Abstract
Purpose
Spinal sarcoidosis, referring to involvement of the spine in sarcoidosis, is relatively rare and may mimic other neurological disease affecting the spine. The authors present a clinic radiological review of 18 spinal sarcoidosis patients who presented to a tertiary hospital, with emphasis on initial imaging and radiological response to treatment.
Materials and methods
We retrospectively reviewed our departmental imaging archives over a 15-year period and found 49 cases of neurosarcoidosis out of which 18 patients had spinal magnetic resonance imaging.
Results
Approximately 72% (13/18) of the neurosarcoidosis patients showed some form of spinal involvement. The clinical, epidemiological and imaging data were reviewed for these 13 patients at presentation and follow-up. The findings on magnetic resonance imaging included leptomeningeal enhancement (61%), pachymeningeal (23%), intramedullary enhancing lesions (38%) and bony involvement (15%). The cervical segment was most frequently involved followed by the thoracic segment. Involvement was often long segment (4.2 spinal segments) with proclivity for the dorsal cord. Mean follow-up was 23.2 months. A complete or near-complete radiological response occurred in 66% while partial response was seen in 25% patients. Four patients had isolated central nervous system involvement including one with isolated spinal cord involvement. On diffusion-weighted imaging, the apparent diffusion coefficient of intramedullary lesions was increased compared to normal-appearing cord on baseline and subsequent follow-up scans.
Conclusions
Spinal sarcoidosis was previously considered uncommon but is being increasingly recognized with widespread use of magnetic resonance imaging. Proclivity for dorsal surface involvement is characteristic, although not necessarily pathognomonic. Also, quantitative diffusion studies may serve as a biomarker for the disease activity and parenchymal injury.
Keywords: Neurosarcoidosis, spinal sarcoidosis, MRI, bone sarcoidosis
Introduction
Sarcoidosis is a chronic idiopathic, multisystem inflammatory disorder with a global annual incidence of approximately 10–20 per 100,000 persons.1 Sarcoidosis most frequently involves the lungs, skin and lymph nodes. Central nervous system (CNS) involvement is uncommon, occurring symptomatically in 5–15% of sarcoidosis patients and in about 25% of patients with systemic sarcoidosis on autopsy studies.2 Spinal cord involvement occurring together with CNS involvement has traditionally been considered uncommon, while isolated spinal sarcoidosis (SS) is much rarer. Spinal cord involvement is non-specific, and the diagnosis is often difficult when the spinal involvement is the first clinical presentation especially in the absence of systemic sarcoidosis. The true prevalence of SS is unknown, although it has traditionally been reported in less than 1% of all sarcoidosis patients. Scattered case reports and small retrospective studies have previously described imaging findings in SS which include intramedullary lesions, cord edema, arachnoiditis and pachymeningitis.3–14 These studies have reviewed the follow-up spinal imaging for any intramedullary T2 signal changes and contrast enhancement with no other well-defined imaging biomarkers which may reflect clinical severity, response to therapy or residual neurological deficits. However, most of these studies only have a smaller number of patients and are retrospective in nature.3–14 In addition to T2 signal changes and contrast enhancement we have also evaluated the diffusion properties of intramedullary lesions which has not been attempted by previously reported studies to our knowledge.
The goal of the present study was to determine the prevalence of spinal involvement in patients with neurosarcoidosis at the time of initial diagnosis, to describe the imaging findings at presentation and on follow-up and to evaluate the apparent diffusion coefficient (ADC) values of intramedullary lesions compared to normal-appearing cord on initial and subsequent follow-up scans. We hypothesized that the spinal involvement in neurosarcoidosis patients is likely to be significantly higher than previously reported in the literature. We also assumed that despite the resolution of imaging findings with therapy, patients may show persistent neurological deficits which could reflect either ongoing inflammation at the microscopic level versus permanent injury.
Materials and methods
The study was approved by the institutional review board and involved a retrospective search of the departmental imaging archives using specific keywords, and has previously been described in more detail elsewhere.15 The clinical diagnosis of neurosardoidosis depends on the ‘Zajicek criteria’ according to which it is ‘definite’ if neural biopsy is positive for typical sarcoid granuloma with Langerhans-type giant cells, ‘probable’ if CNS inflammation is associated with systemic disease and ‘possible’ in the presence of typical clinical presentation with the exclusion of other potential causes.16 Briefly, we found 49 cases of neurosardoidosis (11 ‘definite’, 34 ‘probable’ and four ‘possible’) who presented to our institute over a 15-year period. Of these, spine magnetic resonance imaging (MRI) scans were available in a total of 18 patients out of which 13 patients showed some form of spinal involvement. Imaging findings of these 13 patients were co-reviewed at presentation and on follow-up by two fellowships trained radiologists(GB, NS) on the standard PACS workstation with a combined experience of greater than 10 years in neuroimaging. In addition to imaging findings, clinical and demographic data were also recorded (Table 1).
Table 1.
Demographic, clinical and imaging features of reported 13 patients with spinal sarcoidosis.
