Short abstract
Obesity may attenuate the expression of brain-derived neurotrophic factor (BDNF), thereby increasing the risk of cognitive dysfunction. High-intensity interval exercise (HIIE) has been shown to be as or more effective than continuous moderate-intensity exercise (CME) in promoting the expression of BDNF in normal-weight individuals. Therefore, the primary purpose of this study was to examine whether or not acute HIIE could be utilized as a practical model to explore the BDNF response in obese versus normal-weight subjects when compared to acute CME. The potential relationship of exercise-induced BDNF with blood lactate and cortisol was also examined. Twelve male subjects (six obese and six normal-weight) participated in a counterbalanced and caloric equated experiment: HIIE (30 min, 4 intervals of 4 min at 80%–90% of VO2max with 3 min of active recovery at 50–60% VO2max) and CME (38 min at 50%–60% VO2max). Blood samples were collected prior to, immediately following exercise, and 1 h into recovery for measurements of serum BDNF, blood lactate, and plasma cortisol. Our results showed that the BDNF response to acute HIIE was greater than CME in obese subjects when compared to normal-weight subjects. Similarly, although acute HIIE induced greater blood lactate and plasma cortisol levels than CME, obese subjects produced less blood lactate, but no difference in cortisol than normal-weight subjects. These findings suggest that acute HIIE may be a more effective protocol to upregulate BDNF expression in an obese population, independent of increased lactate and cortisol levels.
Impact statement
High-intensity interval exercise (HIIE) has been shown to be a time-efficient exercise strategy that provides similar or superior physiological benefits as traditional continuous moderate-intensity exercise (CME). Our previous study demonstrated an equivalent elevation on the BDNF response in both obese and normal-weight individuals following 30 min of acute CME. To discover a time-efficient exercise strategy to improve brain health in an obese population, the present study found that obese individuals elicit a greater level of BDNF following acute HIIE versus CME than normal-weight individuals. These findings indicate that acute HIIE may be an effective strategy to upregulate BDNF expression in obese individuals.
Keywords: Brain-derived neurotrophic factor, obesity, high-intensity interval exercise, cortisol, blood lactate
Introduction
Brain-derived neurotrophic factor (BDNF) is a neurotrophic protein mediator for neuron survival, neuronal growth and plasticity, and memory.1 A lower expression of circulating BDNF has been shown in patients with inflammatory conditions and diseases, including Alzheimer’s disease, Parkinson’s disease,2 and obesity.3 Specifically, research has demonstrated that obesity is associated with increased risk of cognitive dysfunction, although the mechanism(s) of this relationship is not fully elucidated.4
Aerobic training has been shown to not only provide beneficial anti-inflammatory and cardiovascular adaptations,5 but also reduce age-related cognitive decline, preserve brain volume, and potentially improve cerebral blood flow.6 In addition, aerobic training can increase peripheral levels of BDNF,7 which has been shown to reflect an increase in BDNF within the rodent brain to potentially promote a neuroprotection.8,9 Although the BDNF response to acute aerobic exercise demonstrates an intensity-dependent nature,10 the mechanism(s) of this relationship is not fully understood. Specifically, exercise-induced blood lactate has been shown to correlate with increased peripheral level of BDNF.10 Furthermore, increased levels of glucocorticoids (e.g. cortisol) have been demonstrated to directly downregulate BDNF expression through various mechanisms, including decreased levels of BDNF mRNA via suppression of AP-1-mediated transcriptional activation.11
Thus far, most exercise studies examining the BNDF response in an obese population have utilized a continuous moderate-intensity exercise (CME) protocol rather than a high-intensity interval exercise (HIIE) protocol. Specifically, our laboratory has recently shown that acute CME elicits increased plasma BDNF expression in both obese and normal-weight individuals.12 While HIIE training has been shown to be as or more effective than CME in improving body composition, insulin sensitivity,13 and cognitive functioning in obese individuals,14 Saucedo-Marquez et al.15 have demonstrated that acute HIIE is more effective than CME in promoting the expression of BDNF in normal-weight individuals. Therefore, the primary purpose of this study was to examine whether or not acute HIIE could be utilized as a practical model to explore the BDNF response in obese versus normal-weight subjects when compared to acute CME. The potential relationship of exercise-induced BDNF with blood lactate and cortisol was also examined. It was hypothesized that obese subjects would elicit a greater BDNF response than normal-weight individuals following acute HIIE versus CME. Furthermore, the level of serum BDNF following acute HIIE would be positively correlated with blood lactate, but a negative relationship would be observed with plasma cortisol in both groups.
