Abstract
Immunotherapy techniques designed to engage T cells against tumor cells can generate sustained and complete responses in patients whose cancers were resistant to more traditional treatment options. Powering the T cell response – how a T cell gets its energy – plays an important role in the effectiveness of T cell destruction of cancer. Recent work in the field indicates that the modulation of T cell metabolism may allow for improved anti-cancer activity by generating T cells with optimal characteristics for the elimination of tumor cells. In this review, we discuss the key metabolic properties of anti-cancer T cells, along with potential methods to improve T cell immunotherapy through metabolic modulation.
Introduction
The progression of cancer from localized tumor to metastatic disease is associated with reduced patient survival and poor efficacy of further therapeutic interventions [1]. There is currently a lack of therapeutic options to successfully treat metastatic solid tumors with surgery, chemotherapy and radiation strategies often failing to fully arrest disease progression [2, 3]. Increasingly, immunotherapy can be used to treat patients with cancer. This includes the adoptive transfer of naturally-occurring tumor infiltrating lymphocytes (TIL) or genetically-engineered T cells and the use of immune checkpoint inhibitors to boost the function of T cells [4–6•]. Cancer immunotherapy has been successfully utilized to mediate complete and durable clinical responses in patients with several types of cancer including melanoma and acute lymphoblastic leukemia (ALL) [7–9], and is currently being explored as a potential therapeutic strategy in numerous other types of cancer [10]. Recent research has begun to elucidate some of the mechanisms by which T cell mediated cancer immunotherapy works to eliminate disseminated tumor cells and indicates that T cell differentiation status and the metabolic properties of T cells may play an important role in regulating their anti-tumor functionality [11••].
The contextual basis for much of our current understanding of the role of metabolism in regulating tumor immunity is derived from a series of studies on CD8+ T cell differentiation. CD8+ T cells can be divided into subsets such as naïve (TN), stem cell memory (TSCM), central memory (TCM), effector memory (TEM) and terminally differentiated effector cells (TEFF) [12]. Importantly, it has been clearly established that various subsets of T cells have distinct metabolic profiles that regulate function [13, 14]. Interestingly, there is a negative correlation between the degree of differentiation of T cells and their capacity for anti-tumor function [15]. In human patients that have undergone TIL therapy, increased telomere length and CD27 expression in infused T cells have been correlated with improved tumor clearance [16]. Consistent with these findings, the adoptive transfer of fully-differentiated terminal effectors (TEFF) was found to be less effective in controlling tumor growth than utilizing less-differentiated TSCM or TCM subsets in mouse models of large vascularized melanoma [17, 18]. These studies suggest that the acquisition of a fully-differentiated terminal effector phenotype limits the in vivo expansion and survival capacity of T cells following adoptive transfer, which likely limits the effectiveness of their anti-tumor response. Conversely, cells with increased self-renewal potential appear to possess increased therapeutic activity [17, 19].
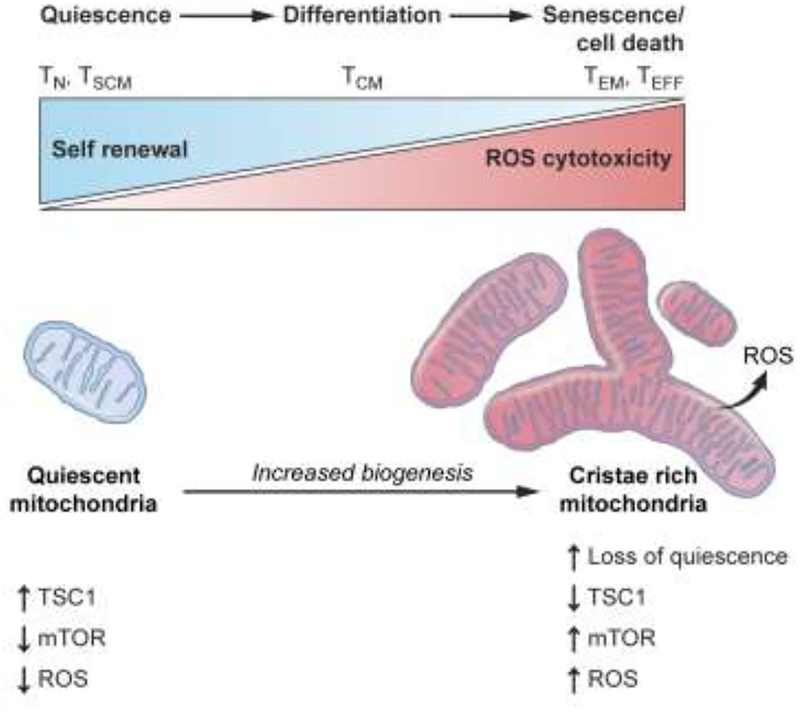
Cellular metabolic processes regulate self-renewal capacity, as evidenced by studies in the settings of hematopoietic stem cells (HSC) and memory [20–22••]. In the HSC setting, increased metabolic activity may directly contribute to the loss of quiescence through the generation of high reactive oxygen species (ROS) levels that can impair long-term self-renewal properties [23–25]. Similarly, the increased mitochondrial metabolism and ROS generation driven by T cell activation is necessary for effector function and proliferation [26, 27] but also may compromise the long-term self-renewal capacity of memory T cell subsets (TSCM and TCM). (Figure 1).