| Patient number | Age/ sex/race | Zajicek criteria/initial presentation – acute, subacute, chronic/symptoms | Signs of myelopathy – 1. sensory; 2. motor; 3. sphincter | Available spine MRI | MRI spine findings | Diffusion-weighted image findings | Intramedullary lesion/normal cord ADC values: 1. on presentation; 2. on follow-up | Follow-up MRI studies: duration (months)/complete, partial and no resolution of imaging findings/treatment | Immunosuppressive treatment used | Brain MRI findings | Extra CNS involvement: 1. pulmonary; 2. extrapulmonary; 3. Both pulmonary and extrapulmonary; 4. isolated CNS | Extraneural and neural biopsy sites |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/F/C | 2/Acute/painful tight sensation in a band across chest; diminished temperature sensation; episode of abrupt numbness and weakness in left arm and face; acute left side motor deficits 2nd episode: increasing back pain | 1 &2 | C+T | 1st episode: multiple foci of enhancement throughout the vertebral bone marrow. Pathological collapse of T7. No abnormal cord signal and enhancement 2nd episode: near complete resolution of enhancement | No DWI abnormalities | DWI not available | 11 month/complete/long-term treatment related complications- multiple chronic vertebral fractures Patient had total 11 follow-ups MRI spine over a period of 12 years for long-term steroid-related complications | 1st episode: prednisone 2nd episode: maintenance methotrexate and tapering prednisone | Multiple FLAIR signal foci in the subcortical, deep, and periventricular white matter | 3/Vertebra & mediastinal lymph node involvement | T7 Vertebral bone, mediastinal lymph node |
| 2 | 35/F/C | 2/Chronic/neck swelling for past 2years, migratory myalgias, chronic numbness and tingling in arm and leg | 1 | C+T+L | 1st episode: multiple enhancing lesions throughout spine including pelvic bones. LME of conus 2nd episode: complete resolution | Slight restriction on DWI, increased ADC | DWI not available | 28 month/complete imaging resolution clinical improvement | 1st episode: high dose prednisone 2nd episode: tapering prednisone | Osteolytic foci and asymmetric abnormal enhancement of calvarium and clivus. No brain parenchymal abnormalities | 3/Bone, lungs | Cervical lymph node, iliac and vertebral bones |
| 3 | 54/F/C | 1/Acute/blurry vision, diplopia, ataxia and disequilibrium, bilateral lower leg weakness 2nd episode: new left-sided weakness, urinary incontinence | 2,3 | C+T+L | 1st episode: whole spinal cord patchy nodular pachymeningeal enhancement. 2nd episode: L spine MRI: normal cord with minimal residual LME | No restriction on DWI | DWI not available | 5 month/ minimal residual LME/Mild lower extremity weakness | 1st episode: high dose prednisone 2nd episode: maintenance prednisone | Few periventricular FLAIR signal, two foci of subacute infarction, nodular LME of the posterior fossa | 4 | Lumbar arachnoid and dura |
| 4 | 31/F/AA | 2/Subacute/left lower extremity weakness and numbness in L5 distribution, right Horner's syndrome. 2nd episode: right upper and left lower extremity painful paresthesia with intermittent blurry vision, intensity of these issues had magnified with numerous bursts and lapses of symptoms | 1,2 | 1. T+L (non-contrast, pregnant patient) | 1st episode: extramedullary mass along left L5 nerve (reported as neurofibroma first), normal cord. 2nd episode: diffuse LME from T12–S1 and along the L4–L5 and L5–S1 nerve roots | No restriction on DWI | DWI not available | 24 month/Clinical worsening MRI: partial response with residual diffuse LME along cauda equina | 1st episode: high dose prednisone 2nd episode: infliximab and high dose prednisone | Diffuse LME and pachymeningeal enhancement Cranial nerve 3, 5, 7, 8th enhancement | 3/Abdominal lymphadenopathy | Right pericarotid soft tissue/intraparotid lymphnode in 2008 |
| 5 | 48/F/C | 2/Acute/numbness and tingling sensation in right arm followed by left arm and mild leg weakness | 1/2 | 1. C+T+L | C7-T2 intramedullary (panmedullary) granulomas with edema and cord expansion | No restriction on DWI | 1. 1.40 ± .17/1.01 ± .15 2. 1.64 ± .07/1.23 ± .04 | 4mth/Mild residual lower extremity weakness and numbness. MRI: complete resolution | High dose prednisone followed by maintenance prednisone | Enhancement along the right fornix | 3 | Mediastinal lymph nodes |
| 6 | 67/M/C | 2/Chronic/light headedness, right eye proptosis, balance and gait problem, lower extremity weakness | 2 | 1. C+T+L | Diffuse LME and pachymeningeal enhancement | No restriction on DWI | DWI not available | 9 month/Residual symptoms of mild lower extremity weakness/MRI: complete resolution | High dose prednisone followed by maintenance prednisone | Multifocal FLAIR abnormalities, granulomas in left occipital, midbrain and pons and diffuse LME | 3 | Lung endobronchial, Right conjunctiva |
| 7 | 42/M/C | 1st episode: 1/subacute; intermittent cramping in legs, shooting sensation in lower back down to feet, urinary retention 2nd episode: continued to have fatigue and lower extremity weakness | 1,2,3, | 1. C+T+L | Enhancing granuloma (panmedullary) in the distal cord T11–L2 with edema and expansion/scattered LME throughout most prominent along cauda equina | Slight DWI restriction, increased ADC | 1. 1.27 ± .09/1.19 ± .18 2. 1.31 ± .01/1.23 ± .02 | 4mth/Residual weakness and neurogenic bladder/residual intramedullary lesion with partial resolution | 1st episode: high dose pulse dexamethasone despite on full dose of methotrexate plus hydroxychloroquine and low dose of prednisone | Parenchymal hemorrhage, subtle LME in posterior fossa and occipital lobes | 4 | Cervical cord biopsy |
| 8 | 48/F/C | 1/Subacute/syncope, dizziness, upper motor neuron signs bilateral lower extremity weakness and bowel/bladder incontinence | 2,3 | 1.C+T+L | Diffuse LME and enhancement of the nerve roots and cauda equina | No DWI abnormalities | DWI not available | 38 month/Significant clinical improvement on steroids and methotrexate/complete imaging resolution | Prednisone and methotrexate, weaned off after being stable | Extensive T2/FLAIR hyperintensities/ perivascular enhancement/ intraparenchymal bleed, LME in posterior fossa and occipital lobes | 4 | Conus |
