Abstract
Background and Objectives:
Women with unilateral early-stage breast cancer are increasingly choosing contralateral prophylactic mastectomy (CPM) despite the absence of survival benefits and increased risk of surgical complications. Data are lacking on whether this trend extends to women with clinically locally advanced non-metastatic (cT4M0) cancer. This study aims to estimate national CPM trends in women with unilateral cT4M0 breast cancer.
Methods:
Women aged ≥ 18 years, who underwent mastectomy during 2004–2014 for unilateral cT4M0 breast cancer were identified using the National Cancer Database and grouped as all locally advanced (T4), chest wall invasion, skin nodule/ulceration, or both (T4abc), and inflammatory (T4d) cancer. Poisson regression for trends and logistic modeling for predictors of CPM were performed.
Results:
Of 23,943 women, 41% had T4abc disease and 35% T4d. Cumulative CPM rates were 15%, 23% and 18%, for the T4abc, T4d and all T4 groups, respectively. Trend analysis revealed a significant upsurge in CPM demonstrating 12% annual growth for T4abc tumors, 8% for T4d and 9% for all T4 (all P < 0.001).
Conclusions:
Increasing numbers of women with unilateral cT4M0 breast cancer, are undergoing CPM. This rising trend warrants further research to understand stakeholders’ preferences in surgical decision-making for women with locally advanced breast cancer.
Keywords: breast cancer, contralateral prophylactic mastectomy, contralateral risk-reducing mastectomy, locally advanced breast cancer, immediate breast reconstruction
Synopsis:
National trends of contralateral prophylactic mastectomy (CPM) were computed among women with non-metastatic locally advanced clinical AJCC T4 breast cancer using the National Cancer Database. The study results revealed that an increasing number of women with unilateral clinical T4 are undergoing CPM. These findings warrant further investigations to better understand stakeholders’ preferences and factors involved in the surgical decision-making process for women with locally advanced breast cancer.
INTRODUCTION
Over the past two decades, an increasing number of women in the United States have been choosing to undergo contralateral prophylactic mastectomy (CPM) for in situ and early-stage invasive breast cancer (T1–3), despite a decline in contralateral breast cancer (CBC) incidence secondary to the use of adjuvant systemic therapy [1–4]. The American Joint Committee on Cancer (AJCC) defines locally advanced (T4) breast cancer as a tumor of any size with direct extension to the chest wall (beyond or other than the pectoralis major) and or to the skin (with ulceration or nodules), or inflammatory (T4d) breast carcinoma [5]. While chest wall invasion (T4a), skin nodule or ulceration (T4b), and both chest wall invasion and skin nodule or ulceration extension (T4c) breast cancer subtypes vary by extent of local invasion, the inflammatory (T4d) breast cancer subtype, is a rare, aggressive form of rapid-onset (shorter than 6 months) carcinoma that presents with erythema and or edema characterized by “peau d’orange” of the skin involving at least one-third of the breast [6]. Classification of inflammatory breast cancer is clinically significant as it has a worse prognosis compared to all other T4 classifications [7, 8].
Surgical management of locally advanced clinical T4abc tumors depends on extent of disease and response to neoadjuvant chemotherapy therapy, with standard therapy consisting of upfront systemic therapy followed by mastectomy and radiation, while patients who are exceptional responders might be considered for breast conservation [9, 10]. The standard of care for inflammatory breast cancer (T4d) remains modified radical mastectomy and postmastectomy radiation therapy following neoadjuvant chemotherapy according to national and international expert panel recommendations [6] as well as National Comprehensive Cancer Network (NCCN) guidelines [7, 11]. There are no current guidelines or recommendations regarding the use of CPM for women with locally advanced breast cancer. As noted in the NCCN guidelines, immediate breast reconstruction (IBR) is contraindicated in the setting of inflammatory breast cancer secondary to the high risk of recurrence rate, need to resect a large skin paddle of skin, and desire to proceed expeditiously to postmastectomy radiation therapy.
The literature demonstrates upward trends of CPM among women with in situ and early-stage breast cancer [1–3, 12, 13], although the data do not support a survival benefit for women with sporadic breast cancer [14]. National-level data on whether this CPM trend extends beyond early-stage cancer, particularly among women with locally advanced (cT4) breast cancer, has not been explicitly established. The aim of this study is to determine the rate of CPM among women with clinical T4 breast cancer and to analyze the temporal trend of CPM in this population using a national database. The hypothesis is that the rate of CPM among women with clinical locally advanced (cT4) breast cancer is increasing.
METHODS
A retrospective longitudinal study for 2004–2014 was conducted using the National Cancer Database (NCDB) following institutional review board approval. The data were awarded after approval of our NCDB participant user file application submitted for the February 2017 cycle. The NCDB, established in 1989, is a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons (ACS). The ACS has executed a Business Associate Agreement that includes a data use agreement with each of its CoC-accredited hospitals. The NCDB is a nationwide, facility-based, comprehensive clinical surveillance oncology dataset that captures 70% of all newly diagnosed malignancies in the United States. In each CoC-accredited hospital, the data are collected and recorded by the hospital’s cancer registrar in close collaboration with its surgeons. Facility Oncology Registry Data Standards (FORDS) is used to define patient and tumor-specific variable definitions [15]. The physician-recorded AJCC tumor-node-metastasis (TNM) elements of the clinical stage are noted prior to surgery, and if the patient undergoes resection, pathological AJCC TNM stage is entered separately. Data reporting to the NCDB is meticulously standardized with annual data quality monitoring, and validity reviews [16].