Figure 1. Mitochondrial ROS levels may impair long-term self-renewal program in CD8+ T cells.
T cell activation and differentiation are accompanied by an increase in mTOR activity that results in loss of quiescence and gain of metabolic activity such as increased mitochondrial biogenesis, mitochondrial ROS and oxidative stress. This model includes observations that cellular differentiation, i.e. the acquisition of effector functions are associated with increase in ROS production within mitochondria. Cellular differentiation is accompanied by loss of ‘stemness’ – the capacity of cells to be multipotent and self-renewing. In this model, less-differentiated T cell subsets such as stem cell memory (TSCM) and central memory (TCM) have reduced levels of ROS whereas terminally differentiated effector memory and effector T cells (TEM and TEFF) display increased ROS levels that are required for their effector function such as cytotoxicity. Increased oxidative stress and DNA damage as a result of ROS accumulation may directly drive CD8+ T cells towards terminal differentiation with characteristics of loss of T cell proliferation, T cell effector function and impaired self-renewal.
In this review, we discuss the metabolic requirements for T cells used in immunotherapy, nutrient competition between T cells and tumor, a metabolic checkpoint in the tumor microenvironment, and the metabolic properties of T cells that successfully eliminate tumors. We summarize and appraise recent reports that have highlighted the importance of metabolic maintenance in T cells by the mTOR signaling pathway in self-renewal [28–30] and in T-cell quiescence [31], memory versus effector T-cell differentiation [32, 33], ROS levels [20, 34] and anti-tumor activity [35]. Because T cell differentiation and functional activity are metabolically linked [36•], it is possible for the first time to characterize the metabolic properties of T cells that associated with enhanced anti-tumor efficacy and to program metabolic pathways to optimize the clinical efficacy of T cell-based therapies.
Metabolic requirements of immunotherapy
The lifespan of T cells employed in immunotherapy encompasses several phases, each with different functional and metabolic demands. Tumor-specific T cells must be successfully isolated from a patient’s tumor and then cultured in vitro to generate large numbers of cells [16]. Upon transfer, tumor-specific T cells must be able to engraft, localize to the tumor, survive in the harsh microenvironment it is likely to encounter there, finally, mount a sustained attack against tumor cells by proliferating and producing inflammatory cytokines and molecules that trigger tumor cell lysis. At each stage of this process, the T cells must balance the metabolic demands of energy maintenance, cell survival and persistence with those of rapid proliferation and inflammatory function. This balancing act requires the utilization of diverse metabolic programs [36].
Studies have shown aerobic glycolysis is required for T cells to achieve full inflammatory functionality [37–39], while T cells with a memory phenotype of increased persistence typically utilize an oxidative metabolic program characterized by increased mitochondrial fatty acid oxidation and spare respiratory capacity (SRC) [13, 40]. These metabolic programs can determine not only function but also fate. T cells with enforced glycolysis may become terminally differentiated [41] and the imposition of an oxidative metabolic program may drive T cell quiescence and loss of effector function [42•].
Thus, the metabolic requirements for successful T cell tumor immunotherapy are dynamic and likely to require the metabolic machinery for both long-term survival (characterized by quiescence and fatty acid-based metabolism) and a vastly more energetically demanding effector function that is appears to involve high levels of glycolysis as well as oxidative phosphorylation. Recent work has begun to shed light on how the metabolic programs of T cells can be harnessed to promote improved anti-tumor response.
