Abstract
Erlotinib is a targeted anticancer therapy used for treating epidermal growth factor receptor (EGFR) mutation positive lung cancer in advanced stage as well as for other malignancies. The most common cutaneous side effect of erlotinib, are well documented; however the number of reports regarding cutaneous leukocytoclastic vasculitis (CLCV) are limited. We report a case, a 58-year-old, 60 kg weight, non-smoking woman suffering of lung adenocarcinoma and brain metastases treated with erlotinib monotherapy with 150 mg/day dose, who presents cutaneous leukocytoclastic vasculitis after 8 months of initiating the treatment. The administration of the drug was discontinued and oral prednisolone treatment was introduced at 1 mg/kg body weight dose for two weeks, decreasing the dose with 5 mg, at every 3 days. The treatment was combined with topical potent steroid and antibiotic therapy used once, daily. The lesions cleared within 7 weeks without recurrence. The treatment with erlotinib was restarted after 14 days with a lower dose of 100 mg/day. The skin lesions have not occurred anymore. Unfortunately the evolution was unfavorable, our patient died 3 months after the vasculitis healing, due to the complications of new metastases that occurred. This may indicate the inefficiency of erlotinib. The late onset of 240 days of the vasculitis and the presumed inefficiency of the drug lead to the speculation that the appearance of cutaneous vasculitis could be a worsening clinical marker of the tumor response. This limited number of cases precludes any meaningful interpretation of data about the erlotinib induced cutaneous vasculitis. Further investigations are needed to assess cutaneous vasculitis.
Keywords: EGFR inhibitors, lung adenocarcinoma, erlotinib, cutaneous side effects, vasculitis
Introduction
Erlotinib is a targeted anticancer therapy with selective inhibitory activity for tyrosine kinase of the epidermal growth factor receptor (EGFR) (1). This potent drug is used for treating EGFR mutation positive lung cancer in advanced stage as well as for other malignancies, such as hepatic or pancreatic malignant tumors (1). Cutaneous side effects of erlotinib, such as papulopustular rash, xerosis, pruritus, eczema craquele, rosacea like dermatitis or paronychia, are well documented (2); however, the number of reports regarding cutaneous leukocytoclastic vasculitis (CLCV) are limited (3). We have found eight published cases of cutaneous vasculitis induced by erlotinib treatment.
Case report
In 2016, a 58-year old, 60 kg weight, non-smoking woman was diagnosed with advanced stage of lung adenocarcinoma and brain metastases. She was treated at the Oncology Clinic with radiotherapy for the brain and lung lesions, and then received multiple cycles of platinum-based chemotherapy. Due to worsening evolution and the new brain metastases, erlotinib monotherapy was initiated with 150 mg/day dose. After 8 months of initiating the treatment without any complications, the patient was consulted at the Dermatology Department for the developing of multiple palpable round-ovular purpuric lesions, erosions and ulcerations which appeared bilaterally on the lower legs, and dorsal on the forearm. Moderate xerosis of skin was also found, especially on the lower limbs area. No periungual clinical signs were found (Figs. 1 and 2). The lesions were between 3 mm and 1.5 cm in size, gradually increased in number, became more distributed and were associated with moderate pruritus and pain. The patient denied having a fever, abdominal pain, arthralgia or other relevant subjective symptoms. The presumptive clinical diagnosis of vasculitis was made. Laboratory results showed moderately elevated inflammatory analyses, elevated transaminase levels and discrete anemia. The number of eosinophils was within normal range. The renal function was unimpaired, and the results of urinalysis were in normal limits. No other clinical signs or symptoms and laboratory findings possibly related to an infection or to inflammatory diseases were noted. For an accurate diagnosis, it is recommended to perform the lymphoblastic transformation test to erlotinib, but unfortunately, it was not possible. Skin biopsy was performed. The histopathology revealed a dense perivascular neutrophilic infiltration, fibrinoid necrosis of the vessel walls, leukocytoclastic and red blood cell extravasation, confirming the diagnosis of cutaneous leukocytoclastic vasculitis (Figs. 3 and 4). The appearance of cutaneous vasculitis was attributed to erlotinib toxicity. The administration of the drug was discontinued and oral prednisolone treatment was introduced at 1 mg/kg body weight dose for two weeks, decreasing the dose with 5 mg, at every 3 days. The treatment was combined with topical potent steroid and antibiotic therapy used once, daily. The lesions cleared within 7 weeks without recurrence. The treatment with erlotinib was restarted after 14 days with a lower dose of 100 mg/day, based on the literature data (4). The skin lesions have not recurred. Unfortunately, the evolution of the metastatic lung cancer was unfavorable. At 3 months after vasculitis healing, the patient died due to the complications of new metastases that occurred.
