Abstract
Established tumors are complex masses that contain not only neoplastic cells but also nontransformed cellular elements such as stromal cells, the neovasculature, and the full gamut of immune cells. However, evidence suggests that, unlike cells found in lymphoid organs that productively respond to acute infections, immune cells in tumors are dysregulated and functionally impaired. Tumor masses can contain regulatory lymphocytes, myeloidderived suppressor cells, alternatively activated macrophages, and dendritic cells. Ablation or reprogramming of this aberrant microenvironment might dramatically augment cancer therapies, and this strategy is currently being deployed in a variety of clinical trials. A better understanding of the cellular constituents of tumors and the mechanisms involved in immune evasion may help guide the next generation of innovative cancer immunotherapies.
Introduction
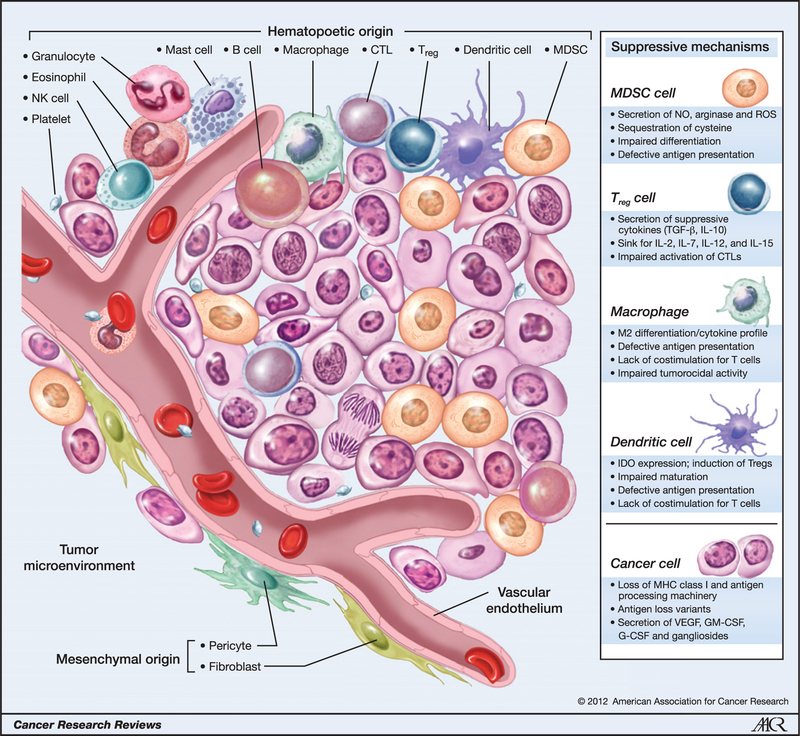
For years, scientists have focused almost exclusively on transformed cells within solid tumor masses and believed that deciphering the biology of these cells could entirely explain tumor formation. Although many cancer biologists retain this view, we now have considerable evidence that nonneoplastic host elements, such as mesenchymal-derived cells and cellular components of the vascular and immune systems, contribute substantially to carcinogenesis, tumor progression, and the metastases of transformed cells (1–9). These cells form critical components of a stromal network that fosters neovascularization and provide optimal cytokine and inflammatory support to drive the proliferation of transformed cells into solid masses (10–12). The cellular constituents of a tumor can include immune cells that are normally found in secondary lymphoid organs, and studies have provided clear evidence of infiltrating lymphocytes, natural killer (NK) cells, macrophages, dendritic cells (DC), eosinophils, mast cells, and immature myeloid cells or myeloid-derived suppressor cells (MDSC) in both murine and human tumors (Fig. 1; refs. 6, 7, 13). In contrast to the case of a productive immune response to a pathogen, immune cells that reside within tumors are dysregulated and functionally impaired. The complex interactions among the various cell types in the tumor microenvironment are only now beginning to be fully understood as an emerging hallmark of cancer (7). In addition, the plasticity of cells within tumors is now being appreciated. For example, evidence now suggests that immature myeloid cells possess the ability to differentiate into macrophages, DCs, and endothelial cells in response to strong proangiogenic stimuli induced by tumor cells (14–17). Furthermore, infiltrating immune cells become alternatively activated with a perturbed phenotype and a functional profile, creating an environment that is conducive to T-cell suppression (2). Growing evidence now suggests that even targeted agents and chemotherapies require an endogenous immune response to induce tumor regression (18, 19). In this review, we describe the immunologic constituents of the tumor microenvironment, define the mechanisms that contribute to escape of tumors from immune recognition, and discuss therapeutic interventions to alter the tightly coordinated regulatory networks within tumors.