| 9 | 60/F/C | 2/Acute/experienced whiplash like effect with unsteadiness and weakness of lower extremity | 2 | C | Cervicomedullary to C5 intramedullary (central and posterior) granulomas. Diffuse LME | Slight DWI restriction, increased ADC | 1. 1.18 ± .02/1.16 ± .10 2. 1.21 ± .07/1.23 ± .04 | 28 month/Clinically improved with mild vertigo/complete imaging resolution | High dose IV methylprednisolone with tapering dose | Diffuse LME, cerebellar granulomas | 3 | Mediastinal lymphnode |
| 10 | 56/M/C | 1/Chronic daily headache with recent worsening of headache and vision | - | C | Cervical pachymeningeal enhancement | No DWI abnormalities | DWI not available | 5 month/Clinically improved/residual mild enhancement | Steroid & methotrexate | Pachymeningeal enhancement in temporo-occipital region | 3 | Brain Lymphnode and liver |
| 11 | 34/M/C | 2/Chronic/poor balance, weakness, Left foot drop | 2 | C+T+L | Posterior intramedullary lesion at C3–C7, LME | Slight DWI restriction, increased ADC | DWI not available | 108 month/Progressive increase in clinical symptoms/worsening of imaging findings Total two follow-ups in 02/2008–06/2012–06/2017 | Increased/steroid & methotrexate/ infliximab | Normal | 3 | Lung lesion biopsy |
| 12 | 70/F/C | 2/Acute/acute left 6th nerve palsy, left vision loss, headache, left leg weakness, bladder and bowel incontinence | 2,3 | C+T+L | Posterior intramedullary lesion at C6–C7, LME along dorsal and cauda equina | No DWI abnormalities | DWI not available | No follow-up images/slight improvement in neurological symptoms | High dose steroids | Multifocal FLAIR signal abnormalities/ bilateral II, III, VI, V cranial nerve enhancement | 3 | Mediastinal lymphnode biopsy |
| 13 | 55/M/C 06/08–09/11 | 1/Subacute: progressive paresthesia and sensory loss in the left upper extremity and truncal area | 1 | C+T | Posterior intramedullary lesion at C1–T2 | DWI not available | DWI not available | 12 month/Clinically stable/residual enhancement, partial response | Stable neurological symptoms continued methotrexate | Normal | 4 (Isolated spinal) | Spinal cord biopsy |
M: male; F: female; C: caucasian; AA: African-American. On the basis of the Zajicek criteria, cases may be definite (neural tissue biopsy), probable (presence of CNS inflammation with evidence of systemic disease), or possible (clinical presentation consistent with neurosarcoidosis with exclusion of alternate diagnosis); LME: leptomeningeal enhancement; C: cervical; T: thoracic; L: lumbar; DWI: diffusion-weighted imaging.
Images were acquired on either a 1.5 or 3T system. Spine MRI included standard T1-weighted imaging, T2-weighted imaging, short tau inversion recovery, and post-contrast sequences in at least one axial or sagittal plane. At the time of initial presentation all the spinal MRIs were done with contrast except for one patient who was pregnant at the time of diagnosis. Available diffusion-weighted imaging (DWI) sequences were also evaluated to assess the diffusivity of the intramedullary lesions compared with normal-appearing cord. We measured the ADC values of intramedullary lesions and normal-appearing spinal cord at initial and follow-up scans. DWI images were acquired in the axial plane with 6 mm of slice thickness and a b value of 0 and 1000 except in one patient in whom DWI was acquired in the sagittal plane. Both qualitative and quantitative evaluation of the intramedullary enhancing lesions and normal spinal cord were done on DWI. Regions of interest (ROI) were defined in areas of increased intramedullary enhancement on post-contrast axial T1-weighted sequence and copied to the DWI and ADC map. The same ROI was placed in the portion of the normal-appearing spinal cord without signal abnormality and the ADC value was measured to serve as an internal reference standard. Follow-up MRIs were evaluated for radiological response after treatment. Duration of follow-up included the time between first and last available spine MRIs. Data were expressed as mean, standard deviation and range. Percentages of positive and negative findings were calculated. We also examined the available brain MRIs of these patients for the presence or absence of associated brain lesions.
Results
Of the 18 patients with available spine MRIs, only eight patients had whole-spine MRI studies, while the remaining 10 patients had limited spine MRI at presentation (cervical and thoracic in four, thoracic and lumbar in three, only cervical in two and only thoracic spine imaging in one patient). Abnormal imaging findings on spinal MRIs were noted in 72% (13/18) of the patients. The clinical summary with imaging findings for all 13 patients is outlined in Table 1. The mean age at presentation was 50 years; age range 31–70 and a male to female ratio of 5:8. Most of the patients were white 92% (12/13) and one was African-American. According to the Zajicek criteria, five patients met ‘definite’ criteria (neural tissue biopsy) and eight patients met ‘probable’ criteria.16 Approximately 69% (9/13) of the patients had co-existing systemic disease, often with either pulmonary or lymph nodal involvement. All patients had neurological signs at presentation and these were acute in 38% (5/13), subacute in 31% (4/13), and chronic in 31% (4/13) patients, defined as neurological clinical presentation within 1 month, 1–3 months and 3 months or more, respectively. Almost all the patients 92% (11/13) except one presented with myelopathic symptoms of motor (77%), sensory (46%) and sphincter (30%) dysfunction. The myelopathy presentation was acute in 42%, subacute in 25% and 33% in chronic. In most of our patients, the histological diagnosis of sarcoidosis was established from extra-neural organ biopsies (69%) including two vertebral bone biopsies. Neurosurgical biopsies were obtained in 38% (5/13) patients including four spinal cord and one brain biopsy. One patient had both neural and extra-neural biopsy. Biopsy revealed non-caseating granulomas in all 13 patients.
