Abstract
The onset of cancer metastasis is the defining event in cancer progression when the disease is considered lethal. The ability of metastatic cancer cells to stay dormant for extended time periods and reawaken at later stages leading to disease recurrence makes treatment of metastatic disease extremely challenging. The tumor microenvironment plays a critical role in deciding the ultimate fate of tumor cells, yet the mechanisms by which this occurs, including dormancy, is not well understood. This mini-review discusses bioengineered models inspired from tissue engineering strategies that mimic key aspects of the tumor microenvironment to study tumor dormancy. These models include biomaterial based three dimensional models, microfluidic based models, as well as bioreactor based models that incorporate relevant microenvironmental components such as extracellular matrix molecules, niche cells, or their combination to study microenvironmental regulation of tumor dormancy. Such biomimetic models provide suitable platforms to investigate the dormant niche, including cues that drive the dormant to proliferative transition in cancer cells. In addition, the potential of such model systems to advance research in the field of tumor dormancy is discussed.
Keywords: Tumor dormancy, Tumor microenvironment, Metastasis, Bioengineered models
Introduction
The progression of cancer from the primary to the metastatic setting usually marks the transition to an incurable diagnosis [1]. Accumulating evidence suggests that disseminated tumor cells can stay in a dormant state for extended periods of time and could reawaken at a later stage resulting in disease relapse and often mortality [2]. For instance, greater than 67% of deaths from breast cancer occur beyond the 5-year survival window and disease recurrence is noted after almost a decade of being “cancer-free” in many patients [3, 4]. In addition, dormant tumor cells can also persist at the primary tumor site, following surgical resection of the primary tumor [5]. Tumor cells can also metastasize and stay dormant even prior to the evolution of the primary tumor [6]. While drug treatments exist, resistance to treatment is noted in many patients and the dormant/resistant tumor cells surviving treatment reactivate and contribute to disease progression at the primary and/or metastatic site [7] (i.e., in organs such as bone, liver, lung, and the brain). These observations highlight the need to understand the cellular and molecular mechanisms associated with tumor cell dormancy.
It is now well appreciated that the tumor microenvironment plays a significant role in controlling the dormant phenotype in tumor cells in addition to genetic alterations [2, 8–10]. In the context of metastatic disease, this is consistent with Paget’s “seed and soil” hypothesis proposed over a century ago, which states that metastasis occurs only when the organ environment (soil) is conducive to metastatic tumor cell (seed) growth [11–14]. Thus, experimental models to study and understand the mechanisms associated with dormancy must capture the bidirectional tumor cell-microenvironment interactions. In early work elucidating the role of microenvironment on tumor dormancy, Aguirre-Ghiso and colleagues showed that growth signals from fibronectin (an extracellular matrix (ECM) protein) via the urokinase plasminogen activator receptor (uPAR)-α5β1-integrin complex was critical, and thus reduction in the level of uPAR in human epidermoid cancer cells induced tumor dormancy when tested using standard tissue culture polystyrene (TCPS) substrates (routinely employed two dimensional (2D) culture models) in vitro as well as using mouse models in vivo [15]. Studies utilizing these models have also defined several key molecular features of tumor cell dormancy, including a high signaling ratio of p38/ERK [16–19].
A variety of in vivo mouse models, including genetically engineered mouse models, orthotropic /subcutaneous tumor models, tumor resection models, as well as experimental metastasis mouse models have been used to gain insight into tumor dormancy [20–23]. For instance, experimental metastasis mouse models have revealed the existence of a dormant state in cancer cells delivered to a metastatic organ site in vivo [24, 25]. However, mouse models provide limited control of the organ environment for controlled investigations. In addition, animal-animal variations, difficulties associated with imaging dormant cells in internal tissues, as well as high costs, can make the use of such models a challenging undertaking. In recent years, there has been a growing interest in utilizing components typically employed in tissue engineering (e.g., biomaterial scaffolds, tissue specific cells, and bioreactors) to study the tumor microenvironment and its role in governing tumor dormancy. These systems not only enable better recapitulation of the tumor microenvironment by capturing the relevant microenvironmental cues such as biophysical cues compared to the traditionally studied 2D culture models but also the study of tumor cell phenotype in a physiological relevant and controlled setting.
This review focuses on various tissue engineering inspired strategies that have been employed to elucidate microenvironmental regulation of tumor cell dormancy. In particular, we discuss biomaterial based models, microfluidic based models, as well as bioreactor based models and how these bioengineered models have been utilized to study the dormant phenotype as well as the transition from a dormant to proliferative phenotype in cancer cells. Collectively, such microenvironment mimicking model systems provide useful tools to probe the dormant niche as well as elucidate the molecular mechanisms regulating tumor dormancy.
Bioengineered models mimicking the tumor microenvironment to study tumor cell dormancy
Biomaterial based models
Biomaterial scaffolds commonly employed in tissue engineering such as hydrogels, porous scaffolds, and electrospun fibrous scaffolds have been used as models to study tumor cell dormancy. Such three dimensional (3D) culture systems could be engineered to mimic specific features of the tumor microenvironment (e.g., stiffness, topography) as well as incorporate other relevant noncancerous cells. In this section, we discuss the various types of biomaterial based models that have been employed to study microenvironmental regulation of tumor dormancy.
Natural biomaterial based models
A variety of natural biomaterials have been used to study tumor cell dormancy and maintenance of this state via targeting the cytoskeletal organization [26], incorporating relevant niche cells [27, 28], modulation of stiffness [29], or via modulation of signaling pathways (e.g., Src family kinase (SFK) inhibition [30]). Specifically, hydrogels composed of Collagen-I [31], hyaluronic acid [32], fibrin [29], and Matrigel [26, 30, 31, 33] have been employed (studies summarized in Table 1). Barkan et al., utilized Basement Membrane Matrix (BME) (or Matrigel) and found that this matrix maintained the dormant state of D2.0R cancer cells that were observed to be dormant in vivo as opposed to traditionally studied 2D models (e.g., TCPS) and that the transition to the proliferative state was mediated via β-1 integrin signaling [26]. Further, myosin light chain kinase (MLCK) activation was also necessary for this transition as inhibition of MLCK or β-1 integrin impeded the dormant to proliferative state transition. Similarly, A549 lung cancer cells cultured in Matrigel underwent dormancy and exhibited drug resistance compared to standard 2D culture (TCPS) [34].
Table 1.