Figure 1.
Cellular infiltrates within the tumor microenvironment. Established cancers consist of a wide array of immune cells that contribute to the tumor stroma of a growing malignancy. Tumors possess infiltrating cells of both innate and acquired immunity, such as MDSCs, macrophages, DCs, mast cells, eosinophils, neutrophils, NK cells, and lymphocytes. These cells coordinately form a complex regulatory network that fosters tumor growth by creating an environment that enables cancers to evade immune surveillance and destruction. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; NO, nitric oxide; ROS, reactive oxygen species.
T-Regulatory Lymphocytes
The initial observation that populations of T cells possess the ability to suppress immune responses was made more than 30 years ago (20, 21). Subsequently, these T-regulatory cells (TReg) were rediscovered, and the importance of CD4+ CD25+ TRegs for inhibiting antitumor immunity was established in a series of experiments by multiple groups showing that CD25+ T-cell depletion significantly improved antitumor immunity in different mouse models (22–24). More recently, studies showed that the transcription factor forkhead box P3 (Foxp3) is critical for the development of the functional characteristics of TRegs (25, 26). We now have an abundance of data from studies in mice and humans that show an increase in the frequency of TRegs both in the periphery and within tumors from cancers of different histologies, including melanomas, lung cancers, esophageal cancers, breast cancers, ovarian cancers, gastric cancers, colorectal cancers, and lymphomas (27, 28).
Several mechanisms for TRegs-mediated immune evasion have been reported. Of importance, the secretion of TGF-β and interleukin-10 (IL-10) by TRegs in tumors helps create an immunosuppressive environment that blunts antitumor effector responses by CD4+, CD8+, and NK cells. TRegs also express a heterotrimeric receptor (CD25: α-chain; CD122: β-chain; and CD132: γ-chain) that has a 100-fold higher affinity for IL-2 than the dimeric form lacking the CD25 α-chain (29–31). This functional difference likely results in TRegs acting as competitive sinks for IL-2 within the tumor microenvironment (29, 30). Furthermore, it is likely that they function as sinks for other important homeostatic and antitumor cytokines, such as IL-7, IL-12, and IL-15 (32).
TRegs also express several surface molecules (e.g., CTLA4, PD-1-L, and GITR-L) that interact with their respective ligands (CD80/CD86, PD-1-L, and GITR-L) on antigen-presenting cells. Cross-talk between these cell types within tumors likely maintains and propagates a coordinated suppressive and tolerogenic environment (27, 28). In various murine tumor models and more recently in human clinical trials (33, 34), antibodies that were designed to inhibit CTLA-4– and PD-1–mediated signaling showed the ability to induce endogenous antitumor immunity that resulted in the regression of established cancers. However, the mechanistic link between CTLA-4 and PD-1 blockade and altered TReg function in humans still remains to be fully understood and may help guide future combination-based immunotherapies.
Regulatory Myeloid Cells
It is now well established that subpopulations of myeloid cells are able to inhibit immune responses to cancer using a variety of mechanisms. Pioneering work describing the inhibitory properties of myeloid cells in cancer was published more than 2 decades ago by groups led by Diana Lopez and Rita Young (35–38). Myeloid-derived cells represent numerous distinct and heterogeneous populations of cells of hematopoietic origin (39). The marker CD11b (also known as αM-integrin) represents an important myeloid cell lineage differentiation antigen. Collectively, myeloid cells create a chronic inflammatory environment and are critical mediators of tumor initiation, angiogenesis, and metastasis (11, 12, 40, 41). They also contribute to the formation of the tumor stroma and shape the immune environment in expanding tumor masses (13, 40). Important myeloid cell subsets that contribute to immune dysfunction within the tumor stroma include MDSCs, macrophages, and DCs.