Study inclusion criteria were women 18 years of age and older diagnosed with unilateral breast cancer of clinical AJCC tumor stage T4: T4a (chest wall invasion), T4b (skin nodule or ulceration), T4c (T4a and T4b), T4d (inflammatory carcinoma), or T4 not otherwise specified (NOS). Clinical tumor stage T4 was defined using AJCC 6th and 7th edition criteria provided by the NCDB. Women who underwent any type of unilateral or bilateral mastectomy––subcutaneous (skin/nipple-sparing), radical, modified radical, extended, or NOS with or without IBR––were identified using the codes provided in the participant user file by NCDB and included in the analysis (http://ncdbpuf.facs.org/content/breast) (Table S2). Women with metastatic breast cancer, who underwent breast-conservation surgery, or without a report available on their surgery type were excluded (Figure 1).
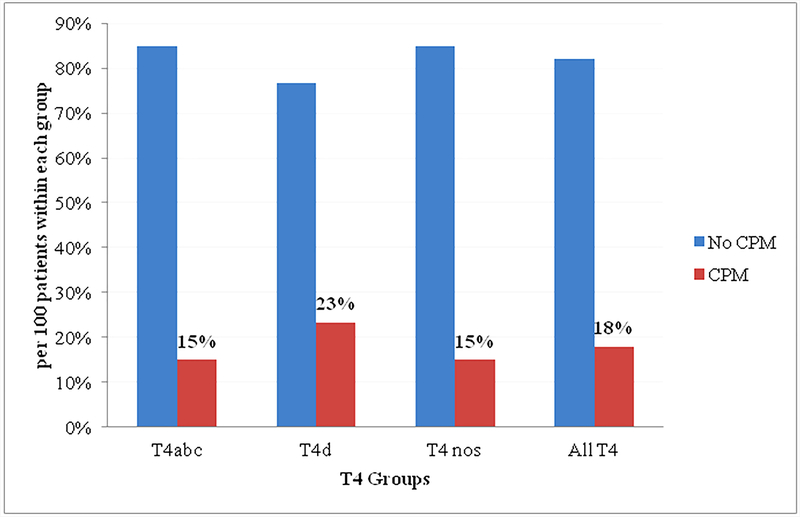
Figure 1.
Rate of CPM in women with T4M0 breast cancer stratified by clinical AJCC T4 subtypes T4abc, T4d, and NOS. All T4 includes T4abcd and NOS.
AJCC, American Commission on Cancer; CPM, contralateral prophylactic mastectomy; NOS, not otherwise specified
The total eligible cohort was classified into T4abc and T4d categories according to the AJCC tumor staging system listed above and studied separately. Patient demographics, clinical characteristics, oncologic factors, and treatment modalities were analyzed for each group. Types of surgery, including unilateral mastectomy, CPM, IBR, and reconstruction method were identified using site-specific surgery codes provided in the participant-user file containing NCDB data dictionary. From the overall cohort, patients were categorized into CPM and no-CPM groups. The annual CPM rate was determined separately for patients in each group and presented as CPM per 100 mastectomies. The annual rate of CPM with IBR was calculated separately and presented as IBR per 100 CPMs. Trend analyses were performed, and incidence rate ratios (IRRs) were calculated for the entire cohort and each group: T4abc and T4d. Predictors of CPM were explored using univariate and multivariate analyses. In patients who received neoadjuvant therapy, clinical tumor size was measured before initiating therapy. Neoadjuvant therapy was defined as receipt of systemic chemotherapy before surgery.
Chi-square statistics or Fisher’s exact test was used to compare categorical variables. Trends were analyzed using Poisson regression. Multivariate logistic regression analysis controlling for potential confounders was performed to determine independent predictors of CPM. All tests were two-sided, and a P value of < 0.05 was considered significant. All analyses were performed using IBM SPSS Statistics for Windows, Version 24.0. (Armonk, NY: IBM Corp).
RESULTS
A total of 23,943 women met study inclusion criteria. Of all the women studied, 41% had a clinical diagnosis of T4abc (n = 9,824), 35% had a clinical diagnosis of T4d (n = 8,321), and 24% had a clinical diagnosis of T4 NOS (n = 5,798). Twenty-two percent of the cohort had lymphovascular invasion, and 70% had positive regional lymph nodes. Fifty-eight percent of the cohort received neoadjuvant therapy, 17% received adjuvant systemic chemotherapy, and 65% received postmastectomy radiotherapy (Table 1).
