Short abstract
Dietary copper supplementation reverses pressure overload-induced cardiac hypertrophy by copper replenishment in the heart. A copper-selective chelator, trientine (triethylenetetramine [TETA]), reverses left ventricular hypertrophy associated with diabetes also by copper replenishment in the heart. The present study was undertaken to address the critical issue how TETA delivers copper to the heart. Adult male Sprague-Dawley rats were subjected to transverse aortic constriction (TAC) to induce cardiac hypertrophy. Eight weeks after the TAC surgery, cardiac hypertrophy was developed and copper content in the heart was reduced. TETA was then administrated by gavage in two different dosages (21.9 or 87.6 mg/kg day) for six weeks. The results showed that in the lower dosage, TETA replenished copper contents in the heart, along with a decrease in the copper concentration in the blood and kidney, and an increase in the urine. In the higher dosage, TETA did not replenish copper contents in the heart, but markedly increased copper concentrations in the urine and decreased those in the blood and kidney. Neither lower nor higher TETA dosage altered copper concentrations in other organs. Corresponding to myocardial copper replenishment, the lower dose TETA suppresses cardiac hypertrophy, as judged by a reduction in the left ventricle wall thickness and a decrease in the heart size, and diminished cardiac fibrosis, as reflected by a decrease in collagen I content. TETA in the higher dose not only did not suppress cardiac hypertrophy, but also caused cardiac hypertrophy in sham-operated rats. TETA-mediated myocardial copper restoration is independent of copper transporter-1 or -2 but related to an energy-dependent transportation. This study demonstrates that low-dose TETA functions as a copper chaperone, selectively delivering copper to the copper-deprived heart through an active transportation; in higher doses, TETA simply retains its chelator function, removing copper from the body by urinary excretion.
Impact statement
Our study reveals that TETA, traditionally regarded as a copper chelator, in lower doses delivers copper selectively to the heart through a mechanism independent of copper transporter-1 or -2. Copper supplementation by a lower dose of TETA suppresses pressure overload-induced cardiac hypertrophy. Since ischemic heart disease and hypertrophic cardiomyopathy are accompanied by myocardial copper loss, this approach of using a lower dose of TETA to supplement copper to the heart would help treat the disease condition of patients with such cardiac events.
Keywords: Copper, cardiac selective, cardiac hypertrophy, suppression, copper transporters, trientine
Introduction
Trientine (triethylenetetramine [TETA]), a copper-selective transition metal chelator, has been shown to improve cardiac structure and function in rat model of streptozotocin-induced diabetes.1–4 It was also shown that TETA restored indexes of systemic copper homeostasis and left ventricle mass in type 2 diabetic patients with cardiac hypertrophy.5–7 Considering the increased urinary copper excretion following TETA administration and the level of copper extracted from the coronary arteries by TETA infusion, it was initially suggested that TETA might confer clinical benefit because of its elimination of excess cardiac copper accumulation.1,3,8 However, further study has shown that despite diabetic animals and patients display signs of a systemic copper overload state, copper deficiency appears in the heart. Importantly, total copper level in the heart was fully restored to the normal level after TETA treatment.2,9
Dietary copper supplementation reverses cardiac hypertrophy induced by pressure overload.10 Copper is required for cardiac structure and function,11–15 and pressure overload-induced cardiac hypertrophy is accompanied by copper depletion in the heart.10 Thus, it becomes a reasonable and clinically sound approach to improve cardiac structural and functional recovery from pathological hypertrophy by copper supplementation.10,16–18
It appears contradictory that both copper chelator and dietary copper supplementation can improve cardiac structure and function by copper restoration in the heart. A critical issue that needs to be resolved is how TETA delivers copper to the heart. An important consideration is the relationship between the concentration and the affinity to copper of TETA. Based on stoichiometry and Le Chatelier’s principle,19 the shift of chemical equilibrium between Cu–TETA and other copper carriers and the TETA intramolecular interactions would be affected by the variation in TETA concentrations. It is thus speculated that on certain dose ranges, TETA may behave as a copper chaperone to deliver copper to the needed target organs, and otherwise as a chelator to eliminate copper from the body. In addition, the potential regulation of copper cross-membrane transportation by TETA is another consideration. There are two well-known transporters for cellular copper acquisition in the heart tissue, namely copper transporter 1 (CTR-1)20–22 and its homologous protein copper transporter 2 (CTR-2).23,24 The present study was thus undertaken to demonstrate (1) whether TETA can deliver copper to the copper-deprived heart in response to pressure overload, and (2) if yes, how does this take place?
Materials and methods
Animals and animal care
Male Sprague-Dawley rats, 6–8 weeks old and weighing 218 ± 9 g, were obtained from Chengdu Da-Shuo Experimental Animal Breeding and Research Center, a Chinese government-accredited rodent animal center in Sichuan province, China. The animals were acclimatized to laboratory conditions for a period of at least one week in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility. The rats were housed in standard laboratory cages with ad libitum access to standard chow and tap water in a temperature-controlled room at 22 ± 1°C with a humidity of 50 ± 10% and a 12 h dark–light cycle (lights on at 8:00 and off at 20:00) as approved by the Laboratory Animal Management Committee of Sichuan province. To ameliorate pain after surgery, the analgesic dezocine (0.8 mg/kg) was given intramuscularly and once daily for the next two days. All animal procedures were approved by the Institutional Animal Care and Use Committee at Sichuan University West China Hospital.
Experimental design
Rats were randomly divided into sham-operated control and transverse aortic constriction (TAC) surgery groups. Rats were subjected to TAC to produce pressure overload-induced pathological cardiac hypertrophy. Eight weeks after the surgery procedure, rats were screened according to Doppler ultrasound of carotid arteries and echocardiography. The criteria for TAC rats included for further study were that blood flow velocity of right carotid artery had to be at least twice higher than that of the left one and the increase of diastolic left ventricular anterior wall depth (LVAWd) and left ventricular posterior wall depth (LVPWd) needed to be more than 40%. The selected rats based on the above criteria were randomly divided into normal saline (NS) treatment groups (sham–NS group and TAC–NS group) and TETA treatment groups (sham–TETA group and TAC–TETA group). During the six weeks’ treatment described below, echocardiography, and blood and urine sample collection were performed every two weeks. At the end of treatment, all the rats were sacrificed for organ tissue collection. Two batches of experiments were carried out to demonstrate the biological functions of lower and higher dosages of TETA.