MDSCs
MDSCs are classically described as a population of CD11b+ Gr-1+ cells in tumor-bearing mice with demonstrated abilities to suppress CD8+ T-cell antitumor immunity (35, 42). It was discovered early on that granulocyte-macrophage colonystimulating factor (GM-CSF) is a key factor in driving the expansion of these cells (43). Subsequent studies have shown that MDSCs are a heterogeneous population of cells consisting of CD11b+ Ly6Chigh Ly6Glow cells of monocytic origin and CD11b+ Ly6Ghigh Ly6Clow cells of granulocytic origin (44). It is important to note that the Gr-1 antibody recognizes both Ly6C and Ly6G, and it is possible that cells that stain bright or high for Gr-1 represent a population of cells distinct from those that show less intense or Gr-1 intermediate staining for MDSCs. In humans, the markers for MDSCs are not clearly defined, due in large part to the lack of a Gr-1 homolog. On the basis of early studies, it is thought that the phenotype for human MDSCs likely resides within a population of LIN−HLA-DR−CD33+ cells (44).
MDSCs bear the same markers as nonsuppressive myeloid cells that are found under normal physiologic conditions in hosts. However, in tumor-bearing mice and patients with cancer, myeloid cells are induced into a suppressive state and found with altered differentiation profiles that resemble an immature phenotype (14, 35, 45, 46). These immature myeloid cells possess the capacity to differentiate into macrophages, DCs, and neutrophils (44). Furthermore, they display plasticity between different lineages, as studies have highlighted the ability of CD11b+ Gr-1+ MDSCs to differentiate into CD31+ endothelial cells (17).
The multiple mechanisms by which myeloid cells mediate immune evasion are being elucidated in mouse models. Most studies have highlighted the requirement for direct cell–cell contact for MDSCs to mediate T-cell suppression, suggesting that short-lived mediators or direct cross-talk between cells induces immune dysfunction. It is now well accepted that MDSCs express high levels of inducible nitric oxide synthase and arginase 1 (14, 44). The production of nitric oxide imparts a direct suppressive role to T cells by the induction of T-cell apoptosis, inhibition of STAT5 signaling, and the formation of peroxynitrite, a potent oxidant that induces nitration and nitrosylation of amino acids that are essential for T-cell function (14, 44). The increased activity and production of arginase 1 depletes L-arginine from the tumor microenvironment and impairs the local proliferative capacity of T cells (47). Other mechanisms of MDSC-mediated immune suppression that have been described include the sequestration of cysteine leading to the limited availability of this essential amino acid for T cells, the secretion of suppressive cytokines (e.g., IL-10), and the overproduction of reactive oxygen species (48). Of interest, recent work also suggested that MDSCs have the ability to skew the differentiation of CD4+ T cells into TRegs (49), although further studies are needed to show a direct immunoregulatory link. Taken together, these studies reveal that MDSCs facilitate tumor growth not only by producing proangiogenic factors but also by employing a myriad of immunosuppressive mechanisms that blunt effector T-cell responses.
Alternatively activated macrophages
Although much of the focus on the suppressive capacity of myeloid cells is directed toward MDSCs, macrophages also play a crucial role in immune evasion within tumors. These stromal cells, which are marked by the expression of CD11b and F4/80 in mice and CD11b, CD14, CD33, and CD68 in humans, are classically described as being skewed toward an M2-altered functional profile (2, 16, 41). M2-polarized macrophages produce lower levels of proinflammatory cytokines, such as IL-1β, TNF-α, and IL-12, and higher levels of immunosuppressive mediators, such as IL-10, TGF-β, and VEGF (2, 16, 41). Macrophages also have the inherent ability to present antigen to T lymphocytes and provide costimulatory support under the right environment. This ability to cultivate an adaptive immune response is perturbed within the tumor microenvironment and likely plays a role in the shutdown of Tcell–mediated immunity.
Dysfunctional macrophages are also stunted in their ability to mediate direct lysis of malignant cells. Studies have shown that following activation under inflammatory conditions, macrophages can mediate direct cytotoxicity against malignant cells, likely through soluble mediators such as TNF-α, oxygen radicals, and matrix metalloproteinases (50). Thus, devising strategies to reeducate tumor-infiltrating macrophages into an activated M1-type cell may improve both direct killing and indirect licensing of adaptive antitumor immune responses (51).