Summary of the studies utilizing bioengineered models to study tumor dormancy
| Type of Cancer | Cancer Cell Lines | Niche cells | Biomaterial | Key Findings | References |
|---|---|---|---|---|---|
| Biomaterial based models | |||||
| Breast Cancer and Osteosarcoma | D2.0R, D2A1, MCF-7, MDA-MB-231, 4 T1, K7 M2, K7M2AS1.46 | – | Basement Membrane Matrix | Inhibition of MLCK maintained the dormant phenotype of cancer cells and reduced metastatic outgrowth | Barkan et al., [26] |
| Breast Cancer and Osteosarcoma | D2.0R, D2A1, MCF-7, MDA-MB-231, K7 M2, K7M2AS1.46 | – | Basement Membrane Matrix | Cells can be maintained in dormant state by inhibiting MLCK phosphorylation | Barkan et al., [33] |
| Breast Cancer | D2.0R, D2A1 | – | Basement Membrane Matrix | Collagen-I enriched fibrotic environment revoked dormant phenotype in cancer cells | Barkan et al., [35] |
| Breast Cancer and Osteosarcoma | MDA-MB-231, K7 M2, 4 T1, D2.0R, D2A1 | – | Basement Membrane Matrix | Joint inhibition of SFK and MEK1/2 prevented switch from dormant to proliferative phenotype in cancer cells while simultaneously suppressing their survival | EL Touny et al., [30] |
| Breast Cancer | MDA-MB-231 | HMEC-1 | Hyaluronic acid hydrogel | Hyaluronic acid environment lowers the ERK/p38 ratio in cancer cells in 3D co-culture, which is indicative of a dormant phenotype | Kassim et al., [32] |
| Breast, Prostate and Ovarian Cancer | MCF-7, MDA-MB-231, MDA-MB-468, OVCAR-5, MCF10ADCIS.COM, LnCAP | – | Silica-PEG hydrogel | Physical immobilization of cancer cells in a stiff mechanical environment induces dormancy | Preciado et al., [54] |
| Melanoma, Liver, Lung and Breast Cancer | B16,4 T1, H22, A375, A549, HepG2 | – | Fibrin gel | Nuclear translocation of Cdc42 in a stiff mechanical environment induces dormancy in B16 cells by activating Cdc42-tet2 pathway | Liu et al., [29] |
| Breast Cancer | MDA-MB-231, MCF-7, T4–2 | HUVEC, Lung Fibroblasts, Bone marrow mesenchymal stem cells | Laminin rich ECM | Stable microvascular endothelium induces quiescent phenotype in disseminated tumor cells (DTC) which is mediated by Thrombospondin-1 whereas, sprouting endothelium promote proliferation in DTCs by producing periostin & TGF-β1 | Ghajar et al., [28] |
| Breast Cancer | SUM159, SUM149, MDA-MB-231, MDA-MB-435, BT474, MCF-7, T47D, ZR75–1 | HUVEC, Bone marrow derived mesenchymal cells (HS-5), hFOB, BMSC | Collagen biomatrix | Niche cells HUVEC, HS-5, and hFOB induce quiescence in cancer cells and this phenotype can be reversed by inhibiting p38, ALK5, and RTK | Marlow et al., [27] |
| Breast and Ovarian Cancer | MCF-7, MDA-MB-231, OVCAR-3 | – | Collagen gel | Chemically induced hypoxia through CoCl2 treatment induces dormancy in MCF-7 cells by increasing p38/pERK ratio | Lee et al., [51] |
| Breast, Colon and Pancreatic Cancer | MDA-MB-231, HCT-116,CFPAC-1 | – | Collagen gel | Stiff mechanical environment coaxed the cancer cells towards dormancy which resulted in increased drug resistance | Fang et al., [31] |
| Liver Cancer | Huh7, HepG2 | – | PA gel | Soft environments induced dormancy in cancer cells by downregulating the phosphorylation of FAK, ERK and STAT3 resulting in increased drug resistance and stem-like characteristics | Schrader et al., [53] |
| Prostate Cancer | M12, P69, M12mac25, LNCaP C4–2 | – | pHEMA scaffolds | pHEMA scaffolds suppressed the proliferative phenotype in LNCaP cells in vivo whereas, they promoted the growth of M12mac25 cells by triggering the dormant to proliferative switch | Long et al., [55] |
| Breast Cancer | MDA-MB-231, T47D | – | PCL fibrous scaffold | Cancer cells cultured on PCL scaffolds and treated with carboplatin exhibited a quiescent phenotype | Guiro et al., [56] |
| Bladder, Prostate, Lung, Breast Cancer and Neuroblastoma | T24, UMUC-3, PC3, PC3-PSMA, MCF-7, MDA-MB-231, H1975, SH-SY5Y | NIH3T3 mouse fibroblasts, BJ-5ta human foreskin fibroblasts & WPMY-1 human prostate stromal cells | Amikagel | Bladder cancer cells on ~ 215 kPa Amikagels were growth arrested and resistant to docetaxel | Pavan Grandhi et al., [57] |
| Lung Cancer | A549 | – | Matrigel | Matrigel induced dormancy in A549 cells via inhibition of PI3K/Akt and ERK1/2 pathways. | Keeratichmroen et al., [34] |
| Bladder Cancer | SV-HUC-1, TCCSUP, RT4, J82, 253JP, 253JB-V | – | Matrigel, SIS gel | SIS gel suppressed the growth and malignant phenotype of bladder cancer cells by driving them towards dormancy whereas, Matrigel supported the malignant and invasive phenotype | Hurst et al., [46] |
| Bladder Cancer | SV-HUC-1, TCCSUP, RT4, J82, 253JP, 253JB-V | – | Matrigel, SIS gel | SIS gel suppresses malignant phenotype in cancer cells as indicated by gene expression pattern whereas Matrigel promotes the expression of growth associated genes | Dozmorov et al., [47] |
| Bladder Cancer | J82, JB-V | – | Matrigel, Collagen, SIS gel | Dormant phenotype was observed in J82 or JB-V cells injected with SIS gel in nude mice | Hurst et al., [48] |
| Breast, Bladder, Prostate, Pancreatic, Gastric Cancer and Glioblastoma | MDA-MB-231, MDA-MB-435, MDA-MB-235, 4 T1, J82, PC-3, DU145, U251, AGS, Capan-1 | – | SIS gel | Identified drugs to target dormant cells | Hurst et al., [49] |
| Microfluidic based models | |||||
| Breast Cancer | MDA-MB-231 | Human hepatocytes, NPCs | PEG hydrogel | Dormant phenotype was observed in cancer cells co-cultured with hepatic niche on the liver-chip incorporating PEG hydrogel | Clark et al., [61] |
| Breast Cancer | MDA-MB-231, MCF-7 | Human hepatocytes, NPCs | – | A subpopulation of cancer cells underwent dormancy on the liver chip and this was associated with a differential cytokine profile | Wheeler et al., [58] |
| Breast Cancer | MDA-MB-231, MCF-7 | Human mammary epithelial cells, human hepatic stellate cells (LX-1, LX-2, TWNT-1), rat hepatic stellate cells (HSC-T6) human endothelial cells (TMNK-1) | – | IL-8 revoked the hepatocyte induced dormancy in MDA-MB-231 cells | Khazali et al., [59] |