Table 1:
Patient Demographics and Clinical Characteristics by CPM Status in AllT4a Cohort
| Characteristics | Categories | Totalb N=23943 | UM N=19641 | CPM N=4302 | P-value |
|---|---|---|---|---|---|
| Age, year | [≥65] | 8930 (37.3) | 8219 (41.8) | 711 (16.5) | <0.001 |
| [50–64] | 9134 (38.1) | 7312 (37.2) | 1822 (42.4) | ||
| [40–49] | 4225 (17.6) | 3049 (15.5) | 1176 (27.3) | ||
| [18–39] | 1654 (6.9) | 1061 (5.4) | 593 (13.8) | ||
| Race | White | 18841 (78.7) | 15159 (77.2) | 3682 (85.6) | <0.001 |
| Black | 4023 (16.8) | 3535 (18.0) | 488 (11.3) | ||
| Other/unknown | 1079 (4.5) | 947 (4.8) | 132 (3.1) | ||
| Ethnicity | H/L | 1505 (6.7) | 1301 (7.1) | 204 (5.0) | <0.001 |
| Non-H/L | 20958(87.5) | 17116(87.1) | 3842(89.3) | ||
| Education: % of No HSDc | [≥21] | 4551 (19.3) | 3916 (20.2) | 635 (14.9) | <0.001 |
| [13–20.9] | 6447 (27.3) | 5390 (27.8) | 1057 (24.8) | ||
| [7–12.9] | 7491 (31.7) | 6018 (31.1) | 1473 (34.5) | ||
| [< 7] | 5147 (21.8) | 4047 (20.9) | 1100 (25.8) | ||
| Insurance status | None | 1181 (4.9) | 1016 (5.2) | 165 (3.8) | <0.001 |
| Medicare | 8593 (35.9) | 7838 (39.9) | 755 (17.5) | ||
| Medicaid | 2964 (12.4) | 2444 (12.4) | 520 (12.1) | ||
| Government | 188 (0.8) | 141 (0.7) | 47 (1.1) | ||
| Private/MC | 10684 (44.6) | 7924 (40.3) | 2760 (64.2) | ||
| Unknown | 333 (1.4) | 278 (1.4) | 55 (1.3) | ||
| Facility type | CCP | 2772 (12.4) | 2426 (13.1) | 346 (9.3) | <0.001 |
| CCCP | 10398 (45.7) | 8587 (46.2) | 1811 (48.8) | ||
| INCP | 2356 (10.6) | 1891 (10.2) | 465 (12.5) | ||
| Academic/Research | 6763 (30.3) | 5676 (30.5) | 1087 (29.3) | ||
| Charlson/Deyo score | 0 | 19433 (81.2) | 15728 (80.1) | 3705 (86.1) | <0.001 |
| 1 | 3603 (15.0) | 3094 (15.8) | 509 (11.8) | ||
| 2 | 907 (3.8) | 819 (4.2) | 88 (2.0) | ||
| Tumor size, mm | [>20] | 21157 (88.4) | 17474 (89.0) | 3683 (85.6) | <0.001 |
| [≤20] | 2786 (11.6) | 2167 (11.0) | 619 (14.4) | ||
| LVI | Absent | 4272 (17.8) | 3328 (16.9) | 944 (21.9) | <0.001 |
| Present | 5262 (22.0) | 4177 (21.3) | 1085 (25.2) | ||
| NA | 14409 (60.2) | 12136 (61.8) | 2273 (52.8) | ||
| Clinical AJCC N | 0 | 6652 (29.7) | 5618 (30.7) | 1034 (25.2) | <0.001 |
| 1 | 9427 (42.0) | 7469 (40.8) | 1958 (47.7) | ||
| 2 | 4044 (18.0) | 3362 (18.3) | 682 (16.6) | ||
| 3 | 2309 (10.3) | 1877 (10.2) | 432 (10.5) | ||
| Clinical AJCC T | T4abc | 9824 (41.0) | 8349 (42.5) | 1475 (34.3) | <0.001 |
| T4d | 8321 (34.8) | 6322 (32.4) | 1949 (45.3) | ||
| T4 NOS | 5798 (24.2) | 4920 (25.0) | 878 (20.4) | ||
| ER Status | Negative | 8978 (37.5) | 7264 (37.0) | 1714 (39.8) | |
| Positive | 14178 (59.2) | 11688 (59.5) | 2490 (57.9) | <0.001 | |
| Unknown | 787(3.3) | 689(3.5) | 98(2.3) | ||
| PR Status | Negative | 11571 (48.3) | 9384 (47.8) | 2187 (50.8) | <0.001 |
| Positive | 11494 (48.0) | 9492 (48.3) | 2002 (46.5) | ||
| Unknown | 878 (3.7) | 765 (3.9) | 113 (2.6) | ||
| HER2 Statusd | Negative | 5484 (23.1) | 4350 (22.4) | 5484 (23.1) | |
| Positive | 2405 (10.2) | 1810 (9.3) | 2405 (10.2) | <0.001 | |
| Unknown | 16054(67.1) | 13481(68.6) | 2573(59.8) | ||
| Margins | Negative | 20834 (87.0) | 16911 (86.1) | 3923 (91.2) | <0.001 |
| Positive | 2688 (11.2) | 2363 (12.0) | 325 (7.6) | ||
| Chemotherapy | None | 1716(7.2) | 1659(8.4) | 57(1.3) | <0.001 |
| Neoadjuvant | 13970(58.3) | 10794(55.0) | 3176(73.8) | ||
| Adjuvant | 4126(17.2) | 3635(18.5) | 491(11.4) | ||
| Unknown | 4131(17.3) | 3553(18.1) | 578(13.4) | ||
| PMRT | None | 8320(34.7) | 7257(36.9) | 1063(24.7) | <0.001 |
| Yes | 15243(64.5) | 12053 (62.2) | 3190 (74.9) | <0.001 | |
| Unknown | 1045(4.4) | 912(3.7) | 133(2.0) | ||
| Endocrine | None | 9831(41.1) | 8137(41.4) | 1694(39.4) | 0.031 |
| Therapy | Yes | 11703(48.9) | 9502(48.4) | 2201(51.2) | |
| Unknown | 2409(10.0) | 1997(10.2) | 407(9.5) | ||
| Immunotherapy | None | 22262(93.0) | 18358(93.5) | 3904(90.7) | <0.001 |
| Yes | 1345(5.6) | 989(5.0) | 356(8.3) | ||
| Unknown | 336(1.4) | 294(1.5) | 42(1.0) | ||
AllT4 = Tabc+T4+NOS (not otherwise specified)
Column % may not add up to 100 due to missing information
No HSD: Percentage of no high school degree by zip codes
Routine HER2neu status was available after 2010
CPM, contralateral prophylactic mastectomy; UM, unilateral mastectomy; H/L, Hispanic/Latino; HSD, high-school diploma; CCP, community cancer program; CCCP indicates comprehensive community cancer program; INCP, integrated network cancer program; LVI, lymphovascular invasion; NA, not available; AJCC, American Joint Committee on Cancer; NOS, not otherwise specified; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; PMRT Post-mastectomy radiotherapy
Trends of Contralateral Prophylactic Mastectomy
Cumulative CPM rates for the entire period of 2004–2014 are illustrated in Figure 1. The overall CPM rate among women with T4 breast cancer was 18%. Results of the stratified analysis showed that the CPM rate in the clinical T4d group was higher than that in the T4abc group (23% vs. 15%; P < 0.05) (Figure 1).