Metabolic programs that promote successful therapy
Effector T cells require a metabolic program of aerobic glycolysis to proliferate and generate effector cytokines such as IFNγ [43, 44], however the utilization of glycolytic metabolism during in vitro priming and expansion of T cells can result in reduced functional activity in vivo. Blocking glucose metabolism using the hexokinase inhibitor 2-deoxyglucose (2-DG) [41] or by restricting the activity of Akt [45] during in vitro priming limits T-cell differentiation. These metabolic manipulations resulted in more potent anti-tumor activity and increased metabolic fitness in mouse models of melanoma. While causative proof has not been shown, increased mitochondrial spare respiratory capacity was also observed as a result of these metabolic interventions. Inhibition of cholesterol metabolism can also promote anti-tumor activity [46]. Recent work in the chimeric antigen receptor (CAR) T cell setting has similarly shown that oxidative metabolism is associated with cell persistence and longevity [47].
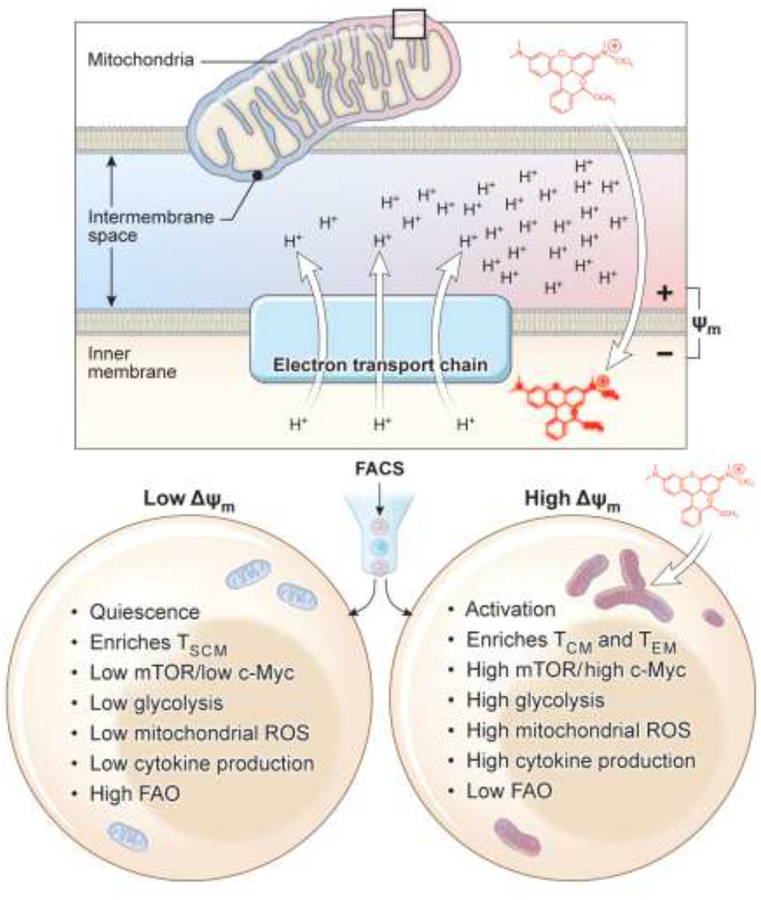
T cells with elevated mitochondrial membrane potential (Δψm) and ROS are associated with reduced anti-tumor efficacy [20]. Interestingly, T cells with low-Δψm during in vitro priming had phenotypes consistent with memory formation such as high spare respiratory capacity (SRC) and expression of memory genes, and also exhibited superior persistence compared with high Δψm cells. In contrast, cells with high Δψm were more glycolytic, expressed effector genes and had increased in vitro functionality (Figure 2). It seems plausible that increased ROS abundance, observed in T cells with high Δψm, is detrimental to the long-term survival of T cells [20]. The degree of metabolic activity of T cells may be regulated by the mitochondrial dynamics of the cell, with mitochondrial fusion promoting cell longevity and improved anti-tumor response, while mitochondrial fission drives terminal effector differentiation [48••]. Elevated metabolic activity during ex vivo expansion may result in T cells that have low ability to persist in vivo and consequently generate a poor anti-tumor response. A recent study indicated that arginine supplementation in the culture media during ex vivo expansion can promote oxidative metabolism and inhibit glycolytic metabolism to yield more effective anti-tumor T cells [49]. Thus, culture conditions in which the metabolic activity of anti-tumor T cells is limited during ex vivo expansion may result in the production of cells with beneficial characteristics for immunotherapy.
Figure 2. Metabolic sorting of therapeutic T cells based on mitochondrial activity for T-cell based immunotherapy.