| Breast Cancer | MDA-MB-231 | Human hepatocytes, NPCs | – | Introduction of inflammatory stimuli promoted growth of dormant cancer cells | Clark et al., [60] |
| Lung Cancer | H1975 | Human primary airway and alveolar epithelial cells, Human lung microvascular epithelial cells | – | Breathing motion incorporated in engineered lung chip decreased cancer cell growth leading to a dormant phenotype | Hassel et al., [62] |
| Bioreactor based model | |||||
| Breast Cancer | MDA-MB-231, MCF-7, MDA-MB-231BRMS1 | Mouse MC3T3-E1 osteoblasts, Normal Human Osteoblast cells | – | Remodeling cytokines TNFα and IL-1β promoted metastatic outgrowth of dormant MDA-MB-231BRMS1 cells | Sosnoski et al., [63] |
MLCK Myosin light chain kinase, SFK Src family kinases, MEK Mitogen activated protein kinases, ERK Extracellular-signal regulated kinases, Cdc42 Cell division control protein 42, Tet-2 Tet methylcytosine dioxygenase 2, TGF-β1 Transforming growth factor beta 1, ECM Extracellular matrix, HUVEC Human umbilical vein endothelial cells, FAK Focal adhesion kinase, STAT3 Signal transducer and activator of transcription 3, BMSC Bone marrow stromal cells, RTK Receptor tyrosine kinase, pHEMA poly (2-hydroxyethyl methacrylate), PCL Polycaprolactone, PEG Polyethylene glycol, PA Polyacrylamide, SIS Small intestine submucosa, HMEC Human microvascular endothelial cells, TNFα Tumor necrosis factor alpha, NPCs Nonparenchymal cells, hFOB Human fetal osteoblasts, IL Interleukin, PI3K Phosphoinositide 3-kinase, Akt Protein kinase B
In contrast to BME inducing a dormant state, incorporating Collagen-I within BME lead to a proliferative phenotype in dormant mouse breast cancer D2.0R cells in vitro [35]. Activation of β-1 integrin was responsible for the emergence of this phenotype and thus inhibiting β-1 integrin and the associated downstream signaling pathway components (Src, extracellular-signal regulated kinase (ERK), or MLCK) significantly inhibited proliferation. Modulation of signaling pathways to control the dormant vs. proliferative phenotype has also been investigated using natural biomaterial based models. Specifically, SFK inhibition caused localization of p27 (cyclin dependent kinase inhibitor) to the nucleus and inhibited proliferation that was induced by incorporating Collagen-I into BME [30]. Further, combined targeting of SFK and mitogen activated protein kinase (MEK) was shown to induce apoptosis in dormant cancer cells, thereby demonstrating the efficacy and potential of this combinatorial treatment for treating recurrent disease.
Niche cells present in the tumor microenvironment have been incorporated into natural biomaterial scaffolds to create a model of dormancy for bone metastatic breast cancer cells. For example, Marlow et al., employed a 3D collagen biomatrix that were seeded with either primary bone marrow stromal cells (BMSC) or a mix of osteoblasts, mesenchymal, and endothelial cell lines (BMCL-Bone marrow cell lines) [27]. In this system, breast cancer cells co-cultured with BMSCs proliferated whereas those cultured with BMCL remained in a dormant state and this phenomenon was observed both in vitro and in vivo. Moreover, breast cancer cells retrieved from BMCL co-cultures began proliferating when co-cultured with BMSCs. The dormant state observed in this model was also reversible when p38, and receptor tyrosine kinase (RTK) (pathways involved in dormancy [36–38]) was inhibited. These observations were also validated in vivo by subcutaneously implanting cell-laden biomaterial constructs in murine models. Such “hybrid in vivo models” wherein biomaterial scaffolds are integrated with murine models have been recently utilized in several investigations to study the metastatic niche [39–45]. Similarly, Ghajar et al., demonstrated that endothelial cells influenced the dormant phenotype in breast cancer cells in a laminin-rich ECM [28]. Specifically, established or stable endothelium induced a dormant state via endothelial-derived thrombospondin-1 (TSP-1). In contrast, the authors showed that cancer cell growth was accelerated at sprouting neovascular tips (i.e., sprouting endothelium), which was associated with enhanced expression of Transforming growth factor beta 1 (TGF-β1) and periostin, and with the loss of TSP-1. In a hyaluronic acid hydrogel model, when breast cancer cells were co-cultured with a human microvascular endothelial cell line (HMEC-1), expression of ERK/p38 was reduced in co-culture compared to breast cancer cell monoculture indicating the emergence of a dormant state in breast cancer cells [32].
Similar to the utilization of Matrigel, Hurst et al., [46] utilized SIS gel (derived from small intestine submucosa (SIS) representative of a normal basement membrane matrix) to study phenotype regulation in bladder cancer cells and compared it with Matrigel (representative of a remodeled tumor matrix). In these studies, Matrigel promoted a more invasive phenotype as opposed to a non-aggressive phenotype that was observed in the SIS gel. Further, cells isolated from Matrigel when grown on SIS gel demonstrated growth characteristics similar to cells grown on SIS gel and vice versa demonstrating that this phenotype regulation was dependent on the gel composition. These results were further supported via comparative gene expression studies [47]. In a follow up study, these observations were further validated using hybrid in vivo models [48]. In particular, when J82 or JB-V bladder cancer cells were subcutaneously injected with SIS gel in nude mice, cancer cells were observed to be in a dormant state with no sign of tumor formation. However, in some cases, cells transitioned from a dormant to a proliferative state. Tumor growth was noted in 40% of SIS gel xenografts following a 4–18 week dormancy period. Specifically, the transition from a dormant to a proliferative phenotype was dependent on the number of implanted tumor cells, with tumors more likely to form when more than 3 million tumor cells were implanted [48]. These models have also been utilized to identify therapeutics that target dormant cells [49] .