Methods
Subjects
Twelve (six obese [25.54 years ± 1.67 SEM] and six normal-weight [22.58 yrs. ± 0.69 SEM]) healthy male subjects were recruited from flyers posted around the Florida Atlantic University Boca campus and the surrounding community (see anthropometric characteristics in Table 1). All recruited participants were categorized as either obese or normal-weight, with a body mass index (BMI) of at least 30 kg/m2 or between 18.5 and 24.9 kg/m2, respectively. Informed consent and medical history forms were completed by all subjects upon the first visit, as well as a seven-day physical activity questionnaire. An approval to conduct this study was obtained from the Institutional Review Board.
Table 1.
Participant anthropometric and metabolic characteristics.
| Variable | Obese (n = 6) | Normal-weight (n = 6) | P |
|---|---|---|---|
| Age (years) | 25.54 ± 1.67 | 22.58 ± 0.69 | 0.147 |
| Height (cm) | 178.17 ± 2.40 | 177.75 ± 1.47 | 0.885 |
| Weight (kg) | 121.70 ± 6.05 | 69.0 ± 3.36 | <0.001* |
| BMI (kg/m2) | 38.25 ± 1.36 | 21.78 ± 0.74 | <0.001* |
| HIIE rSBP (mmHg) | 145.33 ± 5.55 | 115.0 ± 3.49 | 0.001* |
| CME rSBP (mmHg) | 139.0 ± 4.46 | 118.0 ± 3.69 | 0.005* |
| HIIE rDBP (mmHg) | 84.33 ± 4.42 | 75.33 ± 1.43 | 0.082 |
| CME rDBP (mmHg) | 87.33 ± 2.62 | 70.47 ± 3.15 | 0.002* |
| HIIE rHR (BPM) | 75.0 ± 2.37 | 64.67 ± 3.22 | 0.027* |
| CME rHR (BPM) | 76.0 ± 2.27 | 67.67 ± 6.39 | 0.263 |
| VO2max (mL/kg/min) | 33.88 ± 2.09 | 51.77 ± 1.94 | <0.001* |
| Waist circumference (cm) | 114.37 ± 6.57 | 77.67 ± 1.72 | <0.001* |
| Hip circumference (cm) | 121.0 ± 3.10 | 94.25 ± 1.74 | <0.001* |
| WHR | 0.94 ± 0.03 | 0.82 ± 0.02 | 0.010* |
BMI: body mass index; rSBP: resting systolic blood pressure; rDBP: resting diastolic blood pressure; rHR: resting heart rate; VO2max: maximal oxygen consumption; WHR: waist/hip ratio.
Note: The * indicates the difference between obese and normal-weight groups. Data are presented as means ± standard error of mean (SEM).
Subject exclusion criteria were identified using the health history questionnaire and included the presence or suspicion of diseases, such as any cardiovascular, inflammatory, metabolic, or rheumatologic disease. Any subjects who used any medication, supplements, or tobacco/nicotine products such as cigarettes, chewing tobacco, and vaporizers were excluded. Subjects were also excluded from the study if an average of alcohol consumption exceeded more than 10 drinks per week. These exclusion criteria were determined using the health history questionnaire. Prior to each visit, all subjects were instructed to have an overnight fasting and also refrain from any alcohol or caffeine consumption, and any high-intensity physical activity for at least 24 h. Due to the fact that training may affect the physiological outcomes following acute exercise, any subject who participated in moderate or vigorous physical activity of at least 150 min per week were also excluded from the study.16 The subjects in this study are a subset of the cohort in our recent published paper.17
Experimental protocol
Data collection for the study consisted of three exercise sessions beginning at 7:00–7:30 a.m. in the Exercise Biochemistry Laboratory. Informed consent, a medical history questionnaire, and a seven-day physical activity recall were administered and completed during the first visit as well as measurements of subjects’ height, weight (SECA 769, Chino, CA), and hip/waist circumferences. Resting heart rate (HR) and blood pressure were obtained using a digital monitor (Polar T31, Polar Electro, Kempele, Finland) and a sphygmomanometer (752M-Mobile Series, American Diagnostic Corporation, Hauppauge, NY) after resting quietly for 20 min. Subjects then participated in a graded maximal exercise test lasting approximately 12 to 15 min on a treadmill (Norditrack X11i) specifically develop for assessing maximal heart rate (HRmax) and maximal oxygen consumption (VO2max) obtained using open-circuit spirometry (ParvoMedics Metabolic Measurement System, ParvoMedics, Sandy, UT, USA). The specific protocol of this maximal exercise test has been previously published.17
Two caloric equated and counterbalanced exercise protocols (HIIE and CME) comprised the second and third visits utilizing a previously published treadmill protocol.18,19 The HIIE protocol consisted of a 5-min walking or jogging warm-up at 50–60% of the subject’s VO2max, followed by four high-intensity intervals lasting 4 min each at a speed and grade that elicited 80–90% of VO2max; 3 min of active recovery at 50–60% VO2max was performed between each high-intensity interval. To equate the total caloric expenditure between the two protocols, the CME protocol included a 5-min walking or jogging warm-up at 50–60% VO2max for a total of 38 min. During each visit, a trained phlebotomist collected a 10 mL sample of whole blood from a superficial vein of the antecubital space with a closed IV catheter system (BD Nexiva 20GA, REF 383516, Franklin Lakes, NJ, USA) prior to (Pre), immediately post (Post), and 1 h following the completion of both acute HIIE and CME (recovery one hour [R1h]). All samples were collected into appropriate collection tubes and stored at −80°C for further analyses.