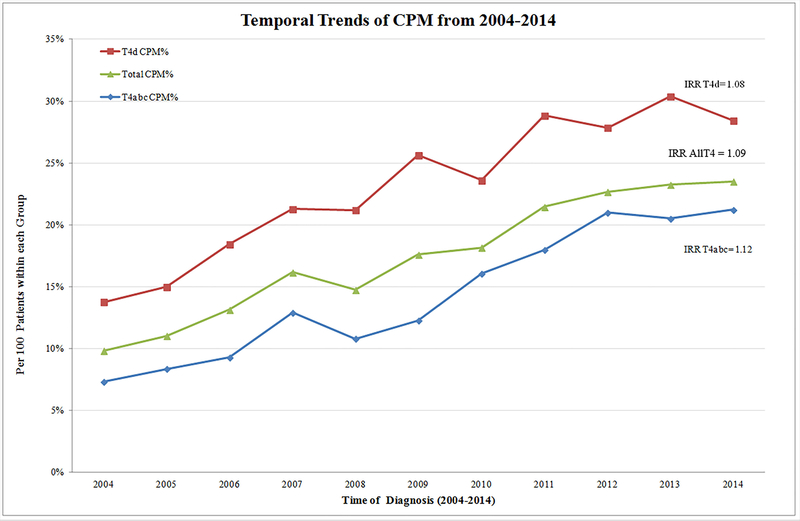
Temporal CPM trends for the period of study are charted in Figure 2. The overall CPM rate among women with clinical T4 tumors demonstrated a significant upward trend, with an annual 9% increase over the study period (IRR: 1.09; P < 0.001). Stratified analyses for T4abc and T4d revealed a significant rising trend in the rate of CPM in both groups, demonstrating the annual growth of 12% and 8%, respectively (both P < 0.001) (Figure 2).
Figure 2.
Temporal trends of CPM in women with T4M0 breast cancer stratified by subtype groups. IRRT4d: 1.08 (95% CI: 1.06–1.09); IRRAllT4: 1.09 (95% CI: 1.08–1.10); IRRT4abc: 1.12 (95% CI: 1.10–1.14); all P < 0.001.
CPM, contralateral prophylactic mastectomy; IRR, incidence rate ratio
Contralateral Prophylactic Mastectomy Versus Unilateral Mastectomy: Univariate Analyses
Univariate analyses of factors associated with CPM are presented in Table 1; women of younger age, white race, and higher education were more likely to have chosen CPM. Women with payers consisting of private insurance, managed care, or other government insurance were more likely to undergo CPM (P < 0.001). Contrastingly, non-white women with a higher comorbidity index and Medicare as the primary payer were less likely to have undergone CPM (P < 0.05). An analysis of oncologic factors revealed that women with smaller clinical tumor size and positive lymph nodes were also more likely to have undergone CPM. Women in the CPM group were also more likely to have negative surgical margins and to have received neoadjuvant or adjuvant systemic chemotherapy as well as postmastectomy radiotherapy (all P < 0.05) (Table 1). In an attempt to account for tumor downstaging by neoadjuvant chemotherapy, we assessed CPM rates among women with a clinical T4abc disease who had AJCC pathologic tumor stage pT4 vs. pT0–3 tumors following neoadjuvant chemotherapy (n = 5,111). Those women who had a decrease in pathologic tumor stage (pT ≤ 3) following neoadjuvant chemotherapy were significantly more likely to undergo CPM (64 vs. 53%; P < 0.001). When analyzing the type of cancer care facility, community cancer programs were less likely to perform CPM compared with other cancer programs (P < 0.001) (Table 1).
Independent Predictors of Contralateral Prophylactic Mastectomy
Table 2 depicts a multivariable regression model showing independent predictors of CPM. Factors including younger age, white race, higher education, and private insurance remained significant predictors of CPM after adjusting for potential confounders. Comprehensive community programs and integrated network cancer programs also demonstrated a significantly higher CPM rate compared with the community cancer centers. Interestingly, women with a clinical diagnosis of T4d breast cancer were more likely to have undergone CPM than women with T4abc subtypes (Adjusted odds ratio (AOR): 1.35; P < 0.001) (Table 2). Receipt of adjuvant or neoadjuvant chemotherapy, as well as postmastectomy radiotherapy, was also associated with an increase in CPM rate (All P < 0.05) (Table 2). Among the women who received neoadjuvant chemotherapy, a post-hoc sub-analysis was performed. Women with T4abc breast cancer whose AJCC pT stage decreased to ≤ 3 demonstrated an increased rate of CPM compared to those who maintained T4abc stage following neoadjuvant therapy (64 vs. 53 %; P < 0.001).