Figure 1.
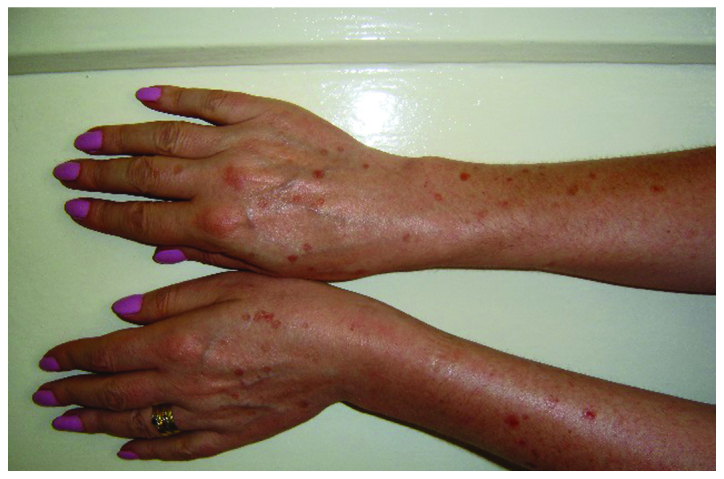
Clinical aspect dorsal on the forearm. Multiple palpable round-ovular purpuric lesions, erosions and ulcerations.
Figure 2.
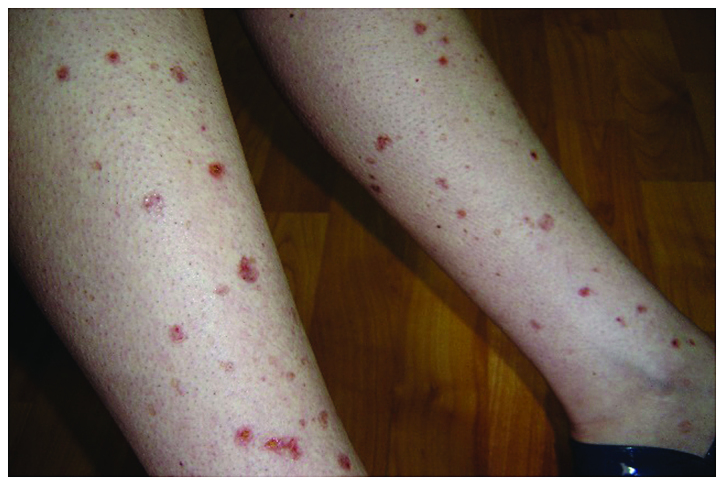
Clinical aspect on the lower legs. Multiple palpable round-ovular purpuric lesions, erosions and ulcerations.
Figure 3.
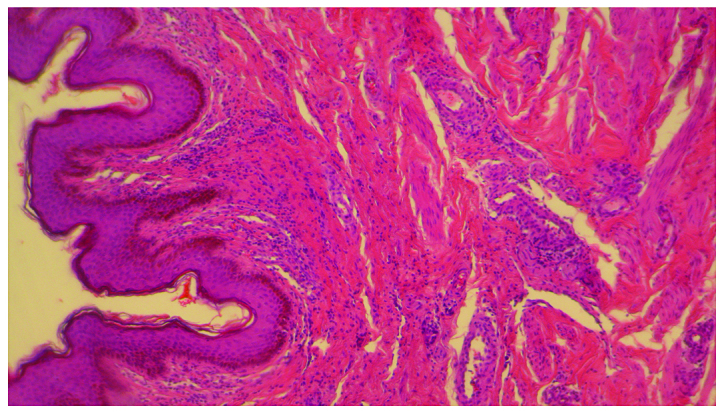
Leukocytoclastic and red blood cell extravasation, in the dermis. Hematoxylin and eosin staining; magnification, ×10.
Figure 4.
