Abstract
Recent data suggest that severe psoriasis is an independent risk factor for chronic renal disease. In the present study, we investigated the role of related-purine derivatives as predictors of renal dysfunctions in patients with psoriasis. A prospective study was conducted on a group of 45 patients with psoriasis vulgaris and 45 control cases, monitored over a 5-year period. Alterations of renal function, albumin/creatinine ratio (ACR, mg/g) and UA/creatinine ratio (UACR, mg/mg) were determined in spontaneous urine samples. The status of related-purine derivatives was evaluated by quantification of uric acid (UA, mg/dl), adenosine deaminase (ADA, UI/mg protein), xanthine oxidase (XO, UI/mg protein) and 8-hydroxy-deoxy-guanosine levels (8-OHdG, ng/ml) in serum samples. Compared to the controls, in patients with psoriasis there was an increase in related-purine derivatives levels, which was demonstrated by the elevated serum levels of UA (5.1±0.4 vs. 5.4±1.0, P=0.066), ADA (0.14±0.08 vs. 0.29±0.12, P=0.052), XO (0.22±0.11 vs. 0.42±0.21, P=0.011) and 8-OHdG (3.1±0.05 vs. 8.3±4.7, P=0.002). The serum levels of related-purine derivatives were associated with the severity of psoriasis. In addition, there was a link between the serum levels of related-purine derivatives and markers of renal impairment. There were positive correlations between 8-OHdG and ACR (r=0.452, P=0.028) and between ADA, XO, UA, 8-OHdG and UACR (r=0.297 and P=0.032, r=0.301 and P=0.002, r=0.431 and P=0.027, r=0.508 and P=0.002) and negative correlations between UA, 8-OHdG and the estimated glomerular filtration rate (r=−0.301 and P=0.036, r=−0.384 and P=0.002). Thus, severe psoriasis is a risk factor for the development of renal disease.
Keywords: psoriasis, purine derivatives, uric acid, kidney disease, serological markers
Introduction
Psoriasis is a chronic immunologically mediated dermatosis, affecting 2–3% of the general population (1–3). Multiple mechanisms are involved in its pathogenesis, such as the hyper-reactivity of T-lymphocytes and dendritic cells, excessive inflammatory cytokine synthesis, accelerated epidermal turnover, epidermal hyperproliferation, reduced keratinocyte differentiation, overexpression of angiogenesis and oxidative stress (4–14). The extent of skin involvement is variable, ranging from several psoriatic plaques to generalized forms. The disease evolves with periods of exacerbation and remission (15,16).
It seems that severe psoriasis is an independent risk factor for chronic renal disease (17,18). The mechanisms that mediate kidney failure in patients with psoriasis are controversial. In a retrospective study, it was estimated that patients with psoriasis develop chronic renal disease at a higher percentage compared to controls (5 vs. 2%). Moreover, the risk of kidney disease was higher in the young patients (18). The treatment should be adapted to meet the individual needs of the patients with psoriasis. Non-pharmacological interventions (diet, cessation of smoking and alcohol intake, weight loss, physical exercise) may improve the response to therapy. Potentially nephrotoxic drugs should be used with caution and renal function should be periodically monitored in patients with psoriasis in order to minimize the risk of adverse renal events (13,15,19,20).
In medical literature, there are few studies on the relationship between uremic toxins and the decline of renal function in patients with psoriasis vulgaris. Recent data suggest the role of several serum and urinary markers in the early detection and monitoring of renal disease. The urinary levels of creatinine, albumin, uric acid (UA) and the estimated glomerular filtration rate (eGFR) can be evaluated, as well as cystatin C, neutrophil gelatinase-associated lipocalin, kidney injury molecule 1, cytokines and chemokines (21). The progressive increase in the level of uremic toxins exerts a negative impact on kidney function (22–25).
Purine derivatives pertain to the class of low molecular weight uremic toxins, which frequently accumulate in the body. Low molecular weight uremic toxins are water-soluble compounds with a molecular weight below 0.5 kDa, and consequently are easily removed by dialysis, and do not exert harmful effects on the body (26,27). The most important uremic toxins classified as purine derivatives are: Products resulted from the degradation of purines (adenosine, inosine, xanthine, guanosine, hypoxanthine, guanine, UA), products of guanosine triphosphate catabolism (neopteri n) and oxidative DNA base damage product (8-hydroxy-deoxy-guanosine) (28–30). The metabolic pathways of purine catabolism imply several phases (31).
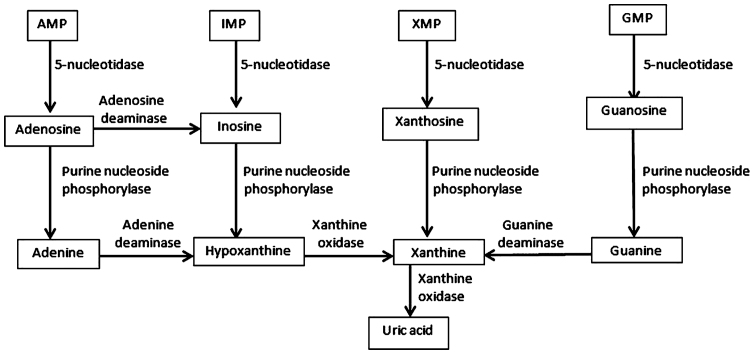
The first step involves the transformation of purine mononucleotides (AMP-adenosine monophosphate, IMP-inosine monophosphate, XMP-xanthine monophosphate and GMP-guanine monophosphate) into purine nucleosides (adenosine, inosine, xanthosine and guanosine), a reaction catalyzed by 5-nucleotidase (E.C.3.1.3.5). Subsequently, conversion of purine nucleosides occurs which frees purine bases (adenine, hypoxanthine, xanthine and guanine), a reaction catalyzed by purine nucleoside phosphorylase (E.C.2.2.2.1). The next step involves the conversion of adenine to hypoxanthine (a reaction catalyzed by adenine deaminase - E.C.3.5.4.4) and then to xanthine (a reaction catalyzed by xanthine oxidase - E.C.1.17.3.2). Guanine is converted to xanthine by guanine deaminase. The final product of purine metabolism is UA, obtained through the enzymatic oxidation of xanthine (a reaction catalyzed by xanthine oxidase) (Fig. 1).