Similar to cell surface markers that identify superior T cells for clinical applications, sorting T cells based on mitochondrial ROS and membrane potential (Δψm) can be explored for cell-based therapy. After T cell priming, sorting activated T cells based on mitochondrial activity into low-Δψm or high-Δψm would allow us to enrich for metabolic fit T cells for sustained T cell effector function, long-term persistence and anti-tumor activity.
Once T cells are transferred in vivo and have made their way to the tumor site, the utilization of a much more active metabolic phenotype is likely required for anti-tumor efficacy [41, 50]. Enforced glycolysis through genetic or pharmacological enhancement of hypoxia inducible factor (HIF) such as mutation or knockout to the Von Hippel Lindau (VHL) gene [51] or the prolyl hydroxylases (PHD) [52••] can result in elevated anti-tumor activity. Additionally, glycolytic metabolism may directly support the generation of inflammatory cytokines that are necessary for anti-tumor activity through epigenetic regulation [43]. The association of high Δψm with cytokine production by T cells [20] may indicate that sustained mitochondrial activity by T cells at the tumor site would be required for tumor control. Conversely, engagement of PD1 at the tumor site [42] or an excess of tumor-derived potassium in the tumor microenvironment can act to inhibit the functionality of anti-tumor T cells [53]. Two recent studies have shown that the loss of metabolic activity through Akt-mediated inhibition of PPAR-gamma coactivator 1α (PGC-1α) can reduce effector function and result in poor anti-tumor activity in T cells [54, 55••]. Furthermore, overexpression of PGC-1α in T cells has been shown to improve anti-tumor activity, suggesting that reprogramming mitochondrial biogenesis of T cells might represent an alternative strategy to improve TIL function for cancer treatment. In contrast, a recent report has demonstrated that mitochondrial FAO is not necessary for memory T cell formation and function. Sustained glycolysis through conditional knockout of von Hippel Lindau tumor suppressor protein (Vhl), that increases HIF activity, supports increased T cell persistence [56••]. This finding seems to contradict the view that memory cells have low glycolytic rates and may point to the complexities of HIF signaling [57, 58].
Nutrient Competition of T cells and tumor, a metabolic checkpoint in the Tumor Microenvironment
It is increasingly appreciated that tumors are not merely a homogeneous mass of malignant cells, but rather a complex structure containing vascular and stromal cells that support the tumor as well as diverse array of infiltrating immune cells including lymphocytes and myeloid-derived cells [16, 59]. Thus, success in T cell-mediated cancer therapy is not only achieved by adequate trafficking of T cells within the tumor deposit, but may also be largely determined by whether T cells successfully compete for nutrients in an immunosuppressive environment. Effector T cells appear to compete with tumor cells for glucose that enables them secrete IFN-γ and eradicate established tumor. Glucose deprivation suppresses TCR-dependent activation of Ca2+ and NFAT signaling and this leads to T cell hypo-responsiveness. PD-1 ligand (PD-L1) expression by tumor cells activates the AKT/mTOR pathway to promote tumor cell glycolysis [50, 59••]. Antibodies that block the PD-1/PD-L1 checkpoint may restore glucose in tumor microenvironment, permitting T cell glycolysis and IFN-g production. A new study also reported that ovarian cancers imposed glucose restriction on T cells and dampened their effector function [60]. Taken together, it has become increasingly clear that glucose availability within the tumor microenvironment regulates T cell effector function.