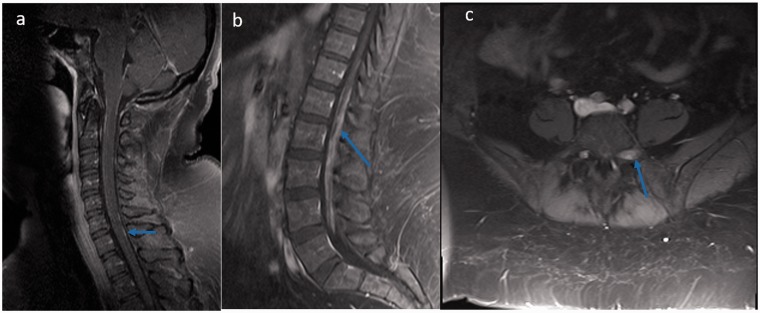
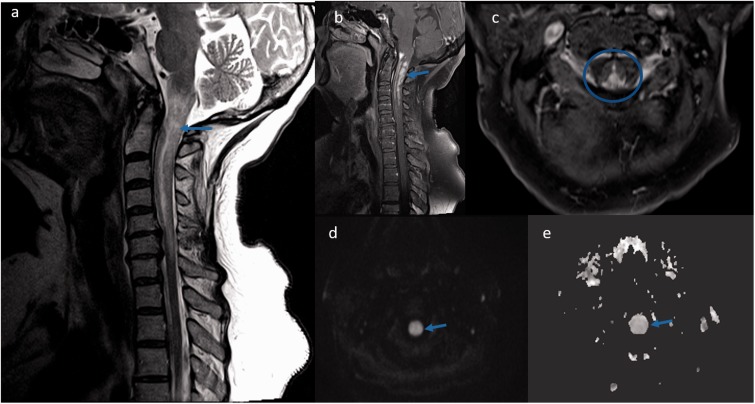
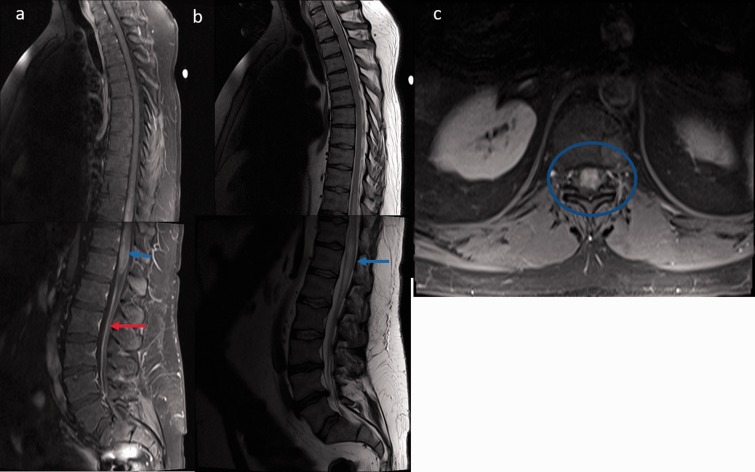
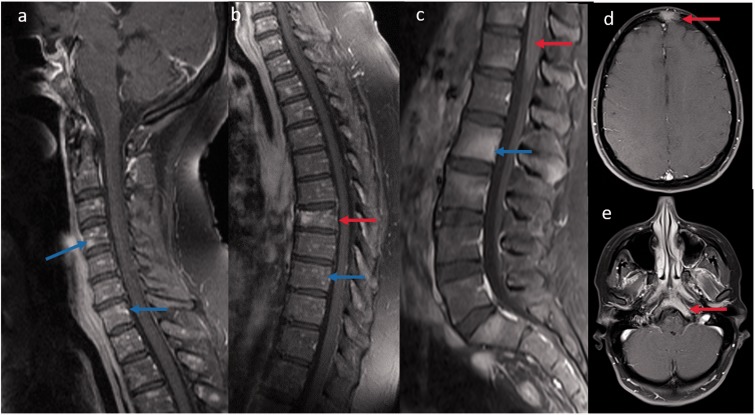
The most common spine MRI findings were reported as leptomeningeal enhancement followed by enhancing intramedullary granulomas and less common vertebral bone marrow enhancement (Table 2). According to the classification of Junger et al., six patients were in stage 1 and another six patients were in stage 2.11 Leptomeningeal and pachymeningeal enhancement (Figure 1(a,b)) was noted in 61% and 23% of patients, respectively, involving more frequently the cervical segment (76%), followed by the thoracic (53%) and conus segments (38%). Intramedullary granulomas with cord edema were noted in 46% (6/13) of patients, with exclusive cervical cord involvement in three patients, exclusive thoracic cord involvement in one, and combined cervical and thoracic cord involvement in two patients (Figures 2 and 3). All the intramedullary lesions demonstrated contrast enhancement (Figures 2 and 3) and had exclusive dorsal cord location in three patients (Figure 2(c)), panmedullary in two (Figure 3(c)) and combined centrodorsal in one patient. The average length of intramedullary lesions spanned for 4.2 spinal segments (expressed as vertebral bodies). One patient also showed the enlargement and enhancement of the left L5 nerve root (Figure 1(c)) which resolved on follow-up imaging. Biopsy-confirmed vertebral column involvement was seen in 15% (2/13) of patients with exclusive bony involvement and pathological vertebral collapse in one patient. In both cases, MRI of the spine demonstrated multifocal areas of marrow involvement and abnormal marrow enhancement (Figure 4).
Table 2.
Spine and brain MRI characteristics of 13 spinal sarcoidosis patients.
| Findings | Number of patients |
|---|---|
| Abnormal spinal MRI | 13 |
| Leptomeningeal enhancement | 8 |
| Pachymeningeal enhancement | 3 |
| Spinal cord localization | |
| Cervical | 8 |
| Thoracic | 6 |
| Conus | 6 |
| Whole cord | 4 |
| Intramedullary enhancing lesions | 6/13 |
| Cervical | 3 |
| Thoracic | 1 |
| Cervicothoracic | 2 |
| Axial localization for intramedullary lesions | |
| Anterior | 0 |
| Posterior | 3 |
| Centrodorsal | 1 |
| Panmedullary | 2 |
| Average length of intramedullary involvement (measure against vertebral bodies) | 4.2 vertebral segments |
| Vertebral bony involvement | 2 |
| Brain parenchymal abnormality – MRI | 10/13 |
| Leptomeningeal enhancement | 8 |
| Pachymeningeal enhancement | 2 |
| Cranial nerve involvement | 2 |
| FLAIR hyperintensities | 5 |
| Intraparenchymal granulomas | 2 |
| Intraparenchymal bleed | 2 |
| Ischemic changes | 1 |
| Skull bone involvement | 1 |
MRI: magnetic resonance imaging; FLAIR: fluid-attenuated inversion recovery.
Figure 1.
(a, b) Sagittal T1-weighted post-contrast images reveal linear leptomeningeal enhancement of cervical and nodular pachymeningeal enhancement in the conus region (blue arrows). (c) Axial T1-weighted post-contrast image reveals dorsal, extramedullary mass along left L5 nerve (blue arrow).
Figure 2.
