Abstract
The promyelocytic leukemia (PML)-retinoic acid receptor α (RARA) fusion is hypothesized to serve a vital role in the pathogenesis of acute promyelocytic leukemia (APL), which results from a reciprocal translocation between chromosomes 15 and 17, t(15;17)(q24;q21). A minority of APL cases lack the classical t(15;17) and have been identified to have cryptic or masked t(15;17) or complex translocations. The present study reports on a case of a 37-year-old male with APL harboring a complex three-way translocation t(1;17;15)(q21;q21;q24). This karyotypic interpretation was further confirmed by fluorescence in situ hybridization, and 98% of the bone marrow cells analyzed were positive for the PML-RARA fusion gene. After combined treatment with all-trans retinoic acid and arsenic trioxide, the patient achieved complete remission with no recurrence for 3 years to date. To the best of our knowledge, the present study is the first to report on the novel variant of t(15;17) involving the breakpoint 1q21.
Keywords: complex translocation, t(1;17;15), acute promyelocytic leukemia, promyelocytic leukemia-retinoic acid receptor α, chromosome 1
Introduction
Acute promyelocytic leukemia (APL) with promyelocytic leukemia (PML)-retinoic acid receptor α (RARA) gene fusion, a distinct subtype of acute myeloid leukemia (AML) accounting for ~10% of cases (1). The PML-RARA fusion is hypothesized to serve a vital role in the pathogenesis of APL. Usually, patients with APL with the PML-RARA fusion gene are sensitive to molecular target-based agents, including all-trans retinoic acid (ATRA) and arsenic trioxide (ATO).
The fusion gene usually results from classical t(15;17)(q24;q12) rearrangements. However, a minority of APL cases actually lacks this classical chromosomal aberration and is additionally associated with the formation of the PML-RARA fusion gene. APL cases lacking classical t(15;17) arise from insertion events or more complex rearrangements, and the latter account for 10% of APL lacking classical t(15;17) (2). Due to its rarity, the outcome of complex translocations has remained to be characterized.
Analysis of these complex translocations is of great interest, as they may occur in clusters around particular chromosomal bands. These rare translocations may provide insight that may be useful for identifying novel gene rearrangements in APL. In the present study, a case report of a patient with APL harboring three-way translocations in a complex karyotype that was determined by molecular cytogenetic approaches is provided.
Case report
Case presentation
A 37-year-old man was admitted to the Second Hospital of Jilin University (Changchun, China) in September 2014. He had an intermittent fever and a sore throat for 6 days, as well as a nosebleed for 2 days and hematuria for 1 day. A complete blood examination demonstrated a hemoglobin (Hb) count of 104 g/l (normal range, 115–150 g/l), a white blood cell (WBC) count of 45.8×109/l (normal range, 3.5–9.5×109/l) with 89% blasts and a platelet count of 10×109/l (normal range, 125–350×109/l). Coagulation tests identified a prothrombin time of 16.0 sec (normal, 9.4–12.5 sec), a normal prothrombin international ratio of 1.35 (normal, 0.8–1.2), a prothrombin activity of 61% (normal, 80–150%), an activated partial thromboplastin time of 33.6 sec (normal, 22.0–42.0 sec), a fibrinogen level of 1.31 g/l (normal, 2.0–4.0 g/l) and a D-dimer of 57.20 µg/ml (normal, 0–1.0 µg/ml). The patient had no hepatosplenomegaly.
Morphologic analysis
Analysis of the bone marrow (BM) aspirate demonstrated a markedly hypercellular marrow with the absence of megakaryocytes and 88% abnormal promyelocytes. Cytochemical staining of the BM aspirate specimen was performed as described previously (3), at room temperature. The film preparation was covered with sufficient 0.3% benzidine solution (Baso Biotech Co., Ltd., Wuhan, China) for 1 min. An equal amount of 0.03% H2O2 solution (Baso Biotech Co., Ltd.) was added. After 4–5 min, the stained smears were washed with tap water. The smears were counterstained with Wright-Giemsa solution (Baso Biotech Co., Ltd.) for 10 min. The results demonstrated that the abnormal promyelocytes were strongly positive for myeloperoxidase. However, analyses of esterases were not performed. The core biopsy, which consisted of sheets of abnormal promyelocytes, of the bone marrow demonstrated a cellularity of >95%.
