Abstract
IgG4-related disease (IgG4-RD) is a multi-organ chronic inflammatory process caused by infiltration of IgG4-positive plasma cells in one or more organs. Intracranial involvement has only recently become better recognized. Our case series adds to the growing literature on the varying presentations of intracranial IgG4 by describing the clinical and imaging findings of three patients who presented to our institution with intracranial involvement. Our first patient presented with a mass-forming IgG4 pachymeningitis mimicking a sphenoid wing meningioma, which is to our knowledge the largest mass-forming pachymeningitis published in the literature. Our second case depicts another presentation of extensive IgG4 pachymeningitis involving both cavernous sinuses and surrounding Meckel’s caves. The third case describes a patient with presumed lymphocytic hypophysitis, which was later determined to be IgG4-related hypophysitis with concomitant pachymeningitis and perineural spread along the optic nerves. The delayed diagnoses in our cases illustrates the diagnostic challenge that clinicians face in differentiating intracranial IgG4-RD from other infiltrative diseases such as sarcoidosis, granulomatous disease, tuberculosis and lymphoma. Earlier consideration of IgG4-related hypophysitis and hypertrophic pachymeningitis in the differential diagnosis can prevent significant morbidity including unnecessary surgical intervention and organ failure secondary to extensive fibrosis.
Keywords: Hypophysitis, IgG4 disease, intracranial, pachymeningitis
Introduction
Immunoglobulin G4 (IgG4)-related disease (IgG4-RD) is an increasingly recognized multi-systemic, chronic inflammatory process characterized by sclerosing inflammation and fibrosis caused by infiltration of IgG4-positive plasma cells in one or multiple organs.1 The pancreas is the most commonly affected organ but involvement of other organs including the kidneys, lungs, thyroid, salivary glands and orbits has been well documented.1,2
A number of review articles have been published on the various manifestations of IgG4-RD in the head and neck.3,4 Intracranial IgG4-RD has been described in patients presenting with clinical and/or imaging features of hypophysitis, pachymeningitis, inflammatory pseudotumors, perineural spread and cerebral parenchymal involvement.5–8
Establishing a diagnosis of intracranial IgG4-RD is often delayed in part due to its non-specific imaging features mimicking more common infiltrative processes including sarcoidosis, granulomatous disease, tuberculosis and lymphoma.9 We report three cases of intracranial IgG4 in patients who presented to our institution. The cases illustrate a spectrum of intracranial involvement and the time course from initial presentation to diagnosis highlights the diagnostic challenge that IgG4-RD presents clinicians with.
Case series
Among the 32 patients who presented to our institution between 2004 and 2016 with IgG4-RD, three (9%) were identified to have intracranial involvement. The clinical course and imaging follow-up of these three patients are summarized.
Case 1
A 70-year-old man with no previous medical history presented with acute onset of diplopia and was admitted for stroke evaluation. He had no headache, speech disturbance, or motor dysfunction. Initial computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated no evidence of acute cerebral ischemia but revealed a large avidly enhancing extra-axial mass overlying the left fronto-temporal region with associated mild overlying hyperostosis (Figure 1). This lesion was thought to be a meningioma based on its imaging appearance. The patient was initially managed conservatively but developed seizures and ultimately underwent resection of the lesion. The intraoperative appearance of the mass was also felt to be consistent with a meningioma. However, histopathological evaluation revealed mass-forming IgG4 pachymeningitis (Figure 2). Serum IgG4 levels were found to be elevated at 1.52 g/L (normal range 0.039–0.864 g/L). Investigation for other systemic manifestations of IgG4 disease was negative. Corticosteroid treatment was initiated but was not tolerated by the patient. Follow-up MRI one year later did not reveal recurrence.
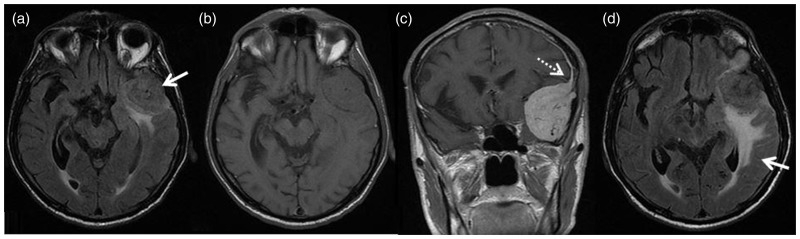
Figure 1.