Surgical procedure for animals
Prior to the surgical procedure, all subjects were anesthetized in an induction chamber with 1.5–2.5% isoflurane mixed with 0.8 L/min 100% O2. Then endotracheal intubation was introduced for ventilation. As described in our previous studies, the TAC model subjects were established with the novel knot method.25,26 Briefly, the aortic arch of the rat was exposed via the left second intercostal space incision on the chest wall. The aortic arch was gently separated from the thymus and fat tissue. Following identification of the transverse aorta, a prepared piece of a 6-0 silk suture which can decrease the aortic diameter by one half was placed between the innominate and left carotid arteries. Once the two knots meet, further constriction was not possible. Finally, the chest cavity was closed by bringing together the second and third ribs with 3-0 nylon sutures and all layers of muscle and skin were closed with 5-0 nylon sutures. In sham control rats, the entire procedure was identical except for the banding of the aorta.
Doppler ultrasound of carotid arteries and echocardiography
Rats were anesthetized in an induction chamber with 1.5–2.5% isoflurane mixed with 0.8 L/min 100% O2 for all measurements. A 5.8 MHz transducer (Vivid 7 Dimension, GE) was placed on the left and right sides of the neck to detect flow velocity in the left and right carotid arteries. At each site, optimal Doppler flow velocity signals were obtained by adjusting the position of the transducer.27 A series of echocardiograms were performed using an 11.5 MHz transducer (Vivid 7 Dimension, GE) as previously described.28–30 Diastolic LVAWd and LVPWd were obtained using two-dimensional mode by taking the measurements of short-axis cross-sectional areas.31
TETA dosages selection and TETA treatment
As previous studies reported, dosages employed for the treatment of Wilson’s disease in adults typically vary from 750 to 2000 mg/day (equivalent to ∼ 10.7–28.6 mg/kg day in 70 kg adults) and in children vary from 500 to 750 mg/day (equivalent to ∼ 12.5–18.8 mg/kg day in 40 kg children).32 Based on these concentrations employed in known clinical applications of TETA, we administered two dosages of TETA to rats at 21.9 and 87.6 mg/kg day (respectively, equivalent to ∼ 4.6 and 18.4 mg/kg day in 60 kg adults).33 Lower dosage is equivalent to ∼ 375 mg/day, almost one half of recommended minimum dosage, and higher dosage was equivalent to ∼ 1400 mg/day.
All rats were weighed before the morning treatment every day. The rats of sham–TETA and TAC–TETA groups were given one of the two doses of TETA by gavage twice a day (9:00 and 17:00). The same volume (10 mL/kg day) of 0.9% NS was administered to rats of sham–NS and TAC–NS groups twice a day. All rats were treated continuously for six weeks.
Sample and tissue preparation
Blood samples were collected through tail vein with an intravenous catheter and 1 mL whole blood was collected and stored in heparin sodium vacuum blood collection tubes for each animal and blood plasma was collected after being centrifuged (3000 r/min, 10 min, 20°C). All plasma samples were stored in −80°C refrigerator for Cu concentration assay.
Rats were placed individually in metabolism cages and 24 h urine samples collected from different groups in metal-free propylene tubes. Supernatant of urine was retained after being centrifuged (3000 r/min, 10 min, 20°C). Excretion samples were kept at −80°C until Cu concentration assay.
Rats were sacrificed and organs were excised after being perfused with ice-cold PBS with 0.1 mL of 1% heparin, including heart, liver, kidney, spleen, lung, and duodenum. Hearts were cut through cross-sectional plane into three parts. Apex cordis and other organ tissues were stored at −80°C for Cu content assay. Middle part of heart, right lobe of liver, and right kidney were embedded in the OCT gel (Tissue-Tek, SAKURA) and then frozen in liquid nitrogen to prepare frozen sectioning for histological examination. Basis cordis was cut into several pieces and frozen in liquid nitrogen for the detection of protein and mRNA levels.
Immunohistochemistry analysis
Briefly, the slides were blocked by 3% hydrogen peroxide for 20 min and followed by incubation with 1% bovine serum albumin at 37°C for 1 h under dark conditions. After being washed with PBS 3× at 5 min for each washing, the slides were further incubated with the rabbit anti-rat type I collagen polyclonal primary antibody (1:200, Abcam) overnight in a 4°C refrigerator. Then, the slides were incubated with HRP-labeled mouse anti-rabbit secondary antibody (CST) at 37°C for 1 h. Finally, freshly mixed diaminobenzidine (CST) was added according to the manufacturer’s directions, whereas the nucleus was counterstained by hematoxylin.
The staining of the tissue sections was detected by a light microscope (Nikon, 80i) and the images were digitized (SPOT FLEX). Under 100× magnification, four visual fields were randomly observed from each slide, defining the positive expression area (positive area/1000 pixel) for semiquantitative analysis.
Measurement of average size of cardiomyocytes
All the sections were fixed and incubated with 20 µg/mL FITC-conjugated lectin collected from Triticum vulgaris (1:100, Sigma, L4895) to label the cell membrane at 37°C for 1 h and then photographed under a confocal microscope (ECLIPSE Ti, Nikon). Under 200× magnification, five visual fields of each part of left ventricle were collected, and the average size in each part was determined by IPP 6.0 software.
Cu content detection
Total Cu was detected using graphite furnace atomic absorption spectrophotometry (ICE3500, Thermo Fisher). Solid samples (organ tissue) of 10 mg were weighed after 24 h lyophilization and were subjected to nitrification with 1 mL nitric acid in 60°C dryer overnight, and then 10 µL of the prepared samples was diluted to 1 mL with ultrapure water for Cu detection. Liquid samples (blood plasma and urine) of 20 µl were added to 100 µL nitric acid and stored at 60°C overnight, then 20 µL of the samples was diluted to 1 mL with ultrapure water for detection.
Western blotting analysis
Aimed to isolate total proteins, tissues were dissolved in TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The protein concentrations were determined using a BCA protein assay kit (Thermo Fisher, USA) and then mixed with 5× loading buffer and denatured in boiling water (10 min at 100°C). Thirty-microgram proteins of each sample were separated by 10% SDS-PAGE. Proteins were then transferred to a polyvinylidene fluoride membrane (Bio-Rad, USA). Membranes were blocked by 5% non-fat dry milk in Tris–HCl buffer solution (10 mM Tris–HCl, pH 8.0, 150 mM NaCl, and 0.1% Tween-20) for 1 h at room temperature, and then the blotting membranes were incubated overnight at 4°C with respective primary antibodies (anti-CTR1, sc-66847, Santa Cruz, USA; anti-CTR2, sc-104852, Santa Cruz, USA; anti-GAPDH, Zhongshan, China) in blocking solution according to the manufacturer’s recommendation. After incubation, the blotting membranes were washed with Tris–HCl buffer solution for six times for 5 min each, and next, blots were incubated with appropriate secondary antibody for 1 h at 37°C. Finally, target protein bands were observed by adding a chemiluminescent horseradish peroxidase substrate (Millipore Corporation, Germany) and analyzed using vilber fusion software (Bio-Rad Laboratories, USA). Specific protein bands results are consistent with the data of manufacture instruction and previous studies used the same antibodies to detect CTR-1 and -2 protein levels in rat cardiac tissue.9
qRT-PCR analysis
Total RNA was extracted from rat left ventricle tissue with TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instruction. The integrity of RNA was measured by agarose gel electrophoresis, and the concentration was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher, USA). RNA was reverse transcribed to complementary DNA (cDNA) using a PrimeScripts RT reagent kit (TaKaRa, Japan) in an MJMini Personal Thermal Cycler (Bio-Rad, USA). The amount of cDNA corresponding to 100 ng of RNA was amplified using a SYBR Green PCR kit (Bio-Rad Laboratories) with the primers for CTR-1, CTR-2, and β-actin. The primer sequences (Table 1) were designed and synthesized by Invitrogen.