Spinal cord magnetic resonance imaging displaying lesions in cervical cord sarcoidosis: (a) Sagittal T2-weighted image displaying heterogeneous cord hyperintensities and edema in the cervical area (blue arrow); (b) T1-weighted post-contrast image showed intramedullary gadolinium enhancement in the cervico-medullary region (blue arrow); (c) Axial T1-weighted post-contrast image showed predominant posterior cord involvement (blue circle). The corresponding lesions exhibited normal to slightly increased diffusion on axial diffusion-weighted image (d, arrow) and axial apparent diffusion coefficient map image (e, arrow), reflecting vasogenic edema and inflammation.
Figure 3.
Spinal cord magnetic resonance imaging displaying lesions in spinal cord sarcoidosis: (a) Sagittal T1-weighted post-contrast imaging of whole spine revealed intramedullary gadolinium enhancement in the dorsal (T10–T12) and conus region (blue arrow) with diffuse leptomeningeal enhancement along the cauda equina (red arrow); (b) Sagittal T2-weighted image displaying heterogeneous cord hyperintensities with edema (blue arrow); (c) Axial T1-weighted post-contrast image shows panmedullary cord involvement (blue circle).
Figure 4.
(a, b) Sagittal T1-weighted post-contrast images reveal diffuse peppered enhancement of the cervical and thoracic vertebrae bone marrow (blue arrow) without any leptomeningeal and cord involvement. Associated T7 vertebral pathological collapse (red arrow). Biopsy revealed non-caseating granuloma. (c) Sagittal T1-weighted post-contrast image in another patient reveals multifocal areas of bone marrow enhancement (blue arrow) along with leptomeningeal enhancement (red arrow). Axial T1-weighted post-contrast brain images of the same patient also showed bony enhancement of frontal and clivus (red arrow) regions.
DWI images were available in three of the six patients at baseline and subsequent follow-up. Table 3 represents the ADC values from intramedullary lesions and normal-appearing cord in these three patients at initial presentation and subsequent follow-ups. Two of these patients showed complete resolution of contrast enhancement on follow-up. In the remaining three patients, baseline DWI scans were unavailable in one patient and at follow-up in two patients. All the intramedullary lesions exhibited normal to slightly increased diffusivity with corresponding increased ADC map image, reflecting vasogenic edema and inflammation (Figure 2 (d and e)). ADC of intramedullary lesions was calculated as mean ± SD at baseline (1.28 ± 0.092) and last available follow-up (1.38 ± 0.055). Similarly, we measured the normal-appearing cord ADC at baseline (1.16 ± 0.014) and on subsequent follow-up (1.23 ± 0.033). We also examined the uninvolved normal-appearing spinal cord for any area of diffusion restriction with low ADC suggesting cord ischemia and found none.
Table 3.
Diffusion-weighted imaging – ADC values from intramedullary lesions and normal appearing cord on initial presentation and subsequent follow-ups in three patients.
| Patient number | Intramedullary lesion/normal cord ADC values 1. On presentation (mean ± SD) | Intramedullary lesion/normal cord ADC values On follow-ups (mean ± SD) |
|---|---|---|
| 5 | 1.40 ± 0.17/1.01 ± 0.15 3. | 1.64 ± 0.07/1.23 ± 0.04 |
| 7 | 1.27 ± 0.09/1.19 ± 0.18 | 1.31 ± 0.01/1.23 ± 0.02 |
| 9 | 1.18 ± 0.02/1.16 ± 0.10 | 1.21 ± 0.07/1.23 ± 0.04 |
| Average | 1.28 ± 0.092/1.16 ± 0.014 | 1.38 ± 0.055/1.23 ± 0.033 |
ADC: apparent diffusion coefficient.
All the patients received corticosteroids as initial treatments with the addition of immunosuppressive agents when required. Follow-up imaging was available in 93% (12/13) of patients with a total 27 follow-up scans of which one patient had 11 follow-ups for treatment-related complications. The mean follow-up period was 23.2 months with range of 4–108 months. The clinical summary for all patients is outlined in Table 1. Corticosteroids (methylprednisolone, prednisone or dexamethasone) were given to all patients as the initial treatment followed by other immunotherapeutic agents such as methotrexate (n = 5) and infliximab (n = 2) in non-responsive cases or corticosteroid-induced complications. Complete or near-complete imaging resolution with minimal residual leptomeningeal/pachymeningeal/cord enhancement was seen in eight patients (Table 1), with mean onset of imaging improvement occurring at 16 months (range 4–38 months). At the study conclusion, residual neurological deficits were present in all patients, being relatively mild in six patients. Partial response with moderate resolution of imaging findings was seen in three patients over a period of 14 months (range 4–24 months) with persistent sphincter dysfunction and significant lower extremity weakness. One patient who is currently clinically stable had numerous bursts and lapses of clinical symptoms in between and showed progressive worsening of clinical and imaging findings despite being on a full dose of methotrexate and infliximab over a follow-up duration of 108 months.
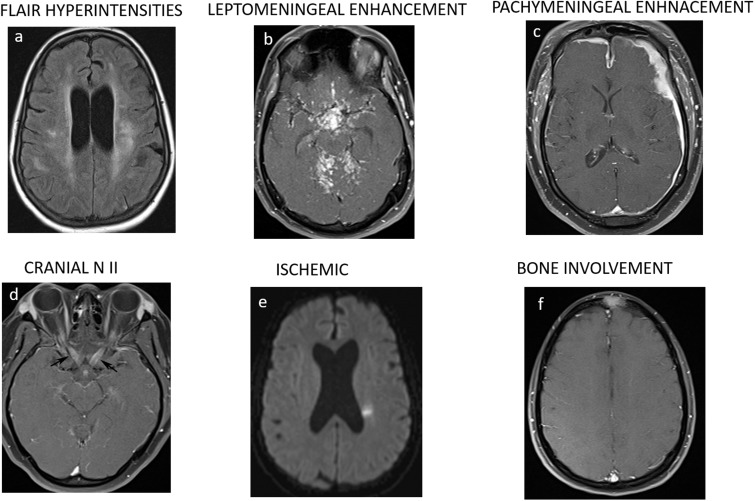
Concurrent brain MRIs showed parenchymal abnormalities in 77% (10/13) of patients. These abnormal MRI findings (Figure 5) were most commonly leptomeningeal enhancement (80%), abnormal periventricular white matter fluid-attenuated inversion recovery (FLAIR) hyperintensities (50%), cranial nerve involvement (20%), enhancing intraparenchymal granulomas, bleed (20% each), and subacute infarct (10%). MRI brain findings are summarized in Table 2.