Table 2:
Multivariable Regression Analysis for Independent Predictors of CPM in AllT4a Cohort
| Independent Predictors | AOR | 95% Confidence Interval | P-Valueb |
|---|---|---|---|
| Age, year: [≥65] | Reference | ||
| [50–64] | 1.97 | 1.70–2.28 | <0.001 |
| [18–49] | 3.14 | 2.67–3.69 | <0.001 |
| Race: White | Reference | ||
| Black | 0.48 | 0.42–0.55 | <0.001 |
| Other/unknown | 0.44 | 0.35–0.57 | <0.001 |
| Ethnicity H/L vs. non-H/L | 0.54 | 0.44–0.65 | <0.001 |
| Education: % of No HSDc: [≥21] | Reference | ||
| [13–20.9] | 1.06 | 0.93–1.21 | 0.238 |
| [7–12.9] | 1.17 | 1.03–1.32 | 0.002 |
| [< 7] | 1.24 | 1.09–1.42 | <0.001 |
| Insurance status: None | Reference | ||
| Medicare | 1.21 | 0.95–1.54 | 0.122 |
| Medicaid | 1.09 | 0.86–1.36 | 0.485 |
| Other Government | 1.60 | 1.04–2.47 | 0.034 |
| Private | 1.57 | 1.28–1.94 | <0.001 |
| Facility type: CCP | Reference | ||
| Academic/research | 0.97 | 0.84–1.13 | 0.724 |
| CCCP | 1.24 | 1.08–1.42 | 0.002 |
| INCP | 1.37 | 1.16–1.63 | <0.001 |
| Clinical AJCC T4abc | Reference | ||
| Clinical AJCC T4d | 1.35 | 1.23–1.48 | <0.001 |
| Clinical AJCC NOS | 1.00 | 0.90–1.11 | 0.939 |
| Tumor size ≤20 vs >20 mm | 1.23 | 1.09–1.38 | 0.001 |
| Clinical AJCC N Status: 0 | Reference | ||
| 1 | 1.04 | 0.94–1.14 | 0.502 |
| 2 | 0.95 | 0.84–1.08 | 0.413 |
| 3 | 0.93 | 0.80–1.07 | 0.306 |
| ER Positive vs negative | 1.05 | 0.94–1.18 | 0.409 |
| PR Positive vs negative | 0.91 | 0.81–1.02 | 0.111 |
| HER2 Positive vs negative | 1.09 | 0.96–1.25 | 0.190 |
| Chemotherapy: None | |||
| Neoadjuvant | 3.79 | 2.79–5.13 | <0.001 |
| Adjuvant | 2.51 | 1.83–3.43 | <0.001 |
| PMRT: None | Reference | ||
| Yes | 1.17 | 1.07–1.29 | 0.001 |
| IBR vs no IBR | 2.24 | 2.01–2.50 | <0.001 |
AllT4 = Tabc+T4+NOS (not otherwise specified)
Adjusted for age, race, ethnicity, education, payer and facility type, tumor size, T4d and nodal status, chemotherapy, radiotherapy, and IBR
No HSD: Percentage of no high school degree by zip codes
CPM, contralateral prophylactic mastectomy; AOR, adjusted odds ratio; H/L Hispanic/Latino; HSD, high-school degree; CCP, community cancer program; CCCP, comprehensive community cancer program; INCP, integrated network cancer program, AJCC, American Joint Committee on Cancer, ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; PMRT Post-mastectomy radiotherapy; IBR Immediate b
Women who had IBR were significantly more likely to have undergone CPM (AOR: 2.24; P < 0.001). A second multivariable model was performed to assess the impact of reconstruction trends. The regression model adjusted for the year of diagnosis plus all the above variables used in the first model and demonstrated greater than threefold increase in the CPM rates (AOR: 3.65, 95% confidence interval: 2.69–4.94; P < 0.001) from 2004–2015 irrespective of IBR status.
Contralateral Prophylactic Mastectomy and Immediate Breast Reconstruction
Among all women who underwent CPM, the cumulative IBR rate was 22% for the study period (n = 948/4,302) (Table 3). An upward trend in the IBR rate from 14% to 27% was observed across the study period, at an annual growth rate of 16% (P < 0.001) (Table 3). The overall IBR rate in women who underwent CPM for T4abc and T4d was 26% and 18%, respectively (Table 1). Trend analyses of the women with IBR showed increasing annual rates of CPM of 18% and 13% for T4abc and T4d, respectively (both P < 0.001; data not shown).