Table 1.
Gene names and primer sequences used for qRT-PCR analysis.
| Gene | Primer |
|---|---|
| CTR-1 | Forward Primer: CCCACGAGATGATGATGCCT |
| Reverse Primer: AGCCATTTCTCCAGGTGTGTT | |
| CTR-2 | Forward Primer: CCGATGCACTTCATCTTCTCAG |
| Reverse Primer: GCCAACCTTGATGCCCTCATA | |
| β-actin | Forward Primer: TGGCTCCTAGCACCATGAAG |
| Reverse Primer: AAACGCAGCTCAGTAACAGT |
CTR-1: copper transporter 1; CTR-2: copper transporter 2; qRT-PCR: quantitative real time polymerase chain reaction.
Statistical analysis
All of the data were presented as mean ± SD and analyzed by SPSS 22.0 and Prism 5.0. Student’s t-test was used to analyze the differences in copper contents in organ tissues between TAC rats and sham controls. Echocardiography parameters and copper concentrations, including blood plasma and urine values, at different times were analyzed by repeated measurement analysis of variance. Two-way ANOVA was applied to analyze the differences of heart weight to body weight ratio, heart weight to tibial length ratio, copper contents in organ tissues, type I collagen, average size of cardiomyocytes, and the protein and mRNA levels of CTR-1 and -2 among all groups. P < 0.05 was considered statistically significant.
Results
Copper reduction in the pressure overload-induced hypertrophic heart
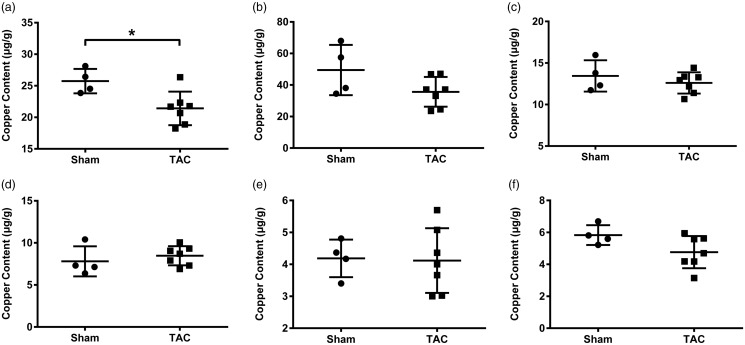
Eight weeks after TAC surgery, heart, kidney, liver, duodenum, spleen, and lung samples were collected for the measurement of copper content. The total level of copper in the heart of rats subjected to TAC was significantly decreased (Figure 1(a)). Copper content in the kidney appeared to be downtrend in the TAC rats, but not significantly different from those of sham-operated controls (Figure 1(b)). Copper content in the liver and duodenum in the TAC rats remained at the same level as that in the sham-operated controls (Figure 1(c) and (d)). Copper level in the spleen and lung was very low and not changed in rats subjected to TAC (Figure 1(e) and (f)).
Figure 1.
Changes in total copper levels in several organs eight weeks’ post-TAC surgery. (a) Heart, (b) kidney, (c) liver, (d) duodenum, (e) spleen, and (f) lung. Data are presented as mean ± SD, n = 4 for sham group and n = 7 for TAC group, *P < 0.05. TAC: transverse aortic constriction.
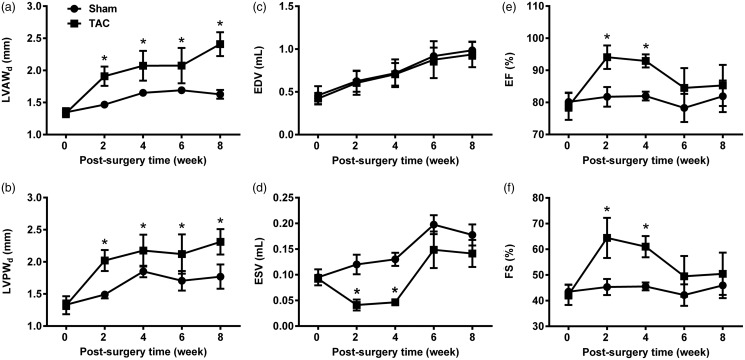
Cardiac hypertrophy was developed, as measured by echocardiography; left ventricular wall thickness (LVAWd and LVPWd) was significantly and gradually increased from two to eight weeks after the TAC surgery (Figure 2(a) and (b)), left ventricular volume (ESV) was significantly decreased from two to four weeks, followed by a recovery from six to eight weeks after the TAC surgery (Figure 2(d)), along with no changes in EDV (Figure 2(c)). Cardiac contractile function (EF and FS) was also significantly enhanced following a recovery, eight weeks after the TAC surgery (Figure 2(e) and (f)).
Figure 2.
Changes in cardiac structure and function in hypertrophic hearts. (a) Diastolic LVAWd, (b) diastolic LVPWd, (c) end-diastolic volume (EDV), (d) end-systolic volume (ESV), (e) ejection fraction (EF), and (f) fractional shortening (FS). Data are presented as mean ± SD, n = 5–9, *P < 0.05. LVAWd: left ventricular anterior wall depth; LVPWd: left ventricular posterior wall depth; TAC: transverse aortic constriction.