Measurements of serum BDNF, plasma cortisol, and blood lactate
The level of blood lactate was measured from the whole blood immediately following each blood collection using a digital portable lactate analyzer (Lactate Scout+; EKF Diagnostics, Cardiff, UK). Subsequently, a 5 mL sample of whole blood was collected into a serum separation tube for analysis of BDNF, which was assayed in duplicate with enzyme-linked immunosorbent assays (Promega Corporation, Madison, WI). A 5 mL blood sample was collected into a tube containing EDTA for analysis of plasma cortisol, and centrifuged for 15 min at 2000 × g at 4°C. Plasma cortisol was assayed in duplicate using ELISA kits (Calbiotech Inc., El Cajon, CA). Both serum and plasma were batch processed for further analyses of BDNF and cortisol. All analyses were conducted according to the manufacturer’s instructions. Finally, inter- and intra-assay coefficients of variation for BDNF were 7.4% and 5.8%, respectively. Inter- and intra-assay coefficients of variation for cortisol were 6.5% and 7.3%, respectively. The sensitivity levels of BDNF and cortisol ELISA kits were 15.6 pg/mL and 0.44 ng/mL, respectively.
Statistical analyses
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS version 24.0). Differences between obese and normal-weight groups in baseline variables were conducted by independent t tests. A 2 (group) × 2 (treatment) × 3 (time points: Pre, Post, and R1h) repeated measures analyses of variance (ANOVA) was utilized to examine the effect of acute HIIE and CME on the levels of serum BDNF, plasma cortisol and blood lactate. The Greenhouse-Geisser correction of degrees of freedom was used when sphericity assumptions were violated. Significant effects were further analyzed with Bonferroni post hoc comparisons. Furthermore, Pearson’s product–moment correlations were used to examine the relationship of serum BDNF with plasma cortisol and blood lactate following both HIIE and CME protocols. Finally, normality of the data was confirmed with a Shapiro–Wilk test. A post hoc power analysis was conducted using the program G*Power (version 3.1.9.2) for primary outcome measures. Based on the difference of mean and standard deviation (baseline to immediately post-exercise) with an alpha level of 0.05 for serum BDNF, blood lactate, and plasma cortisol in both HIIE and CME, the overall sample size of 12 subjects in this study achieved an adequate power (> 80%) for all outcome variables. Statistical significance was set at P ≤ 0.05.
Results
Anthropometric and metabolic measurements of the study participants
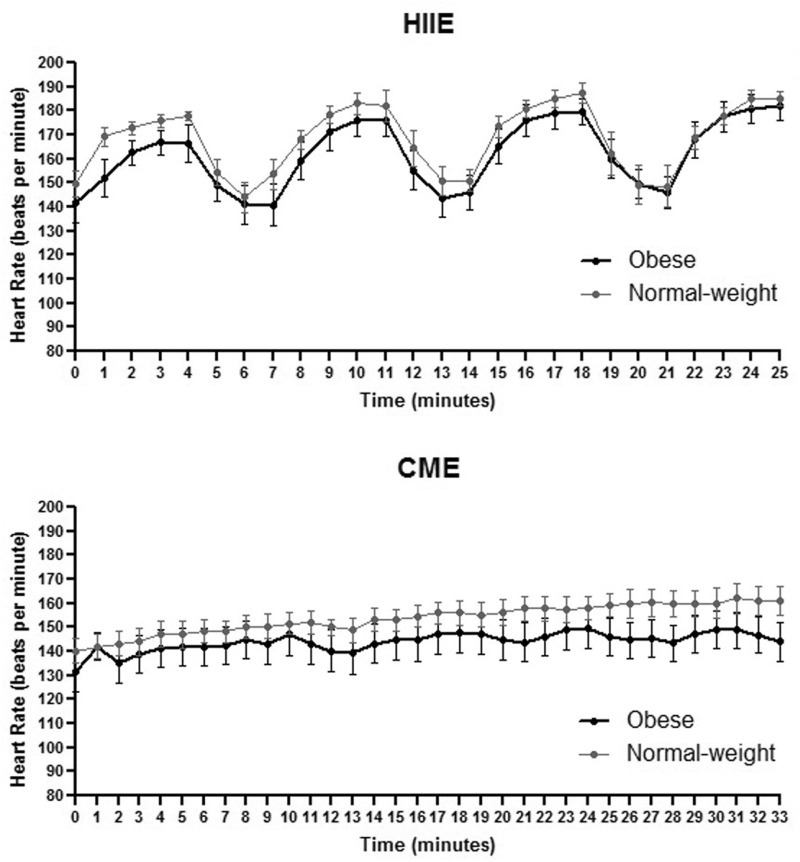
Anthropometric measurements and baseline metabolic characteristics for both obese and normal-weight groups are reported in Table 1. Statistical significance was found between groups for body weight, BMI, systolic and diastolic blood pressures, waist/hip circumferences and ratio, heart rate, and relative VO2max. HR following both acute HIIE and CME in both groups was recorded (Figure 1).