Figure 5.
(a–f) Axial brain magnetic resonance imaging showing associated abnormal brain findings.
Discussion
Spinal involvement was traditionally considered rare in sarcoidosis patients but can encompass a large spectrum of manifestations such as leptomeningeal, pachymeningeal, intramedullary and rarely vertebral bodies. The clinical presentation of SS mimics other myelopathies and manifests with sensory, motor, bladder and bowel dysfunction. MRI is very sensitive to localize the lesions; however, imaging appearances are less specific and highly variable. The diagnosis of SS is challenging especially in the absence of systemic sarcoidosis; therefore, the evaluation of extra-neural sarcoidosis is usually performed to avoid invasive neural tissue biopsy.7,9,10 In the present study, we retrospectively analyzed the clinical and imaging manifestations of 13 SS patients including subsequent follow-ups to evaluate the long-term imaging response to treatment. The clinical presentation of the 13 SS cases was in concordance with the previously reported studies in the literature.3–14 As reported earlier, there was a female dominance and most patients were in the 35–45 year age group. All the patients in the current study experienced neurological symptoms at the time of the initial sarcoidosis diagnosis, and 92% of patients presented with myelopathic symptoms of sensory, motor and sphincter dysfunction including two patients with characteristic ‘corset-like’ neuropathy presentation.17
Junger et al.11 classified the MRI manifestations of the intramedullary lesions into four stages, and suggested the correlation between inflammation and gadolinium enhancement. Stage 1: linear leptomeningeal enhancement representing early inflammation. Stage 2: spread of the inflammation to the parenchyma through the perivascular (Virchow–Robin) spaces leading to intramedullary enhancing lesions with cord enlargement. Stage 3: presents with relatively normal-size cord with focal or multifocal enhancement. Stage 4: relative atrophy of the cord and no enhancement. It led to the assumption that enhancement starts from the meninges towards the cord parenchyma.10,11 Leptomeningeal enhancement reported in 61% of patients predominantly involving the cervical cord, similar to the 48–71% reported in the literature (Table 3). We reported relatively fewer numbers of enhancing intramedullary granulomas associated with cord swelling in 46% of patients compared to 57–100% reported in the literature (Table 4). The reason may be cases with dorsal surface involvement were considered parenchymal while we probably considered some of them as leptomeningeal or pachymeningeal only. On MRI, spinal cord lesions demonstrated low signal intensity on T1, high signal intensity on T2 and nodular enhancement on contrast administration.
Table 4.
Cases of spinal sarcoidosis in the reported English language literature.
| Author | Year of study | No of patients | Age group (years) | Neural tissue biopsy | MRI | Systemic disease | Enhancing intramedullary granulomas | Leptomeningeal enhancement |
|---|---|---|---|---|---|---|---|---|
| Present study | 13 | 31–70 | 38% | 13 | 69% | 46%/cervical in 83%/>4.2 segments | 61% | |
| Durel et al.3 | 2016 | 20 | 20–67 | 50% | 20 | 95% | 70%/cervical in 70%/>3 segments | 60% |
| Sohn et al.4 | 2014 | 27 | 22–63 | 14% | 27 | All | 81%/cervical/3.9 segments | 48% |
| Sakushima et al.6 | 2011 | 6 | 42–76 | None | 6 | All | All | – |
| Cohen-Aubart et al.7 | 2010 | 31 | Mean age 41.6+12.5 | 19% | 26 | All | 74%/cervical in 58%/2.7+2.47 | – |
| Varron et al.8 | 2009 | 7 | 26–73 | None | All | All | 100%/cervical in 57% | – |
| Kobayashi et al.12 | 2013 | 9 | 22–65 | None | 9 | All | 89%/cervical/3.7 ± 2.6 segments | 67% |
| Wang et al.13 | 2015 | 7 | 39–63 | None | 7 | All | 100%/thoracic/6–8 segments | 71% |
MRI: magnetic resonance imaging.
Spinal cord sarcoidosis can occur at any spinal level, although cervical (56%) and thoracic segment (37%) involvement was found to be more common compared to lumbosacral involvement, as reported by previous studies.3–14 The average length of the spinal cord involvement by enhancing intramedullary granulomas was found to be long and spanned for 4.2 vertebral segments, which may be an important clue in differentiating SS from other neurological disease mimics.3,4,6 Furthermore, axial images may be of importance in localizing the intramedullary granulomas and a propensity for the dorsal cord.3,4,12,13
All the intramedullary lesions exhibited normal to slightly increased diffusivity with correspondingly increased values at the ADC map image, reflecting vasogenic edema and inflammation. Kobayashi et al. also reported iso to high signal intensity with normal to slightly increased diffusion on DWI in two patients with intramedullary lesions, reflecting vasogenic edema and inflammation.12 We also evaluated quantitative diffusion in a small subset of patients with intramedullary lesions on first and subsequent follow-up scans. We noticed increased ADC in the intramedullary lesions compared to the normal cord on baseline and subsequent follow-up scans despite being imaging resolution of enhancement and edema. This suggests either continued ongoing neural inflammation versus underlying tract injury, and probably requires further assessment with high resolution MRI and quantitative diffusion tensor imaging (DTI) studies of the spinal cord. Despite the presence of DWI in most of the MRI studies in the current series, we did not find any acute cord infarcts, even though spinal infarcts have previously been documented on autopsy studies. This may be because of the high slice thickness on our diffusion-weighted images (6 mm). It is possible that higher resolution imaging may show such infarcts which likely to be are currently underdiagnosed on imaging.