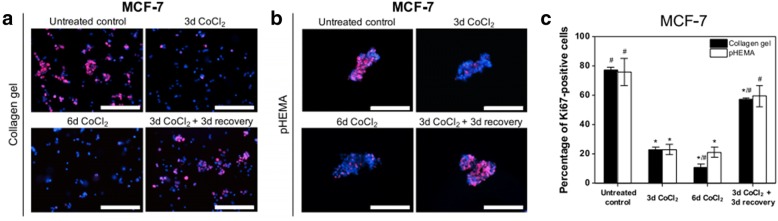
Hypoxia, a characteristic feature of the tumor microenvironment [50], has also been incorporated with natural biomaterials such as Collagen to develop dormancy models. For example, Lee et al., utilized cobalt chloride (CoCl2) (a hypoxia mimicking agent) with Collagen gels to induce dormancy in breast cancer cells [51]. They found that MCF-7 breast cancer cells exhibited a dormant phenotype in this model system and this phenotype was reversible when the cells were grown in CoCl2 free media. These results were also observed when the cells were grown on non-adhesive poly(2-hydroxyethyl methacrylate) (pHEMA) coated tissue culture plates (Fig. 1).
Fig. 1.
In a Collagen hydrogel incorporating hypoxia mimicking agent CoCl2 (300 μM) or pHEMA coated culture plates, MCF7 breast cancer cells exhibited a dormant phenotype, which was reversible after treatment with CoCl2 free growth media. Fluorescence images of MCF7 cells stained for Ki67 (red) and nuclei (blue) for untreated control, 3 day treatment with CoCl2, 6 day treatment with CoCl2 and 3 day treatment with CoCl2 followed by 3 day recovery period in (a) Collagen hydrogels and (b) pHEMA coated culture plates and (c) quantification of Ki-67 status in these conditions. Scale bar = 200 μm. Figure taken from [51] and reprinted with permission of BioMed Central (Springer Nature)
More recently, fibrin gels were employed to elucidate the impact of matrix stiffness on tumor cell dormancy. Specifically, Liu et al., employed [29] fibrin gels of 90, 450 and 1050 Pa bracketing the range of stiffness noted for many tissues (100–3000 Pa [52]). In this system, murine B16 & human melanoma A375 cells embedded within 1050 Pa fibrin gels remained dormant as opposed to those in 90 Pa gels. This induced dormancy was reversible, as cells isolated from 1050 Pa fibrin gel proliferated when cultured in 90 Pa gels. Maintenance of the dormant state with increasing stiffness in this system was mediated via translocation of cell division control protein 42 (Cdc42) from the cytosol to the nucleus, in turn, promoting tet methylcytosine dioxygenase 2 (Tet-2) expression, and subsequently activating cell-cycle inhibiting p21 and p27 genes.
Synthetic biomaterial based models
In addition to natural biomaterial-based models, synthetic biomaterial systems such as polyacrylamide (PA), silica-polyethylene glycol (silica-PEG), polycaprolactone (PCL), and pHEMA have been utilized to study the impact of tumor microenvironment on the dormant phenotype. Synthetic biomaterials provide a highly tunable platform and are more reproducible compared to natural biomaterial-based models. Schrader and colleagues utilized PA hydrogels to study the influence of matrix stiffness on the behavior of hepatocellular carcinoma cells [53]. They found these cancer cells cultured on stiff hydrogels (12 kPa) rapidly proliferated compared to soft hydrogels (1 kPa) as indicated via increased Ki67 (a proliferation marker) positivity, with the soft hydrogels promoting a more dormant-like phenotype. Inhibition of β1-integrin or Focal adhesion kinase (FAK) significantly reduced Ki-67 status on stiff hydrogels (12 kPa), thereby implicating these pathways in the observed cellular response.
Physical immobilization of cancer cells in synthetic biomaterials has also been shown to induce a dormant phenotype in cancer cells. For instance, MCF-7 breast cancer cells encapsulated in a porous silica-PEG hydrogel system underwent cell-cycle arrest, but resumed proliferation when they were retrieved from the hydrogel and cultured on TCPS [54]. Similarly, Long et al., employed sphere-templated porous pHEMA hydrogels to develop prostate cancer xenografts [55]. Using this system, they demonstrated that M12mac25 prostate cancer cells subcutaneously inoculated into athymic nude mice using Matrigel stayed largely dormant. However, with pHEMA scaffolds (with or without Matrigel) tumor formation was noted providing a model of dormancy escape in prostate cancer cells.
In addition to hydrogels, synthetic electrospun fiber-based biomaterials have been used to study tumor dormancy. To this end, random or aligned electrospun PCL fibrous scaffolds were used to examine the behavior of Carboplatin (a chemotherapy) treated vs. non treated breast cancer cells [56]. Non treated breast cancer cells exhibited a more dormant phenotype on fibrous scaffolds as evidenced using cell cycle analysis whereas the treated breast cancer cells exhibited this phenotype when cultured on fibrous scaffolds as well as TCPS.
Semi-synthetic biomaterial based models
Semi-synthetic scaffolds fabricated using a combination of natural and synthetic materials have also been investigated to develop models of tumor dormancy. For example, Pavan Grandhi et al., utilized amikacin hydrate and poly (ethylene glycol) diglycidyl ether (PEGDE) to develop a new hydrogel termed as “Amikagel” that was used to study dormancy in bladder cancer [57]. They found that 90% of T24 bladder cancer cells cultured on ~ 215 kPa Amikagels were cell cycle arrested in G0/G1 phase and were resistant to chemotherapeutic drugs such as docetaxel. However, when cells from the ~ 215 kPa Amikagels were transferred to ~ 36 kPa Amikagels, a sub-population of cells escaped dormancy and began proliferating. Overall, such biomimetic biomaterial based models provide useful tools to better understand the dormant niche. For instance, biomaterial based models are well suited to probe the impact of biophysical cues (such as matrix stiffness) on tumor dormancy versus traditional 2D culture models. These tools would also subsequently allow the study of molecular mechanisms governing the dormant phenotype as well as the dormant-to-proliferative switch.