Axial T2 fluid-attenuated inversion recovery (FLAIR); large left anterior temporal extra-axial mass (arrow) with associated vasogenic edema (a). Axial T1WI; the lesion is isointense to grey matter with no evidence of acute hemorrhage (b). Post-gadolinium injection coronal T1WI; the lesion demonstrates avid homogeneous enhancement with an apparent “dural tail” (dashed arrow) extending superiorly (c). Axial T2 FLAIR one year later; redemonstrates the anterior temporal extra-axial mass with significant increase in vasogenic edema in the temporal lobe (arrow) (d).
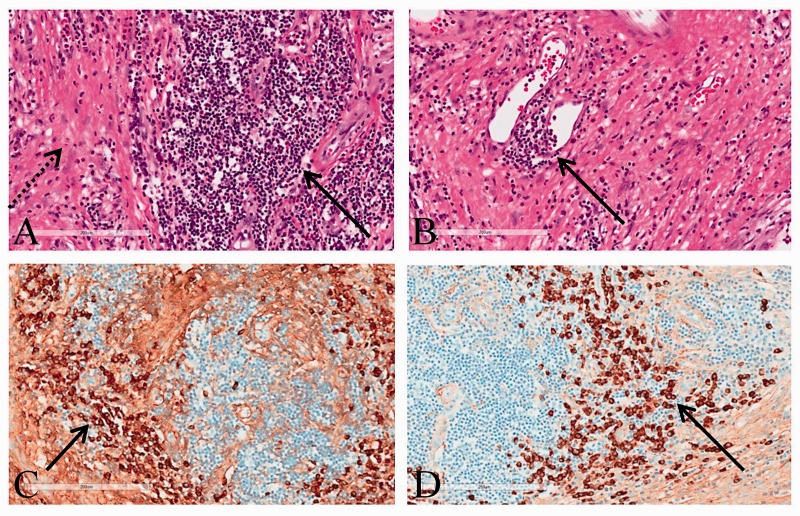
Figure 2.
Hematoxylin-eosin stain demonstrates a mixed inflammatory cell infiltrate of lymphocytes, plasma cells and eosinophils forming nodules (solid arrow), on a background of a sclerotic fibrocollagenous stoma (dashed arrow) (a). There is obliterative phlebitis, with inflammatory cells grouped around venules (arrow) (b). Immunostaining demonstrates many of the plasma cells label for IgG (arrow) (c). Immunostaining stain demonstrates most of the plasma cells label for IgG4 (with the IgG4/IgG ratio 91%) (d).
Case 2
A 54-year-old man with a past history of chronic sagittal sinus thrombosis, remote right frontal infarction with resulting left-sided weakness, and seizures presented with a one-year history of headaches. MRI demonstrated T2 hypointense and avidly enhancing infiltrating tissue within both cavernous sinuses and surrounding Meckel’s caves (Figure 3(a) and (b)) and dural thickening around the chronically occluded sagittal sinus (Figure 3(c) and (d)). There was narrowing of both cavernous internal carotid arteries (ICAs) (Figure 3(e)). A dural tail was visualized along the dorsum sellae and along the anterior tentorium cerebelli. Extension into the left orbital apex resulted in mild medial displacement of the left optic nerve.
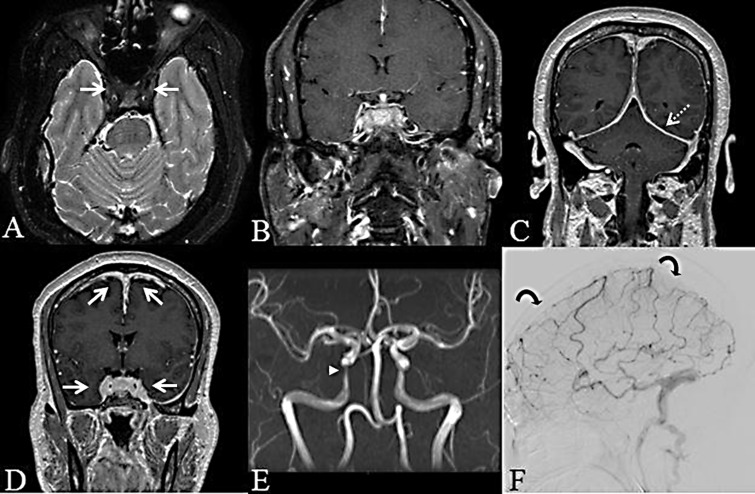
Figure 3.