Functionally impaired DCs
DCs are often described as professional antigen-presenting cells because of their ability to robustly license T cells in secondary lymphoid organs (52). However, in the setting of cancer, DCs in close contact with malignant cells develop functional impairments and fail to prime T cells to the same degree as DCs residing within lymphoid organs under nonpathologic conditions (15, 36). Several tumor-derived mediators, such as VEGF, macrophage CSF (M-CSF), GM-CSF, IL-6, IL-10, and gangliosides, have been reported to contribute to the altered differentiation of DCs (53, 54). These immature DCs often express no or low levels of costimulatory molecules, such as CD40, CD80, and CD86, and have been described to express indoleamine 2,3-dioxygenase, an enzyme that degrades the essential amino acid tryptophan that leads to the suppression of T-cell immunity (55). Tumor-associated DCs also possess defects in the machinery to effectively present antigen and downregulate MHC class I and II molecules in addition to genes associated with antigen presentation, such as transporter associated with antigen processing (TAP) and low-molecular-weight proteins (54). Thus, tumors have evolved an intricate network that alters the differentiation of DCs and impairs the ability of these cells to license effective adaptive immune responses against tumor antigens.
Cancer Immunotherapy
In the era of modern medicine, proof of the effectiveness of immune-based treatments against cancer was established with the development of high-dose IL-2 for patients with metastatic renal cell carcinoma and metastatic melanoma (56). Recently, ipilimumab (Yervoy; Bristol-Myers Squibb), a monoclonal antibody targeting CTLA-4, received approval from the U.S. Food and Drug Administration (FDA) for the treatment of patients with metastatic melanoma, representing a major milestone in the immunotherapy of cancer (33, 57). Furthermore, early-phase trials with a monoclonal antibody targeting PD-1 are also showing promising results for patients with metastatic melanoma and colon cancer (34). Both CTLA-4– and PD-1–based therapies target pathways that negatively regulate T-cell function, and recent studies have highlighted the ability of anti-CTLA-4 and antiPD-1 antibodies to synergize and reverse T-cell anergy within tumors by enhancing the local proliferation of effector T cells (58).
Another promising immunotherapy modality involves the adoptive transfer of tumor-infiltrating lymphocytes and T-cell receptor– or chimeric antigen receptor–redirected peripheral blood lymphocytes in patients with metastatic cancer (59–63). In early phase I and II trials for patients with metastatic melanoma, adoptive T-cell therapies were shown to mediate high durable response rates of 50% to 70% when combined with preconditioning regimens designed to ablate suppressive elements of the immune system. The precedence for an FDAapproved cellular therapy was recently set by the development of sipuleucel-T (Provenge; Dendreon Corporation), a cancer vaccine that requires the transfer of autologous ex vivo sensitized peripheral blood monocytes in patients with metastatic prostate cancer.
One of the ultimate goals for cancer therapeutics is to administer an i.v. product to patients with widespread metastatic cancer and achieve high local concentrations of antitumor mediators directly at the tumor site. T cells represent the ideal vehicle for delivering such products to the tumor microenvironment because of the exquisite specificity of T-cell receptors for antigens expressed by cells in tumors, and the ability of high-affinity chimeric antigen receptors to recognize surface proteins expressed on malignant or stromal cells. Two-photon in vivo imaging techniques have enabled the visualization of T cells moving through tissues with high instantaneous velocities, with an arrest in migration upon contact with cognate antigen. This understanding of immunobiology, combined with our ability to genetically engineer T cells ex vivo, allows investigators to design tumor-specific T cells that are capable of secreting wide arrays of therapeutic agents.
Therapeutic Use of IL-12 Engineered T Cells
We recently described our ability to treat large, established B16 melanomas with a single dose of 10,000 tumor-specific CD8+ T cells engineered to secrete a functional single-chain IL-12 molecule following a lymphodepleting regimen that reduced the number of intratumoral TRegs (64, 65). Our initial hypothesis was that IL-12 licenses T cells and NK cells to directly kill tumor targets. Surprisingly, however, our experiments revealed that direct ligation of receptors on T cells and NK cells was not necessary, and IL-12 triggered a programmatic change in naturally occurring dysfunctional myeloidderived cells within the tumor microenvironment (51, 66). IL12 induced a cascade of molecular danger signals that sensitized tumor-infiltrating macrophages, DCs, and MDSCs to potently stimulate the activation of CD8+ T cells (66, 67). We next examined the role of IFN-γ, an important downstream mediator of IL-12, and showed that the direct immunologic effects of IFN-γ are necessary to achieve the full therapeutic potential of the treatment.