Improve metabolic fitness of human T cells for cancer immunotherapy
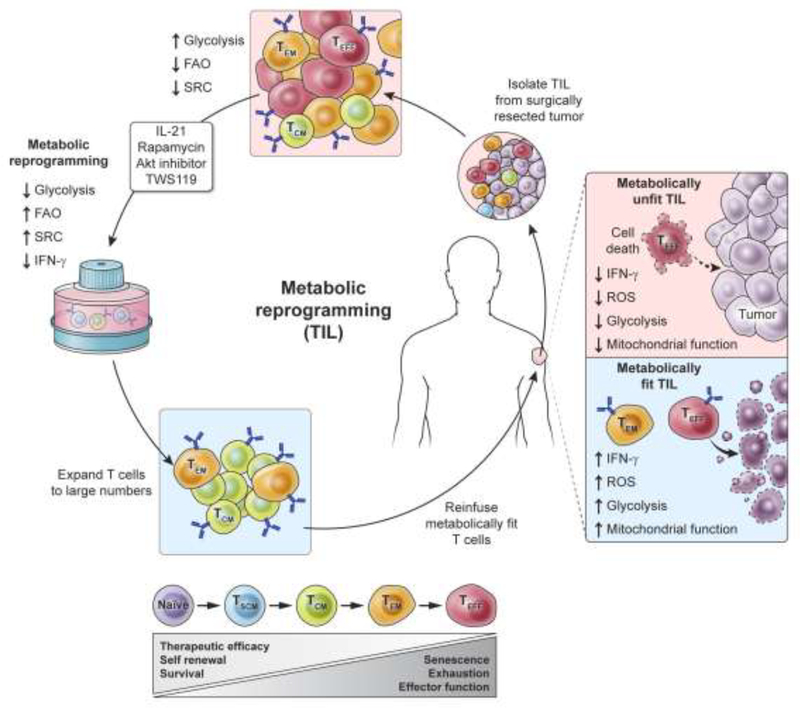
Immunotherapy using adoptive transfer of naturally-occurring, tumor-infiltrating T cells (TIL) can mediate the complete regression in some patients with metastatic melanoma [16]. TIL are heterogeneous with respect to their state of differentiation (as illustrated in Figure 3) and the generation of large numbers of autologous TIL for adoptive transfer requires in vitro expansion. Current methods of expansion of TIL can trigger Akt and mTOR activity, driving the terminal differentiation of T cells. We and others have demonstrated that restraining Akt [45] or mTOR [30] activity during T cell priming or enhancing STAT3 activity [61] and Wnt-β-catenin signaling in human T cells [18], can arrest T cell development, maintaining stem cell-like memory T cells. This arrested development of effector T cells is associated with enhanced metabolic properties including reduced glycolysis and increased utilization of fatty acid oxidation, improving long-term survival and anti-tumor activity of human T cells.
Figure 3. Clinical approaches to enhance “metabolic fitness” of anti-tumor T cells for advanced cancer.
Immunotherapy using adoptive transfer of tumor-specific T cells can mediate dramatic tumor regression in patients with advanced cancer. Animal models have revealed that “metabolically fit” anti-tumor T cells—those with enhanced fatty acid oxidation (FAO) and spare-respiratory capacity (SRC) and lower rates of glycolytic activity—have improved efficacy in eradicating established tumor. Because these metabolic features in mouse models are associated with improved long-term persistence and tumor regression, we propose enhancing “metabolic fitness” of T cells with pharmacologic agents targeting key metabolic pathways. This novel immunometabolic approach may improve the clinical efficacy of T cell-based therapies for patients with advanced cancer.
Model of T cell differentiation based on metabolic activity
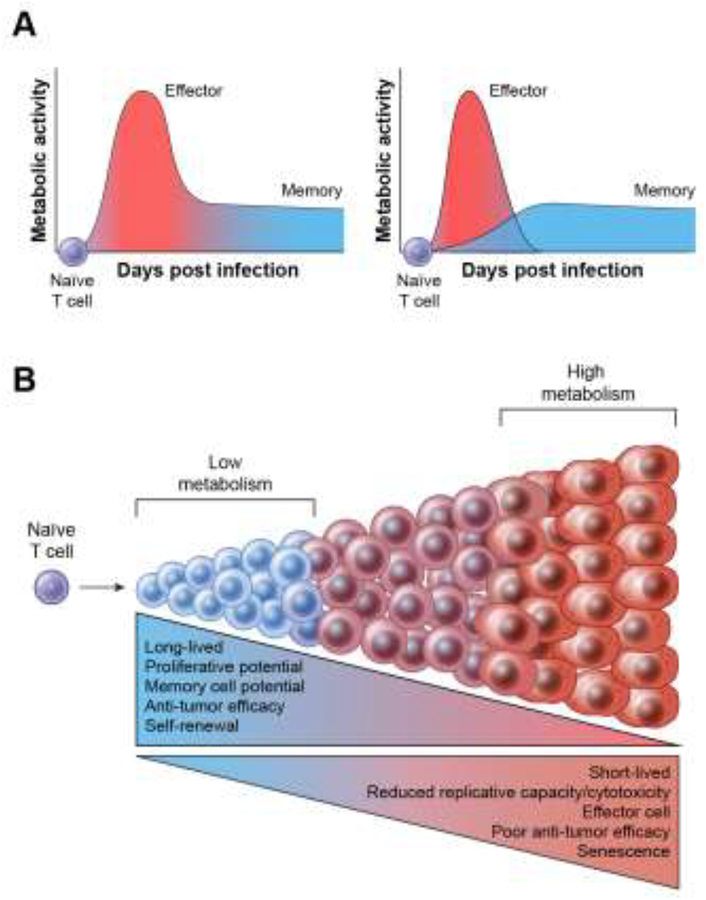
We therefore propose a model for anti-tumor T cell immunometabolism, extending our previous work with T cell differentiation, in which cells with high metabolic activity during in vitro expansion and priming results in the acquisition of a terminal effector phenotype. High metabolic activity, with concomitant high Δψm and ROS levels leads to short-lived cells with poor anti-tumor efficacy (Figure 4). In contrast, cells with restrained metabolic activity in vitro are preserved in a functional state where increased self-renewal and persistence are favored, allowing for long-lived and improved anti-tumor function. A critical aspect of this model is that restraints on the metabolic activity of T cells at the tumor site must be released to enable effector function and tumor destruction.