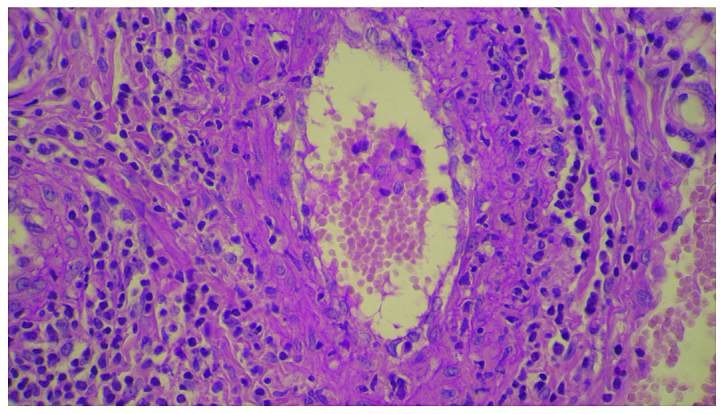
Perivascular neutrophilic infiltration, fibrinoid necrosis of the vessel walls. Hematoxylin and eosin staining; magnification, ×40.
The Ethics approval was obtained from the Commission of the Mures County Clinical Hospital and the Ethics Committee for Research of the University of Medicine and Pharmacy of Tirgu Mures (approval nos. 1537/2016 and 24/2016, respectively), and written informed consent was obtained from the patient.
Discussion
A thorough review of the literature was performed using international database search. Available case reports and current review articles were investigated to provide up-to-date information about vasculitis induced by erlotinib treatment. Cutaneous side effects of protein kinase inhibitors, such as maculopapular rash, hand-foot syndrome, pruritus, bullous dermatitis, target like purpura, xerosis and vasculitis, are well documented (3,5,6). Faye et al in 2013 analyzing ninety-four cases of patients treated with protein kinase inhibitors, showed that sorafenib was responsible in 40% of the cases for serious cutaneous adverse reactions, followed by erlotinib in 25.2% of the cases (2). Regarding the appearance of vasculitis induced by these drugs, only in one case erlotinib treatment was found responsible. Zhu et al in 2018 reported on different adverse cutaneous reactions associated with erlotinib treatment at 20 Chinese patients with cancer. None of them suffered of cutaneous vasculitis (7). Cutaneous vasculitis is a well-recognized side effect of many common drugs including penicillin, sulfonamides, thiazides and oral contraceptives (10–15% of vasculitis). Other new anticancer targeted therapies have been found to induce vasculitis, as gefitinib, sorafenib, sunitinib, bortezomib and everolimus (1,8,9). We searched PubMed/MEDLINE, Google Scholar, and Web of Science databases and found eight published cases of cutaneous vasculitis induced by erlotinib treatment (1,3,4,10–14), one of the authors presented two clinical cases (4). Erlotinib treatment was indicated in six cases of lung carcinoma, in two cases of hepatic and in one case of pancreatic malignancy. In all cases the clinical aspect was of vasculitis, excepting a case that appeared as Henoch-Schőnlein purpura (13). In seven cases the onset of vasculitis occured at the age over 70 years, in two cases the patients were under 55 years old. Two cases were males, and seven females. The onset of the vasculitis occurred between 14 days and 80 days after initiating erlotinib therapy, in our case the onset was after 240 days. In all the cases the treatment with erlotinib was stopped and restarted with a lower dose of 100 mg/day, on average after 14 days, or after the disappearance of the cutaneous lesions. In one case systemic treatment with prednisolone was introduced similarly to our case. The cutaneous vasculitis healed between 21 and 80 days. In all cases, systemic complications, such as renal failure, abdominal discomfort, and arthralgia, were not mentioned. Boeck et al was the first to report two cases of cutaneous vasculitis; in both, erlotinib treatment was stopped, and skin lesions improved with oral steroid therapy, similarly to our case (4). In all cases, after a short period of erlotinib withdrawal, the vasculitis did not appear and we continued with the administration of a reduced dose of erlotinib.
The mechanism of erlotinib-induced vasculitis remains unknown, and it is probably a dose-dependent phenomenon, as the reduced-dose of erlotinib re-administration did not produce vasculitis. Concerning the evolution of the basic disease and the efficacity of the lower dose of erlotinib, only Brandi mentioned that his patient died after 65 months after the occurrence of the vasculitis (1). In none of the cases has a new flare of vasculitis appeared. Several studies have reported so far a connection between the antitumor efficacy of EGFR inhibitors and cutaneous adverse effects (15–17). Jin et al reports that multiple cutaneous toxicities could indicate a good tumor response (14). In our case the fatal evolution, at 3 months after the cutaneous vasculitis healed, possibly indicating the inefficiency of erlotinib. The late onset of 240 days of the vasculitis in our case, and the presumed inefficiency of the drug lead to the speculation that the appearance of cutaneous vasculitis could be a worsening clinical marker of the tumor response.