TETA, in lower dosage, increases copper content in the copper-deprived heart
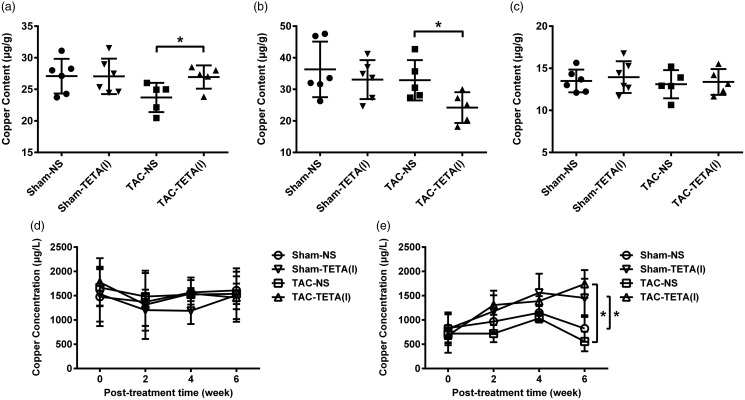
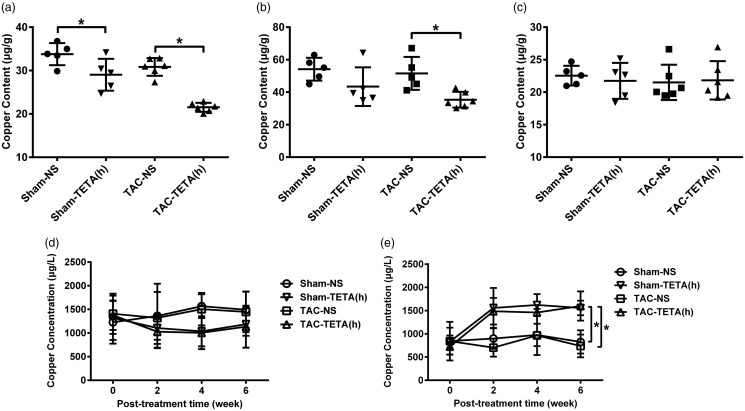
After six weeks of lower dosage TETA (21.9 mg/kg day) treatment, copper content in the hypertrophic hearts was significantly elevated, reaching to the level comparable to that of sham-operated controls (Figure 3(a)). In contrast, after six weeks of higher dosage TETA (87.6 mg/kg day) treatment, copper content in the hypertrophic hearts not only failed to increase, but also was further reduced to 64% of sham-operated control (Figure 4(a)). It was further observed that in lower dosage, TETA did not alter copper content in the heart of sham-operated controls, but in higher dosage, it significantly reduced (more than 14%) copper content in the heart of sham-operated controls (Figures 3(a) and 4(a)).
Figure 3.
Changes in copper distribution in the heart and extracardiac tissues after treatment with a lower dose (21.9 mg/kg day) TETA for six weeks. (a) Heart, (b) kidney, (c) liver, (d) blood plasma, and (e) urine. Data are presented as mean ± SD, n = 5–9, *P < 0.05. NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine.
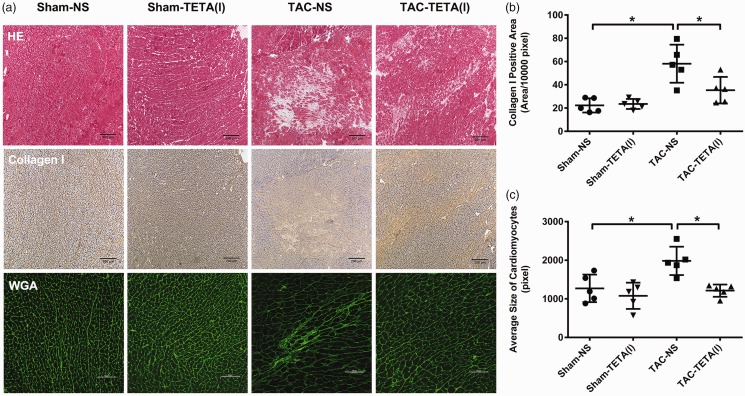
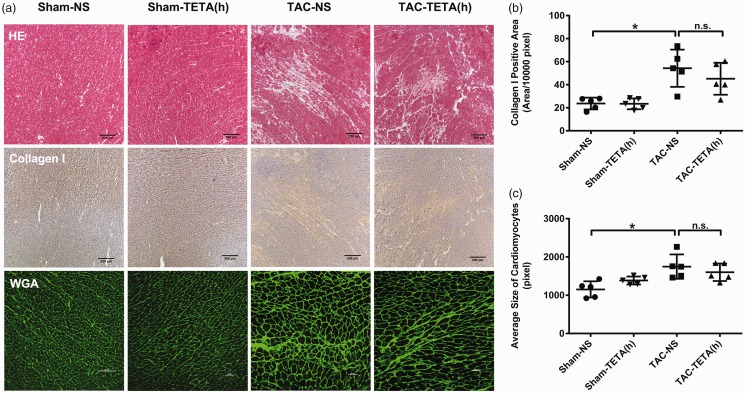
Figure 6.
Changes in cardiac histology after treatment with a lower dose of TETA (21.9 mg/kg day) for six weeks. (a) Top row, necrosis and fibrosis observed by H&E staining, bar = 200 µm. Middle row, type I collagen deposition in the interstitial substance shown by immunohistochemical staining (brown), bar = 200 µm. Bottom row, cardiomyocytes size indicated by FITC-conjugated WGA (green) staining for cell membrane, bar = 100 µm; (b) comparison of the type I collagen deposition; and (c) comparison of the average size of cardiomyocytes. Data are presented as mean ± SD, n = 5 in each group, *P < 0.05. NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine. (A color version of this figure is available in the online journal.)
TETA exerts the same effects on copper redistribution in extracardiac tissues in both lower and higher dosages
Pressure overload by TAC increased copper concentrations in the blood, which were reduced by TETA treatment both in lower (by 21.5%) and higher (by 26.1%) dosages after two weeks’ treatment (Figures 3(d) and 4(d)). This TETA-induced copper reduction in the blood was also observed in the sham-operated controls. TETA, in both lower and higher dosages, significantly increased copper concentrations in the urine in both TAC and sham-operated rats after two to six weeks’ treatment (Figures 3(e) and 4(e)). This increase in urinary copper concentrations was accompanied by a significant decrease in copper content in the kidney after the treatment with TETA (Figures 3(b) and 4(b)). Copper content in the liver slightly fluctuated after the treatment with TETA (Figures 3(c) and 4(c)). Copper contents in other organs including lung, duodenum, and spleen were not altered by either TAC or TETA treatment (data not shown).