A few studies have also reported sarcoid vasculitis-related rare cerebrovascular complications of infarct and bleeding in neurosardoidosis patients.15,18–20 Nagai et al. reported an autopsy finding of an intramedullary sarcoidosis with marked atrophy in the cervicothoracic segments and loss of normal grey and white matter structures.21 Microscopically, the parenchyma of the spinal cord was necrotic and studded by numerous granulomatous nodules in a perivascular distribution, with intense perivascular infiltration of lymphocytes which contributes to spinal cord necrosis due to ischemia from arterial obliteration.21 A rare published case report of spinal cord hemorrhage in a neurosardoidosis patient who had been on long-term corticosteroid treatment, recently highlighted the possibilities of spinal cord hemorrhage secondary to sarcoid vasculitis. In our study, we have not encountered any similar case of intramedullary hemorrhage, although susceptibility sequences sensitive for hemorrhage detection was not available in any of the patients.22
Isolated involvement of the spinal cord by sarcoidosis in the absence of brain and systemic involvement is extremely rare. This needs to be neurologically and radiologically differentiated from other mimickers such as neuromyelitis optica spectrum disorders to prevent delayed diagnosis and treatment. Neuroimaging findings suggestive of SS are subpial gadolinium enhancement extending two or more vertebral segments and persistent enhancement for over 2 months after corticosteroid treatment, whereas ring-like enhancement is more common in neuromyelitis optica spectrum disorders.23
In our series, we encountered one case of isolated intramedullary sarcoidosis in which the patient presented with cervical myelopathy. The diagnosis was established after spinal cord biopsy and treated with high-dose steroids followed by methotrexate. After 12 months of adequate treatment, the patient was clinically stable with persistent sensorimotor paraparesis and the neuroradiological findings partially remitted.7,24 Another study by Saleh et al. found only one biopsy-confirmed case of true isolated spinal cord sarcoidosis, further highlighting the rarity of the disease.25
Brain MRIs were more frequently reported to be abnormal in SS, and we found brain parenchymal abnormalities in 70% and bony calvarium involvement in 10% of our cohort of patients.3,7,12 Such findings are important in narrowing the differential diagnosis and guiding the diagnostic algorithm. In our study, leptomeningeal enhancement and FLAIR signal abnormalities were predominantly reported, and our results were concurrent with the previously reported literature.3–7,12,13 CNS bleeding and ischemia are rare complications of neurosarcoidosis and we found two such cases of subacute infarct and parenchymal bleed in this cohort.15,21
In general, osseous involvement in sarcoidosis ranges from 1% to 13% and is felt to be underestimated as most of the lesions are clinically asymptomatic. It most commonly involves the tubular bones of the hands and feet, with less frequent involvement of the skull and vertebral column, located mainly in the thoracolumbar region.26 The radiographic pattern of involvement varies between purely lytic to mixed lytic sclerotic lesions on computed tomography. MRI is sensitive for bone lesion detection but is not specific for the diagnosis of sarcoidosis. Lesions are usually T1 hypointense with variable T2 signal intensity and enhance on contrast administration. Histological proof is required to exclude the numerous other possibilities such as metastasis, lymphoma and infections.27,28 In our case series, we have found two biopsy-confirmed cases of vertebral bone involvement, with exclusive bone involvement in one patient (Figure 4) including the skull and pelvic bones. One patient had peppered lesions (Figure 4(a,b)), while the other had more ill-defined lesions (Figure 4(c)) on MRI which were both lytic and sclerotic on computed tomography.
SS may be refractory to treatment and frequently requires immunosuppressants in addition to corticosteroids in non-responsive refractory cases. In our study, additional immunosuppressants were given to seven patients who were resistant to steroids. We reported complete or near-complete imaging resolution in eight patients, with residual mild neurological deficits in six patients. Partial imaging response with persistent sphincter dysfunction and significant lower extremity weakness was reported in three patients. One patient had progressive worsening of clinical and imaging findings despite being on a full dose of methotrexate and infliximab. Clinical findings do not always parallel MRI findings and despite improvements in MRI findings over the follow-up period, a sufficient number of the patients displayed functional disabilities.4,7,29 The question as to how disease activity in neurosardoidosis should be monitored is not clear and no reliable imaging biomarkers have yet been identified. Our observations of alterations in ADC values which persist despite resolution of contrast enhancement, although noted in a small subset of patients, raise the possibility that ADC values and DTI may be helpful as an imaging biomarker. We proposed a larger, prospective quantitative diffusion study to explore further the possibility of DTI as an imaging biomarker.
The limitations of our study include its retrospective nature and a small sample size, which are not uncommon given the rarity of this disease. Due to the small sample size and absence of any comparison group we were not able to conclude any statistical significance. Other limitations include referral bias as the study was performed at a tertiary care center, and also a geographical bias of a mid-west population base which was reflected in the composition of our cohort. Similarly, whole-spine MRIs were not available in all the patients which might have biased the distribution of findings. Finally, DWI sequences were acquired with slice thickness of 6 mm which probably precluded the detection of ischemic lesions.
Despite these limitations, our study observations support the previously reported findings and strengthen the existing literature on this rare disease. Given the positive imaging findings in a large number of patients with SS, these also argue for routine spinal imaging in patients with neurosarcoidosis. The strengths of our study include the presence of a tissue diagnosis in all patients and evaluation of ADC values in intramedullary lesions. The observation that the ADC values did not return to baseline despite the resolution of contrast enhancement raises interesting questions which need to be addressed in future studies.
Conclusions
SS was previously considered uncommon but now is being increasingly recognized with the widespread use of MRI. The predominance of dorsal cord involvement is characteristic, although not necessarily pathognomonic. Other imaging findings include diffuse leptomeningeal and nerve root enhancement, with concomitant intramedullary enhancing granulomas spanning more than three vertebral segments with predilection for cervicothoracic segments. In line with previous studies, these findings are helpful to distinguish SS from other neurological disease primarily affecting the spinal cord. Also, evaluation of ADC values may serve as an important imaging biomarker and its role needs to be explored further.