Flow cytometry
A total of 50 µl bone marrow (1×106 cells) and antibodies (listed below) were added to four test tubes at room temperature for 15 min in the dark. Optilyse C lysing solution (cat. no. 349202; BD Biosciences, San Jose, CA, USA) was added to the tubes for 10 min at room temperature in the dark. Then, 1 ml PBS was added to three of the tubes, which were centrifuged at 300 × g for 5 min; the supernatant was removed and then the cells were washed with PBS. PBS (500 µl) was added to the three tubes and the samples were incubated with the following antibodies: Fluorescein isothiocyanate (FITC)-conjugated anti-CD15 (cat. no. 332778), R-phycoerythrin (PE)-conjugated anti-CD117 (cat. no. 340529), PerCP-Cy™ 5.5-conjugated anti-CD34 (cat. no. 347203), PE-conjugated anti-CD13 (cat. no. 347837), PerCP-Cy5.5-conjugated anti-CD19 (cat. no. 340951; all 1:25), allophycocyanin (APC)-H7-conjugated anti-HLA-DR (cat. no. 641393), BD Horizon™ V450-conjugated anti-CD38 (cat. no. 646851), V450-conjugated anti-CD11b (cat. no. 560480), APC-conjugated anti-CD4 (cat. no. 340443), APC-H7-conjugated anti-CD14 (cat. no. 641394), V500-conjugated anti-CD45 (cat. no. 560777; all 1:100), PE-Cy7-conjugated anti-CD33 (cat. no. 333946; 1:50; all BD Biosciences), PE-Cy7-conjugated anti-CD123 (cat. no. 306010), APC-conjugated anti-CD7 (cat. no. 343108; both Biolegend, Inc., San Diego, CA, USA), APC-conjugated anti-CD56 (cat. no. IM2474; all 1:50), FITC-conjugated anti-CD64 (cat. no. IM1604U), FITC-conjugated anti-CD36 (cat. no. IM0766U), PE-conjugated anti-CD10 (cat. no. A07760; all 1:25), PE-Cy7-conjugated anti-CD20 (cat. no. IM3629U; 1:100; Beckman Coulter, Inc., Brea, CA, USA). The first test tube was contained anti-CD15, anti-CD117, anti-CD34, anti-HLA-DR, anti-CD38, anti-CD45, anti-CD33 and anti-CD7 antibodies. The second test tube was contained anti-CD64, anti-CD56, anti-CD13, anti-CD34, anti-HLA-DR, anti-CD45, anti-CD11b and anti-CD123 antibodies. The third test tube was contained anti-CD36, anti-CD10, anti-CD20, anti-CD19, anti-CD4, anti-CD14, anti-CD38 and anti-CD45 antibodies.
The sample in the fourth test tube was incubated with 100 µl intraprep permeabilization reagent A (cat. no. 641776; BD Biosciences) for 5 min at room temperature. Following the centrifugation of the fourth test tube at 300 × g for 5 min at room temperature, the supernatant was removed. A total of 50 µl intraprep permeabilization reagent B and 20 µl cytoplasmic antibodies (listed below) were added to the tube and agitated for 15 min. PBS (1 ml) was added to the tube, which was centrifuged at 300 × g for 5 min at room temperature, then the supernatant was removed. PBS (500 µl) was added to the fourth test tubes and the samples were incubated with the following antibodies: FITC-conjugated TDT (cat. no. F7139), PE-conjugated MPO (cat. no. R7209; both Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), PE-Cy7-conjugated anti-CD2 (cat. no. 335786; all 1:50), PerCP-Cy5.5-conjugated anti-CD5 (cat. no. 341109), PerCP-Cy5.5-conjugated anti-CD9 (cat. no. 341649), APC-H7-conjugated anti-CD3 (cat. no. 641397), V450-conjugated anti-cCD3 (cat. no. 558117), V500-conjugated anti-CD45 (cat. no. 560777; all 1:100; BD Biosciences), APC-conjugated anti-CD79a (cat. no. 333506; 1:50; Biolegend, Inc.). All four samples were then examined using a BD FACSCanto II flow cytometer and the data were analyzed using BD FACSDIVA™ 6.1.3 software (both BD Biosciences). The results demonstrated that the samples were positive for CD13, CD33 and myeloperoxidase (MPO), and some cells expressed CD38, CD64 and CD117.