Figure 1.
The metabolic pathways of purine degradation.
In psoriasis patients, the severity of the skin disease, assessed by the Psoriasis Area Severity Index (PASI), has been analyzed in relation to the prevalence of renal dysfunctions (3,16,18,32,33), purine catabolism (4) and the oxidative stress level (5,6,34). Alteration of purine degradation may be associated with the extension of psoriasis lesions, stimulation of epidermal proliferation and increased DNA peroxidation (6,35–38).
These reactions produce a wide range of reactive oxidants: hydrogen peroxide (H2O2), superoxide anion (O2•−), hydroxyl radical (OH•), nitric oxide (NO•), peroxynitrite (ONOO−), and carbonate radical (CO3•−). Reactive oxygen species and reactive nitrogen species, formed under the action of xanthine oxidase, act on proteins, lipids and nucleic acids, causing damage and cell toxicity (39–42). 8-Hydroxy-deoxyguanosine, a representative metabolite for purine derivatives toxins (43), is a useful indicator for early diagnosis and management of patients with psoriasis (6).
Based on these considerations, we aim to establish characteristic patterns of related-purine derivatives that could represent specific serological markers for the identification of those patients with psoriasis vulgaris at a higher risk of developing renal dysfunctions. In this context, the serum profile of related-purine derivatives was analyzed in psoriasis patients in correlation with the severity of the skin disease and markers of renal impairment. The biomarkers carried out in this study, grouped under the name of related-purine derivatives, include UA (the final product of purine catabolism), adenosine deaminase (ADA, an enzyme which converts irreversibly adenosine and deoxyadenosine to inosine and deoxy-inosine), xanthine oxidase (XO, which catalyzes the oxidation of hypoxanthine to xanthine as well as the oxidation of xanthine to UA) and 8-hydroxy-deoxy-guanosine (8-OHdG, a biomarker of oxidative DNA damage). Renal function was assessed through the serum creatinine level, urinary determinations of eGFR (ml/min/1.73 mp), albumin/creatinine ratio (ACR, mg/g) and UA-creatinine ratio (UACR, mg/mg).
Materials and methods
Study participants
All the study participants provided consent to the use of their biological samples in research studies. The Ethics Committee of ‘Victor Babes’ Clinical Hospital for Infectious Diseases (Bucharest, Romania) approved the study protocol.
A prospective study was conducted on 45 patients with psoriasis vulgaris and 45 control cases, monitored over a 5-year period. Inclusion criteria for the study were: Adults, normolipidemic, normoponderal, with a balanced diet. Exclusion criteria for the study were: Cardiovascular disease, metabolic syndrome, diabetes, anemia, urinary tract infections, nephrolithiasis, leukemia, Lesch-Nyhan syndrome, Wilson's disease, viral hepatitis, sickle-cell disease, chronic kidney disease, xanthinuria, lead toxicity, treatment with nephrotoxic drugs, folic acid deficiency, pregnant women and breastfeeding women.
The severity of psoriasis was assessed using the PASI score, which assesses the severity of three clinical signs (erythema, thickness, scaling) on a scale from 0 to 4 and the percentage of the skin area involved. The PASI score interpretation was as follows: <7, mild chronic plaque-type psoriasis; 7–12, moderate chronic plaque-type psoriasis; >12, severe chronic plaque-type psoriasis (41).
Changes of glomerular permeability were detected by determining eGFR, ACR and UACR. To assess alterations of renal function, ACR and UACR were determined in spontaneous urine samples. The status of related-purine derivatives was evaluated by quantification of the serum levels of UA (mg/dl), adenosine deaminase (UI/mg protein), XO (UI/mg protein) and 8-OHdG (ng/ml).
Biological samples were a spontaneous urine sample, preferably first morning urine collected in sterile, preservative-free containers. Urine specimens were centrifuged at 3,000 × g for 10 min at room temperature 20°C. The supernatant was used for measurement of the biological parameters. Venous blood samples (7 ml) were collected in a vacutainer without an anticoagulant and centrifuged at 6,000 × g for 10 min at 20°C. The supernatant was used for biochemical determinations.
Laboratory methods
The determination of creatinine was performed using the colorimetric technique. The method is based on the reaction between creatinine and picric acid in alkaline medium. The absorbance measured at a wavelength of 492 nm is directly proportional to the amount of creatinine in the sample.
The determination of albuminuria was performed via turbidimetric immunoassay using polyclonal antibodies against human albumin. The determination of UA was performed by the colorimetric technique, using the reaction catalyzed by uricase. The concentration of quinonimine resulted from the reaction was determined by measuring the absorbance at a wavelength of 546 nm. The determination of xanthine-oxidase was performed using a spectrophotometer (HumaStar 300; HUMAN Gesellschaft für Biochemica und Diagnostica mbH, Germany, Wiesbaden). Absorbance of the obtained coloured complex was read at a wavelength of 570 nm. The determination of adenosine deaminase was performed via spectrophotometry and the evaluation of the resulting product was measured at a wavelength of 550 nm.
The quantitative determination of hs 8-OHdG (highly sensitive oxidative DNA adduct 8-OHdG) was produced in serum using an enzyme-linked immunosorbent assay (ELISA). The principle of the method is based on the ability of DNA oxidation products to interact with 3,3,5,5-tetramethylbenzidine. The method uses DNA-specific monoclonal antibodies, which cross-react with the oxidative degradation products of DNA (8-hydroxy-guanine, 8-hydroxy-guanosine). The final product of the reaction is colorimetrable via microplate reader (Tecan Austria GmbH, Grodig, Austria) at a wavelength of 450 nm.