Microfluidic based models
Microfluidic based models have also been used to study tumor dormancy. Such models allow for incorporation of nutrient/growth factor gradients. In addition, niche cells present in the tumor microenvironment are also typically incorporated in these models. One of the microfluidic based models is the commercially available LiverChip® wherein hepatocytes and non-parenchymal cells (NPCs) can be co-cultured to form an ex vivo microphysiologic model of the liver that could be used to study dormancy in cancer cells, including those that metastasize to the liver [58]. In this system, hepatocytes can be cultured for ~ 15 days without losing their functionality. This setup also contains an oxygen sensor and micro-reactor pumps to control the flow of nutrients and growth factors. In this system, a sub population of MDA-MB-231 and MCF7 breast cancer cells underwent dormancy (Fig. 2) that was associated with an increase in cancer attenuation signals (i.e., follistatin) and decrease in the pro-inflammatory signals (Insulin like growth factor binding protein 1 (IGFBP-1), Macrophage inflammatory protein 1 alpha (MIP-1α), Monocyte chemoattractant protein (MCP-1) & Interleukin-6 (IL-6)) for MDA-MB-231 cells, whereas in the case of MCF-7 cells, increase in cancer associated (e.g., Vascular endothelial growth factor A (VEGF-A), Epidermal growth factor (EGF)) and pro-inflammatory signals (IL-6, MCP-1) was noted. More recently, Khazali et al., tested if inflammatory signals present in the hepatic niche (from hepatic stellate cells) stimulated escape from the dormancy phenotype using the LiverChip® [59]. Indeed, introduction of IL-8 promoted proliferation of otherwise dormant MDA-MB-231 breast cancer cells as tested using EdU incorporation assay. This was also associated with an increase in phosphorylated ERK levels. Similarly, Clark et al., demonstrated that introduction of an inflammatory stimuli such as EGF or lipopolysaccharide (LPS) promoted proliferation of dormant MDA-MB-231 breast cancer cells [60].
Fig. 2.
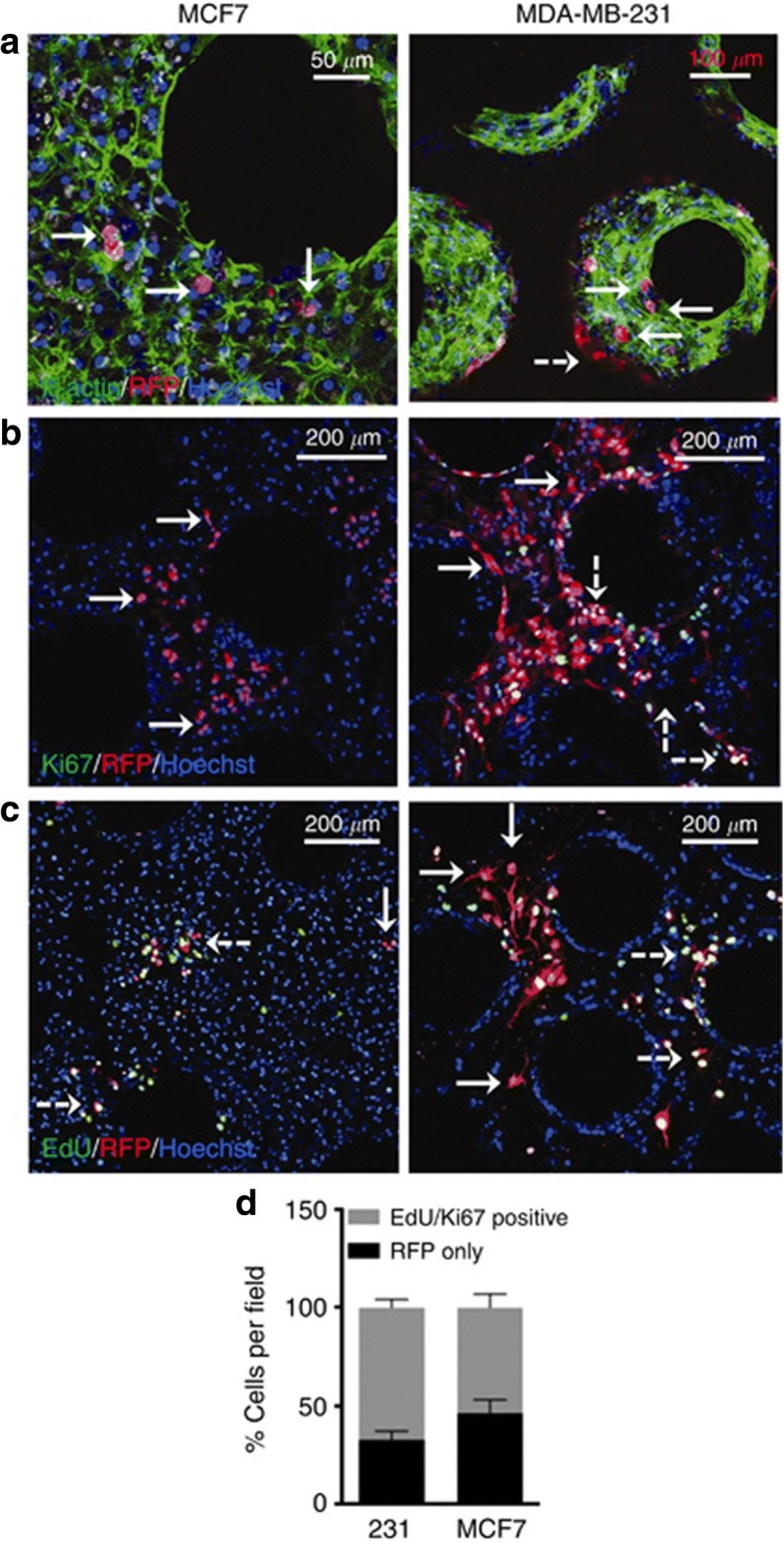
In a liver chip model, a subpopulation of MCF7 and MDA-MB-231 breast cancer cells underwent growth arrest. a Fluorescence image of MCF7 and MDA-MB-231 cells seeded with hepatocytes and non-parenchymal cells (F-Actin = green; Hoechst = blue, tumor cells = red (RFP) (b) Ki67 staining (green) and (c) EdU staining (green) of tumor cells and (d) Quantification of Ki67 and EdU status for both cell lines. Solid arrows indicate dormant cells and dashed white arrows indicate proliferating cells. Figure taken from [58] and reprinted with permission of Springer Nature
Biomaterial scaffolds have also been incorporated into microfluidic based models for studies of tumor dormancy. For example, a PEG based hydrogel was incorporated into the liver microphysiological system by Clark et al., in a follow up study [61]. In this model, MDA-MB-231 breast cancer cells exhibited a dormant phenotype on the PEG based hydrogel as compared to the polystyrene. Further, these cells were also found to be resistant to high doses of chemotherapy drugs such as Cisplatin and Doxorubicin on the hydrogel as opposed to polystyrene supported cultures.