Figure 4. Model of T cell differentiation based on metabolic activity of lymphocytes.
Upon encountering cognate antigen, naïve CD8+ T cells (TN) undergo massive clonal expansion and differentiate into short-lived effector and long-lived memory CD8+ cells. This cell fate commitment is accompanied by changes in metabolic activity (increase in glycolysis, mitochondrial membrane potential and mitochondrial ROS) after T cell activation. Two dominant competing metabolic models of T cell differentiation are shown (Panel A). The left side of the panel depicts the off-on-off model in which quiescent cells become effector cells with a high metabolic rate which gradually de-differentiate into memory cells with more quiescent metabolism. The right side of Panel A depicts a model of progressive differentiation in which T cells with a highly active metabolic state die, in large part because of their production of ROS and granzymes. In this ‘developmental model,’ long-lived memory T cells can only originate from cells that retain relative metabolic quiescence. Panel B. We propose that high levels of metabolic activity may drive CD8+ T cells toward a terminally differentiated effector state that is associated with limited lifespan and replicative potential, impaired antitumor efficacy and ultimately cell senescence. In contrast, low levels of metabolic activity during T cell priming favors the formation of long-lived memory CD8+ T cells that have enhanced long-term survival and anti-tumor immunity.
Conclusions
The emerging interface between immune response and metabolism ‘immunometabolism’ helps us understand the bio-energetic requirements of T cell differentiation, cell-fate decision and function of T cells. Current methods for generating T cell products for adoptive immunotherapy have the pitfall of driving cells toward terminal differentiation and senescence. Approaches that are informed by a knowledge of the metabolic requirements for the optimal function of anti-tumor T cells are likely to be the subject of intense work in the field of immunometabolism.
Highlights.
Memory T cells have quiescent metabolism.
Activation of T cells triggers both glycolysis and oxidative phosphorylation.
Elevated T cell metabolic activity is necessary at the tumor site to promote tumor killing
High mitochondrial membrane potential (ΔΨm) is associated with cytokine production and capacity for cytotoxic function.
Metabolic reprogramming of T cells may improve TCR and chimeric antigen receptor (CAR) based immunotherapy.
Acknowledgements and funding.
The authors of this review were supported by the Center for Cell-Based Therapy, NCI, NIH (Bethesda, MD), NIH-Center for Regenerative Medicine, the Milstein Family Foundation and by the Intramural Research Program of the NCI (ZIA BC010763). We thank Gautam Mehta, Joe Crompton and Devikala Gurusamy for insightful input of this review. Finally, we thank Alan Hoofring and Ethan Tyler for creating the illustrations used in this piece.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Mehlen P and Puisieux A, Metastasis: a question of life or death. Nat Rev Cancer, 2006. 6(6): p. 449–58. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, et al. , Second and subsequent lines of chemotherapy for metastatic breast cancer: what did we learn in the last two decades? Ann Oncol, 2002. 13(2): p. 197–207. [DOI] [PubMed] [Google Scholar]
- 3.Spaans JN and Goss GD, Drug resistance to molecular targeted therapy and its consequences for treatment decisions in non-small-cell lung cancer. Front Oncol, 2014. 4: p. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Rosenberg SA and Restifo NP, Adoptive cell transfer as personalized immunotherapy for human cancer. Science, 2015. 348(6230): p. 62–8.An update on recent developments in the field of adoptive immunotherapy using conventional T cell receptors to treat melanoma and other common epithelial cancers.