Statistical analysis
A comparison of the obtained results between the groups for quantitative variables was performed using the t-test. The correlations between variables were determined by linear regression. The relationship between pairs of two parameters was assessed by Pearson's correlation coefficient (r). We chose a significance level (p) of 0.05 (5%) and a confidence interval of 95% for hypothesis testing.
Results
Patient characteristics
We performed a prospective observational study on 45 patients with psoriasis vulgaris (duration of psoriasis 6.4±3.1 years, PASI 10.4±5.3) and 45 healthy volunteers, who met the inclusion criteria (Table I).
Table I.
Characteristics of study participants.
| Items | Psoriasis vulgaris | Control | P-value |
|---|---|---|---|
| Age (years) | 40.3±11.3 | 39.4±8.3 | 0.763 |
| Male/female ratio | 24/21 | 25/20 | 0.923 |
| Smokers/non-smokers ratio | 11/34 | 9/36 | 0.755 |
| BMI (kg/m2) | 20.4±1.3 | 19.8±1.1 | 0.899 |
| Systolic blood pressure (mmHg) | 117.3±0.7 | 115.5±0.5 | 0.677 |
| Diastolic blood pressure (mmHg) | 72.3±0.2 | 73.5±0.4 | 0.546 |
BMI, body mass index.
Serum determinations
The serum concentration of UA was 5.4±1.8 mg/dl in patients with psoriasis vulgaris and 5.1±0.4 mg/dl in the control group, with statistically insignificant variations between the two groups. There was an important difference between the activity of ADA in patients with psoriasis vulgaris and controls (0.29±0.12 UI/mg protein vs. 0.14±0.08 UI/mg protein, P=0.052). The activity of XO was also significantly increased in psoriasis patients versus controls (0.42±0.21 UI/mg protein vs. 0.22±0.11 UI/mg protein, P=0.011). The serum level of 8-OHdG in patients with psoriasis vulgaris was significantly higher compared to controls (8.3±4.7 ng/ml vs. 3.1±0.05 ng/ml, P=0.002) (Table II).
Table II.
Serum determinations in psoriasis patients and controls.
| Items | Psoriasis vulgaris | Control | P-value |
|---|---|---|---|
| Glucose (mg/dl) | 88.2±5.3 | 81.4±5.1 | 0.654 |
| ASAT (U/l) | 18.4±3.5 | 21.8±6.3 | 0.341 |
| ALAT (U/l) | 16.0±7.2 | 17.3±4.3 | 0.549 |
| Cholesterol (mg/dl) | 155.3±11.5 | 146.3±12.6 | 0.388 |
| Tryglicerides (mg/dl) | 82.5±5.3 | 76.9±10.4 | 0.426 |
| Urea (mg/dl) | 39.3±4.5 | 30.4±5.3 | 0.088 |
| CRP (mg/dl) | 0.37±0.24 | 0.19±0.19 | 0.072 |
| Creatinine (mg/dl) | 0.88±0.12 | 0.73±0.07 | 0.068 |
| UA (mg/dl) | 5.4±1,8 | 5.1±0.4 | 0.066 |
| ADA (U/mg protein) | 0.29±0.12 | 0.14±0.08 | 0.052 |
| XO (U/mg protein) | 0.42±0.21 | 0.22±0.11 | 0.011 |
| 8-OHdG (ng/ml) | 8.3±4.7 | 3.1±0.05 | 0.002 |
ASAT, aspartate transaminase; ALAT, alanine aminotransferase; U/L, unit level; CRP, C reactive protein; UA, uric acid; ADA, adenosine deaminase; XO, xanthine oxidase; 8-OHdG, 8-hydroxy-deoxy-guanosine.
Urinary determinations
Since the creatinine excretion is a relatively constant variable, the determination of urinary creatinine may be valuable in estimating the renal function. Therefore, the urinary creatinine concentration can be used as a reference parameter for albuminuria and UA. Statistically significant variations were observed for ACR and UACR between patients with psoriasis vulgaris and controls (Table III).
Table III.
Urinary determinations in psoriasis patients and controls.
| Items | Psoriasis vulgaris | Control | P-value |
|---|---|---|---|
| eGFR (ml/min/1.73 mp) | 95.6±7.4 | 102.1±5.6 | 0.127 |
| ACR (mg/g) | 19.3±11.8 | 7.8±5.2 | 0.050 |
| UACR (mg/mg) | 0.39±0.17 | 0.27±0.11 | 0.048 |
eGFR, estimated glomerular filtration rate; ACR, albumin/creatinine ratio; UACR, uric acid/creatinine ratio.
Correlation of serum levels of related-purine derivatives with the severity of psoriasis
Compared to the controls, UA, ADA, XO and 8-OHdG levels were significantly higher in patients with psoriasis with PASI >12 (P<0.05) (Table IV).
Table IV.
Serum related-purine derivatives levels in psoriasis patients and controls.
| Psoriasis vulgaris PASI score | ||||
|---|---|---|---|---|
| Items | Control | <7 | 7–12 | >12 |
| ADA (UI/mg protein) | 0.14±0.08 | 0.20±0.04 | 0.24±0.06 | 0.38±0.11 |
| XO (UI/mg protein) | 0.22±0.11 | 0.29±0.07 | 0.39±0.12 | 0.53±0.23 |
| UA (mg/dl) | 5.1±0.4 | 3.7±0.6 | 5.1±1.1 | 5.7±1.9 |
| 8-OHdG (ng/ml) | 3.1±0.5 | 5.2±2.1 | 7.9±2.2 | 12.4±7.3 |
ADA, adenosine deaminase; XO, xanthine oxidase; UA, uric acid; 8-OHdG, 8-hydroxy-deoxy-guanosine; PASI, psoriasis area and severity index.