Axial fat-suppressed T2WI; diffuse hypointense material filling both cavernous sinuses (arrow) (a). Post-gadolinium coronal fat-suppressed T1WI; avid diffuse enhancement of the infiltrative lesion that is low in T1 signal intensity prior to contrast (not shown) and ring enhancement of Meckel’s cave bilaterally, with “collapse” of the right Meckel’s cave (b). Coronal post-gadolinium T1WI; diffuse pachymeningeal enhancement involving the falx cerebri and tentorium cerebelli (dashed arrow) (c). Coronal post-gadolinium T1WI; diffuse nodular thickening around the superior sagittal and cavernous sinuses (arrows) (d). Time-of-flight magnetic resonance angiography (MRA); progressive narrowing of the petrocavernous portion of both internal carotid arteries more marked on the right (arrowhead) (initial MRA not shown) (e). Digital subtraction angiography venous phase; occlusion of the superior sagittal sinus (curved arrows), proximal transverse sinus and deep venous system (f).
The differential diagnosis based on imaging was broad including inflammatory pseudotumor, granulomatous inflammation (such as sarcoidosis), idiopathic hypertrophic pachymeningitis, metastases, meningioma and lymphoma. Infection screen was negative. A dural and arachnoid excisional biopsy via a right parietal craniotomy was negative for neoplasia and infection. Collagenous tissue was present within the specimen and there were scattered positive IgG plasma cells in the dura.
The disease was quiescent for five years. The patient then developed progressive left eye ptosis, vision loss and headaches. Neuro-ophthalmologic assessment revealed chronic left central retinal artery occlusion. Repeat MRI demonstrated disease progression with increased soft tissue infiltration in the left cavernous sinus, left middle cranial fossa and left optic canal. A cerebral angiogram excluded an arteriovenous shunt and redemonstrated smooth narrowing of the bilateral cavernous ICAs and the known chronic dural venous sinus thrombosis (Figure 3(f)).
Rheumatologic and infectious disease workup showed increased erythrocyte sedimentation rate at 65 mm/h (normal range 0–15 mm/h) and C-reactive protein at 95 mg/L (normal range 0–5 mg/L). Lumbar puncture was normal apart from an elevated protein of 1.55 g/L (normal range 0.15–0.45 g/L). Serum IgG4 was mildly elevated at 0.927 g/L.
Given the elevated serum IgG4 and presence of IgG-staining plasma cells in the dura at the time of open biopsy, the unifying diagnosis was that of IgG4 pachymeningitis. Management with oral prednisone and long-term tapering immunosuppression with azathioprine was initiated, which suppressed the inflammatory markers and serum biochemistry. Two months following initiation of treatment, there was reduction in the degree of dural enhancement and resolution of the temporal lobe vasogenic edema. Notably, the degree of pachymeningitis was stable. Clinically, there was an improvement in the patient’s headache with no new neurological symptoms and no left-sided visual loss.
Case 3
A 28-year-old woman presented with progressive headaches and sudden loss of vision in the left eye. MRI of the brain revealed a sellar lesion with suprasellar extension compressing the optic nerve and chiasm (Figure 4(a)). Endocrine studies did not reveal any pituitary hormonal abnormalities. The patient underwent left frontal craniotomy for biopsy and decompression of the optic nerve, which demonstrated a lymphocytic hypophysitis. Oral tapering prednisone was initiated and the patient’s headaches resolved. Post-operatively, she could count fingers with the left eye.