Table 3:
Volume and Rate of CPM with Immediate Breast Reconstruction in AllT4a Cohort
| Diagnosis Year | CPMa, N | CPM with IBR, n (%) |
|---|---|---|
| 2004 | 163 | 22 (13.5) |
| 2005 | 184 | 29 (15.8) |
| 2006 | 234 | 46 (19.7) |
| 2007 | 309 | 52 (16.8) |
| 2008 | 352 | 64 (18.2) |
| 2009 | 440 | 90 (20.5) |
| 2010 | 452 | 101 (22.3) |
| 2011 | 529 | 115 (21.7) |
| 2012 | 551 | 128 (23.2) |
| 2013 | 558 | 156 (28.0) |
| 2014 | 530 | 145 (27.4) |
| Total, (n) | 4,302 | 948 (22) |
| IRRb | 1.09 | 1.16 |
AllT4 = Tabc+T4+NOS (not otherwise specified)
P <0.001 for all
CPM, contralateral prophylactic mastectomy; IBR immediate breast reconstruction
IRR, incidence rate ratio
DISCUSSION
This study reports, for the first time, national rates and trends in the utilization of CPM among women in the United States diagnosed with unilateral locally advanced (T4) breast cancer using the NCDB. We found that the rate of performing CPM for T4 disease increased annually by 9% (Figure 2), demonstrating growth of 139% since 2004. These results are surprising given the lack of survival benefit and an increased breast cancer-specific mortality risk among women with more advanced-stage disease [8] [14] [17]. The CPM trends in our study closely mirror CPM rates in early-stage cancer, as reported in an NCDB study showing a 13% annual rise during 1998–2011 [18], and others [1–3, 19, 20]. A SEER data study of women with stages I–III breast cancer demonstrated a tripling of the CPM rate from 7.7% to 28.3% during 2000–2010; however, the authors did not separately analyze trends in T4 cancer [16]. When comparing women with T4d breast cancer to those with T4abc subtypes, CPM was more common in the T4d group for the overall study period (23% vs. 15%; P < 0.05). The Mayo Clinic has reported an institutional rate of CPM as high as 36% and 45% among women with T4 and T4d breast cancer, respectively, for the period 2008–2015 [20], although it did not analyze temporal trends.
The increasing CPM rates observed among women with T4d tumors is of particular interest, as inflammatory breast cancer has historically been associated with a more aggressive disease course, higher local recurrence, and mortality rates, compared with earlier-stage disease [8–10, 21, 22]. In our study, the overall cross-sectional rate of CPM was 23% in the T4d group, which showed an 8% annual growth during 2004–2014 (Figure 2). A limited analysis of the SEER database for inflammatory breast cancer also found an increase in the 4-year periodic rate of CPM from 6% to 23% [23].
Recent studies have reported improvements in survival among patients with inflammatory breast cancer, especially in women with HER2 overexpressing disease managed with targeted anti-HER2 therapy [24, 25]. However, survival remains poor compared to non-inflammatory cancers, with 5-year disease-free survival for patients with estrogen/progesterone hormone receptor positive/HER2 negative tumors, HER2 overexpressing tumors, and triple-negative tumors reported at 46%, 82%, and 33%, respectively [24]. Although survival appears to be improving over time for some women with locally advanced breast cancer, there remains a concern about advocating CPM or IBR for this high-risk cohort.
In theory, CPM offers oncologic benefit to women with a low-risk index unilateral breast cancer, long life expectancy, and high CBC risk that could have an impact on overall survival. Contrastingly, women with clinical T4 breast cancer who underwent CPM in our study had a high-risk index breast lesion with an increased risk for both locoregional and distant recurrence [9]. Moreover, performing CPM is associated with a 2- to 3-fold increase in the risk of surgical complications, such as postoperative infections and wound-related complications [26, 27], which carries the potential risk of delaying adjuvant therapy. Additional breast surgery may also lead to long-term complications, such as chronic pain and the need for additional procedures. Counseling women on CPM warrants consideration of both the potential benefits as well as the risks associated with the operation. Our findings raise concern that surgical decisions are being made without considering the potential surgical complications and the poor prognosis associated with the locally advanced T4 breast cancer, as well as the lack of survival benefit associated with the use of CPM [13, 20, 21].
Patient-related factors for women with T4 breast cancer choosing CPM mirror those reported by others for women with early-stage disease. The results from our multivariate analysis show that women who were younger, white, had higher education, and had private or other government insurance were more likely to choose CPM [12, 15]. Women who received IBR were significantly more likely to have undergone CPM than women who did not––a finding consistent with the literature on CPM among early-stage breast cancer patients [13, 18, 28]. In a study exploring predictors of CPM among women with in situ and early-stage invasive breast cancer, young age and availability of IBR explained the highest variation, with coefficients of determination of 32% and 29%, respectively [18]. Although speculative, one potential factor affecting the rising trend of IBR in women with clinically T4 non-metastatic breast cancer may be the Women’s Health and Cancer Right Act (WHCRA) of 1998, which requires all women undergoing surgery for any stage of breast cancer be offered reconstruction for the native breast removed as well as the contralateral breast; studies have reported this association for women with early-stage breast cancer undergoing IBR [15]. Our multivariable model also demonstrated that women with the T4 disease who received neoadjuvant therapy were over 3 times more likely to have chosen CPM than unilateral mastectomy (AOR: 3.79; P < 0.001) (Table 2). Surprisingly, a sub-cohort of women with T4abc breast cancer whose AJCC pT stage decreased to ≤ 3 following neoadjuvant chemotherapy were also more likely to have undergone CPM compared to those who maintained T4abc prior to the surgery (64 vs. 53 %; P < 0.001). In the era of neoadjuvant therapy, which primarily aims to potentially increase the possibility of breast conservation [10], a paradoxical trend, which resembles that among women with early-stage cancer[29] [27], hints toward non-medical drivers of CPM. These robust results seen across disease stages in the literature including our study may suggest that sociodemographic rather than isolated medical factors may sway the decision to proceed with CPM. While the number of women who did not receive chemotherapy is small, it is possible that this group represents a population with greater co-morbidities, which may also have an impact on CPM decision-making.