Molecular analysis
The PML-RARA rearrangement was confirmed by reverse transcription-qualitative polymerase chain reaction (RT-qPCR). Total RNA was extracted from bone marrow samples using TRIzol reagent (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Complementary DNA was synthesized using the total RNA, random hexamer primers (cat. no. 3801), dNTP mixture, 5X M-MLV buffer and reverse transcriptase M-MLV (cat. no. 2641Q; both Takara Bio, Inc., Otsu, Japan). The conditions for reverse transcription reaction consisted of 10 min at 30°C, 60 min at 42°C, 15 min at 70°C, and 4°C indefinitely. Subsequently, a qPCR analysis was performed using Premix Ex Taq™ (cat. no. RR390A; Takara Bio, Inc.), and the following primers: PML-RARA forward, 5′-CCGTCATAGGAAGTGAGGTCT-3′ and reverse, 5′-GGCTGGGCACTATCTCTTCA-3′; and GAPDH forward, 5′-AATGGAAATCCCATCACCATCT-3′ and reverse, 5′-CATCGCCCCACTTGATTTTG-3′. The thermocycling conditions were as follows: An initial denaturation at 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 58°C for 40 sec on the Applied Biosystems 7500 Fast PCR System (Thermo Fisher Scientific, Inc.). The values were normalized to GAPDH transcript levels and expressed as a ratio of PML-RARA to GAPDH by absolute quantification method (Table I). The results demonstrated that only the short-type chimeric transcript was expressed, while RARA-PML was not expressed. The patient was positive for FMS related tyrosine kinase 3 internal tandem duplication (FLT3-ITD) gene mutation, which was detected as previously described (4).
Table I.
Reverse transcription-qualitative polymerase chain reaction analysis results.
| Test | Results |
|---|---|
| PML-RARA (Long-type) | Negative |
| PML-RARA (Variant-type) | Negative |
| PML-RARA (Short-type) | Positive |
| PML-RARA (copy numbers) | 186,700 |
| GAPDH (copy numbers) | 183,500 |
| PML-RARA/GAPDH | 101.74% |
The cut-off for the number of PML-RARA transcripts was 50.
Treatment
Based on these results, a diagnosis of APL with PML-RARA was made. The patient started induction therapy with oral ATRA [40 mg/(m2day)], intravenous ATO [10 mg/(m2day)] and cytarabine [200 mg/(m2day)] treatment. On the 3rd day after therapy initiation, the WBC count reached 61.8×109/l with double lower limb edema and bone pain syndrome, which are symptoms of APL differentiation (5). Subsequently, the oral ATRA therapy was stopped and the patient received treatment with dexamethasone (10 mg for 3 days) and daunorubicin (20 mg for 3 days); the APL differentiation symptoms gradually disappeared. The patient repeatedly received ATRA induction therapy; the WBC count gradually decreased to a normal count. The patient received intrathecal dexamethasone (10 mg) and cytarabine (50 mg) treatment for prevention. The patient demonstrated hematological recovery after 39 days. A complete hematological remission was achieved and BM aspiration demonstrated a regenerating marrow without morphologic evidence of the malignant disease. The patient was negative for PML-RARA fusion gene. The patient in complete remission (CR) received consolidation courses every month, with no recurrence for 4 years to date.