Positive correlations between ADA, XO, UA and PASI >12 (r=0.498, P=0.004; r=0.601, P<0.001 and r=0.421, P=0.017) were obtained. There was a strong positive correlation between the serum levels of 8-OHdG and PASI (r=0.406, P=0.008 for PASI ranging from 7 to 12, r=0.782, P=0.000 for PASI >12) (Table V).
Table V.
Statistical correlations between serum related-purine derivatives and PASI score.
| <7 | 7–12 | >12 | ||||
|---|---|---|---|---|---|---|
| Items | r | p | r | p | r | p |
| ADA | 0.094 | 0.353 | 0.137 | 0.062 | 0.498 | 0.004 |
| XO | 0.103 | 0.281 | 0.095 | 0.413 | 0.601 | <0.001 |
| UA | 0.087 | 0.988 | 0.105 | 0.243 | 0.421 | 0.017 |
| 8-OHdG | 0.122 | 0.078 | 0.406 | 0.008 | 0.782 | <0.001 |
ADA, adenosine deaminase; XO, xanthine oxidase; UA, uric acid; 8-OHdG, 8-hydroxy-deoxy-guanosine; PASI, psoriasis area and severity index.
Correlations between serum related-purine derivatives and markers of renal impairment
Serum markers of related-purine derivatives in patients with psoriasis were associated with markers of renal impairment. Positive correlations between 8-OHdG and ACR (r=0.452, P=0.028), between ADA, XO, UA, 8-OHdG (r=0.297 and P=0.032; r=0.031 and P=0.002; r=0.431 and P=0.027; r=0.508 and P<0.001) and UACR. Negative correlations between UA, 8-OHdG and eGFR (r=−0.301 and P=0.036; r=−0.384 and P=0.002) were registered (Table VI).
Table VI.
Statistical correlations between serum related-purine derivatives and markers of renal impairment in patients with psoriasis.
| ACR | UACR | eGFR | ||||
|---|---|---|---|---|---|---|
| Items | r | p | r | p | r | p |
| ADA | 0.111 | 0.564 | 0.297 | 0.032 | −0.088 | 0.512 |
| XO | 0.076 | 0.102 | 0.301 | 0.002 | −0.302 | 0.121 |
| UA | 0.341 | 0.098 | 0.431 | 0.027 | −0.301 | 0.036 |
| 8-OHdG | 0.452 | 0.028 | 0.508 | <0.001 | −0.384 | 0.002 |
ADA, adenosine deaminase; XO, xanthine oxidase; UA, uric acid; 8-OHdG, 8-hydroxy-deoxy-guanosine; ACR, albumin/creatinine ratio; UACR, uric acid/creatinine ratio; eGFR, estimated glomerular filtration rate.
Discussion
In the human body, the progressive increase in the level of uremic toxins exerts a negative impact on all organs, tissues and systems, causing acute and chronic renal dysfunctions, cardiovascular, respiratory and hepatic diseases, atherosclerosis, fibrosis or metabolic alterations (22–25). The presence of UA derivatives is associated with the induction of specific dysfunctions and the normalization of UA levels results in the resolution of the clinical manifestations (26,27,44–46). In terms of chemical structure, uremic toxins are purine, pyrimidine, methylamine, phenyl or indole derivatives, guanidine, polyols, ribonucleosides, peptides, cytokines, advanced glycation end products, advanced lipoxidation end products and reactive carbonyl compounds (26,27,46).
The role played by purine degradation in the pathogenesis of psoriasis is an exciting research topic. The results of the present study on the status of related-purine derivatives in patients with psoriasis indicate changes of the serum level of UA and 8-OHdG and alteration of the enzymatic activities of adenosine deaminase and xanthine oxidase depending on the clinical severity of psoriasis and the stage of renal chronic disease. These results draw attention to the cumulative toxic effect of purine derivatives on the kidney.
In the present study, we obtained slightly increased levels of UA, without statistical significance, in patients with psoriasis versus controls. A significant variation in serum UA levels correlated with the severity of psoriasis. In patients with severe psoriasis, with a PASI score higher than 12, a significantly increased level of UA was obtained compared for both controls and the group of patients with mild or moderate psoriasis. This was also supported by the positive relationship established between serum UA levels and PASI score and between serum UA levels and the results of the tests assessing the renal function in patients with psoriasis.
Stimulation of epidermal proliferation and increased DNA damage may be associated with hyperuricemia, and the normal UA levels could be explained by the selection criteria of patients [normal body mass index (BMI), lack of inflammation, balanced nutritional status]. In addition, the results of this study show that the risk of kidney disease is more evident in patients with severe psoriasis. Our findings are consistent with several reports that have emphasized harmful effects of UA on kidney, the target organ of hyperuricemia (26–28). Data related to the correlation between serum UA levels and the severity of psoriasis are inconsistent. In some studies, elevated serum values were obtained in patients with psoriasis versus controls (38), whereas other studies reported normal UA levels in psoriasis (35,37). Positive correlations between UA levels and the following parameters have been reported: PASI score, cutaneous extension of psoriatic lesions, and BMI (35).
Elevated levels of serum UA promote endothelial dysfunction and renal lesions by decreasing the availability of nitrogen monoxide and inducing oxidative stress. UA-induced endothelial damage could be caused by the reduction in intracellular ATP due to the inactivation of aconitase-2 and enoyl CoA-hydratase-1, decreased mitochondrial DNA/nuclear DNA ratio, increased mitochondrial calcium, resulting in the alteration of membrane potential and generation of reactive oxygen species (47). Consequently, endothelial dysfunction can be associated with systemic manifestations (arteriosclerosis, insulin resistance) and renal damage (hypoxia, inflammation, glomerulosclerosis, tubulointerstitial fibrosis) (47,48).