Non-medical drivers for CPM include a desire of obtaining chest symmetry, recall fatigue, and disease-related anxiety. Patients must weigh these factors against the increased risk associated with bilateral mastectomies compared to unilateral mastectomy. While postoperative complications [27, 30, 31], the length of hospitalization, and blood transfusion rates increase following bilateral mastectomies, the quality-of-life measurements including satisfaction with breast appearance, symmetry, and body image improve following reconstruction [32]. Whereas non-medical drivers such as anxiety, fear of future cancer risk, desire for symmetry, and wish to avoid surveillance mammograms are cited reasons for choosing CPM [33], surgeons must counsel women on their oncological and procedure-related risks. In contrast to the management of early-stage breast cancer, for T4d tumors, a conventional total mastectomy without IBR to allow resection of a large skin paddle given the underlying pathophysiology of dermal lymphatic infiltration––a classic presentation in inflammatory breast cancer—remains the standard of care. Whereas, we observed that IBR, which requires a skin-sparing mastectomy, was associated with increasing CPM utilization, the majority of women in our cohort undergoing CPM (88%) did not undergo IBR.
Future studies, exploring the drivers of CPM using innovative research methodologies, may help illustrate the reasons behind the decision to perform CPM. One such investigation is being employed at our institution using a market-research tool–conjoint analysis, which emulates real-life scenarios confronting patients while they are making the critical decision for unilateral breast cancer. Furthermore, there are ongoing efforts, such as published consensus statements from the American Society of Breast Surgeons and the Society of Surgical Oncology, geared to better educate women on CPM decision-making [34, 35]. However, there are no strict guidelines or any widely used decision tools to foster shared decision making regarding CPM [36].
Our study has strengths and limitations. The NCDB is not a population-based database; hence, overall rates and trends may not be generalizable. Use of large database data is subject to the accuracy of the data. Nonetheless, the data do represent 70% of the inpatient population who underwent mastectomy for locally advanced breast cancer; consequently, a pattern providing the national landscape of treatment trends among representative cancer patients is discernible. There is no information on delayed surgeries in the NCDB; therefore, overall CPM and breast reconstruction rates may be higher than those observed in this study. Additionally, the unavailable data elements, such as indications for CPM, family history, genetic predisposition, prior biopsies and magnetic resonance imaging of breast, limit our ability to explain the trend. A survey design incorporating various trade-offs may help elucidate factors associated with the decision to undergo CPM.
CONCLUSION
Increasing numbers of women with non-metastatic locally advanced unilateral breast cancer are choosing to undergo bilateral mastectomy. The increasing trend of CPM warrants further research investigating factors driving the trend including preferences of all stakeholders involved in the surgical decision making for women with locally advanced breast cancer.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations:
- AOR
adjusted odds ratio
- AJCC
American Joint Committee on Cancer
- ACS
American College of Surgeons
- CBC
contralateral breast cancer
- CPM
contralateral prophylactic mastectomy
- CoC
Commission on Cancer
- IBR
immediate breast reconstruction
- IRR
incidence rate ratio
- NCCN
National Comprehensive Cancer Network
- NCDB
National Cancer Database
- NOS
not otherwise specified
- OR
odds ratio
- pT
pathologic AJCC tumor stage
- SEER
Surveillance, Epidemiology, and End Results
- TNM
tumor-node-metastasis
- PUF
participant user file
Footnotes
Financial disclosures: None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Conference proceeding: This study was presented in part in poster format at the Annual Meeting of American Society for Reconstructive Microsurgery, January 13–16, 2018, Phoenix, AZ.
REFERENCES
- 1.Tuttle TM, et al. , Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend Toward More Aggressive Surgical Treatment. Journal of Clinical Oncology, 2007. 25(33): p. 5203–5209. [DOI] [PubMed] [Google Scholar]
- 2.Tuttle TM, et al. , Increasing Rates of Contralateral Prophylactic Mastectomy Among Patients With Ductal Carcinoma In Situ. Journal of Clinical Oncology, 2009. 27(9): p. 1362–1367. [DOI] [PubMed] [Google Scholar]
- 3.Arrington AK, et al. , Patient and Surgeon Characteristics Associated with Increased Use of Contralateral Prophylactic Mastectomy in Patients with Breast Cancer. Annals of Surgical Oncology, 2009. 16(10): p. 2697–2704. [DOI] [PubMed] [Google Scholar]
- 4.Nichols HB, et al. , Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol, 2011. 29(12): p. 1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edge, S.B.A.J.C.o.C., American Cancer Society, AJCC Cancer Staging Handbook: from the AJCC Cancer Staging Manual. 7th ed. 2010, New York, NY: Springer. [Google Scholar]
- 6.Dawood S, et al. , International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol, 2011. 22(3): p. 515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson FM, et al. , Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin, 2010. 60(6): p. 351–75. [DOI] [PubMed] [Google Scholar]
- 8.Dawood S, et al. , Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: a large population-based study. Cancer, 2011. 117(9): p. 1819–26. [DOI] [PubMed] [Google Scholar]
- 9.McIntosh SA, et al. , Local recurrence in patients with large and locally advanced breast cancer treated with primary chemotherapy. Am J Surg, 2003. 185(6): p. 525–31. [DOI] [PubMed] [Google Scholar]
- 10.Shen J, et al. , Effective local control and long-term survival in patients with T4 locally advanced breast cancer treated with breast conservation therapy. Ann Surg Oncol, 2004. 11(9): p. 854–60. [DOI] [PubMed] [Google Scholar]
- 11.Network, N.C.C., NCCN Clinical Practice Guideline in Oncology: Breast Cancer. 2016, National Comprehensive Cancer Network: Fort Washington(PA). [Google Scholar]
- 12.Morrow M, Prophylactic mastectomy of the contralateral breast. Breast, 2011. 20 Suppl 3: p. S108–10. [DOI] [PubMed] [Google Scholar]
- 13.Yao K, et al. , Trends in contralateral prophylactic mastectomy for unilateral cancer: a report from the National Cancer Data Base, 1998–2007. Ann Surg Oncol, 2010. 17(10): p. 2554–62. [DOI] [PubMed] [Google Scholar]
- 14.Pesce C, et al. , Contralateral prophylactic mastectomy provides no survival benefit in young women with estrogen receptor-negative breast cancer. Ann Surg Oncol, 2014. 21(10): p. 3231–9. [DOI] [PubMed] [Google Scholar]
- 15.Standards., F.O.R.D. Commission on Cancer. 2013; Available from: http://www.facs.org/cancer/coc/fordsmanual.html.