Figure 1.
Heart rate during acute HIIE and CME in obese and normal-weight subjects. Data are presented as means ± SEM.
Measurements of serum BDNF, blood lactate, and plasma cortisol
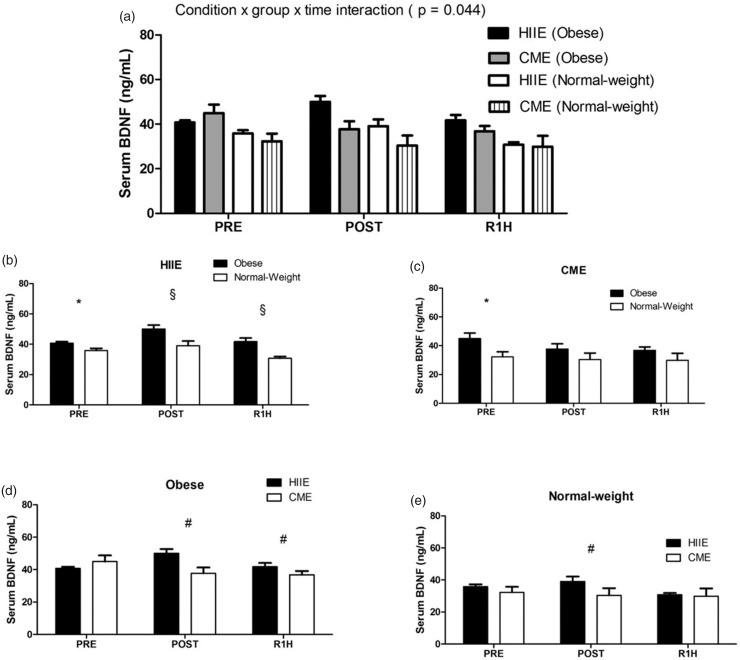
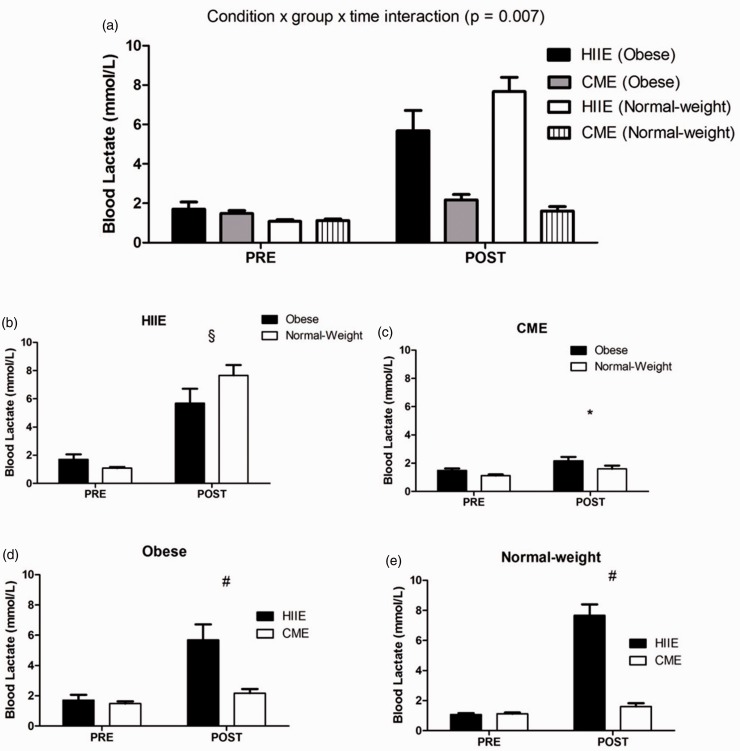
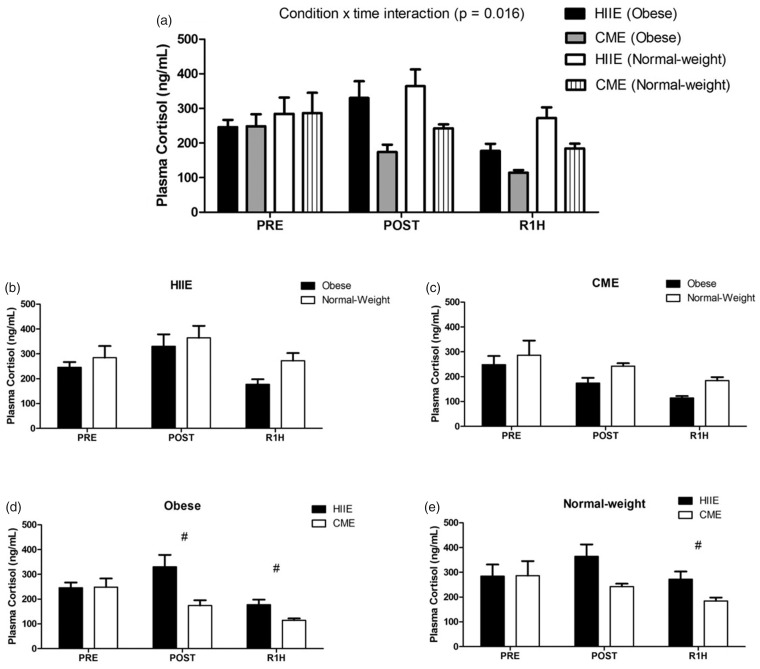
At baseline, obese subjects showed significantly higher levels of serum BDNF than normal-weight subjects in both acute HIIE and CME sessions [t (10) = −2.905, P = 0.016; t (9.938) = −2.470, P = 0.033; respectively] (see Figure 2(b) and (c)), whereas the concentrations of blood lactate and plasma cortisol did not differ between both groups in either acute HIIE or CME session (see Figures 3(b) and (c) and 4(b) and (c)). In addition, the baseline level of serum BDNF was not correlated with either blood lactate or plasma cortisol.
Figure 2.
The expression of serum BDNF following acute HIIE and CME in obese and normal-weight subjects. Acute HIIE exhibited a greater serum BDNF response in obese subjects vs. normal-weight subjects compared to CME. The * indicates a significant difference at baseline between groups in both exercise protocols ((b), P = 0.016; (c): P = 0.033). The § indicates a significant difference from baseline (pre-exercise) between both groups following acute HIIE ((b), P = 0.042). The # indicates a significant difference from the baseline between exercise protocols in both groups ((d): P = 0.008; (e): P = 0.048). Data are presented as means ± SEM.
Figure 3.
The expression of blood lactate following acute HIIE and CME in obese and normal-weight subjects. Acute HIIE elicited a greater blood lactate in normal-weight subjects vs. obese subjects compared to CME (a). The § indicates a significant difference from baseline (pre-exercise) between both groups following acute HIIE ((b): P = 0.044). The * indicates a significant time effect in both groups in response to acute CME ((c): P = 0.007). The # indicates a significant difference from the baseline between exercise protocols in both groups ((d): P = 0.003; (e): P < 0.001). Data are presented as means ± SEM.
Figure 4.
The expression of plasma cortisol following acute HIIE and CME in obese and normal-weight subjects. Acute HIIE exhibited a higher concentration of plasma cortisol compared to CME with no difference between groups (a). The # indicates a significant difference from the baseline between exercise protocols in both groups ((d): P = 0.007; (e): P = 0.010). Data are presented as means ± SEM.