In addition to breast cancer, microfluidics based models have been employed to study dormancy versus growth in lung cancer. A lung cancer-on-a-chip, specifically, lung airway chip and lung alveolus chip, was developed by Hassell and colleagues utilizing microfluidics [62]. Both chips utilize a two channel microfluidic set-up separated via a porous membrane coated with ECM proteins and incorporate airway or lung alveolar epithelial cells interfaced with endothelial cells. In this model, they found that non-small-cell lung cancer cells stayed relatively dormant in the lung airway chip as opposed to the lung alveolus chip wherein significant growth was observed.
Bioreactor based models
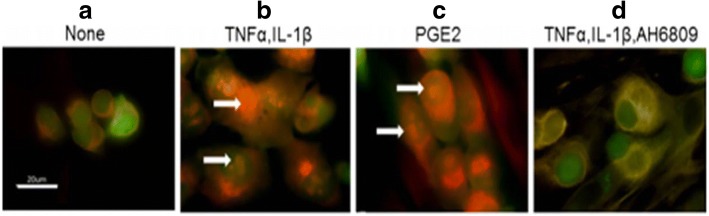
In addition to biomaterial and microfluidic based models, bioreactor based models have been used to investigate dormancy. Niche cells are also incorporated in such models as they allow long term culture. Such a model was utilized by Sosnoski et al. [63], to study breast cancer cell dormancy in a bone mimetic environment as breast cancer cells are known to metastasize to the bone [64, 65]. In this model, a bioreactor was employed to culture bone cells (murine MC3T3-E1 and human osteoblast cells) for up to 120 days. During this culture period, osteoblasts generated tissue that contained 6 or more layers of cells mimicking the pericellular environment [66]. Two month old bioreactor cultures were employed to which cytokines involved in bone remodeling were added, followed by addition of breast cancer cells. Specifically, a metastasis-suppressed MDA-MB-231BRMS1 human breast cancer cell line was used. Addition of cytokines tumor necrosis factor alpha (TNFα) and IL-1β to the bioreactor co-cultures allowed these cells to grow, which otherwise were largely growth arrested. This behavior was also seen when prostaglandin E2 (PGE2) was added to the cultures and addition of PGE2 receptor inhibitor suppressed tumor cell proliferation as seen via Ki67 staining (Fig. 3). The authors also observed a significant enhancement in focal adhesion kinase plaque formation in cancer cells in TNFα and IL-1β treated bioreactor co-cultures. While only few studies have utilized bioreactor based platforms, such platforms provide a better in vitro model system for co-culturing cancer cells as well as niche cells (e.g., breast cancer cells and osteoblasts) for longer time periods. This is advantageous as cancer cells typically stay dormant for extended periods of time in vivo and such models could be employed to capture these characteristic features.
Fig. 3.
In a bioreactor model, addition of TNFα and IL-β1 or PGE2 enabled proliferation of MDA-MB-231BRMS1 cells that were otherwise growth arrested as indicated via Ki67 staining. Fluorescence images of cells stained for Ki67 in (a) untreated control, (b) TNFα and IL-β1 treatment, (c) PGE2 treatment, and (d) TNFα, IL-1β and AH6809 (PGE2 receptor inhibitor) treatment conditions. White arrows indicate positive nuclear Ki67 staining. Scale bar = 20 μm. Figure taken from [63] and reprinted with permission of Springer Nature
Conclusions and perspectives
To elucidate the mechanisms governing dormancy, bioengineered models such as biomaterials, microfluidics, and bioreactor- based models are being increasingly utilized as biomimetic in vitro culture systems to model tumor dormancy. Unlike in vivo models, bioengineered models highlighted herein allow us to pursue a reductionist approach and thereby study how individual microenvironmental cues regulate dormancy in cancer cells owing to their versatility and tunability. To this end, these models have been largely utilized to investigate the impact of mechanical cues, biochemical cues, as well as cellular cues on tumor cell dormancy. Specifically, the cellular cues incorporated in current models largely consist of stromal and vascular cells. However, in addition to stromal and vascular cells, immune cells play a key role in cancer progression and metastasis [67–69]. Future studies should aim at incorporating immune cells such as macrophages in bioengineered models for studying immune-mediated dormancy. Further, 3D in vitro models have recently been utilized to study the microenvironmental regulation of stem-like phenotype in cancer cells [70]. There are striking parallels between cancer stem-like cells (CSCs) and dormant cancer cells. For instance, CSCs exhibit behaviors similar to dormant cancer cells such as increased drug resistance and the ability to repopulate the tumor mass in response to certain microenvironmental cues [71]. However, it is not clear whether they belong to the same dormant population or consist of a distinct population. Bioengineered models could be employed to clarify the extent of overlap between the cancer stem-like phenotype and the dormant phenotype. In addition, these models could be utilized to study the role of fundamental biological processes such as epithelial-to-mesenchymal transition and mesenchymal-to-epithelial transition in regulating cancer cell dormancy as they are known to be involved in cancer metastasis [72, 73]. Finally, current bioengineered models largely focus on single cell (cellular) dormancy, however, balance between proliferation and apoptosis could also lead to tumor dormancy (also called tumor mass dormancy) [2, 74]. It would be worthwhile to model these mechanisms in vitro using biomimetic culture systems as it will further our understanding of tumor mass dormancy. Overall, in the short term, bioengineered models could provide key scientific insight into microenvironmental regulation of the dormant phenotype and, in the long term, may enable the development of therapeutic strategies targeting dormant or active metastatic disease.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Science Foundation (CBET 1749837 to S.R.), The University of Alabama Graduate Council Fellowship (to R.K.), and an Alabama EPSCoR Graduate Research Fellowship (to A.N.)