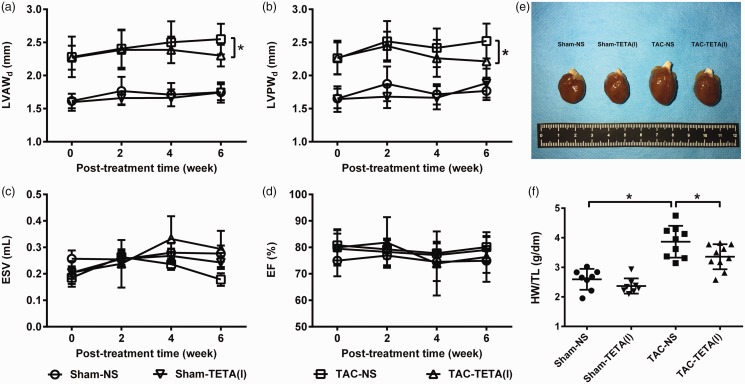
TETA, in lower dosage, suppresses pressure overload-induced cardiac hypertrophy
After six weeks of lower dosage TETA treatment, the heart weight to tibia length ratio was significantly reduced in the rats with cardiac hypertrophy (Figure 5(e) and (f)). This suppression of cardiac hypertrophy was further revealed by the reduction in LVAWd and LVPWd (Figure 5(a) and (b)), as measured by echocardiography. In addition, the measurements of ESV and EF showed an improvement in cardiac contractility in rats with cardiac hypertrophy after the treatment with lower dosage of TETA for six weeks (Figure 5(c) and (d)). Finally, histological examination revealed that after the treatment with lower dosage of TETA (Figure 6(a)), the content of collagen I deposition and the size of cardiomyocytes were significantly reduced in the hypertrophic hearts (Figure 6(b) and (c)).
Figure 4.
Changes in copper distribution in the heart and extracardiac tissues after treatment with a higher dose of TETA (87.6 mg/kg day) for six weeks. (a) Heart, (b) kidney, (c) liver, (d) blood plasma, and (e) urine. Data are presented as mean ± SD, n = 5–6, *P < 0.05. NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine.
Figure 5.
Changes in cardiac structure and function after treatment with a lower dose (21.9 mg/kg day) TETA for six weeks. (a) to (d) Echocardiographic detection of LVAWd, LVPWd, ESV, and EF; (e) representative gross anatomic changes of the hearts among four groups; and (f) heart weight to tibial length ratio. Data are presented as mean ± SD, n = 8–9, *P < 0.05. EF: ejection fraction; ESV: end-systolic volume; HW/TL: HW/TL: heart weight to tibial length ratio; LVAWd: left ventricular anterior wall depth; LVPWd: left ventricular posterior wall depth; NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine. (A color version of this figure is available in the online journal.)
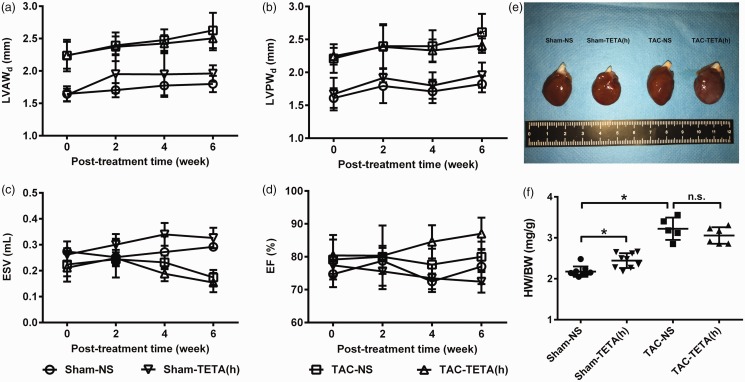
These improvements in the structure and function of the hypertrophic hearts were not observed in the rats treated with higher dosage of TETA (Figure 7(a) to (d)). Furthermore, higher dosage of TETA caused cardiac hypertrophy in the sham-operated rats, as measured by heart weight to body weight ratio (Figure 7(e) and (f)). Histological examination showed no difference after the treatment with higher dosage of TETA (Figure 8(a)), including the content of collagen I deposition and the size of cardiomyocytes (Figure 8(b) and (c)). Other organs did not show pathological changes after the treatment with different doses of TETA (data not shown).
Figure 7.
Changes in cardiac structure and function after treatment with a higher dose of TETA (87.6 mg/kg day) for six weeks. (a) to (d) Echocardiographic detection of LVAWd, LVPWd, ESV, and EF; (e) representative gross anatomic changes of the hearts among four groups; and (f) heart weight to body weight ratio. Data are presented as mean ± SD, n = 5–9, *P < 0.05. EF: ejection fraction; ESV: end-systolic volume; HW/BW: heart weight to body weight ratio; LVAWd: left ventricular anterior wall depth; LVPWd: left ventricular posterior wall depth; NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine. (A color version of this figure is available in the online journal.)
Figure 8.
Changes in cardiac histology after treatment with a higher dose of TETA (87.6 mg/kg day) for six weeks. (a) Top row, necrosis and fibrosis stained by H&E, bar = 200 µm. Middle row, type I collagen deposition in the interstitial substance shown by immunohistochemical staining (brown), bar = 200 µm. Bottom row, cardiomyocytes size indicated by FITC-conjugated WGA (green) staining for cell membrane, bar = 100 µm; (b) comparison of the type I collagen deposition; and (c) comparison of the average size of cardiomyocytes. Data are presented as mean ± SD, n = 5 in each group, *P < 0.05. NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine. (A color version of this figure is available in the online journal.)
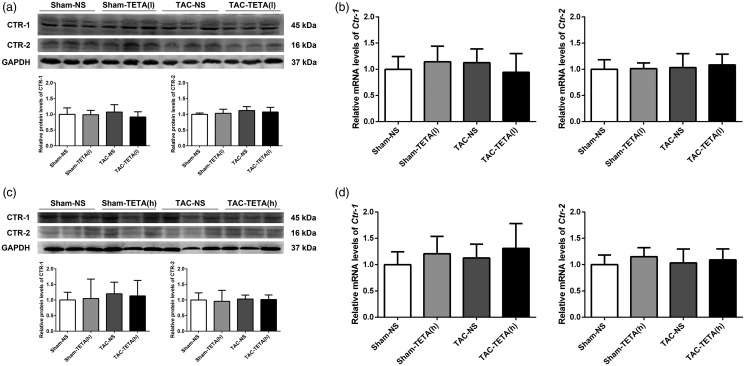
TETA does not affect CTR-1 and -2 in normal and hypertrophic hearts
We investigated whether or not CTR-1 or -2 is involved in the restoration of Cu content in the heart. After six weeks of lower dosage TETA treatment, the protein level of CTR-1 or -2 was not changed in either normal or hypertrophic hearts, as measured by western blotting (Figure 9(a)). In addition, qRT-PCR analysis also detected no changes in either Ctr-1 or -2 mRNA levels in normal or hypertrophic hearts after six weeks’ treatment with TETA (Figure 9(b)). The stable protein and mRNA levels of CTR-1 and -2 were also observed in the rats treated with higher dosage of TETA (Figure 9(c) and (d)).
Figure 9.