In response to exercise, repeated measures ANOVA analysis demonstrated a significant condition by group by time interaction in serum BDNF [F (2, 20) = 3.675, P = 0.044] (see Figure 2(a)), with a greater level in obese subjects than normal-weight subjects following acute HIIE (see Figure 2(b)), whereas acute CME did not elicit any change in both groups (see Figure 2(c) to (e)). Furthermore, a condition by group by time interaction was also observed in blood lactate [F (1, 10) = 11.319, P = 0.007] (see Figure 3(a)), with a higher level in normal-weight subjects than obese subjects in response to acute HIIE (see Figure 3(b)) vs. CME, while acute CME only demonstrated a time effect in both groups (see Figure 3(c)). As expected, acute HIIE elicited a greater level of blood lactate in both groups than CME (Figure 3(d) and (e)). In addition, acute HIIE exhibited a higher concentration of plasma cortisol compared to CME, with no difference in both groups [F (1.269, 12.688) = 7.040, P = 0.016] (see Figure 4(a) to (e)). Finally, the percent change (baseline to immediately post-exercise) of serum BDNF was not correlated with the percent change of blood lactate and plasma cortisol in either acute HIIE (r = −0.480, P = 0.114; r = −0.475, P = 0.118; respectively) or CME (r = −0.393, P = 0.206; r = −0.125, P = 0.698; respectively).
Discussion
This study examined the effect of acute HIIE vs. CME on serum BDNF expression in obese and normal-weight subjects. Our results demonstrate that obese subjects exhibited a greater serum BDNF response than normal-weight subjects following acute HIIE vs. CME. However, acute HIIE elicited a greater blood lactate in normal-weight subjects than obese subjects when compared to CME. Furthermore, the concentration of plasma cortisol was higher following acute HIIE than CME, with no difference between groups. To the best of our knowledge, this study is the first to examine the modulatory role of obesity on exercise-induced BDNF release and to utilize an acute HIIE protocol as a practical model to examine the phenomena of BDNF release in both obese and normal-weight subjects. These findings indicate that acute HIIE may be an effective strategy to upregulate BDNF expression in obese individuals.
The results of this study showed that obese subjects elicited a higher baseline level of serum BDNF when compared to normal-weight individuals. This baseline difference is in concordance with existing literature, demonstrating a positive correlation between BMI and BDNF levels.12,20 The mechanisms of increased BDNF in obesity include the role of energy balance/expenditure and eating behavior (food intake) to resist the disturbance in energy homeostasis.20,21 Specifically, an intravenous injection of BDNF in mice has been demonstrated to improve energy expenditure via increased body temperature and oxygen consumption.22 However, these findings are equivocal to other studies, showing reduced peripheral levels of BDNF in obese patients with insulin resistance.1 Importantly, the current study only recruited obese individuals without history of metabolic diseases, which may have eliminated some confounding factors when compared to the normal-weight counterparts.
Our laboratory has recently examined the effect of acute CME on the BDNF response in obese individuals, showing an equivalent elevation when compared to normal-weight individuals.12 The literature has proposed several mechanisms of exercise-mediated BDNF expression. For example, the release of BDNF in skeletal muscle has been demonstrated to increase fat oxidation via the AMP-activated protein kinase pathway during exercise.23 However, this intramuscular BDNF during exercise does not significantly contribute to the increased peripheral levels.23 In fact, evidence suggests that 70–80% of the circulating BDNF following exercise is derived from the brain.24 Furthermore, the store of BDNF in platelets also contributes to increase peripheral level due to transiently increased platelet counts in response to exercise.25,26 Importantly, this study demonstrated that acute HIIE enhanced a greater concentration of serum BDNF in obese subjects than normal-weight subjects when compared to CME. One possible explanation of this increased BDNF may be due to a higher platelet aggregation and activation following HIIE when compared to CME,27 although the existing literature only shows increased baseline levels of platelet counts in obese individuals when compared to normal-weight individuals.28 In addition, administration of BDNF in mice has been shown to increase the expression of glucose transporter 4 (GLUT4) in skeletal muscle,29 suggesting that the potential effect of BDNF on substrate utilization (e.g., glucose metabolism) following acute HIIE may enhance the modulation of obesity-related metabolic impairment. It is important to note that although the level of BDNF following acute CME (50–60% VO2max) did not change in both groups, an elevation following 12 weeks of aerobic exercise was observed at a similar exercise intensity.30
Finally, increased levels of cortisol have been shown to downregulate BDNF expression;11 however, this relationship in response to exercise still remains equivocal. Specifically, the current study and others31 did not observe any correlation between cortisol and BDNF following either acute HIIE or CME protocol; yet the report of such is opposite.32 Additionally, an injection of sodium-lactate in humans results in an alkalosis effect and increased peripheral levels of BDNF.33 Although Ferris et al.10 found that exercise-induced blood lactate was positively correlated with increased peripheral level of BDNF, the accumulation of this blood lactate in response to exercise leads to acidosis as opposed to alkalosis. The current study found a higher level of blood lactate in normal-weight subjects than obese subjects. Previous literature has demonstrated a lower exercise-induced catecholamine response in obese individuals,34 which would result in less phosphorylase activity and therefore less glycogen breakdown and lactate production. Irrespective of these observations, the fact that obese individuals had lower lactate with no difference in cortisol concentration suggests that increased BDNF following acute HIIE in obesity is most likely independent of increased lactate and/or cortisol levels.
