestantriol screening. EBioMedicine 4, 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Rousson R, Pentchev PG, Louisot P, Vanier MT, 1994. Free sphingoid bases in tissues from patients with type C Niemann-Pick disease and other lysosomal storage disorders. Biochim. Biophys. Acta 1226 (2), 138–144. [DOI] [PubMed] [Google Scholar]
- Rusconi B, Jiang X, Sidhu R, Ory DS, Warner BB, Tarr PI, 2018. Gut sphingolipid composition as a prelude to necrotizing enterocolitis. Sci. Rep 8 (1), 10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvioli R, Scarpa S, Ciaffoni F, Tatti M, Ramoni C, Vanier MT, Vaccaro AM, 2004. Glucosylceramidase mass and subcellular localization are modulated by cholesterol in Niemann-Pick disease type C. J. Biol. Chem 279 (17), 17674–17680. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Carroll B, Buganim Y, Maetzel D, Ng AH, Cassady JP, Cohen MA, Chakraborty S, Wang H, Spooner E, Ploegh H, Gsponer J, Korolchuk VI, Jaenisch R, 2013. Impaired autophagy in the lipid-storage disorder Niemann-Pick type C1 disease. Cell Rep 5 (5), 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Maetzel D, Korolchuk VI, Jaenisch R, 2014. Restarting stalled autophagy a potential therapeutic approach for the lipid storage disorder, Niemann-Pick type C1 disease. Autophagy 10 (6), 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Giordano F, Wu Y, Chan J, Zhu C, Milosevic I, Wu X, Yao K, Chen B, Baumgart T, Sieburth D, De Camilli P, 2014. Coupling between endocytosis and sphingosine kinase 1 recruitment. Nat. Cell Biol 16 (7), 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu R, Jiang H, Farhat NY, Carrillo-Carrasco N, Woolery M, Ottinger E, Porter FD, Schaffer JE, Ory DS, Jiang X, 2015. A validated LC-MS/MS assay for quantification of 24(S)-hydroxycholesterol in plasma and cerebrospinal fluid. J. Lipid Res 56 (6), 1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol J, Blanchette-Mackie J, Kruth HS, Dwyer NK, Amende LM, Butler JD, Robinson E, Patel S, Brady RO, Comly ME, et al. , 1988. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J. Biol. Chem 263 (7), 3411–3417. [PubMed] [Google Scholar]
- Stein VM, Crooks A, Ding W, Prociuk M, O’Donnell P, Bryan C, Sikora T, Dingemanse J, Vanier MT, Walkley SU, Vite CH, 2012. Miglustat improves purkinje cell survival and alters microglial phenotype in feline Niemann-Pick disease type C. J. Neuropathol. Exp. Neurol 71 (5), 434–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Ninomiya H, Ohsaki Y, Higaki K, Davies JP, Ioannou YA, Ohno K, 2001. Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells. Proc. Natl. Acad. Sci. U. S. A 98 (22), 12391–12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Vruchte D, Lloyd-Evans E, Veldman RJ, Neville DC, Dwek RA, Platt FM, van Blitterswijk WJ, Sillence DJ, 2004. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J. Biol. Chem 279 (25), 26167–26175. [DOI] [PubMed] [Google Scholar]
- te Vruchte D, Speak AO, Wallom KL, Al Eisa N, Smith DA, Hendriksz CJ, Simmons L, Lachmann RH, Cousins A, Hartung R, Mengel E, Runz H, Beck M, Amraoui Y, Imrie J, Jacklin E, Riddick K, Yanjanin NM, Wassif CA, Rolfs A, Rimmele F, Wright N, Taylor C, Ramaswami U, Cox TM, Hastings C, Jiang X, Sidhu R, Ory DS, Arias B, Jeyakumar M, Sillence DJ, Wraith JE, Porter FD, Cortina-Borja M, Platt FM, 2014. Relative acidic com-partment volume as a lysosomal storage disorder-associated biomarker. J. Clin. Invest 124 (3), 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharkeshwar AK, Trekker J, Vermeire W, Pauwels J, Sannerud R, Priestman DA, Te Vruchte D, Vints K, Baatsen P, Decuypere JP, Lu H, Martin S, Vangheluwe P, Swinnen JV, Lagae L, Impens F, Platt FM, Gevaert K, Annaert W, 2017. A novel approach to analyze lysosomal dysfunctions through subcellular proteomics and lipidomics: the case of NPC1 deficiency. Sci. Rep 7, 41408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Song F, 2015. Intracellular calcium signaling regulates autophagy via calcineurin-mediated TFEB dephosphorylation. Autophagy 11 (7), 1192–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Lisman Q, 2002. Sphingolipid transport: rafts and translocators. J. Biol. Chem 277 (29), 25855–25858. [DOI] [PubMed] [Google Scholar]
- Vanier MT, 2015. Complex lipid trafficking in Niemann-Pick disease type C. J. Inherit. Metab. Dis 38 (1), 187–199. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Gissen P, Bauer P, Coll MJ, Burlina A, Hendriksz CJ, Latour P, Goizet C, Welford RW, Marquardt T, Kolb SA, 2016. Diagnostic tests for Niemann-Pick disease type C (NP-C): a critical review. Mol. Genet. Metabol 118 (4), 244–254. [DOI] [PubMed] [Google Scholar]
- Vanier MT, Revol A, Fichet M, 1980. Sphingomyelinase activities of various human tissues in control subjects and in Niemann-Pick disease - development and evaluation of a microprocedure. Clin. Chim. Acta 106 (3), 257–267. [DOI] [PubMed] [Google Scholar]
- Vienken H, Mabrouki N, Grabau K, Claas RF, Rudowski A, Schomel N, Pfeilschifter J, Lutjohann D, van Echten-Deckert G, Meyer Zu Heringdorf D, 2017. Characterization of cholesterol homeostasis in sphingosine-1-phosphate lyase-deficient fibroblasts reveals a Niemann-Pick disease type C-like phenotype with enhanced lysosomal Ca(2+) storage. Sci. Rep 7, 43575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitner EB, Platt FM, Futerman AH, 2010. Common and uncommon pathogenic cascades in lysosomal storage diseases. J. Biol. Chem 285 (27), 20423–20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL, 2010. Identification of surface residues on Niemann-Pick C2 essential for hydrophobic handoff of cholesterol to NPC1 in lysosomes. Cell Metabol 12 (2), 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford RW, Garzotti M, Marques Lourenco C, Mengel E, Marquardt T, Reunert J, Amraoui Y, Kolb SA, Morand O, Groenen P, 2014. Plasma lyso-sphingomyelin demonstrates great potential as a diagnostic biomarker for Niemann-Pick disease type C in a retrospective study. PLoS One 9 (12), e114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Brown MS, Shelton JM, Richardson JA, Goldstein JL, Liang G, 2011. Amino acid substitution in NPC1 that abolishes cholesterol binding reproduces phenotype of complete NPC1 deficiency in mice. Proc. Natl. Acad. Sci. U. S. A 108 (37), 15330–15335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Tokoro T, Eto Y, 1994. The attenuated elevation of cytoplasmic calcium concentration following the uptake of low density lipoprotein in type C Niemann-Pick fibroblasts. Biochem. Biophys. Res. Commun 198 (2), 438–444. [DOI] [PubMed] [Google Scholar]
- Young MM, Takahashi Y, Fox TE, Yun JK, Kester M, Wang HG, 2016. Sphingosine kinase 1 cooperates with autophagy to maintain endocytic membrane trafficking. Cell Rep 17 (6), 1532–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M, Somers KL, Thrall MA, Walkley SU, 2001. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol 11 (16), 1283–1287. [DOI] [PubMed] [Google Scholar]