Conventional cytogenetic analysis and fluorescence in situ hybridization (FISH)
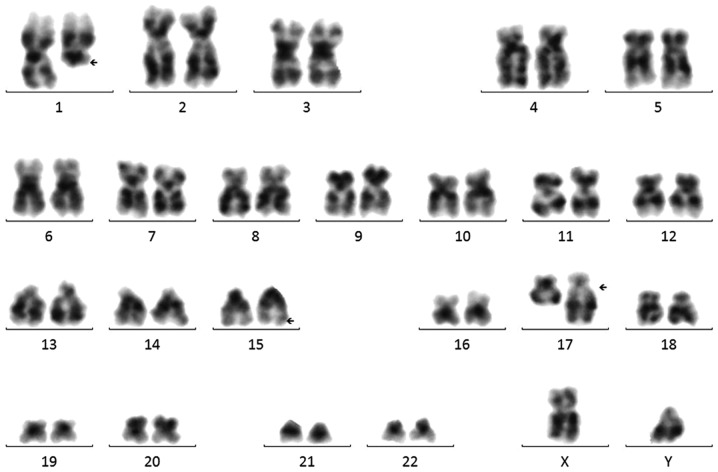
The BM sample of the patient was analyzed for metaphase karyotyping by G-banding after 48 h of unstimulated culture. Of the 20 metaphase cells examined using CytoVision system (Leica Microsystems, San Jose, CA, USA), an apparent complex translocation was identified, which involved chromosome 1 in addition to chromosomes 15 and 17 with breakpoints at 1q21, 15q24 and 17q21. No other consistent structural or numerical abnormalities were detected (Fig. 1). To confirm this complex chromosomal rearrangement and determine the diagnosis, FISH was performed using the Vysis dual-color, dual-fusion probe (Abbott Laboratories, Abbott Park, IL, USA) according to the manufacturer's protocol. The cells were counterstained with DAPI II in the dark at −20°C for 30 min prior to observation. Fluorescent signals were visualized using a fluorescence microscope at a magnification of ×1,000. Typically, in APL with PML-RARA, two yellow signals, one red and one green signal are visible, which indicate the classical t(15;17). In the patient, 392 out of 400 cells (98%) exhibited two red signals, two green signals and one yellow fusion signal (Fig. 2), which were the result of the complex translocation. Taken together, the cytogenomic findings can be described as: 46,XY, t(1;17;15)(q21;q21;q24)[20].nuc ish(PML, RARA)×3(PML con RARAx1)[400].
Figure 1.
G banded karyotype analysis revealed a complex translocation involving chromosome 1;17;15. The arrows indicate the breakpoints on 1q21,15q24 and 17q21.
Figure 2.
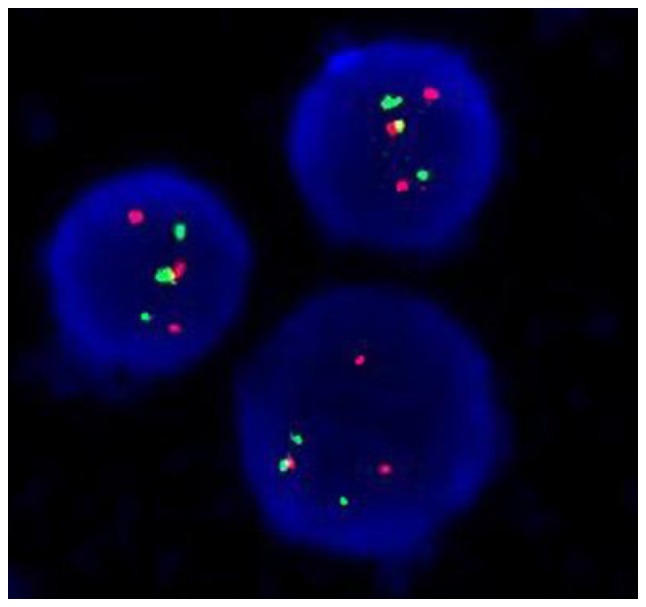
FISH analyses of the patient. Interphase FISH demonstrated a fusion signal pattern of chromosome 15 and 17 in the patient. The PML gene in the chromosome 15 is labelled red, the RARA gene in the chromosome 17 is labelled green and the PML-RARA fusion gene is labelled yellow. Cells were counterstained with DAPI II. Magnification, ×1,000. FISH, fluorescence in situ hybridization; PML, promyelocytic leukemia; RARA, retinoic acid receptor α.
Discussion
To date, to the best of our knowledge, 46 cases with complex translocation have been previously reported (6–8). Among these, only four cases of three-way translocations involved chromosome 1, and the breakpoint occurred at 1p31, 1p32, 1p36 and 1q23 (Table II) (2,9–11). The present study identified the fifth case of APL harboring a three-way translocation involving chromosome 1;17;15. The clinical characterization of the five cases of APL with t(1;17;15) is summarized in Table II. All of the patients were male and the median age at diagnosis was 42 years. No distinct clinical features were observed in APL with complex translocation regarding the three-way t(1;17;15) translocation.