Measurement of protein and mRNA levels of copper transporters CTR-1 and CTR-2 in heart tissue after treatment with two different doses of TETA. (a) Representative Western blots for CTR1 and CTR-2 after treatment with a lower dose of TETA for six weeks, (b) qRT-PCR analysis of Ctr-1 and Ctr-2 mRNA levels after the same treatment above, (c) representative Western blots for CTR1 and CTR-2 after treatment with a higher dose of TETA for six weeks, and (d) qRT-PCR analysis of Ctr-1 and Ctr-2 mRNA levels after the same treatment above. Data are presented as mean ± SD, n = 5–9. CTR-1: copper transporter 1; CTR-2: copper transporter 1; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; NS: normal saline; TAC: transverse aortic constriction; TETA: triethylenetetramine.
Discussion
The role of TETA in copper metabolism and transportation has been well documented in relation to its selective copper chelating capacity.7,34,35 This copper-selective chelator, however, has been shown to be able to deliver copper to the heart that had been subjected to pressure overload36 or with diabetic cardiomyopathy.9 How does a copper chelator deliver copper to, instead of remove copper from, the heart? The present study specifically addressed this question. There are at least two critical conditions that were defined here for TETA to function as a copper delivery agent. The first is that the concentration of TETA has to be at a certain range, far lower than that used for removing copper from the body. The second is that the heart must be under copper deprivation conditions. This effect of a lower dose of TETA to supplement copper to the copper-deprived heart under the pressure overload condition is thus of significant clinical relevance.
It was clearly shown in the present study that pressure overload-induced cardiac hypertrophy was accompanied by copper loss from the heart during pathogenesis. This observation is in an agreement with the result obtained from mouse model of pressure overload-induced cardiac hypertrophy10 and from rat model of diabetic cardiomyopathy.2,9 In our previous studies, we used dietary copper supplementation as a tool to restore copper content in the heart in the mouse model.10,13 However, we observed, under pressure overload condition, that copper concentrations in the blood were actually elevated, as presented here. Dietary copper supplementation would further increase the elevated level of copper in the blood, which would lead to untoward effects, although these have not been defined. Therefore, a novel procedure that can avoid copper overload in the blood but efficiently increase copper content in the heart is advantageous. This lower dose of TETA effectively fulfilled the clinical need for decreasing copper levels in the blood but increasing copper levels in the copper-deprived heart simultaneously. How would the lower dose of TETA function as a cardiac-selective copper delivery therapy?
A lower dose of TETA would alter copper-binding speciation, leading to a change in the availability of labile copper from one group to another group of molecules. Cardiac events are associated with high levels of homocysteine (Hcy)37–39 and copper concentration40,41 in the blood. Our previous studies have demonstrated that phenylephrine-induced hypertrophy of cardiomyocytes was associated with the elevation of Hcy in cultures. Hcy restricts copper availability to cardiomyocytes through its interaction with copper to form Cu–Hcy complexes.42 In the presence of TETA, the formation of Cu–Hcy complexes would be interrupted due to the higher affinity of TETA to copper. Based on stoichiometry, higher concentrations of Cu–TETA would facilitate their intramolecular organometallic interaction, leading to high efficiency of copper removal from the body. But lower concentrations of Cu–TETA would provide opportunities for its substitutive interactions with other copper-binding molecules, functioning as a copper chaperone to deliver copper to other copper-binding molecules in copper-deprived heart tissue (CDHT). On the other hand, according to Le Chatelier’s principle,19 there are two equilibria at play in the situation with CDHT
| (1) |
| (2) |
In the case of a higher dosage of TETA, high Cu–TETA concentration pushes equilibrium (1) to the left, along with reducing the Cu–TETA for entry into equilibrium (2). On the contrary, lower concentrations of Cu–TETA drive equilibrium (2) to the right. The data obtained here would strongly support this speculation, although further analysis is required to define the optimization of the TETA dosage for copper delivery to CDHT.
It is important to note that the TETA-mediated copper delivery to the hypertrophic heart appears specific to the copper-deprived organ. Although the lower dose of TETA increased copper concentrations in the hypertrophic heart, its use at the same dosage did not change copper concentrations in the sham-operated heart. In the sham-operated controls, the Cu–TETA is removed by kidney to reduce blood plasma and increase urine copper concentration because of the absence of CDHT. In the case of a significant amount of CDHT in rats subjected to TAC, Le Chatelier’s principle explains why equilibrium (2) is driven to the right and greater substitution of TETA in producing Cu–CDHT complex, delivering Cu to CDHT, but there is still some elevated amount of Cu–TETA to be removed. Furthermore, both lower and higher doses removed copper from the blood and enhanced copper excretion from the urine and did not change copper concentrations in other organs in either TAC or sham-operated rats. This copper-deprived heart-selective copper delivery would make lower doses of TETA even more clinically relevant.
The next question is, how copper is taken up by the copper-deprived heart? Both CTR-1 and CTR-2 are present in the cardiac tissue,20–24 thus it is reasonable to examine the role of these two copper transporters in the copper acquisition by the cardiac tissue. In previous study, decreased CTR-1 expression and increased CTR-2 expression were observed in rats subjected to diabetic cardiomyopathy. Furthermore, TETA administration did not affect CTR-1, but further enhanced CTR-2 expression in cardiac tissue. Therefore, although CTR-2 is a lower affinity copper transporter than CTR-1, TETA may correct myocardial copper levels by up-regulating CTR-2, thereby increasing copper import.9 However, the observation here showed that neither CTR-1 nor CTR-2 was likely involved in the process of copper uptake by both normal and hypertrophic heart, as indicated by the unchanged levels of either proteins or mRNAs for both CTR-1 and CTR-2. In our recent studies using primary cultures of neonatal rat cardiomyocytes, it was observed that gene silencing of both Ctr-1 and Ctr-2 did not affect copper uptake by the cells. However, depletion of ATP in the cells greatly inhibited copper uptake by the cells, suggesting an energy-dependent process of copper uptake by cardiac tissue (Fu et al., 2018, unpublished observation).
It was also observed that dietary copper supplementation leads to copper repletion in the heart and regression of cardiac hypertrophy.10–13 The mechanism for copper repletion-induced regression of cardiac hypertrophy has been extensively studied. It includes the recovery of VEGF production10,43 and activation of VEGF receptor-1, not receptor-2, in the hypertrophic heart.44,45 More important, copper is required for the transactivation of hypoxia-inducible factor 1α (HIF-1α) regulated gene expression.46,47 HIF-1α regulates the expression of both VEGF and VEGF receptor-1.48–51 Therefore, copper replenishment by a lower dose of TETA in the hypertrophic heart should recapitulate the same effect of dietary copper supplementation, as observed in the present study.