- 5.Robbins PF, et al. , Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol, 2011. 29(7): p. 917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma P and Allison JP, The future of immune checkpoint therapy. Science, 2015. 348(6230): p. 56–61. [DOI] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, et al. , B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood, 2012. 119(12): p. 2709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grupp SA, et al. , Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med, 2013. 368(16): p. 1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, et al. , Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res, 2011. 17(13): p. 4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran E, et al. , T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med, 2016. 375(23): p. 2255–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Chang CH and Pearce EL, Emerging concepts of T cell metabolism as a target of immunotherapy. Nat Immunol, 2016. 17(4): p. 364–8.This comprehensive review covers the basic metabolic pathways in T cells, and discusses about the relationship between metabolism and T cell function and longevity and covers discussions in which T cells might be manipulated by the reprogramming of metabolic pathways for therapeutic purposes.
- 12.Gattinoni L, et al. , A human memory T cell subset with stem cell-like properties. Nat Med, 2011. 17(10): p. 1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearce EL, et al. , Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature, 2009. 460(7251): p. 103–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalek RD, et al. , Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol, 2011. 186(6): p. 3299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sukumar M and Gattinoni L, The short and sweet of T-cell therapy: Restraining glycolysis enhances the formation of immunological memory and antitumor immune responses. Oncoimmunology. 3(1): p. e27573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg SA, et al. , Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer, 2008. 8(4): p. 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klebanoff CA, et al. , Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A, 2005. 102(27): p. 9571–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gattinoni L, et al. , Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med, 2009. 15(7): p. 808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muranski P, et al. , Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood, 2008. 112(2): p. 362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20••.Sukumar M, et al. , Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell Metab, 2016. 23(1): p. 63–76.This study has shown that the level of mitochondrial membrane potential (ΔΨm) in T cells and HSC may regulate the stemness, differentiation and functionality, with low-ΔΨm is associated with enhanced stemness (TSCM and LT-HSC), while high-ΔΨm is associated with increased differentiation, cytokine production, ROS and oxidative stress.
- 21.Luckey CJ, et al. , Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc Natl Acad Sci U S A, 2006. 103(9): p. 3304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vannini N, et al. , Specification of haematopoietic stem cell fate via modulation of mitochondrial activity. Nat Commun, 2016. 7: p. 13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertolo A, et al. , Oxidative status predicts quality in human mesenchymal stem cells. Stem Cell Res Ther, 2017. 8(1): p. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tothova Z, et al. , FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell, 2007. 128(2): p. 325–39. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, et al. , Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature, 2004. 431(7011): p. 997–1002. [DOI] [PubMed] [Google Scholar]
- 26.Sena LA, et al. , Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity, 2013. 38(2): p. 225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinberg SE, Sena LA, and Chandel NS, Mitochondria in the Regulation of Innate and Adaptive Immunity. Immunity, 2015. 42(3): p. 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollizzi KN, et al. , Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol, 2016. 17(6): p. 704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verbist KC, et al. , Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature, 2016. 532(7599): p. 389–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholz G, et al. , Modulation of mTOR Signalling Triggers the Formation of Stem Cell-like Memory T Cells. EBioMedicine, 2016. 4: p. 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang K, et al. , The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol, 2011. 12(9): p. 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Araki K, et al. , mTOR regulates memory CD8 T-cell differentiation. Nature, 2009. 460(7251): p. 108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollizzi KN, et al. , mTORC1 and mTORC2 selectively regulate CD8(+) T cell differentiation. J Clin Invest, 2015. 125(5): p. 2090–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrestha S, et al. , Tsc1 promotes the differentiation of memory CD8+ T cells via orchestrating the transcriptional and metabolic programs. Proc Natl Acad Sci U S A, 2014. 111(41): p. 14858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rao RR, et al. , The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity, 2010. 32(1): p. 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.O’Neill LA, Kishton RJ, and Rathmell J, A guide to immunometabolism for immunologists. Nat Rev Immunol, 2016. 16(9): p. 553–65.An elegant review that discusses the recent advances in the field of immunometabolism. This review covers the complex interplay between metabolic reprogramming and immunity, providing a better understanding of the immune system in health and disease.