In summary, this study demonstrated that acute HIIE elicited a greater serum BDNF response in obese subjects than normal-weight subjects when compared to CME. Importantly, HIIE is a time-efficient strategy with similar or superior physiological benefits that promotes the expression of a growth factor typically associated with brain health, yet that appears to be down regulated in obesity. Thus, the relative simplicity and efficacy of HIIE supports its use as a preventative measure and as an intervention to combat obesity and other chronic disease conditions (e.g. heart, diabetes, neurodegenerative). Although the subject exclusion criteria of >150 min of moderate and vigorous physical activity per week does not completely control for aerobic fitness, it was utilized to reduce any effects that training may have on the physiological outcomes following acute exercise. Moreover, previous research has shown that a higher relative VO2max (mL/kg/min) in obese subjects is mainly due to a higher fat mass, not impaired oxygen uptake or physically inactive.35 In addition, both the obese and normal-weight groups had comparable HR responses during both exercise protocols, demonstrating a similar relative exercise intensity and effort between groups. Therefore, the BDNF response was likely not influenced by disparities between aerobic fitness, with a greater level in obese subjects than normal-weight subjects following acute HIIE vs. CME.
Finally, although this study only included young male subjects, a meta-analysis shows that females have a smaller increase in the level of BDNF following exercise than males36 and the results of this study only provided a model for age-related cognitive decline. Further investigation with a large sample size including a gender analysis may potentially provide a basis to understand the clinical benefits of obesity-associated BDNF in predicting the effectiveness of exercise interventions (i.e. HIIE) for reduced risk of cognitive dysfunction and/or improved mood.
ACKNOWLEDGMENTS
The authors would like to thank the volunteers that donated their time to guarantee the accomplishment of this study.
Authors’ contributions
All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript. Conception and design: ALR, MW, and C-JH. Data collection: ALR, BGF, KMD, PJF, GP, AA, and C-JH. Data analysis and interpretation: ALR, MW, and C-JH. Manuscript writing: ALR, MW, and C-JH. Final approval of manuscript: ALR, MW, BGF, KMD, PJF, GP, AA, and C-JH.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this project was provided by the Department of Exercise Science and Health Promotion at Florida Atlantic University.
References
- 1.Krabbe KS, Nielsen AR, Krogh-Madsen R, Plomgaard P, Rasmussen P, Erikstrup C, Fischer CP, Lindegaard B, Petersen AM, Taudorf S, Secher NH, Pilegaard H, Bruunsgaard H, Pedersen BK. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007; 50:431–8 [DOI] [PubMed] [Google Scholar]
- 2.Bathina S, Das UN. Brain-derived neurotrophic factor and its clinical implications. Arch Med Sci 2015; 11:1164–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, Yanovski JA. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. J Clin Endocrinol Metab 2006; 91:3548–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashrafian H, Harling L, Darzi A, Athanasiou T. Neurodegenerative disease and obesity: what is the role of weight loss and bariatric interventions? Metab Brain Dis 2013; 28:341–53 [DOI] [PubMed] [Google Scholar]
- 5.Warburton D, Nicol C, Bredin S. Health benefits of physical activity: the evidence. CMAJ 2006; 174:801–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tarumi T, Zhang R. The role of exercise-induced cardiovascular adaptation in brain health. Exerc Sport Sci Rev 2015; 43:181–9 [DOI] [PubMed] [Google Scholar]
- 7.Griffin ÉW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly ÁM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav 2011; 104:934–41 [DOI] [PubMed] [Google Scholar]
- 8.Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett 2002; 328:261–4 [DOI] [PubMed] [Google Scholar]
- 9.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 1998; 37:1553–61 [DOI] [PubMed] [Google Scholar]
- 10.Ferris L, Williams J, Shen C. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc 2007; 39:728. [DOI] [PubMed] [Google Scholar]
- 11.Schaaf MJM, DeJong J, DeKloet ER, Vreugdenhil E. Downregulation of BDNF mRNA and protein in the rat hippocampus by corticosterone. Brain Res 1998; 813:112–20 [DOI] [PubMed] [Google Scholar]
- 12.Slusher AL, Whitehurst M, Zoeller RF, Mock JT, Maharaj A, Huang CJ. Brain‐derived neurotrophic factor and substrate utilization following acute aerobic exercise in obese individuals. J Neuroendocrinol 2015; 27:370–6 [DOI] [PubMed] [Google Scholar]
