Retinopathy of prematurity (ROP) is characterized by abnormal vascular development in the retinal periphery.1,2 Here we report macular microvascular anomalies visualized in two infants with treated ROP using an investigational portable optical coherence tomography angiography (OCT-A) system (Spectralis HRA+OCT with Flex and OCT-A module, Heidelberg Engineering, Heidelberg, Germany) with age-appropriate manual segmentation3.
Report of Cases
A Caucasian female born at 24 weeks gestational age (GA) (birth weight 410g) had intravitreal bevacizumab injection in both eyes (35 weeks postmenstrual age, PMA) for Zone II stage 3 ROP, subsequent laser photocoagulation of avascular retina in both eyes, and later vitreoretinal surgery in the right eye for vitreous hemorrhage. Examination under anesthesia (EUA) was performed at 73 weeks PMA. Research OCT-A imaging of the left eye (Zone II stage 3 regressed) showed absence of the foveal avascular zone (FAZ) associated with lack of the foveal pit on the structural OCT scans4. The superficial vascular complex (SVC), which we defined as flow signal between the internal limiting membrane (ILM) and the lower boundary of the inner plexiform layer (IPL), showed an irregular, angular vascular pattern with several large vessels diving into the inner nuclear layer, an area considered deep vascular complex (DVC) territory (Figure 1).
Figure 1.
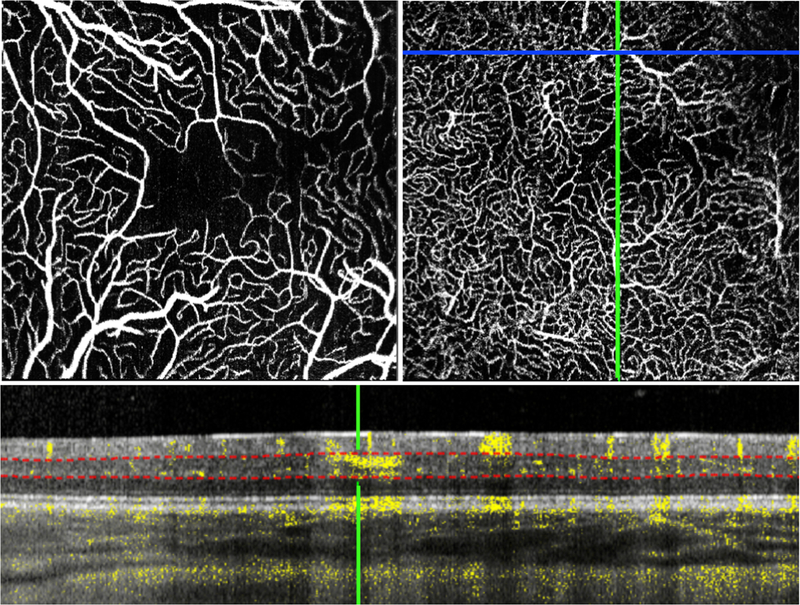
The macula of left eye of an infant with stage 3 ROP, regressed after bevacizumab treatment, imaged at 73 weeks postmenstrual age showed an irregular angular vessel pattern with several large vessels in the superficial vascular complex (A) diving down into the deeper layers of the retina (examples circled in red in B and C). A B-scan shows one of these diving vessels (red arrow at the green crosshair) penetrating into the inner nuclear layer. Red dotted lines indicate the manually placed segmentation boundaries for the DVC.
A Caucasian male born at 24 weeks GA (birth weight 610g) had prior laser photocoagulation for Zone II stage 3 ROP (37 weeks PMA) and developed Zone II stage 4A ROP with plus disease in both eyes at 40 weeks PMA. Structural OCT in the left eye during EUA showed attached macula with a central foveal cystoid space, the boundaries of which corresponded to a small FAZ. OCT-A showed a developed SVC with vessels that appeared to be located deeper than expected within the boundaries that define the SVC, as well as a lack of flow across the corresponding central macula in the DVC (Figure 2).
Figure 2.
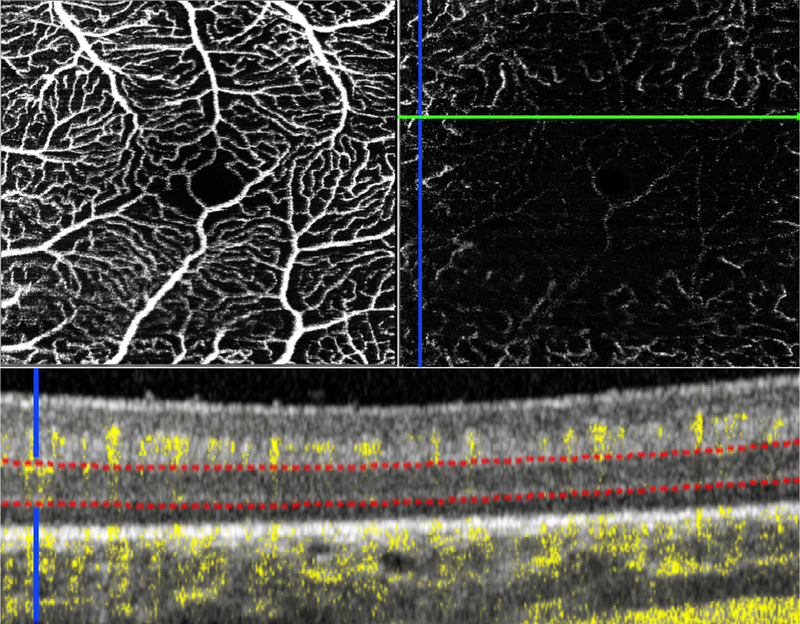
The macula of the left eye of an infant with ROP imaged at 40 weeks postmenstrual age showed a small foveal avascular zone on the en face OCT-A image of the SVC (A) and lack of central macular flow in the DVC (B). A cross-sectional B-scan in the superior perifoveal region (green line on the en face OCT-A image) showed flow signal at the level of DVC only in the peripheral perifoveal region (blue crosshair and upper 1/4 of the DVC in B), and otherwise absence of flow signal in the inner nuclear layer. Red dotted lines indicate the boundaries used to generate the OCT-A image of the DVC. Note the location of the superficial vasculature is deeper than observed in mature retinas; orange brackets highlight the relatively avascular ganglion cell layer.