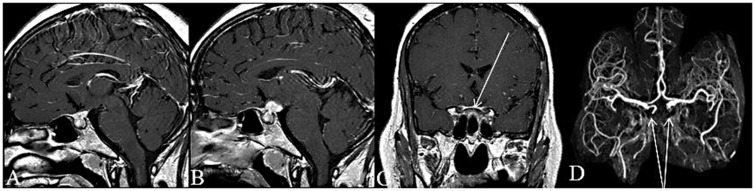
Figure 4.
Post-gadolinium sagittal T1WI; enhancing mass in the sellar/suprasellar region (a). Post-gadolinium sagittal T1WI eight years later; increase in size of the sellar/suprasellar mass (b). Post-gadolinium coronal T1WI; dural enhancement (arrow) extending along to the optic canals bilaterally (c). 3D time-of-flight magnetic resonance angiography; occlusion of the cavernous portion of both internal carotid arteries (arrows) (d).
Two years later, her symptoms returned and MRI brain demonstrated very mild increase in the size of the sellar mass. Oral prednisone was again initiated with tapering over 18 months. At the time, the working diagnosis remained lymphocytic hypophysitis. Evaluation for sarcoidosis was negative.
The patient remained well for seven years and then experienced worsening headaches, deterioration in left eye vision and painful eye movements. MRI brain showed enlargement of the sellar lesion with further suprasellar extension (Figure 4(b)). There was increased compression of the optic chiasm with new associated edema. Extension along the intracranial portion of both optic nerves and further dural enhancement along the tuberculum sellae and planum sphenoidale was seen (Figure 4(c)). Magnetic resonance angiography demonstrated new bilateral cavernous ICA occlusion (Figure 4(d)). Repeat biopsy and debulking of the sellar/suprasellar mass revealed a dense lymphoplasmacytic infiltrate with IgG4-positive plasma cells throughout. Serum IgG4 was mildly elevated at 0.88 g/L. The diagnosis of IgG4-related pachymeningitis was finally made. Oral immunosuppression with methotrexate and rituximab was initiated and the patient had an excellent course, with significant improvement in headaches and few exacerbations over the three years following definitive diagnosis. Serum IgG4 levels returned to normal range and the patient was considered to be in clinical remission.
Discussion
IgG4-RD is an immune mediated fibro-inflammatory disease of unknown cause characterized by infiltration of one or more target organs by IgG4-positive plasma cells and lymphocytes.10,11 The pancreas, which is the most commonly involved organ, was first described as autoimmune pancreatitis in 1995 and found to be associated with elevated levels of IgG4 in 2001.2,12,13 In 2003, Kamisawa et al. postulated that IgG4-RD is a systemic condition affecting many extrapancreatic organs, including but not limited to the bile ducts, gallbladder, lymph nodes, retroperitoneum, kidneys, breasts, prostate, lungs and skin.14
The pathogenesis of IgG4-RD is still not well understood. Out of the four IgG antibodies, IgG4 constitutes <5% of the total IgG in healthy individuals and has traditionally been associated with both autoimmune and allergic diseases.2,10,15
Diagnosis of IgG4-RD is made using a combination of clinical, imaging, serological and pathological information.16,17 In 2011, Umehara et al. proposed the following diagnostic criteria: (a) diffuse or localized swelling or masses in one or more organs; (b) elevated serum IgG4 levels (≥135 mg/dL); (c) dense lymphoplasmacytic infiltration, storiform fibrosis and/or obliterative phlebitis along with IgG4-positive plasma cells (>10–50 per high power field (hpf) depending on the organ or ratio of IgG4+/IgG+ of >40%) on histopathologic examination of the affected organs.11 The diagnosis is definite if all three criteria are fulfilled; probable if (a) and (c) are present and possible if (a) and (b) are met. Lindstrom et al. suggest that a cutoff of >10 IgG4-positive cells/hpf be used for IgG4-related neurological disease.18 Presence of elevated IgG4 in the serum is a weak diagnostic feature as up to 50% of patients with IgG4-RD have normal levels12 and many other diseases have been associated with elevated serum IgG4 levels, such as parasitic infections, autoimmune diseases (i.e. Castleman’s disease, rheumatoid arthritis and antineutrophil cytoplasmic antibody (ANCA) – associated vasculitis), allergic diseases and pancreatic cancer.19 The 2011 diagnostic criteria are also applicable to IgG4 neurological disease with the addition of cerebrospinal fluid (CSF) analysis, which can rule in IgG4 disease imitators and sometimes show intrathecal IgG4. CSF in patients with known IgG4-related pachymeningitis usually shows clear fluid with normal glucose levels, normal to mildly elevated protein concentrations and variable lymphocytosis. Many case reports have also documented oligoclonal bands with intrathecal IgG4 production.16