Table II.
Clinical characterization of previously reported acute promyelocytic leukemia cases harboring a three-way translocation involving chromosome 1;17;15.
| Author (year) | Sex | Age (years) | WBC (×109/l) | Hb (g/l) | DIC | FAB | Karyotype | PML-RARA fusion gene | Treatment | Relapse | Survival (months) | Breakpoints on chromosome 1 | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Present study | M | 37 | 45.8 | 109 | No | M3 | 46, XY, der(1)t(1;17) (q21;q21)t(15;17) (q24;q21),der(15)t(15;17)t(1;17), der(17)t(15;17)t(1;17)[20] | Short type | ATRA+ATO+Ara-C | No | >48 | 1q21 | NA |
| Grimwade (2000) | M | NA | NA | NA | NA | M3 | 46,XY,t(1;17;15)(p32;q21;q22)/46, idem, add(21)(p13)/46, XY | Short type | NA | NA | NA | 1p32 | (2) |
| Osella (1991) | M | 46 | 0.7 | NA | Yes | M3 | 46,XY/46,XY, t(1;15;17)(p36; q22;q21.1)/46,XY, t(1;15;17) (p36;q22;q21.1),+8 | NA | Ara-C+daunorubicin | Yes | >11 | 1p36 | (9) |
| Park and Fairweather (1996) | M | 46 | 4.9 | 94 | Yes | M3 | 46,XY/46, XY, del(1)(p22), del(3)(p25),der(17)t(1;15;17) (17pter→17q21::15q21→ 15q22::1p36→1p31::15q21→ 15q22::17q21→17pter) | NA | ATRA+Ara-C+daunorubicin | Yes | 9 | 1p31 | (10) |
| Galieni (1996) | M | 41 | NA | NA | NA | M3 | 46,XY, t(1;17;15) (q23;q23;q22)/47,idem, +10 | NA | ATRA+C | No | >8 | 1q23 | (11) |
M, male; NA, not available; FAB, French-American-British classification of acute leukemia; DIC, disseminated intravascular coagulation; C, chemotherapy; ATRA, all-trans retinoic acid; ATO, arsenic trioxide; Ara-C, cytarabine; Hb, hemoglobin; WBC, white blood cells; PML-RARA, promyelocytic leukemia-retinoic acid receptor α.
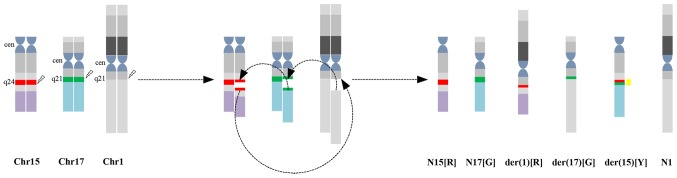
According to karyotyping and FISH analysis, the complex translocation in the present study may be described as follows: 15q24 translocated to 1q21; a small piece of chromosome 17 (17q21) translocated to 15q24; and 1q21 connected to 17q21 that was located in der(15)(q24). As a result of the genomic rearrangement, a part of the PML gene (labeled in red) is present on the derivative of chromosome 1 and a part of the RARA gene (labeled in green) is present on the derivative of chromosome 17. In addition, one yellow signal, one red and one green signal indicates the PML-RARA fusion on the derivative of chromosome 15, and the normal chromosomes 15 and 17, respectively (Fig. 3). Correspondingly, FISH identified two red signals, two green signals and one yellow signal.
Figure 3.
Schematic presentations of karyotyping and fluorescence in situ hybridization analyses for complex translocations. 15q24 lous (promyelocytic leukemia gene) is indicated by a red bar and 17q21 lous (retinoic acid receptor α gene) is indicated by a green bar. Chr, chromosome; N, normal; der, derivative; R, red signal; G, green signal; Y, yellow; cen, centromere.