Acknowledgment
This paper was presented at the American Society of Neuroradiology Annual Meeting 2017.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Fritz D, Van de Beek D, Brouwer MC. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurol 2016; 16: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bathla G, Singh AK, Policeni B, et al. Imaging of neurosarcoidosis: common, uncommon, and rare. Clin Radiol 2016; 71: 96–106. [DOI] [PubMed] [Google Scholar]
- 3.Durel CA, Marignier R, Maucort-Boulch D, et al. Clinical features and prognostic factors of spinal cord sarcoidosis: a multicenter observational study of 20 BIOPSY-PROVEN patients. J Neurol 2016; 263: 981–990. [DOI] [PubMed] [Google Scholar]
- 4.Sohn M, Nozaki K, Culver DA, et al. Spinal cord neurosarcoidosis. Am J Medl Sci 2014; 347: 195–198. [DOI] [PubMed] [Google Scholar]
- 5.Boyaci B, Hornicek F, Rosenthal D, et al. Sarcoidosis of the spine: a report of five cases and a review of the literature. J Bone Joint Surg 2012; 94: e42. [DOI] [PubMed] [Google Scholar]
- 6.Sakushima K, Yabe I, Nakano F, et al. Clinical features of spinal cord sarcoidosis: analysis of 17 neurosarcoidosis patients. J Neurol 2011; 258: 2163–2167. [DOI] [PubMed] [Google Scholar]
- 7.Cohen-Aubart F, Galanaud D, Grabli D, et al. Spinal cord sarcoidosis: clinical and laboratory profile and outcome of 31 patients in a case–control study. Medicine 2010; 89: 133–140. [DOI] [PubMed] [Google Scholar]
- 8.Varron L, Broussolle C, Candessanche JP, et al. Spinal cord sarcoidosis: report of seven cases. Eur J Neurol 2009; 16: 289–296. [DOI] [PubMed] [Google Scholar]
- 9.Scott TF, Yandora K, Valeri A, et al. Aggressive therapy for neurosarcoidosis: long-term follow- up of 48 treated patients. Arch Neurol 2007; 64: 691–696. [DOI] [PubMed] [Google Scholar]
- 10.Nesbit GM, Miller GM, Baker HL, Jr, et al. Spinal cord sarcoidosis: a new finding at MR imaging with Gd-DTPA enhancement. Radiology 1989; 173: 839–843. [DOI] [PubMed] [Google Scholar]
- 11.Junger SS, Stern BJ, Levine SR, et al. Intramedullary spinal sarcoidosis: clinical and magnetic resonance imaging characteristics. Neurology 1993; 43: 333. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi S, Nakata W, Sugimoto H. Spinal magnetic resonance imaging manifestations at neurological onset in Japanese patients with spinal cord sarcoidosis. Intern Med 2013; 52: 2041–2050. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Li Y. Longitudinal ultra-extensive transverse myelitis as a manifestation of neurosarcoidosis. J Neurol Sci 2015; 355: 64–67. [DOI] [PubMed] [Google Scholar]
- 14.Pascuzzi RM, Shapiro SA, Rau AN, et al. Sarcoid myelopathy. J Neuroimag 1996; 6: 61–62. [DOI] [PubMed] [Google Scholar]
- 15.Bathla G, Watal P, Gupta S, et al. Cerebrovascular manifestations in neurosarcoidosis: how common are they and does perivascular enhancement matter?. Clin Radiol 2018; 73(10): 907.e15–907.e23. [DOI] [PubMed] [Google Scholar]
- 16.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis – diagnosis and management. Q J Med 1999; 92: 103–117. [DOI] [PubMed] [Google Scholar]
- 17.Lidar M, Dori A, Levy Y, et al. Sarcoidosis presenting as “corset-like” myelopathy: a description of six cases and literature review. Clin Rev Allerg Immunol 2010; 38: 270–275. [DOI] [PubMed] [Google Scholar]
- 18.Libman RB, Sharfstein S, Harrington W, et al. Recurrent intracerebral hemorrhage from sarcoid angiitis. J Stroke Cerebrovasc Dis 1997; 6: 373–375. [DOI] [PubMed] [Google Scholar]
- 19.Lettau M, Laible M, Treier M. Infarction with subarachnoid hemorrhage from vasculitis with neurosarcoidosis. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2011; 183: 573. [DOI] [PubMed] [Google Scholar]
- 20.Bathla G, Watal P, Gupta S, et al. Cerebrovascular manifestations of neurosarcoidosis: an underrecognized aspect of the imaging spectrum. AJNR Am J Neuroradiol 2018; 39(7): 1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai H, Ohtsubo K, Shimada H. Sarcoidosis of the spinal cord: report of an autopsy case and review of the literature. Pathol Int 1985; 35: 1007–1022. [PubMed] [Google Scholar]
- 22.Pegat B, Drapier S, Morandi X, et al. Spinal cord hemorrhage in a patient with neurosarcoidosis on long-term corticosteroid therapy: case report. BMC Neurol 2015; 15: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flanagan EP, Kaufmann TJ, Krecke KN, et al. Discriminating long myelitis of neuromyelitis optica from sarcoidosis. Ann Neurol 2016; 79: 437–447. [DOI] [PubMed] [Google Scholar]
- 24.Kasliwal MK, Harbhajanka A, Nag S, et al. Isolated spinal neurosarcoidosis: an enigmatic intramedullary spinal cord pathology – case report and review of the literature. J Craniovertebr Junct Spine 2013; 4: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saleh S, Saw C, Marzouk K, et al. Sarcoidosis of the spinal cord: literature review and report of eight cases. J Natl Med Assoc 2006; 98: 965–976. [PMC free article] [PubMed] [Google Scholar]
- 26.Boyaci B, Hornicek F, Rosenthal D, et al. Sarcoidosis of the spine: a report of five cases and a review of the literature. J Bone Joint Surg 2012; 94: e42. [DOI] [PubMed] [Google Scholar]
- 27.Lury KM, Smith JK, Matheus MG, et al. Neurosarcoidosis – review of imaging findings. Semin Roentgenol 2004; 39: 495–504. [DOI] [PubMed] [Google Scholar]
- 28.Ginat DT, Dhillon G, Almast J. Magnetic resonance imaging of neurosarcoidosis. J Clin Imag Sci 2011; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibitoye RT, Wilkins A, Scolding NJ. Neurosarcoidosis: a clinical approach to diagnosis and management. J Neurol 2017; 264: 1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]