Involvement of the central nervous system by IgG4-RD is relatively uncommon but increasingly recognized with hypophysitis and hypertrophic pachymeningitis being the most frequent manifestations. Less commonly, inflammatory pseudotumor, IgG4-related neuropathy and IgG4-related perineural disease have also been documented.16 IgG4-related neurological disease, like the systemic disease, usually has a benign clinical course with excellent response to glucocorticoids.2 Response to glucocorticoids is best early in the course of disease, before extensive fibrosis develops. As a result, accurate and rapid diagnosis of IgG4-related neurological disease is necessary to prevent unnecessary surgical intervention and organ failure. Relapse after steroid withdrawal or during tapering or maintenance doses is common and various steroid-sparing agents may need to be added to the pharmacological regimen.16
Inflammatory pseudotumor is defined as a mass lesion that can present like a neoplastic tumor. Although the orbits, salivary glands, retroperitoneum, lymph nodes, lungs and kidneys are the most common organs of involvement, structures within the central nervous system can be affected on rare occasions. The meninges (imitating meningioma), ventricles, pituitary gland, cranial nerves and brain parenchyma are all sites which have been documented in the literature.16
IgG4-related pachymeningitis is a type of hypertrophic pachymeningitis involving inflammation along with diffuse or focal thickening of the meninges (usually dura mater) in the brain and/or spinal cord.16,20 Symptoms of IgG4-related hypertrophic pachymeningitis may be due to local disease causing mechanical compression/entrapment of adjacent structures and can vary depending on the anatomical location. When the cavernous sinus is involved, as depicted in our second case, cranial nerve palsies, retro-orbital pain, visual disturbance (diplopia or decreased acuity) and extraocular muscle palsies have been reported. IgG4-RD within the middle cranial fossa, cerebellar tentorium and posterior cranial fossa can cause cranial nerve VI–XII palsies and/or cerebellar ataxia. Diffuse disease of the dura mater causes generalized symptoms, such as chronic headaches, seizures and cognitive impairment.6,16,21
On imaging, the differential diagnosis for IgG4-related pachymeningitis is broad and includes vasculitic conditions (such as granulomatosis with polyangiitis (GPA), Behcet’s disease, giant cell arteritis), inflammatory conditions (i.e. rheumatoid arthritis and sarcoidosis), malignancies such as lymphoma and infectious etiologies such as tuberculosis.20 Hypertrophic pachymeningitis can appear as localized or diffuse dural thickening or as a focal mass. On T2-weighted MRI, lesions are usually hypointense consistent with fibrosis and demonstrate homogenous and gradual gadolinium enhancement on T1-weighted images (consistent with inflammation).16,22,23 On CT, dural lesions are hypoattenuating and demonstrate contrast enhancement. Bones adjacent to the lesions are better evaluated on CT and can show remodeling (erosion or sclerosis).22,23 Lymphadenopathy is also often seen although it is not clear whether this is part of the disease or reactive.22
IgG4-related hypophysitis was first diagnosed clinically in 2004 and with pathological evidence in 2007.15 It is characterized by inflammation of the pituitary gland, which may or may not involve connecting structures. Prevalence is highest in middle-aged and elderly males. Patients with IgG4-related hypophysitis commonly present with panhypopituitarism, although diabetes insipidus and anterior hypopituitarism can also occur. Associated symptoms include fatigue, anorexia, weight loss, polyuria, polydipsia, menstrual disturbances and reduced libido.16,24 Differential considerations for IgG4-related hypophysitis include sarcoidosis, GPA, Langerhans cell histiocytosis, tuberculosis and malignancy.15,16,24 Other manifestations of IgG4-RD, such as retroperitoneal fibrosis or lymphadenopathy, if present, can help confirm the diagnosis of IgG4-related hypophysitis although IgG4-hypophysitis can also occur alone.16 On imaging, IgG4-related hypophysitis can present with thickening of the pituitary stalk, enlargement of the pituitary gland and/or presence of sellar mass. Lesions within the pituitary are hypointense on T2-weighted MRI images and enhance homogeneously. Lack of the bright spot in the posterior pituitary on T1 imaging may indicate central diabetes insipidus, although clinical/serological correlation is required.15,16,23