The association between complex translocations and classical t(15;17) remains elusive. Previous studies hypothesized that the complex translocation possibly evolves from the classical t(15;17) (7,12). However, as a cell with t(15;17) alone was not identified, the results of the present suggested that all of the events happened at the same time and not in two steps. A recent study proposed a non-homologous chromosome recombination model as one of the mechanisms that results in chromosome translocations in leukemia (13). It is noteworthy that the breakpoint in the present case occurred in a novel area of the long arm of chromosome 1, 1q21. The breakpoint 1q21 has been identified in AML, predominantly involving two different chromosome translocations. The first was described in a patient with AML with t(1;11)(q21;q23), which leads to the fusion gene MLL-AF1q (14,15), and the second was described in a 1-year-old patient with AML with t(1;21)(q21;q22), which leads to the fusion gene runt-related transcription factor 1-zinc finger protein (ZNF)687 (16). Whether the AF1q or ZNF687 gene is located at the breakpoint and forms a fusion gene in the present case requires clarification.
The incidence of FLT3-ITD mutations in APL is 12–38% (17). FLT3-ITD mutations are associated with higher Hb and WBC levels, as well as short-type PML-RARA fusion transcripts at diagnosis. Hb>9.6 g/dl and WBC≥20×109/l are important factors for predicting the presence of presence FLT3-ITD according to a multivariate analysis (18,19). The results of the present study are consistent with those of the aforementioned study (18), supporting the hypothesis that carriers of FLT3-ITD constitute a biologically distinct group of patients with APL. Previous studies have demonstrated that FLT3-ITD in APL has an adverse prognostic value (17,19). Of note, two previous independent studies demonstrated that the addition of ATO to frontline therapy overcomes the impact of previously described adverse prognostic factors, including FLT3-ITD mutations (20,21). The patient of the present study received treatment with ATO during induction and consolidation courses, with no recurrence for 3 years to date. The outcome of this case is consistent with the results of the aforementioned studies (20,21).
The majority of previous studies observed no difference in clinical outcome between APL with typical t(15;17) and APL with complex translocations (22,23). The results of the present study are consistent with those of previous studies, supporting the hypothesis that the presence of the PML-RARA fusion gene is crucial for achieving the best response to ATRA (24–26). In previous studies, APL with complex translocations, including four cases of three-way translocations involving chromosome 1, appears to be associated with poor prognosis, which may be due to the absence of targeted therapies (27–29). Additional genomic alterations besides the PML-RARA fusion may be implicated in the heterogenicity of therapy outcomes in APL.
In conclusion, the present study was the first, to the best of our knowledge, to identify a novel breakpoint in chromosome 1 in an adult patient with APL using FISH. Furthermore, at the 3-year follow-up, a good response to the combined treatment with ATRA and ATO was revealed, as is observed in typical APL. The significance of complex translocations in APL with PML-RARA requires further investigation.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
LL and LY analyzed the data, performed all of the examinations of the patients and drafted the manuscript. TM, LL and HC contributed to data acquisition and examination of the patient. All authors agree to the submission and have full responsibility for all primary data. No part of this paper has been published or submitted elsewhere.
Ethics approval and consent to participate
The patient provided written informed consent prior to the study according to the Declaration of Helsinki and the study was approved by the Second Hospital of Jilin University.