Conclusion
This study reveals that TETA functions as either a copper chaperone delivering copper to the CDHT or as a copper chelator to remove copper from the body, depending on the dose ranges used. The copper delivery function of a lower dose of TETA is beneficial to the recovery of structure and function of the pressure overload-induced cardiac hypertrophy. Since TETA has been clinically applied in a higher dosage to remove copper from the body under disease conditions such as Wilson’s disease, and was approved to be safe even at higher doses, the finding here that a lower dose of TETA can deliver copper to the copper-deprived heart is of significant clinical relevance. Several cardiac events such as ischemic heart disease and hypertrophic cardiomyopathy are accompanied by myocardial copper loss. This simple and safe approach of using a lower dose of TETA to supplement copper to the heart would be beneficial to patients with such cardiac events.
ACKNOWLEDGMENTS
We thank Xiaoqi Zheng, Shan Zhao, Lin Bai and Zhenghui Luo for technical assistance and Dr Richard Redfearn (University of Tennessee Health Science Center) for assistance with manuscript editing.
Authors’ contributions
JL, CC, XD, LQ, PH, and YJK participated in the design and review of this work; JL and YL performed animal surgery and TETA administration and copper concentration detection; CC performed the protein and mRNA analyses for CTR-1 and CTR-2; XS performed Doppler ultrasound of carotid arteries and echocardiography detection; JL carried out the HE, immunochemistry, and WGA staining; JL collected and analyzed the data; JL and YJK interpreted the results; JL drafted the manuscript; and YJK edited, revised, and approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by National Natural Science Foundation of China (grant number 81230004 to YJK).
References
- 1.Cooper GJ, Phillips AR, Choong SY, Leonard BL, Crossman DJ, Brunton DH, Saafi L, Dissanayake AM, Cowan BR, Young AA, Occleshaw CJ, Chan YK, Leahy FE, Keogh GF, Gamble GD, Allen GR, Pope AJ, Boyd PD, Poppitt SD, Borg TK, Doughty RN, Baker JR. Regeneration of the heart in diabetes by selective copper chelation. Diabetes 2004; 53:2501–8 [DOI] [PubMed] [Google Scholar]
- 2.Zhang L, Ward ML, Phillips AR, Zhang S, Kennedy J, Barry B, Cannell MB, Cooper GJ. Protection of the heart by treatment with a divalent-copper-selective chelator reveals a novel mechanism underlying cardiomyopathy in diabetic rats. Cardiovasc Diabetol 2013; 12:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Pontre B, Pickup S, Choong SY, Li M, Xu H, Gamble GD, Phillips AR, Cowan BR, Young AA, Cooper GJ. Treatment with a copper-selective chelator causes substantive improvement in cardiac function of diabetic rats with left-ventricular impairment. Cardiovasc Diabetol 2013; 12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baynes JW, Murray DB. The metal chelators, trientine and citrate, inhibit the development of cardiac pathology in the Zucker diabetic rat. Exp Diabetes Res 2009; 2009:696378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper GJ, Young AA, Gamble GD, Occleshaw CJ, Dissanayake AM, Cowan BR, Brunton DH, Baker JR, Phillips AR, Frampton CM, Poppitt SD, Doughty RN. A copper(II)-selective chelator ameliorates left-ventricular hypertrophy in type 2 diabetic patients: a randomised placebo-controlled study. Diabetologia 2009; 52:715–22 [DOI] [PubMed] [Google Scholar]
- 6.Cooper GJ, Chan YK, Dissanayake AM, Leahy FE, Keogh GF, Frampton CM, Gamble GD, Brunton DH, Baker JR, Poppitt SD. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation: quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes 2005; 54:1468–76 [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Chan YK, Gamble GD, Poppitt SD, Othman AA, Cooper GJ. Triethylenetetramine and metabolites: levels in relation to copper and zinc excretion in urine of healthy volunteers and type 2 diabetic patients. Drug Metab Dispos 2007; 35:221–7 [DOI] [PubMed] [Google Scholar]
- 8.Gong D, Lu J, Chen X, Choong SY, Zhang S, Chan YK, Glyn-Jones S, Gamble GD, Phillips AR, Cooper GJ. Molecular changes evoked by triethylenetetramine treatment in the extracellular matrix of the heart and aorta in diabetic rats. Mol Pharmacol 2006; 70:2045–51 [DOI] [PubMed] [Google Scholar]
- 9.Zhang S, Liu H, Amarsingh GV, Cheung CC, Hogl S, Narayanan U, Zhang L, McHarg S, Xu J, Gong D, Kennedy J, Barry B, Choong YS, Phillips AR, Cooper GJ. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc Diabetol 2014; 13:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi SC, Eaton JW, Saari JT, Kang YJ. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp Med 2007; 204:657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Wang L, Schuschke DA, Zhou Z, Saari JT, Kang YJ. Marginal dietary copper restriction induces cardiomyopathy in rats. J Nutr 2005; 135:2130–6 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Saari JT, Kang YJ. Weak antioxidant defenses make the heart a target for damage in copper-deficient rats. Free Radic Biol Med 1994; 17:529–36 [DOI] [PubMed] [Google Scholar]
- 13.Elsherif L, Wang L, Saari JT, Kang YJ. Regression of dietary copper restriction-induced cardiomyopathy by copper repletion in mice. J Nutr 2004; 134:855–60 [DOI] [PubMed] [Google Scholar]
- 14.Prohaska JR, Heller LJ. Mechanical properties of the copper-deficient rat heart. J Nutr 1982; 112:2142–50 [DOI] [PubMed] [Google Scholar]
- 15.Kopp SJ, Klevay LM, Feliksik JM. Physiological and metabolic characterization of a cardiomyopathy induced by chronic copper deficiency. Am J Physiol 1983; 245:H855–66 [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulou LC, Sue CM, Davidson MM, Tanji K, Nishino I, Sadlock JE, Krishna S, Walker W, Selby J, Glerum DM, Coster RV, Lyon G, Scalais E, Lebel R, Kaplan P, Shanske S, De Vivo DC, Bonilla E, Hirano M, DiMauro S, Schon EA. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet 1999; 23:333–7 [DOI] [PubMed] [Google Scholar]
- 17.Freisinger P, Horvath R, Macmillan C, Peters J, Jaksch M. Reversion of hypertrophic cardiomyopathy in a patient with deficiency of the mitochondrial copper binding protein Sco2: is there a potential effect of copper? J Inherit Metab Dis 2004; 27:67–79 [DOI] [PubMed] [Google Scholar]
- 18.Witte KK, Nikitin NP, Parker AC, von Haehling S, Volk HD, Anker SD, Clark AL, Cleland JG. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J 2005; 26:2238–44 [DOI] [PubMed] [Google Scholar]
- 19.Allahverdyan AE, Galstyan A. Le Chatelier’s principle in replicator dynamics. Phys Rev E Stat Nonlin Soft Matter Phys 2011; 84:041117. [DOI] [PubMed] [Google Scholar]