Several studies have ascribed the benefits of IL-12 and IFN-γ to unmasking the immunogenicity of transformed cells; however, we showed that sensitization of nonmalignant stromal cells by IL-12 and IFN-γ also plays an important role in antitumor immunity (3, 66). The infiltration of T cells into the tumor microenvironment was shown to be completely dependent on the ability of reprogrammed myeloid cells to cross-present antigen naturally present within the tumor (66). We also witnessed a complete reconfiguration of myeloid-derived infiltrates within regressing lesions, suggesting that the cross-presentation of antigens by myeloid-derived cells within tumors enabled the elimination of stromal cells by effector T cells and triggered the collapse of large vascularized lesions. Furthermore, although direct recognition of antigen on cancer cells by CD8+ T cells was not necessary, recognition of cross-presented antigen was critical for induction of tumor regression. Taken together, our studies suggest that IL-12, partly through IFN-γ, reversed suppressive factors within the tumor microenvironment by enabling antigen-presenting cells to effectively license antitumor CD8+ T cell responses.
This concept has formed the basis for clinical trials that are currently accruing patients at the National Cancer Institute, NIH. We now envision adoptively transferring IL-12 engineered T cells specific for tumors across multiple histologies by redirecting naturally occurring tumor-specific cells or TCR/CAR modified cells (68, 69). The use of IL-12 to increase the immunogenicity of tumors may make it possible to treat cancers that have classically been thought to be unresponsive to immune-based treatments.
Conclusions
One of the emerging hallmarks of cancer is the concept that tumor masses are complex structures composed of both malignant and nonmalignant immune cells that support cancer growth and prevent immune destruction (7). This understanding of the cellular constituents of the tumor microenvironment has helped guide the design of powerful T-cell therapies that are capable of causing the regression of large tumor burdens. However, one of the major obstacles facing tumor immunologists today is the need to find appropriate tumor targets. The heterogeneity among different cancer histologies poses a formidable challenge to scientists attempting to devise broadly applicable treatment regimens. An understanding of the immunologic constituents of the tumor stroma may help guide future cancer therapies. All cancers, regardless of their epithelial origin, are inherently infiltrated by stromal cells. These cells may provide a universal target for the treatment of all solid tumors (70, 71).
Acknowledgments
Grant Support
Center for Cancer Research, National Cancer Institute, National Institutes of Health (ZIA BC 010763).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell 2011;19:429–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010;22:231–7. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 2010;70: 5649–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol 2003;82:539–48. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, et al. Cancer and inflammation: promise for biologic therapy. J Immunother 2010;33:335–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougan M, Li D, Neuberg D, Mihm M, Googe P, Wong KK, et al. A dual role for the immune response in a mouse model of inflammationassociated lung cancer. J Clin Invest 2011;121:2436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8: 618–31. [DOI] [PubMed] [Google Scholar]
- 12.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol 2008;18:372–8. [DOI] [PubMed] [Google Scholar]
- 13.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol 2006;18:226–31. [DOI] [PubMed] [Google Scholar]
- 14.Bronte V Myeloid-derived suppressor cells in inflammation: uncovering cell subsets with enhanced immunosuppressive functions. Eur J Immunol 2009;39:2670–2. [DOI] [PubMed] [Google Scholar]
- 15.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest 2006;35:459–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor GrþCD11bþ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004;6:409–21. [DOI] [PubMed] [Google Scholar]
- 18.Balachandran VP, Cavnar MJ, Zeng S, Bamboat ZM, Ocuin LM, Obaid H, et al. Imatinib potentiates antitumor T cell responses in gastrointestinal stromal tumor through the inhibition of Ido. Nat Med 2011; 17:1094–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011;8: 151–60. [DOI] [PubMed] [Google Scholar]
- 20.Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 1970;18:723–37. [PMC free article] [PubMed] [Google Scholar]
- 21.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 1982;155:1063–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antony PA, Restifo NP. CD4 CD25 T regulatory cells, immunotherapy of cancer, and interleukin-2. J Immunother 2005;28:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res 1999;59:3128–33. [PubMed] [Google Scholar]
- 24.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25 CD4 T cells: a common basis between tumor immunity and autoimmunity. J Immunol 1999;163:5211–8. [PubMed] [Google Scholar]