Patient consent for publication
Written informed consent was obtained from the patient for the publication of their data.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 2.Grimwade D, Biondi A, Mozziconacci MJ, Hagemeijer A, Berger R, Neat M, Howe K, Dastugue N, Jansen J, Radford-Weiss I, et al. Characterization of acute promyelocytic leukemia cases lacking the classic t(15;17): Results of the european working party. Groupe français de cytogénétique hématologique, groupe de français d'hematologie cellulaire, UK cancer cytogenetics group and BIOMED 1 european community-concerted action ‘molecular cytogenetic diagnosis in haematological malignancies’. Blood. 2000;96:1297–1308. [PubMed] [Google Scholar]
- 3.Chen WX, Zhu HL, Xue M, Zhou H, Zhao F, Yan N, Chen Y. Quick staining technique for myeloperoxidase using potassium iodide and oxidized pyronine B. Acta Histochem. 2014;116:292–296. doi: 10.1016/j.acthis.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, Nugent E, Mills KI, Wheatley K, Solomon E, et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106:3768–3776. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]
- 5.Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: Experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3:e2011059. doi: 10.4084/mjhid.2011.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Ma J, Liu X, Liu R, Xu L, Wang L, Cen J, Chu X. A complex translocation (3;17;15) in acute promyelocytic leukemia confirmed by fluorescence in situ hybridization. Oncol Lett. 2016;12:4717–4719. doi: 10.3892/ol.2016.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Kim YM, Wang X, Li Y, Pang H, Lee JY, Li S. Coexistence of t(15;17) and t(15;16;17) detected by fluorescence in situ hybridization in a patient with acute promyelocytic leukemia: A case report and literature review. Oncol Lett. 2014;8:1001–1008. doi: 10.3892/ol.2014.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennour A, Tabka I, Youssef YB, Zaier M, Hizem S, Khelif A, Saad A, Sennana H. A PML/RARA chimeric gene on chromosome 12 in a patient with acute promyelocytic leukemia (M4) associated with a new variant translocation: t(12;15;17)(q24;q24;q11) Med Oncol. 2013;30:409. doi: 10.1007/s12032-012-0409-3. [DOI] [PubMed] [Google Scholar]
- 9.Osella P, Wyandt H, Vosburgh E, Milunsky A. Report of a variant t(1;15;17)(p36;q22;q21.1) in a patient with acute promyelocytic leukemia. Cancer Genet Cytogenet. 1991;57:201–207. doi: 10.1016/0165-4608(91)90153-L. [DOI] [PubMed] [Google Scholar]
- 10.Park JP, Fairweather RB. Complex t(1;15;17) in acute promyelocytic leukemia with duplication of RAR alpha and PML sequences. Cancer Genet Cytogenet. 1996;89:52–56. doi: 10.1016/0165-4608(95)00365-7. [DOI] [PubMed] [Google Scholar]
- 11.Galieni P, Marotta G, Vessichelli F, Diverio D, Minoletti F, Bucalossi A, Lo Coco F, Lauria F. Variant t(1;15;17)(q23;q22;q23) in a case of acute promyelocytic leukemia. Leukemia. 1996;10:1658–1661. [PubMed] [Google Scholar]
- 12.Miyazaki K, Kikukawa M, Kiuchi A, Shin K, Iwamoto T, Ohyashiki K. Complex translocations derived stepwise from standard t(15;17) in a patient with variant acute promyelocytic leukemia. Cancer Genet Cytogenet. 2007;176:127–130. doi: 10.1016/j.cancergencyto.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5:1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Tse W, Zhu W, Chen HS, Cohen A. A novel gene, AF1q, fused to MLL in t(1;11) (q21;q23), is specifically expressed in leukemic and immature hematopoietic cells. Blood. 1995;85:650–656. [PubMed] [Google Scholar]
- 15.Busson-Le Coniat M, Salomon-Nguyen F, Hillion J, Bernard OA, Berger R. MLL-AF1q fusion resulting from t(1;11) in acute leukemia. Leukemia. 1999;13:302–306. doi: 10.1038/sj.leu.2401299. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TT, Ma LN, Slovak ML, Bangs CD, Cherry AM, Arber DA. Identification of novel Runx1 (AML1) translocation partner genes SH3D19, YTHDf2, and ZNF687 in acute myeloid leukemia. Genes Chromosomes Cancer. 2006;45:918–932. doi: 10.1002/gcc.20355. [DOI] [PubMed] [Google Scholar]