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- Akt
Protein kinase B
- BMCL
Bone marrow cell lines
- BME
Basement membrane matrix
- BMSC
Bone marrow stromal cells
- Cdc42
Cell division control protein 42
- CSCs
Cancer stem cells
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- ERK
Extracellular-signal regulated kinase
- FAK
Focal adhesion kinase
- hFOB
Human fetal osteoblasts
- HMEC
Human microvascular endothelial cells
- HUVEC
Human umbilical vein endothelial cells
- IGFBP-1
Insulin like growth factor binding protein 1
- IL
Interleukin
- ILK
Integrin linked kinase
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein 1
- MEK
Mitogen-activated protein kinase
- MIP-1α
Macrophage inflammatory protein 1 alpha
- MLCK
Myosin light chain kinase
- NPCs
Nonparenchymal cells
- PA
Polyacrylamide
- PCL
Polycaprolactone
- PEG
Polyethylene glycol
- PEGDE
Poly (ethylene glycol) diglycidyl ether
- PGE2
Prostaglandin E2
- pHEMA
poly (2-hydroxyethyl methacrylate)
- PI3K
Phosphoinositide 3-kinase
- RTK
Receptor tyrosine kinase
- SFK
Src family kinases
- SIS
Small intestine submucosa
- STAT3
Signal transducer and activator of transcription 3
- TCPS
Tissue culture polystyrene
- Tet-2
tet methylcytosine dioxygenase 2
- TGF-β1
Transforming growth factor beta 1
- TNFα
Tumor necrosis factor alpha
- TSP-1
Thrombospondin-1
- uPAR
Urokinase plasminogen activator receptor
- VEGF-A
Vascular endothelial growth factor A
Authors’ contributions
SR, RK, and AN wrote and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aguado BA, Bushnell GG, Rao SS, Jeruss JS, Shea LD. Engineering the pre-metastatic niche. Nat Biomed Eng. 2017;1:0077. doi: 10.1038/s41551-017-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguirre-Ghiso JA, Bragado P, Sosa MS. Metastasis awakening: targeting dormant cancer. Nat Med. 2013;19(3):276–277. doi: 10.1038/nm.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2011;21(1):42–49. doi: 10.1016/j.gde.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Kottke T, Boisgerault N, Diaz RM, Donnelly O, Rommelfanger-Konkol D, Pulido J, et al. Detecting and targeting tumor relapse by its resistance to innate effectors at early recurrence. Nat Med. 2013;19(12):1625–1631. doi: 10.1038/nm.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature. 2016;540:588. doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells A, Griffith L, Wells JZ, Taylor DP. The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. 2013;73(13):3811–3816. doi: 10.1158/0008-5472.CAN-13-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghajar CM. Metastasis prevention by targeting the dormant niche. Nat Rev Cancer. 2015;15(4):238–247. doi: 10.1038/nrc3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linde N, Fluegen G, Aguirre-Ghiso JA. The relationship between dormant Cancer cells and their microenvironment. Adv Cancer Res. 2016;132:45–71. doi: 10.1016/bs.acr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguirre-Ghiso JA, Sosa MS. Emerging topics on disseminated Cancer cell dormancy and the paradigm of metastasis. Annual Rev Cancer Biol. 2018;2(1):377–393. [Google Scholar]
- 11.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133(3421):571–573. [PubMed] [Google Scholar]
- 12.Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan RN, Rafii S, Lyden D. Preparing the "soil": the premetastatic niche. Cancer Res. 2006;66(23):11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147(1):89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L. Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell. 2001;12(4):863–879. doi: 10.1091/mbc.12.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63(7):1684–1695. [PubMed] [Google Scholar]
- 18.Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64(20):7336–7345. doi: 10.1158/0008-5472.CAN-04-0113. [DOI] [PubMed] [Google Scholar]
- 19.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst RE, Bastian A, Bailey-Downs L, Ihnat MA. Targeting dormant micrometastases: rationale, evidence to date and clinical implications. Ther Adv Med Oncol. 2016;8(2):126–137. doi: 10.1177/1758834015624277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall JC, Collins JW, Nakayama J, Horak CE, Liewehr DJ, Steinberg SM, et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J Natl Cancer Inst. 2012;104(17):1306–1319. doi: 10.1093/jnci/djs319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almog N, Ma L, Raychowdhury R, Schwager C, Erber R, Short S, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69(3):836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 23.Almog N, Henke V, Flores L, Hlatky L, Kung AL, Wright RD, et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006;20(7):947–949. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- 24.Naumov GN, MacDonald IC, Weinmeister PM, Kerkvliet N, Nadkarni KV, Wilson SM, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62(7):2162–2168. [PubMed] [Google Scholar]
- 25.Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56(5):1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 26.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68(15):6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey S, Pipili A, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013;73(23):6886–6899. doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 28.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumor dormancy. Nat Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Lv J, Liang X, Yin X, Zhang L, Chen D, et al. Fibrin stiffness mediates dormancy of tumor-repopulating cells via a Cdc42-driven Tet2 epigenetic program. Cancer Res. 2018;78(14):3926–3937. doi: 10.1158/0008-5472.CAN-17-3719. [DOI] [PubMed] [Google Scholar]
- 30.El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014;124(1):156–168. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang JY, Tan S-J, Wu Y-C, Yang Z, Hoang BX, Han B. From competency to dormancy: a 3D model to study cancer cells and drug responsiveness. J Transl Med. 2016;14(1):38. doi: 10.1186/s12967-016-0798-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassim YL, Al Tawil E, Buquet C, Le Cerf D, PierreVannier J. Three dimensional tumor engineering by co-culture of breast tumor and endothelial cells using a hyaluronic acid hydrogel model. J Clin Exp Oncol. 2017;6(5):1000194.