- 16.Williams RT, Stewart AK, and Winchester DP, Monitoring the delivery of cancer care: Commission on Cancer and National Cancer Data Base. Surg Oncol Clin N Am, 2012. 21(3): p. 377–88, vii. [DOI] [PubMed] [Google Scholar]
- 17.Miller ME, et al. , Contralateral Breast Cancer Risk in Women with Ductal Carcinoma In Situ: Is it High Enough to Justify Bilateral Mastectomy? Ann Surg Oncol, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albornoz CR, et al. , Bilateral Mastectomy versus Breast-Conserving Surgery for Early-Stage Breast Cancer. Plastic and Reconstructive Surgery, 2015. 135(6): p. 1518–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pesce CE, et al. , Changing surgical trends in young patients with early stage breast cancer, 2003 to 2010: a report from the National Cancer Data Base. J Am Coll Surg, 2014. 219(1): p. 19–28. [DOI] [PubMed] [Google Scholar]
- 20.Kummerow KL, et al. , Nationwide Trends in Mastectomy for Early-Stage Breast Cancer. JAMA Surgery, 2015. 150(1): p. 9. [DOI] [PubMed] [Google Scholar]
- 21.Hance KW, et al. , Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst, 2005. 97(13): p. 966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, et al. , Triple-negative subtype predicts poor overall survival and high locoregional relapse in inflammatory breast cancer. Oncologist, 2011. 16(12): p. 1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, et al. , A standard mastectomy should not be the only recommended breast surgical treatment for non-metastatic inflammatory breast cancer: A large population-based study in the Surveillance, Epidemiology, and End Results database 18. Breast, 2017. 35: p. 48–54. [DOI] [PubMed] [Google Scholar]
- 24.Hieken TJ, et al. , Influence of Biologic Subtype of Inflammatory Breast Cancer on Response to Neoadjuvant Therapy and Cancer Outcomes. Clin Breast Cancer, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Rosso KJ, et al. , Improved Locoregional Control in a Contemporary Cohort of Nonmetastatic Inflammatory Breast Cancer Patients Undergoing Surgery. Ann Surg Oncol, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Miller ME, et al. , Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol, 2013. 20(13): p. 4113–20. [DOI] [PubMed] [Google Scholar]
- 27.Momoh AO, et al. , Tradeoffs Associated With Contralateral Prophylactic Mastectomy in Women Choosing Breast Reconstruction: Results of a Prospective Multicenter Cohort. Ann Surg, 2017. 266(1): p. 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawley ST, et al. , Social and Clinical Determinants of Contralateral Prophylactic Mastectomy. JAMA Surgery, 2014. 149(6): p. 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantor O, et al. , The Shifting Paradigm for Breast Cancer Surgery in Patients Undergoing Neoadjuvant Chemotherapy. Ann Surg Oncol, 2018. 25(1): p. 164–172. [DOI] [PubMed] [Google Scholar]
- 30.Silva AK, et al. , The Effect of Contralateral Prophylactic Mastectomy on Perioperative Complications in Women Undergoing Immediate Breast Reconstruction: A NSQIP Analysis. Ann Surg Oncol, 2015. 22(11): p. 3474–80. [DOI] [PubMed] [Google Scholar]
- 31.Wilkins EG, et al. , Complications in Postmastectomy Breast Reconstruction: One-year Outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) Study. Ann Surg, 2018. 267(1): p. 164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berlin NL, et al. , Hospital Variations in Clinical Complications and Patient-reported Outcomes at 2 Years After Immediate Breast Reconstruction. Ann Surg, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Murphy BL, et al. , Contralateral Prophylactic Mastectomy for Women with T4 Locally Advanced Breast Cancer. Ann Surg Oncol, 2016. 23(10): p. 3365–70. [DOI] [PubMed] [Google Scholar]
- 34.Boughey JC, et al. , Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol, 2016. 23(10): p. 3100–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boughey JC, et al. , Contralateral Prophylactic Mastectomy Consensus Statement from the American Society of Breast Surgeons: Additional Considerations and a Framework for Shared Decision Making. Ann Surg Oncol, 2016. 23(10): p. 3106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicholas Z, et al. , A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast, 2016. 26: p. 31–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.