In this study, we revealed that elevated UA levels observed in patients with severe psoriasis were associated with increased xanthine oxidase activity. The serum levels of xanthine oxidase were negatively correlated with eGFR and UACR in urine. The relationship between xanthine oxidase and renal impairment can be explained by the activation of renin-angiotensin system, preglomerular arteriolopathy, induction of oxidative stress and inflammation, and endothelial dysfunctions (49). The role of xanthine oxidase in the terminal differentiation of keratinocytes may be explained by localization of the enzyme in the granular layer of the epidermis (50). The stimulation of the inflammatory process in human keratinocytes by irradiation with UV rays was correlated with the overexpression of xanthine oxidase and increased production of superoxide (51).
Adenosine is another endogenous purine nucleoside, possibly involved in the pathogenesis of psoriasis. It is thought that adenosine may exert anti-inflammatory and immunomodulatory effects through specific receptors expressed on endothelial cells, leukocytes, mast cells, macrophages, dendritic cells and consequently may limit the extension of psoriatic lesions. Anti-inflammatory effects of adenosine may be achieved by increasing intracellular cAMP levels, modulation of apoptosis, reduction in cytokine synthesis, leucocyte recruitment and immune function regulation (52). The catabolism and bioavailability of adenosine may be modulated by adenosine deaminase (53). In our study, higher serum adenosine deaminase levels were obtained in patients with psoriasis compared to the controls. In addition, the activity of adenosine deaminase was associated with the severity of the disease. In patients with mild or moderate psoriasis, an enhanced adenosine deaminase activity was observed, but no correlation with PASI score was revealed. In patients with severe psoriasis significantly elevated levels of adenosine deaminase were determined compared to those with mild and moderate psoriasis and control group. There was also a strong positive correlation between adenosine deaminase activity and PASI score in patients with severe psoriasis. These findings support the hypothesis that adenosine deaminase could be validated by further studies as a useful indicator in monitoring patients with psoriasis, assessing the response to therapy and predicting the prognosis of the disease (52,54,55). In previous studies on patients with psoriasis, adenosine deaminase activity was found to decline after treatment with PUVA, cyclosporine, etanercept, and psoralen, reconfirming the ability of this enzyme to be associated with T-cell activation (55,56). The increase in adenosine deaminase activity could become a predictive factor for identifying patients with psoriasis at risk of developing relapses prior to the occurrence of clinical manifestations.
An accelerated purine catabolism stimulates the production of free oxygen radicals. The accumulation of reactive oxygen species is associated with changes of DNA structure (oxidation, methylation, single and double strand breaks, cross-links to protein, deletions or translocations) (57,58). In our study, a significant increase in serum levels of 8-OHdG was found in patients with psoriasis versus the controls. The strong correlation between serum concentration of 8-OHdG and PASI score in patients with severe psoriasis and the correlation between 8-OHdG levels and markers of renal impairment suggest that 8-OHdG could favour the onset and/or development of renal disease in patients with psoriasis. In medical literature, there are limited data on the role of 8-OHdG in progressive renal fibrosis (59,60), hypertension associated with proteinuria (61), chronic renal failure (62), diabetes associated with proteinuria (63,64), bladder cancer (65), renal cancer (66) and urothelial carcinoma (67).
Psoriatic arthritis is an important condition associated with psoriasis (68). Regarding urate-lowering drugs, the study by Namazi suggested the beneficial role of allopurinol in the treatment of psoriasis given its ability to neutralize free radicals and inhibit both the secretion of tumour necrosis factor alpha and expression of intercellular adhesion molecule-1 (69). The study by Tsuruta et al concluded that hyperuricemia may be considered an independent risk factor for psoriatic arthritis (70). In addition, findings showed that patients with psoriasis and psoriatic arthritis had an important risk of gout (71).
Taken together, our data and those of the aforementioned studies suggest that severe psoriasis is a risk factor for the development of renal disease. In patients with psoriasis, renal and urinary tract abnormalities have been reported, such as IgA nephropathy, secondary renal amyloidosis, proliferative membranous glomerulonephritis, proliferative mesangial glomerulonephritis, focal proliferative glomerulonephritis, nephrolithiasis and recurrent urinary tract infections (32,72). The relationship between psoriasis and renal disease can be explained by three main mechanisms: Immune-mediated renal damage, drug-related renal damage, and chronic-renal damage (33).
In summary, psoriasis vulgaris can be regarded as a cascade of events that starts from inflammation, oxidative stress and a series of comorbidities. Our study indicates that renal impairment is a frequent condition in patients with psoriasis vulgaris. The related-purine derivatives may be specific serological markers for identifying those patients with psoriasis vulgaris at a high risk of developing renal dysfunctions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