- 37.Macintyre AN, et al. , The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab, 2014. 20(1): p. 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerriets VA, et al. , Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest, 2015. 125(1): p. 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang R, et al. , The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity, 2011. 35(6): p. 871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Windt GJ, et al. , Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity, 2012. 36(1): p. 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sukumar M, et al. , Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest, 2013. 123(10): p. 4479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Patsoukis N, et al. , PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun, 2015. 6: p. 6692.This study provided the first evidence that upon PD-1 ligation, activated T cells had increased expression of CPT1a, fatty acid oxidation and are unable to engage in glycolysis. This study suggested that the enhancement of FAO may provide a mechanism for the longevity of T cells receiving PD-1 signals in patients with cancer.
- 43.Peng M, et al. , Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science, 2016. 354(6311): p. 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CH, et al. , Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell, 2013. 153(6): p. 1239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crompton JG, et al. , Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res, 2015. 75(2): p. 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang W, et al. , Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature, 2016. 531(7596): p. 651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawalekar OU, et al. , Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity, 2016. 44(2): p. 380–90. [DOI] [PubMed] [Google Scholar]
- 48••.Buck MD, et al. , Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell, 2016. 166(1): p. 63–76.This study demonstrated that mitochondrial ultrastructure controls T cell fate and metabolism. They showed that memory T cells have fused mitochondria and favored oxidative phosphorylation and FAO in memory T cells. In contrast, mitochondrial fission in effector cells leads to cristae expansion and promoted aerobic glycolysis. This is the first study suggesting that enforcing fusion can improve T cell based adoptive immunotherapy against tumors.
- 49.Geiger R, et al. , L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Ho PC, et al. , Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell, 2015. 162(6): p. 1217–28Together with Ref 44, this paper describes the Warburg metabolism enables tumor cells to restrict glucose availability to T cells, suppressing anti-tumor immunity. Chang CH et al., demonstrated that checkpoint blockade antibodies against CTLA-4, PD-1, and PD-L1, restore glucose in tumor microenvironment, permitting T cell glycolysis and IFN-γ production. HO PC et al. shows that phosphoenolpyruvate carboxykinase 1 (PCK1) overexpression in T cells increased effector function that results in restricted tumor growth.
- 51.Doedens AL, et al. , Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol, 2013. 14(11): p. 1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Clever D, et al. , Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell, 2016. 166(5): p. 1117–1131 e14.This study demonstrated that inhibition of PHD Proteins in CD4+ T cells results in increased HIF1 activity, elevated aerobic glycolysis and heightened effector differentiation. Furthermore, this study highlighted that pharmacological inhibition of PHD proteins in CD4+ T cells can improve T cell effector function and adoptive cell transfer immunotherapy against B16 melanoma.
- 53.Eil R, et al. , Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature, 2016. 537(7621): p. 539–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bengsch B, et al. , Bioenergetic Insufficiencies Due to Metabolic Alterations Regulated by the Inhibitory Receptor PD-1 Are an Early Driver of CD8(+) T Cell Exhaustion. Immunity, 2016. 45(2): p. 358–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Scharping NE, et al. , The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity, 2016. 45(2): p. 374–88.This study demonstrated that tumor infiltrating lymphocytes within mouse tumors display reduced levels of mitochondrial mass and metabolism. They further provided evidence that overexpression of PGC-1a in T cells increased mitochondrial biogenesis and effector function of T cells and anti-tumor immunity.
- 56••.Phan AT, et al. , Constitutive Glycolytic Metabolism Supports CD8+ T Cell Effector Memory Differentiation during Viral Infection. Immunity, 2016. 45(5): p. 1024–1037.Using conditional deletion of hypoxia-inducible factor regulator Vhl, this study shows that constitutive glycolytic metabolism mediated by sustained HIF1 activity may preferentially promote differentiation of CD8+ T Cell effector memory cells. By showing that glycolytic metabolism does not hinder differentiation of memory CD8+ T cells, this study proposes an interesting model where increased spare respiratory capacity and a reliance on OXPHOS are not essential for memory CD8+ T cells.
- 57.Piret JP, et al. , Is HIF-1alpha a pro- or an anti-apoptotic protein? Biochem Pharmacol, 2002. 64(5–6): p. 889–92. [DOI] [PubMed] [Google Scholar]
- 58.Makino Y, et al. , Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. J Immunol, 2003. 171(12): p. 6534–40. [DOI] [PubMed] [Google Scholar]
- 59.Kerkar SP and Restifo NP, Cellular constituents of immune escape within the tumor microenvironment. Cancer Res, 2012. 72(13): p. 3125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao E, et al. , Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat Immunol, 2016. 17(1): p. 95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui W, et al. , An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity, 2011. 35(5): p. 792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]