Discussion
Here we report depth-resolved macular microvascular anomalies in two eyes after treatment of type I ROP: (1) Violation of vascular stratification, with large superficial vessels diving from IPL into INL; (2) Deeper SVC vessels located closer to the IPL associated with (3) incomplete perifoveal DVC development. These findings, not previously reported in children or adults with history of ROP (PubMed search on March 13, 2018: “retinopathy of prematurity” and “optical coherence tomography angiography”), suggest that the retinal perifoveal microvasculature may undergo an active developmental and remodeling process during the critical postnatal period of ROP pathogenesis. Of note, these findings are based on a single imaging session each in two infants, both of whom received ROP treatments prior to imaging. Thus, it is difficult to judge if the incomplete development of the DVC relative to the SVC and the deeper SVC localization are physiological, pathological, or related to treatment or perfusion status, especially with a lack of age-matched controls.
Depth-resolved visualization using OCT-A provides an opportunity to observe the development of perifoveal microvasculature in detail. However, as the overall thickness of the developing retina in infants is different from the adult retina3,5, and the developmental timeline and location of the superficial and deep vascular layers have yet to be explored, the segmentation criteria of SVC and DVC in infants would seem to warrant further study. Further analysis on a larger sample size is needed in order to determine the ideal vascular boundaries for premature infants.
The advent of supine retinal imaging and OCT-A has allowed visualization of vascular details not seen on traditional table-top imaging2,6. The findings in this report may guide future research of human retinal vascular development, and alert us to macular changes in infants with ROP.
Acknowledgements
Funding for this research was provided by the International Association of Government Officials (iGO) Fund (L.V.), Research to Prevent Blindness Unrestricted Grant to Duke Eye Center, Research to Prevent Blindness Career Development Award (X.C.), NIH (RO1 EY25009, P30EY005722), NEI (K23EY028227), and Lions Duke Pediatric Eye Research Endowment. The research equipment (Spectralis with Flex and OCT-A modules) was provided by Heidelberg. None of the above organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, of the manuscript; and decision to submit the manuscript for publication. Heidelberg reviewed and approved the manuscript.
Footnotes
Interest disclosures:
STH, XC, RH, MPK – none
CAT - Alcon (Royalties), Genentech (Recipient)
LV - Knights Templar Eye Foundation (Recipient); PDC’s ENABLE Award (Recipient); Janssen Pharmaceutical (Recipient); Roche (Recipient); DORC (Recipient); Second Sight (Recipient); Alcon (Recipient); Genentech (Recipient)
References
- 1.Campbell JP, Nudleman E, Yang J, et al. Handheld Optical Coherence Tomography Angiography and Ultra-Wide-Field Optical Coherence Tomography in Retinopathy of Prematurity. JAMA ophthalmology 2017;135(9):977–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinekar A, Chidambara L, Jayadev C, Sivakumar M, Webers CA, Shetty B. Monitoring neovascularization in aggressive posterior retinopathy of prematurity using optical coherence tomography angiography. J AAPOS 2016;20(3):271–274. [DOI] [PubMed] [Google Scholar]
- 3.Vajzovic L, Hendrickson AE, O’Connell RV, et al. Maturation of the human fovea: correlation of spectral-domain optical coherence tomography findings with histology. Am J Ophthalmol 2012;154(5):779–789.e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falavarjani KG, Iafe NA, Velez FG, et al. Optical Coherence Tomography Angiography of the Fovea in Children Born Preterm. Retina 2017;37(12):2289–2294. [DOI] [PubMed] [Google Scholar]
- 5.Hendrickson A, Possin D, Vajzovic L, Toth CA. Histologic Development of the Human Fovea From Midgestation to Maturity. Am J Ophthalmol 154(5):767–778.e762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Viehland C, Carrasco-Zevallos OM, et al. Microscope-Integrated Optical Coherence Tomography Angiography in the Operating Room in Young Children With Retinal Vascular Disease. JAMA ophthalmology 2017;135(5):483–486. [DOI] [PMC free article] [PubMed] [Google Scholar]