- 20.Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab 2010; 11:353–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Prohaska JR, Thiele DJ. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc Natl Acad Sci USA 2001; 98:6842–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr 2006; 136:21–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertinato J, Duval S, L’Abbe MR. Copper transporter 2 content is lower in liver and heart of copper-deficient rats. Int J Mol Sci 2010; 11:4741–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohrvik H, Nose Y, Wood LK, Kim BE, Gleber SC, Ralle M, Thiele DJ. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc Natl Acad Sci USA 2013; 110:E4279–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Han P, Xiao Y, Liu J, Kang YJ. A novel knot method for individually measurable aortic constriction in rats. Am J Physiol Heart Circ Physiol 2014; 307:H987–95 [DOI] [PubMed] [Google Scholar]
- 26.deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp 2010;38:e1729 [DOI] [PMC free article] [PubMed]
- 27.Reddy AK, Taffet GE, Li YH, Lim SW, Pham TT, Pocius JS, Entman ML, Michael LH, Hartley CJ. Pulsed Doppler signal processing for use in mice: applications. IEEE Trans Biomed Eng 2005; 52:1771–83 [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, Altman P, Schecter AD, Hajjar RJ, Tarzami ST. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol 2010; 176:1705–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson LE, Sheth M, Denyer RF, Dostal DE. Baseline echocardiographic values for adult male rats. J Am Soc Echocardiogr 2004; 17:161–7 [DOI] [PubMed] [Google Scholar]
- 30.Ferferieva V, Van den Bergh A, Claus P, Jasaityte R, La Gerche A, Rademakers F, Herijgers P, D’Hooge J. Assessment of strain and strain rate by two-dimensional speckle tracking in mice: comparison with tissue Doppler echocardiography and conductance catheter measurements. Eur Heart J Cardiovasc Imaging 2013; 14:765–73 [DOI] [PubMed] [Google Scholar]
- 31.Gueret P, Meerbaum S, Zwehl W, Wyatt HL, Davidson RM, Uchiyama T, Corday E. Two-dimensional echocardiographic assessment of left ventricular stroke volume: experimental correlation with thermodilution and cineangiography in normal and ischemic states. Cathet Cardiovasc Diagn 1981; 7:247–58 [DOI] [PubMed] [Google Scholar]
- 32.Dahlman T, Hartvig P, Lofholm M, Nordlinder H, Loof L, Westermark K. Long-term treatment of Wilson’s disease with triethylene tetramine dihydrochloride (trientine). QJM 1995; 88:609–16 [PubMed] [Google Scholar]
- 33.Cho HY, Blum RA, Sunderland T, Cooper GJ, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of a copper-selective chelator (TETA) in healthy adults. J Clin Pharmacol 2009; 49:916–28 [DOI] [PubMed] [Google Scholar]
- 34.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M, Gusella JF, Evgrafov O, Penchaszadeh GK, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 1993; 5:344–50 [DOI] [PubMed] [Google Scholar]
- 35.Siegemund R, Lossner J, Gunther K, Kuhn HJ, Bachmann H. Mode of action of triethylenetetramine dihydrochloride on copper metabolism in Wilson’s disease. Acta Neurol Scand 1991; 83:364–6 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Xiao Y, Liu J, Feng L, Kang YJ. Copper-induced reduction in myocardial fibrosis is associated with increased matrix metalloproteins in a rat model of cardiac hypertrophy. Metallomics 2018; 10:201–8 [DOI] [PubMed] [Google Scholar]
- 37.Malinow MR, Bostom AG, Krauss RM. Homocyst(e)ine, diet, and cardiovascular diseases: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1999; 99:178–82 [DOI] [PubMed] [Google Scholar]
- 38.Mansoor MA, Bergmark C, Haswell SJ, Savage IF, Evans PH, Berge RK, Svardal AM, Kristensen O. Correlation between plasma total homocysteine and copper in patients with peripheral vascular disease. Clin Chem 2000; 46:385–91 [PubMed] [Google Scholar]
- 39.Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 2003; 551:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeremy JY, Shukla N, Angelini GD, Day A, Wan IY, Talpahewa SP, Ascione R. Sustained increases of plasma homocysteine, copper, and serum ceruloplasmin after coronary artery bypass grafting. Ann Thorac Surg 2002; 74:1553–7 [DOI] [PubMed] [Google Scholar]
- 41.Shukla N, Angelini GD, Jeremy JY. Interactive effects of homocysteine and copper on angiogenesis in porcine isolated saphenous vein. Ann Thorac Surg 2007; 84:43–9 [DOI] [PubMed] [Google Scholar]
- 42.Zuo X, Dong D, Sun M, Xie H, Kang YJ. Homocysteine restricts copper availability leading to suppression of cytochrome C oxidase activity in phenylephrine-treated cardiomyocytes. PLoS One 2013; 8:e67549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anjos-Ramos L, Carneiro-Ramos MS, Diniz GP, Martins-Silva J, Barreto-Chaves ML. Early cardiac hypertrophy induced by thyroxine is accompanied by an increase in VEGF-A expression but not by an increase in capillary density. Virchows Arch 2006; 448:472–9 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Jiang Y, Kang YJ. Copper reverses cardiomyocyte hypertrophy through vascular endothelial growth factor-mediated reduction in the cell size. J Mol Cell Cardiol 2008; 45:106–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Bourcy K, Kang YJ. Copper-induced regression of cardiomyocyte hypertrophy is associated with enhanced vascular endothelial growth factor receptor-1 signalling pathway. Cardiovasc Res 2009; 84:54–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng W, Ye F, Xue W, Zhou Z, Kang YJ. Copper regulation of hypoxia-inducible factor-1 activity. Mol Pharmacol 2009; 75:174–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu L, Ding X, Zhang Z, Kang YJ. Copper is required for cobalt-induced transcriptional activity of hypoxia-inducible factor-1. J Pharmacol Exp Ther 2012; 342:561–7 [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Qiu L, Lin C, Yang H, Fu H, Li R, Kang YJ. Copper-dependent and -independent hypoxia-inducible factor-1 regulation of gene expression. Metallomics 2014; 6:1889–93 [DOI] [PubMed] [Google Scholar]
- 49.Pichiule P, Agani F, Chavez JC, Xu K, LaManna JC. HIF-1 alpha and VEGF expression after transient global cerebral ischemia. Adv Exp Med Biol 2003; 530:611–7 [DOI] [PubMed] [Google Scholar]
- 50.Dai Y, Xu M, Wang Y, Pasha Z, Li T, Ashraf M. HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 2007; 42:1036–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sung KC, Kim KS, Lee S. Hypoxic regulation of VEGF, HIF-1(alpha) in coronary collaterals development. Korean J Intern Med 2005; 20:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]