IN, MT, CM and SRG conceived the study, identified and reviewed the literature. CIM, MIM and MIS collected the data. CDE and CE analyzed and interpreted the data. All authors equally contributed to writing the manuscript, and CDE and CE edited and revised the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of ‘Victor Babes’ Clinical Hospital for Infectious Diseases (Bucharest, Romania). All the participants gave their consent to the use of their biological samples in research studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, van de Kerkhof P, Ståhle M, Nestle FO, Girolomoni G, Krueger JG. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 2.Kölliker Frers RA, Bisoendial RJ, Montoya SF, Kerzkerg E, Castilla R, Tak PP, Milei J, Capani F. Psoriasis and cardiovascular risk: Immune-mediated crosstalk between metabolic, vascular and autoimmune inflammation. IJC Metab Endocr. 2015;6:43–54. doi: 10.1016/j.ijcme.2015.01.005. [DOI] [Google Scholar]
- 3.Sârbu MI, Georgescu SR, Tampa M, Sârbu AE, Simionescu O. Biological therapies in psoriasis - revisited. Rom J Intern Med. 2018;56:75–84. doi: 10.1515/rjim-2017-0045. [DOI] [PubMed] [Google Scholar]
- 4.Murari K, Ray AS, Lodha RS. Adenosine deaminase: A potential biomarker for evaluating the severity of psoriasis. Int J Pharma Bio Sci. 2015;6:629–634. [Google Scholar]
- 5.Shree UGB, Vishal B, Shindhu M, Shenoy MM, Shenoy C. Advanced oxidation protein product in psoriasis and its correlation with disease severity. Int J Sci Stud. 2015;2:156–159. [Google Scholar]
- 6.Basavaraj KH, Vasu Devaraju P, Rao KS. Studies on serum 8-hydroxy guanosine (8-OHdG) as reliable biomarker for psoriasis. J Eur Acad Dermatol Venereol. 2013;27:655–657. doi: 10.1111/j.1468-3083.2011.04441.x. [DOI] [PubMed] [Google Scholar]
- 7.Nwabudike LC, Tatu AL. Reply to Happle R. And al. Koebners sheep in Wolf's clothing: Does the isotopic response exist as a distinct phenomenon? J Eur Acad Dermatol Venereol. 2018;32:336–337. doi: 10.1111/jdv.14899. [DOI] [PubMed] [Google Scholar]
- 8.Tampa M, Sarbu MI, Mitran MI, Mitran CI, Matei C, Georgescu SR. The pathophysiological mechanisms and the quest for biomarkers in psoriasis, a stress-related skin disease. Dis Markers. 2018;2018:5823684. doi: 10.1155/2018/5823684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Căruntu C, Boda D, Căruntu A, Rotaru M, Baderca F, Zurac S. In vivo imaging techniques for psoriatic lesions. Rom J Morphol Embryol. 2014;55:1191–1196. [PubMed] [Google Scholar]
- 10.Batani A, Brănișteanu DE, Ilie MA, Boda D, Ianosi S, Ianosi G, Caruntu C. Assessment of dermal papillary and microvascular parameters in psoriasis vulgaris using in vivo reflectance confocal microscopy. Exp Ther Med. 2018;15:1241–1246. doi: 10.3892/etm.2017.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negrei C, Arsene AL, Toderescu CD, Boda D, Ilie M. Acitretin treatment in psoriasis may influence the cell membrane fluidity. Farmacia. 2012;60:767–772. [Google Scholar]
- 12.Nicolae I, Ene CD, Schipor S, Tampa M, Matei C, Georgescu SR. Dopamine-chemical mediator in atopic dermatitis. Rev Chim. 2013;64:1201–1206. [Google Scholar]
- 13.Negrei C, Ginghină O, Căruntu C, Burcea Dragomiroiu GT, Jinescu GE, Boda DA. Investigation relevance of methotrexate polyglutamates in biological systems by high performance liquid chromatography. Rev Chim. 2015;66:766–768. [Google Scholar]
- 14.Caruntu C, Boda D, Dumitrascu G, Constantin C, Neagu M. Proteomics focusing on immune markers in psoriatic arthritis. Biomarkers Med. 2015;9:513–528. doi: 10.2217/bmm.14.76. [DOI] [PubMed] [Google Scholar]
- 15.Gisondi P, Galvan A, Idolazzi L, Girolomoni G. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne) 2015;2:1. doi: 10.3389/fmed.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbu MI, Tampa M, Matei C, Mitran CI, Mitran MI, Pituru S, Pop CS, Saramet G, Georgescu SR. Infliximab biosimilar versus methotrexate for the treatment of moderate to severe psoriasis. Farmacia. 2017;65:962–967. [Google Scholar]
- 17.Wan J, Wang S, Haynes K, Denburg MR, Shin DB, Gelfand JM. Risk of moderate to advanced kidney disease in patients with psoriasis: Population based cohort study. BMJ. 2013 Oct 15; doi: 10.1136/bmj.f5961. (Epub ahead of print). doi: https://doi.org/10.1136/bmj.f5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi CC, Wang J, Chen YF, Wang SH, Chen FL, Tung TH. Risk of incident chronic kidney disease and end-stage renal disease in patients with psoriasis: A nationwide population-based cohort study. J Dermatol Sci. 2015;78:232–238. doi: 10.1016/j.jdermsci.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Negrei C, Caruntu C, Ginghina O, Dragomiroiu GT, Toderescu CD, Boda D. Qualitative and quantitative determination of methotrexate polyglutamates in erythrocytes by high performance liquid chromatography. Rev Chim. 2015;66:607–610. [Google Scholar]
- 20.Sârbu MI, Tampa M, Mitran MI, Mitran CI, Limbău AM, Georgescu SR. Adverse reactions of biological therapies in patients with psoriasis. J Mind Med Sci. 2017;4:4–12. doi: 10.22543/7674.41.P0412. [DOI] [Google Scholar]
- 21.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif. 2010;29:357–365. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HJ, Yen CH, Wu IW, Hsu KH, Chen CK, Sun CY, Chou CC, Chen CY, Tsai CJ, Wu MS, et al. The association of uremic toxins and inflammation in hemodialysis patients. PLoS One. 2014;9:e102691. doi: 10.1371/journal.pone.0102691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu G, Tu W, Qin S. The relationship between deiodinase activity and inflammatory responses under the stimulation of uremic toxins. J Transl Med. 2014;12:239. doi: 10.1186/s12967-014-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A. European Uremic Toxin Work Group: Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol. 2012;23:1258–1270. doi: 10.1681/ASN.2011121175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boueiz A, Damarla M, Hassoun PM. Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am J Physiol Lung Cell Mol Physiol. 2008;294:830–840. doi: 10.1152/ajplung.00007.2008. [DOI] [PubMed] [Google Scholar]