- 33.Barkan D, Green JE. An in vitro system to study tumor dormancy and the switch to metastatic growth. J Vis Exp. 2011;54. [DOI] [PMC free article] [PubMed]
- 34.Keeratichamroen S, Lirdprapamongkol K, Svasti J. Mechanism of ECM-induced dormancy and chemoresistance in A549 human lung carcinoma cells. Oncol Rep. 2018;39(4):1765–1774. doi: 10.3892/or.2018.6258. [DOI] [PubMed] [Google Scholar]
- 35.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70(14):5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–735. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5(16):1799–1807. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clin Cancer Res. 2011;17(18):5850–5857. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Narkhede AA, Shevde LA, Rao SS. Biomimetic strategies to recapitulate organ specific microenvironments for studying breast cancer metastasis. Int J Cancer. 2017;141(6):1091–1109. doi: 10.1002/ijc.30748. [DOI] [PubMed] [Google Scholar]
- 40.Azarin SM, Yi J, Gower RM, Aguado BA, Sullivan ME, Goodman AG, et al. In vivo capture and label-free detection of early metastatic cells. Nat Commun. 2015;6:8094. doi: 10.1038/ncomms9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao SS, Bushnell GG, Azarin SM, Spicer G, Aguado BA, Stoehr JR, et al. Enhanced survival with implantable scaffolds that capture metastatic breast Cancer cells in vivo. Cancer Res. 2016;76(18):5209–18. doi: 10.1158/0008-5472.CAN-15-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aguado BA, Caffe JR, Nanavati D, Rao SS, Bushnell GG, Azarin SM, et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 2016;33:13–24. doi: 10.1016/j.actbio.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seib FP, Berry JE, Shiozawa Y, Taichman RS, Kaplan DL. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials. 2015;51:313–319. doi: 10.1016/j.biomaterials.2015.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bersani F, Lee J, Yu M, Morris R, Desai R, Ramaswamy S, et al. Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models. Cancer Res. 2014;74(24):7229–7238. doi: 10.1158/0008-5472.CAN-14-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesami P, Holzapfel BM, Taubenberger A, Roudier M, Fazli L, Sieh S, et al. A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin Exp Metastasis. 2014;31(4):435–446. doi: 10.1007/s10585-014-9638-5. [DOI] [PubMed] [Google Scholar]
- 46.Hurst RE, Kyker KD, Bonner RB, Bowditch RD, Hemstreet GP., 3rd Matrix-dependent plasticity of the malignant phenotype of bladder cancer cells. Anticancer Res. 2003;23(4):3119–3128. [PMC free article] [PubMed] [Google Scholar]
- 47.Dozmorov MG, Kyker KD, Saban R, Knowlton N, Dozmorov I, Centola MB, et al. Analysis of the interaction of extracellular matrix and phenotype of bladder cancer cells. BMC Cancer. 2006;6:12. doi: 10.1186/1471-2407-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurst RE, Hauser PJ, Kyker KD, Heinlen JE, Hodde JP, Hiles MC, et al. Suppression and activation of the malignant phenotype by extracellular matrix in xenograft models of bladder cancer: a model for tumor cell "dormancy". PLoS One. 2013;8(5):e64181. doi: 10.1371/journal.pone.0064181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurst RE, Hauser PJ, You Y, Bailey-Downs LC, Bastian A, Matthews SM, et al. Identification of novel drugs to target dormant micrometastases. BMC Cancer. 2015;15:404. doi: 10.1186/s12885-015-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fluegen G, Avivar-Valderas A, Wang Y, Padgen MR, Williams JK, Nobre AR, et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat Cell Biol. 2017;19(2):120–132. doi: 10.1038/ncb3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HR, Leslie F, Azarin SM. A facile in vitro platform to study cancer cell dormancy under hypoxic microenvironments using CoCl(2) J Biol Eng. 2018;12:12. doi: 10.1186/s13036-018-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schrader J, Gordon-Walker TT, Aucott RL, van Deemter M, Quaas A, Walsh S, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53(4):1192–1205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preciado JA, Reátegui E, Azarin SM, Lou E, Aksan A. Immobilization platform to induce quiescence in dormancy-capable cancer cells. Technology. 2017;05(03):129–138. [Google Scholar]
- 55.Long TJ, Sprenger CC, Plymate SR, Ratner BD. Prostate cancer xenografts engineered from 3D precision-porous poly(2-hydroxyethyl methacrylate) hydrogels as models for tumorigenesis and dormancy escape. Biomaterials. 2014;35(28):8164–8174. doi: 10.1016/j.biomaterials.2014.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guiro K, Patel SA, Greco SJ, Rameshwar P, Arinzeh TL. Investigating breast Cancer cell behavior using tissue engineering scaffolds. PLoS One. 2015;10(4):e0118724. doi: 10.1371/journal.pone.0118724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavan Grandhi TS, Potta T, Nitiyanandan R, Deshpande I, Rege K. Chemomechanically engineered 3D organotypic platforms of bladder cancer dormancy and reactivation. Biomaterials. 2017;142:171–185. doi: 10.1016/j.biomaterials.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 58.Wheeler SE, Clark AM, Taylor DP, Young CL, Pillai VC, Stolz DB, et al. Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br J Cancer. 2014;111(12):2342–2350. doi: 10.1038/bjc.2014.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khazali AS, Clark AM, Wells A. Inflammatory cytokine IL-8/CXCL8 promotes tumour escape from hepatocyte-induced dormancy. Br J Cancer. 2018;118(4):566–576. doi: 10.1038/bjc.2017.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark AM, Kumar MP, Wheeler SE, Young CL, Venkataramanan R, Stolz DB, et al. A model of dormant-emergent metastatic breast Cancer progression enabling exploration of biomarker signatures. Mol Cell Proteomics. 2018;17(4):619–630. doi: 10.1074/mcp.RA117.000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clark AM, Wheeler SE, Young CL, Stockdale L, Shepard Neiman J, Zhao W, et al. A liver microphysiological system of tumor cell dormancy and inflammatory responsiveness is affected by scaffold properties. Lab Chip. 2016;17(1):156–168. doi: 10.1039/c6lc01171c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassell BA, Goyal G, Lee E, Sontheimer-Phelps A, Levy O, Chen CS, et al. Human organ Chip models recapitulate Orthotopic lung Cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017;21(2):508–516. doi: 10.1016/j.celrep.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 63.Sosnoski DM, Norgard RJ, Grove CD, Foster SJ, Mastro AM. Dormancy and growth of metastatic breast cancer cells in a bone-like microenvironment. Clin Exp Metastasis. 2015;32(4):335–344. doi: 10.1007/s10585-015-9710-9. [DOI] [PubMed] [Google Scholar]
- 64.Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH, et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med. 2016;8(340) 340ra73-340ra73. [DOI] [PMC free article] [PubMed]
- 65.Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dhurjati R, Liu X, Gay CV, Mastro AM, Vogler EA. Extended-term culture of bone cells in a compartmentalized bioreactor. Tissue Eng. 2006;12(11):3045–3054. doi: 10.1089/ten.2006.12.3045. [DOI] [PubMed] [Google Scholar]
- 67.Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janssen LME, Ramsay EE, Logsdon CD, Overwijk WW. The immune system in cancer metastasis: friend or foe? J Immunother Cancer. 2017;5(1):79. doi: 10.1186/s40425-017-0283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero I, Garrido F, Garcia-Lora AM. Metastases in immune-mediated dormancy: a new opportunity for targeting cancer. Cancer Res. 2014;74(23):6750–6757. doi: 10.1158/0008-5472.CAN-14-2406. [DOI] [PubMed] [Google Scholar]
- 70.He J, Xiong L, Li Q, Lin L, Miao X, Yan S, et al. 3D modeling of cancer stem cell niche. Oncotarget. 2018;9(1):1326–1345. doi: 10.18632/oncotarget.19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kleffel S, Schatton T. Tumor dormancy and cancer stem cells: two sides of the same coin? Adv Exp Med Biol. 2013;734:145–179. doi: 10.1007/978-1-4614-1445-2_8. [DOI] [PubMed] [Google Scholar]
- 72.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jolly MK, Ware KE, Gilja S, Somarelli JA, Levine H. EMT and MET: necessary or permissive for metastasis? Mol Oncol. 2017;11(7):755–769. doi: 10.1002/1878-0261.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2017;11(1):62–78. doi: 10.1016/j.molonc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.