Our first case contributes to the few published cases in the literature of intracranial IgG4-related inflammatory pseudotumors that mimic meningioma on imaging. Nishino et al.7 and Kanno et al.25 were the first to describe supratentorial meningioma-like lesions. Lal et al. report a case series of 16 inflammatory meningiomas in which two cases demonstrated tumors with >10 IgG4-positive plasma cells/hpf and IgG4/IgG ratio was focally increased in two cases up to 31%.8 To our knowledge, our first case is the largest IgG4-related supratentorial mass mimicking a meningioma published in the literature. Further, the imaging features of the mass in our patient appear strikingly similar to the classical imaging appearance of a meningioma including homogeneous enhancement and broad dural tail. Few reports of IgG4-related pachymeningitis have also demonstrated hyperostosis as seen in our case.26
The second case depicts extensive pachymeningitis involving the cavernous sinus with dural extension over the tentorium and surrounding Meckel’s cave bilaterally. Although histopathologic diagnosis was not definitive, IgG4 pachymeningitis was strongly suspected given the mildly elevated serum IgG4 levels along with improvement following steroids. IgG4-related pachymeningitis has been reported6,27 and felt to represent a subset of idiopathic hypertrophic pachymeningitis, which was first described by Naffziger and Stern in 1949.28The dural thickening seen in hypertrophic pachymeningitis may result in compromise of regional cranial nerves or vascular structures, with variable clinical manifestations including headache, raised intracranial pressure, cranial nerve deficits and ischemia.29
The third case highlights the difficulty in and often delayed diagnosis of IgG4-related hypophysitis. A recent case report by Ngaosuwan et al. discusses some features that can be used to distinguish between IgG4-related and lymphocytic hypophysitis and include a relapsing clinical course with steroids and concomitant pachymeningitis.5 Our patient demonstrated clinical relapses and developed pachymeningitis with perineural spread along the optic nerves. IgG4-RD should be more readily considered on the differential diagnosis in patients presenting with symptoms suggestive of pituitary involvement and/or imaging features consistent with hypertrophic hypophysitis.
IgG4-related neurological disease should be considered in the differential diagnosis of diffuse involvement and thickening of the meninges (hypertrophic pachymeningitis) and prominence of the pituitary stalk with or without thickening of the pituitary gland (hypophysitis) on imaging. This case series adds to the growing literature depicting the varying patterns of intracranial involvement of IgG4-RD that remains a diagnostic challenge to both radiologists and clinicians given its non-specific imaging features and clinical presentations.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Vlachou PA, Khalili K, Jang HJ, et al. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics 2011; 31: 1379–1402. [DOI] [PubMed] [Google Scholar]
- 2.Martínez-de-Alegría A, Baleato-González S, García-Figureueiras R, et al. IgG4-related disease from head to toe. Radiographics 2015; 35: 2007–2025. [DOI] [PubMed] [Google Scholar]
- 3.Mulholland GB, Jeffery CC, Satija P, et al. Immunoglobulin G4-related diseases in the head and neck: a systematic review. J Otolaryngol Head Neck Surg 2015; 44: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita A, Sakai O, Chapman MN, et al. IgG4-related disease of the head and neck: CT and MR imaging manifestations. Radiographics 2012; 32: 1945–1958. [DOI] [PubMed] [Google Scholar]