- 26.Lisowska-Myjak B, Skarżyńska E. Role of uremic compounds in organ injury. J Nephrol Ther. 2015;5:1000205. [Google Scholar]
- 27.Lisowska-Myjak B. Uremic toxins and their effects on multiple organ systems. Nephron Clin Pract. 2014;128:303–311. doi: 10.1159/000369817. [DOI] [PubMed] [Google Scholar]
- 28.Xia JF, Liang QL, Hu P, Wang YM, Li P, Luo GA. Correlations of six related purine metabolites and diabetic nephropathy in Chinese type 2 diabetic patients. Clin Biochem. 2009;42:215–220. doi: 10.1016/j.clinbiochem.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Xia J, Wang Z, Zhang F. Association between related purine metabolites and diabetic retinopathy in type 2 diabetic patients. Int J Endocrinol. 2014 Feb 13; doi: 10.1155/2014/651050. (Epub ahead of print). doi: 10.1155/2014/651050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avci E, Cakir E, Cevher SC, Yaman H, Agilli M, Bilgi C. Determination of oxidative stress and cellular inflammation in patients with diabetic nephropathy and non-diabetic nephropathy being administered hemodialysis treatment due to chronic renal failure. Ren Fail. 2014;36:767–773. doi: 10.3109/0886022X.2014.890841. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa T, Aw W, Kaneko K. Metabolic interactions of purine derivatives with human ABC transporter ABCG2: Genetic testing to assess gout risk. Pharmaceuticals (Basel) 2013;6:1347–1360. doi: 10.3390/ph6111347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dervisoglu E, Akturk AS, Yildiz K, Kiran R, Yilmaz A. The spectrum of renal abnormalities in patients with psoriasis. Int Urol Nephrol. 2012;44:509–514. doi: 10.1007/s11255-011-9966-1. [DOI] [PubMed] [Google Scholar]
- 33.Visconti L, Leonardi G, Buemi M, Santoro D, Cernaro V, Ricciardi CA, Lacquaniti A, Coppolino G. Kidney disease and psoriasis: Novel evidences beyond old concepts. Clin Rheumatol. 2016;35:297–302. doi: 10.1007/s10067-015-3126-4. [DOI] [PubMed] [Google Scholar]
- 34.Boda D, Negrei C, Nicolescu F, Bălălău CR. Assessment of some oxidative stress parameters in methotrexate treated psoriasis patients. Farmacia. 2014;62:704–710. [Google Scholar]
- 35.Kwon HH, Kwon IH, Choi JW, Youn JI. Cross-sectional study on the correlation of serum UA with disease severity in Korean patients with psoriasis. Clin Exp Dermatol. 2011;36:473–478. doi: 10.1111/j.1365-2230.2010.03988.x. [DOI] [PubMed] [Google Scholar]
- 36.Christophers E, Mrowietz U. Psoriasis. In: Freedberg IM, Eisen AS, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th. Vol. 1. McGraw-Hill; New York, NY: 2003. pp. 407–427. [Google Scholar]
- 37.Ataseven A, Kesli R, Kurtipek GS, Ozturk P. Assessment of lipocalin 2, clusterin, soluble tumor necrosis factor receptor-1, interleukin-6, homocysteine, and UA levels in patients with psoriasis. Dis Markers. 2014:541709. doi: 10.1155/2014/541709. 2014. doi: 10.1155/2014/541709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gisondi P, Targher G, Cagalli A, Girolomoni G. Hyperuricemia in patients with chronic plaque psoriasis. J Am Acad Dermatol. 2014;70:127–130. doi: 10.1016/j.jaad.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Ciragil P, Kurutas EB, Miraloglu M. New markers: Urine xanthine oxidase and myeloperoxidase in the early detection of urinary tract infection. Dis Markers. 2014:269362. doi: 10.1155/2014/269362. 2014. doi: org/10.1155/2014/269362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vorbach C, Harrison R, Capecchi MR. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003;24:512–517. doi: 10.1016/S1471-4906(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 41.Tampa M, Nicolae I, Ene CD, Sarbu I, Matei C, Georgescu SR. Vitamin C and thiobarbitUA reactive substances (Tbars) in psoriasis vulgaris related to psoriasis area severity index (Pasi) Rev Chim. 2017;68:43–47. [Google Scholar]
- 42.Matei C, Tampa M, Caruntu C, Ion RM, Georgescu SR, Dumitrascu GR, Constantin C, Neagu M. Protein microarray for complex apoptosis monitoring of dysplastic oral keratinocytes in experimental photodynamic therapy. Biol Res. 2014;47:33. doi: 10.1186/0717-6287-47-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dinu LU, Ene CD, Nicolae IL, Tampa M, Matei CL, Georgescu SR. The serum levels of 8-hidroxy-deoxyguanosine under the chemicals influence. Rev Chim. 2014;65:1319–1326. [Google Scholar]
- 44.Barreto FC, Stinghen AE, de Oliveira RB, Franco AT, Moreno AN, Barreto DV, Pecoits-Filho R, Drüeke TB, Massy ZA. The quest for a better understanding of chronic kidney disease complications: An update on uremic toxins. J Bras Nefrol. 2014;36:221–235. doi: 10.5935/0101-2800.20140033. [DOI] [PubMed] [Google Scholar]