- 13.Fisher G, Brown AW, Brown MM, Alcorn A, Noles C, Winwood L, Resuehr H, George B, Jeansonne MM, Allison DB. High intensity interval- vs moderate intensity-training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One 2015; 10:e0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drigny J, Gremeaux V, Dupuy O, Gayda M, Bherer L, Juneau M, Nigam A. Effect of interval training on cognitive functioning and cerebral oxygenation in obese patients: a pilot study. J Rehabil Med 2014; 46:1050–4 [DOI] [PubMed] [Google Scholar]
- 15.Saucedo-Marquez CM, Vanaudenaerde B, Troosters T, Wenderoth N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol 2015; 119:1363–73 [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services. 2008 Physical activity guidelines for Americans. US Department of Health and Human Services (USDHHS). 2008 Physical Activity Guidelines for Americans. Washington, DC: USDHHS; 2008. https://health.gov/paguidelines/2008/ [Google Scholar]
- 17.Ferrandi PJ, Fico BG, Whitehurst M, Zourdos MC, Bao F, Dodge KM, Rodriguez AL, Pena G, Huang CJ. Acute high-intensity interval exercise induces comparable levels of circulating cell-free DNA and Interleukin-6 in obese and normal-weight individuals. Life Sci 2018; 202:161–6 [DOI] [PubMed] [Google Scholar]
- 18.Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA. High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 2004; 11:216–22 [DOI] [PubMed] [Google Scholar]
- 19.Tyldum GA, Schjerve IE, Tjønna AE, Kirkeby-Garstad I, Stølen TO, Richardson RS, Wisløff U. Endothelial dysfunction induced by post-prandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol 2009; 53:200–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med 2004; 66:744–8 [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Bomberg E, Billington C, Levine A, Kotz CM. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Phyhsiol 2007; 293:992–1002 [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Tsuchida A, Itakura Y, Nonomura T, Ono M, Hirota F, Inoue T, Nakayama C, Taiji M, Noguchi H. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes 2000; 49:436–44 [DOI] [PubMed] [Google Scholar]
- 23.Pedersen BK, Pedersen M, Krabbe KS, Bruunsgaard H, Matthews VB, Febbraio MA. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp Physiol 2009; 94:153–60 [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 2009; 94:1062–9 [DOI] [PubMed] [Google Scholar]
- 25.Fujimura H, Altar C, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002; 87:728–34 [PubMed] [Google Scholar]
- 26.Heber S, Volf I. Effects of physical (in) activity on platelet function. Biomed Res Int 2015; 2015:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadizad S, Nouri-Habashi A, Rahmani H, Maleki M, Naderi N, Lotfian S, Salimian M. Platelet activation and function in response to high intensity interval exercise and moderate continuous exercise in CABG and PCI patients. Clin Hemorheol Microcirc 2017; 64:911–9 [DOI] [PubMed] [Google Scholar]
- 28.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract 2005; 59:981–2 [DOI] [PubMed] [Google Scholar]
- 29.Suwa M, Yamamoto K, Nakano H, Sasaki H, Radak Z, Kumagai S. Brain-derived neurotrophic factor treatment increases the skeletal muscle glucose transporter 4 protein expression in mice. Physiol Res 2010; 59:619–23 [DOI] [PubMed] [Google Scholar]
- 30.Jeon YK, Ha CH. The effect of exercise intensity on brain derived neurotrophic factor and memory in adolescents. Environ Health Prev Med 2017; 22:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goekint M, Heyman E, Roelands B, Njemini R, Bautmans I, Mets T, Meeusen R. No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Med Sci Sports Exerc 2008; 40:1990–6 [DOI] [PubMed] [Google Scholar]
- 32.Hotting K, Schickert N, Kaiser J, Roder B, Schmidt-Kassow M. The effects of acute physical exercise on memory, peripheral BDNF, and cortisol in young adults. Neural Plast 2016; 2016:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Strüder HK. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett 2011; 488:234–7 [DOI] [PubMed] [Google Scholar]
- 34.Vettor R, Macor C, Rossi E, Piemonte G, Federspil G. Impaired counterregulatory hormonal and metabolic response to exhaustive exercise in obese subjects. Acta Diabetol 1997; 34:61–6 [DOI] [PubMed] [Google Scholar]
- 35.Ekelund U, Franks PW, Wareham NJ, Aman J. Oxygen uptakes adjusted for body composition in normal-weight and obese adolescents. Obes Res 2004; 12:513–20 [DOI] [PubMed] [Google Scholar]
- 36.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res 2015; 60:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]