- 5.Ngaosuwan K, Trongwongsa T, Shuangshoti S. Clinical course of IgG4-related hypophysitis presenting with focal seizure and relapsing lymphocytic hypophysitis. BMC Endocr Disord 2015; 15: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu LX, Della-Torre E, Stone JH, et al. IgG4-related hypertrophic pachymeningitis: clinical features, diagnostic criteria, and treatment. JAMA Neurol 2014; 71: 785–793. [DOI] [PubMed] [Google Scholar]
- 7.Nishino T, Toda J, Nakatsuka T, et al. IgG4-related inflammatory pseudotumors mimicking multiple meningiomas. Jpn J Radiol 2013; 31: 405–407. [DOI] [PubMed] [Google Scholar]
- 8.Lal A, Dahiya S, Gonzales M, et al. IgG4 overexpression is rare in meningiomas with a prominent inflammatory component: a review of 16 cases. Brain Pathol 2014; 24: 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenderson J, Berenberg J, Tom L, et al. IgG4-related disease: imitating a great imitator. Hawaii J Med Public Health 2015; 74: 22–26. [PMC free article] [PubMed] [Google Scholar]
- 10.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366: 539–551. [DOI] [PubMed] [Google Scholar]
- 11.Umehara H, Okazaki K, Masaki Y, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012; 22: 21–30. [DOI] [PubMed] [Google Scholar]
- 12.Thompson A, Whyte A. Imaging of IgG4-related disease of the head and neck. Clin Radiol 2018; 73: 106–120. [DOI] [PubMed] [Google Scholar]
- 13.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001; 344: 732–738. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol 2003; 38: 982–984. [DOI] [PubMed] [Google Scholar]
- 15.Leporati P, Landek-Salgado MA, Lupi I, et al. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab 2011; 96: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baptista B, Casian A, Gunawardena H, et al. Neurological manifestations of IgG4-related disease. Curr Treat Options Neurol 2017; 19: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto M, Hashimoto M, Takahashi H, et al. IgG4 disease. J Neuroophthalmol 2014; 34: 393–399. [DOI] [PubMed] [Google Scholar]
- 18.Lindstrom KM, Cousar JB, Lopes MB. IgG4-related meningeal disease: clinico-pathological features and proposal for diagnostic criteria. Acta Neuropathol 2010; 120: 765–776. [DOI] [PubMed] [Google Scholar]
- 19.Ebbo M, Grados A, Bernit E, et al. Pathologies associated with serum IgG4 elevation. Int J Rheumatol 2012; 2012: 602809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim EH, Kim SH, Cho JM, et al. Immunoglobulin G4-related hypertrophic pachymeningitis involving cerebral parenchyma. J Neurosurg 2011; 115: 1242–1247. [DOI] [PubMed] [Google Scholar]
- 21.Wallace ZS, Carruthers MN, Khosroshahi A, et al. IgG4-related disease and hypertrophic pachymeningitis. Medicine (Baltimore) 2013; 92: 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katsura M, Mori H, Kunimatsu A, et al. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology 2012; 54: 873–882. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda K, Oba H, Kutomi K, et al. MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol 2012; 33: 2136–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshi D, Jager R, Hurel S, et al. Cerebral involvement in IgG4-related disease. Clin Med (Lond) 2015; 15: 130–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanno H, Tanino M, Watanabe K, et al. Intracranial mass-forming lesion associated with dural thickening and hypophysitis. Neuropathology 2013; 33: 213–216. [DOI] [PubMed] [Google Scholar]
- 26.Lee YS, Lee HW, Park KS, et al. Immunoglobulin G4-related hypertrophic pachymeningitis with skull involvement. Brain Tumor Res Treat 2014; 2: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan SK, Cheuk W, Chan KT, et al. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol 2009; 33: 1249–1252. [DOI] [PubMed] [Google Scholar]
- 28.Naffziger HC, Stern WE. Chronic pachymeningitis; report of a case and review of the literature. Arch Neurol Psychiatry 1949; 62: 383–411. [PubMed] [Google Scholar]
- 29.Schubert RD, Wood M, Levin MH, et al. The severe side of the IgG4-related hypertrophic pachymeningitis disease spectrum. Neurol Neuroimmunol Neuroinflamm 2016; 3: e197. [DOI] [PMC free article] [PubMed] [Google Scholar]