- 45.Glorieux G, Tattersall J. Uraemic toxins and new methods to control their accumulation: Game changers for the concept of dialysis adequacy. Clin Kidney J. 2015;8:353–362. doi: 10.1093/ckj/sfv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semchyshyn HM. Reactive carbonyl species in vivo. Generation and dual biological effects. ScientificWorldJournal 2014. 2014:417842. doi: 10.1155/2014/417842. doi: 10.1155/2014/417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sánchez-Lozada LG, Lanaspa MA, Cristóbal-García M, García-Arroyo F, Soto V, Cruz-Robles D, Nakagawa T, Yu MA, Kang DH, Johnson RJ. UA-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron, Exp Nephrol. 2012;121:71–78. doi: 10.1159/000345509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidovici BB, Sattar N, Prinz J, Puig L, Emery P, Barker JN, van de Kerkhof P, Ståhle M, Nestle FO, Girolomoni G, et al. Psoriasis and systemic inflammatory diseases: Potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785–1796. doi: 10.1038/jid.2010.103. [DOI] [PubMed] [Google Scholar]
- 49.Kim IY, Lee DW, Lee SB, Kwak IS. The role of UA in kidney fibrosis: Experimental evidences for the causal relationship. BioMed Res Int. 2014:638732. doi: 10.1155/2014/638732. 2014. doi: 10.1155/2014/638732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiners JJ, Jr, Rupp T. Conversion of xanthine dehydrogenase to xanthine oxidase occurs during keratinocyte differentiation: Modulation by 12-O-tetradecanoylphorbol-13-acetate. J Invest Dermatol. 1989;93:132–135. doi: 10.1111/1523-1747.ep12277382. [DOI] [PubMed] [Google Scholar]
- 51.Deliconstantinos G, Villiotou V, Stavrides JC. Alterations of nitric oxide synthase and xanthine oxidase activities of human keratinocytes by ultraviolet B radiation. Potential role for peroxynitrite in skin inflammation. Biochem Pharmacol. 1996;51:1727–1738. doi: 10.1016/0006-2952(96)00110-4. [DOI] [PubMed] [Google Scholar]
- 52.Festugato M. Adenosine: An endogenous mediator in the pathogenesis of psoriasis. An Bras Dermatol. 2015;90:862–867. doi: 10.1590/abd1806-4841.20153689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V. Regulation of UA metabolism and excretion. Int J Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 54.Coimbra S, Santos-Silva A. Biomarkers of psoriasis severity and therapy monitoring. World J Dermatol. 2014;3:15–27. doi: 10.5314/wjd.v3.i2.15. [DOI] [Google Scholar]
- 55.Yıldırım FE, Karaduman A, Pinar A, Aksoy Y. CD26/ dipeptidyl-peptidase IV and adenosine deaminase serum levels in psoriatic patients treated with cyclosporine, etanercept, and psoralen plus ultraviolet A phototherapy. Int J Dermatol. 2011;50:948–955. doi: 10.1111/j.1365-4632.2010.04799.x. [DOI] [PubMed] [Google Scholar]
- 56.Bukulmez G, Akan T, Ciliv G. Serum adenosine deaminase levels in patients with psoriasis: A prospective case-control study. Eur J Dermatol. 2000;10:274–276. [PubMed] [Google Scholar]
- 57.Nicolae I, ENE CD, Georgescu SR, Tampa M, Matei C, Ceausu E. Effects of UV radiation and oxidative DNA adduct 8-hydroxy-2′-deoxiguanosine on the skin diseases. Rev Chim. 2014;65:1036–1041. [Google Scholar]
- 58.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 60.Tsai JP, Liou JH, Yeh KT, Tai HC, Cheng YW, Chang HR. Intensity of cytosol expression of 8-OHdG in normal renal tubules is associated with the severity of renal fibrosis. Swiss Med Wkly. 2011;141:w13268. doi: 10.4414/smw.2011.13268. [DOI] [PubMed] [Google Scholar]
- 61.Dincer Y, Sekercioglu N, Pekpak M, Gunes KN, Akcay T. Assessment of DNA oxidation and antioxidant activity in hypertensive patients with chronic kidney disease. Ren Fail. 2008;30:1006–1011. doi: 10.1080/08860220802422044. [DOI] [PubMed] [Google Scholar]
- 62.Domenici FA, Vannucchi MT, Jordão AA, Jr, Meirelles MS, Vannucchi H. DNA oxidative damage in patients with dialysis treatment. Ren Fail. 2005;27:689–694. doi: 10.1080/08860220500242678. [DOI] [PubMed] [Google Scholar]
- 63.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 64.Jeong KH, Lee TW, Ihm CG, Lee SH, Moon JY, Lim SJ. Effects of sildenafil on oxidative and inflammatory injuries of the kidney in streptozotocin-induced diabetic rats. Am J Nephrol. 2009;29:274–282. doi: 10.1159/000158635. [DOI] [PubMed] [Google Scholar]
- 65.Akçay T, Saygili I, Andican G, Yalçin V. Increased formation of 8-hydroxy-2′-deoxyguanosine in peripheral blood leukocytes in bladder cancer. Urol Int. 2003;71:271–274. doi: 10.1159/000072677. [DOI] [PubMed] [Google Scholar]
- 66.Miyake H, Hara I, Kamidono S, Eto H. Prognostic significance of oxidative DNA damage evaluated by 8-hydroxy-2′-deoxyguanosine in patients undergoing radical nephrectomy for renal cell carcinoma. Urology. 2004;64:1057–1061. doi: 10.1016/j.urology.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 67.Chang CH, Yang CM, Yang AH. Renal diagnosis of chronic hemodialysis patients with urinary tract transitional cell carcinoma in Taiwan. Cancer. 2007;109:1487–1492. doi: 10.1002/cncr.22557. [DOI] [PubMed] [Google Scholar]
- 68.Tatu AL, Nwabudike LC. Metoprolol-associated onset of psoriatic arthropathy. Am J Ther. 2017;24:e370–e371. doi: 10.1097/MJT.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 69.Namazi MR. Cannabinoids, loratadine and allopurinol as novel additions to the antipsoriatic ammunition. J Eur Acad Dermatol Venereol. 2005;19:319–322. doi: 10.1111/j.1468-3083.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- 70.Tsuruta N, Imafuku S, Narisawa Y. Hyperuricemia is an independent risk factor for psoriatic arthritis in psoriatic patients. J Dermatol. 2017;44:1349–1352. doi: 10.1111/1346-8138.13968. [DOI] [PubMed] [Google Scholar]
- 71.Merola JF, Wu S, Han J, Choi HK, Qureshi AA. Psoriasis, psoriatic arthritis and risk of gout in US men and women. Ann Rheum Dis. 2015;74:1495–1500. doi: 10.1136/annrheumdis-2014-205212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Singh NP, Prakash A, Kubba S, Ganguli A, Singh AK, Sikdar S, Agarwal SK, Dinda AK, Grover C. Psoriatic nephropathy - does an entity exist? Ren Fail. 2005;27:123–127. doi: 10.1081/JDI-42811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.