- 17.Beitinjaneh A, Jang S, Roukoz H, Majhail NS. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations in acute promyelocytic leukemia: A systematic review. Leuk Res. 2010;34:831–836. doi: 10.1016/j.leukres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Souza Melo CP, Campos CB, Dutra ÁP, Neto JC, Fenelon AJ, Neto AH, Carbone EK, Pianovski MA, Ferreira AC, Assumpcão JG. Correlation between FLT3-ITD status and clinical, cellular and molecular profiles in promyelocytic acute leukemias. Leuk Res. 2015;39:131–137. doi: 10.1016/j.leukres.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Lucena-Araujo AR, Kim HT, Jacomo RH, Melo RA, Bittencourt R, Pasquini R, Pagnano K, Fagundes EM, Chauffaille Mde L, Chiattone CS, et al. Internal tandem duplication of the FLT3 gene confers poor overall survival in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline-based chemotherapy: An International Consortium on Acute Promyelocytic Leukemia study. Ann Hematol. 2014;93:2001–2010. doi: 10.1007/s00277-014-2142-9. [DOI] [PubMed] [Google Scholar]
- 20.Poiré X, Moser BK, Gallagher RE, Laumann K, Bloomfield CD, Powell BL, Koval G, Gulati K, Holowka N, Larson RA, et al. Arsenic trioxide in front-line therapy of acute promyelocytic leukemia (C9710): Prognostic significance of FLT3 mutations and complex karyotype. Leuk Lymphoma. 2014;55:1523–1532. doi: 10.3109/10428194.2013.842985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cicconi L, Divona M, Ciardi C, Ottone T, Ferrantini A, Lavorgna S, Alfonso V, Paoloni F, Piciocchi A, Avvisati G, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukaemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016;30:1987–1992. doi: 10.1038/leu.2016.122. [DOI] [PubMed] [Google Scholar]
- 22.Wiernik PH, Sun Z, Gundacker H, Dewald G, Slovak ML, Paietta E, Kim HT, Appelbaum FR, Cassileth PA, Tallman MS. Prognostic implications of additional chromosome abnormalities among patients with de novo acute promyelocytic leukemia with t(15;17) Med Oncol. 2012;29:2095–2101. doi: 10.1007/s12032-012-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujishima M, Takahashi N, Miura I, Kobayashi Y, Kume M, Nishinari T, Miura AB. A PML/RARA chimeric gene on chromosome 2 in a patient with acute promyelocytic leukemia (M3) associated with a new variant translocation: t(2;15;17)(q21;q22;q21) Cancer Genet Cytogenet. 2000;120:80–82. doi: 10.1016/S0165-4608(99)00238-1. [DOI] [PubMed] [Google Scholar]
- 24.Hernández JM, Martín G, Gutiérrez NC, Cervera J, Ferro MT, Calasanz MJ, Martínez-Climent JA, Luño E, Tormo M, Rayón C, et al. Additional cytogenetic changes do not influence the outcome of patients with newly diagnosed acute promyelocytic leukemia treated with an ATRA plus anthracyclin based protocol. A report of the Spanish group PETHEMA. Haematologica. 2001;86:807–813. [PubMed] [Google Scholar]
- 25.De Botton S, Chevret S, Sanz M, Dombret H, Thomas X, Guerci A, Fey M, Rayon C, Huguet F, Sotto JJ, et al. Additional chromosomal abnormalities in patients with acute promyelocytic leukaemia (APL) do not confer poor prognosis: Results of APL 93 trial. Br J Haematol. 2000;111:801–806. doi: 10.1111/j.1365-2141.2000.02442.x. [DOI] [PubMed] [Google Scholar]
- 26.de Botton S, Coiteux V, Chevret S, Rayon C, Vilmer E, Sanz M, de La Serna J, Philippe N, Baruchel A, Leverger G, et al. Outcome of childhood acute promyelocytic leukemia with all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2004;22:1404–1412. doi: 10.1200/JCO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Freeman CE, Mercer DD, Ye Y, Van Brunt J III, Li MM. Cytogenetic and molecular characterization of complex three-way translocations in acute promyelocytic leukemia. Beijing Da Xue Xue Bao Yi Xue Ban. 2009;41:477–479. [PubMed] [Google Scholar]
- 28.McKinney CD, Golden WL, Gemma NW, Swerdlow SH, Williams ME. RARA and PML gene rearrangements in acute promyelocytic leukemia with complex translocations and atypical features. Genes Chromosomes Cancer. 1994;9:49–56. doi: 10.1002/gcc.2870090109. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese G, Min T, Stuppia L, Powles R, Swansbury JG, Morizio E, Peila R, Donti E, Fioritoni G, Palka G. Complex chromosome translocations of standard t(8;21) and t(15;17) arise from a two-step mechanism as evidenced by fluorescence in situ hybridization analysis. Cancer Genet Cytogenet. 1996;91:40–45. doi: 10.1016/S0165-4608(96)00096-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.