- 25.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 CD25 regulatory T cells. Nat Immunol 2003;4:330–6. [DOI] [PubMed] [Google Scholar]
- 26.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003;299:1057–61. [DOI] [PubMed] [Google Scholar]
- 27.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med 2004;10:900–1. [DOI] [PubMed] [Google Scholar]
- 28.Zou W Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 2006;6:295–307. [DOI] [PubMed] [Google Scholar]
- 29.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol 2003;3:253–7. [DOI] [PubMed] [Google Scholar]
- 30.Fehe´rvari Z, Sakaguchi S. CD4 Tregs and immune control. J Clin Invest 2004;114:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsui S, Ahlers JD, Vortmeyer AO, Terabe M, Tsukui T, Carbone DP, et al. A model for CD8 CTL tumor immunosurveillance and regulation of tumor escape by CD4 T cells through an effect on quality of CTL. J Immunol 1999;163:184–93. [PubMed] [Google Scholar]
- 32.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol 2011;29:4828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, et al. Apoptotic death of CD8 T lymphocytes after immunization: induction of a suppressive population of Mac-1 /Gr-1 cells. J Immunol 1998;161:5313–20. [PMC free article] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 1996;2:1096–103. [DOI] [PubMed] [Google Scholar]
- 37.Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM. Expansion of immunoregulatory macrophages by granulocyte-macrophage colonystimulating factor derived from a murine mammary tumor. Cancer Res 1990;50:227–34. [PubMed] [Google Scholar]
- 38.Young MR, Young ME, Wright MA. Stimulation of immune-suppressive bone marrow cells by colony-stimulating factors. Exp Hematol 1990;18:806–11. [PubMed] [Google Scholar]
- 39.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010;327:656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009;182:4499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 2007;117:1155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci U S A 1995;92:6254–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colonystimulating factor by tumors inhibits CD8 T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999; 162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 44.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8 T cells. J Clin Invest 2006;116: 2777–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother 2001;24:431–46. [DOI] [PubMed] [Google Scholar]
- 47.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010;116:5738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res 2010;70:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1 CD115 immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumorbearing host. Cancer Res 2006;66:1123–31. [DOI] [PubMed] [Google Scholar]
- 50.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 2005;65:3437–46. [DOI] [PubMed] [Google Scholar]
- 51.Chmielewski M, Kopecky C, Hombach AA, Abken H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–706. [DOI] [PubMed] [Google Scholar]
- 52.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- 53.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med 2001;193: 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 2004;4: 941–52. [DOI] [PubMed] [Google Scholar]
- 55.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002;297:1867–70. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg SA. The development of new immunotherapies for the treatment of cancer using interleukin-2. A review. Ann Surg 1988;208:121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011;11:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brenner MK, Heslop HE. Adoptive T cell therapy of cancer. Curr Opin Immunol 2010;22:251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hwu P, Rosenberg SA. The use of gene-modified tumor-infiltrating lymphocytes for cancer therapy. Ann N Y Acad Sci 1994;716:188–97, discussion 197–203. [DOI] [PubMed] [Google Scholar]
- 61.June CH. Principles of adoptive T cell cancer therapy. J Clin Invest 2007;117:1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008;8:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sadelain M T-cell engineering for cancer immunotherapy. Cancer J 2009;15:451–5. [DOI] [PubMed] [Google Scholar]
- 64.Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, et al. Tumor-specific CD8 T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010;70: 6725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther 2011;19:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011;121: 4746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matzinger P. The danger model: a renewed sense of self. Science 2002;296:301–5. [DOI] [PubMed] [Google Scholar]
- 68.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119: 4133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chinnasamy D, Yu Z, Kerkar SP, Zhang L, Morgan RA, Restifo NP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 2012;18:1672–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, et al. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest 2010;120:3953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330: 827–30. [DOI] [PubMed] [Google Scholar]