Erlotinib induced cutaneous vasculitis are very rare. The mechanism of erlotinib-induced vasculitis remains unknown. Some consider that cutaneous vasculitis might reflect better anticancer efficacy. Our case suggests that cutaneous vasculitis could be a worsening clinical marker of the tumor response. This limited number of cases precludes any meaningful interpretation of data on erlotinib induced cutaneous vasculitis. Further investigations are needed to assess cutaneous vasculitis (1). We consider that a complete clinical examination of the skin at regular intervals is mandatory for all patients, no matter the type of cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GLF was responsible for the clinical management of patient, the evaluation and analysis of data, and contibuted to writing the manuscript. LF was responsible for the preparation of biopsy, the analysis of data, and the revision of manuscript for important intellectual content. The final version of the manuscript was approved by all authors.
Ethics approval and consent to participate
The Ethics approval was obtained from the Commission of the Mures County Clinic Hospital and the Ethics Committee for Research of the University of Medicine and Pharmacy Tirgu Mures (approval nos. 1537/2016 and 24/2016, respectively), and written informed consent was obtained from all the patients.
Patient consent for publication
Written informed consent of the patient were obtained.
Competing interests
The authors declare that they have no competing interests.
Authors' information
GLF is the Associate Professor of Dermatology, Department of Dermatology, Dermatology Clinic, University of Medicine and Pharmacy, Târgu Mureş, Romania.
References
- 1.Brandi G, Venturi M, Dika E, Maibach H, Patrizi A, Biasco G. Cutaneous leukocytoclastic vasculitis due to erlotinib: Just an adverse event or also a putative marker of drug efficacy? Cutan Ocul Toxicol. 2013;32:336–338. doi: 10.3109/15569527.2013.780179. [DOI] [PubMed] [Google Scholar]
- 2.Faye E, Bondon-Guitton E, Olivier-Abbal P, Montastruc JL. French Network of Regional Pharmacovigilance Centers: Spontaneous reporting of serious cutaneous reactions with protein kinase inhibitors. Eur J Clin Pharmacol. 2013;69:1819–1826. doi: 10.1007/s00228-013-1532-6. [DOI] [PubMed] [Google Scholar]
- 3.Sawada T, Suehiro M, Hiranuma O. Cutaneous leukocytoclastic vasculitis associated with erlotinib. Indian J Dermatol. 2016;61:238. doi: 10.4103/0019-5154.177793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeck S, Wollenberg A, Heinemann V. Leukocytoclastic vasculitis during treatment with the oral EGFR tyrosine kinase inhibitor erlotinib. Ann Oncol. 2007;18:1582–1583. doi: 10.1093/annonc/mdm420. [DOI] [PubMed] [Google Scholar]
- 5.Rezaković S, Paštar Z, Bukvić Mokos Z, Pavliša G, Kovačević S. Erlotinib-induced rosacea-like dermatitis. Acta Dermatovenerol Croat. 2016;24:65–69. [PubMed] [Google Scholar]
- 6.Rungtrakulchai R, Rerknimitr P. Erlotinib induced target-like purpura. Dermatol Online J. 2014;20:6. [PubMed] [Google Scholar]
- 7.Zhu H, Zhu Z, Huang W, Cheng X, He J, Xiong C, Han J. Common and uncommon adverse cutaneous reactions to erlotinib: A study of 20 Chinese patients with cancer. Cutan Ocul Toxicol. 2018;37:96–99. doi: 10.1080/15569527.2017.1355316. [DOI] [PubMed] [Google Scholar]
- 8.Panebianco M, Ragazzi M, Asensio NM, Pagano M, Gnoni R, Boni C. A case of necrotizing vasculitis with panniculitis, during sorafenib treatment for hepatocellular carcinoma, appeared in disease progression. J Gastrointest Oncol. 2014;5:E121–E124. doi: 10.3978/j.issn.2078-6891.2014.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karadimou A, Migou M, Economidi A, Stratigos A, Kittas C, Dimopoulos MA, Bamias A. Leukocytoclastic vasculitis after long-term treatment with sunitinib: A case report. Case Rep Oncol. 2011;4:385–391. doi: 10.1159/000331419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi Y, Ebi N, Yamaguchi O, Fukusho R, Sugimoto Y, Tsuruno K. A case of cutaneous vasculitis caused by erlotinib treatment and a review of literature. Nihon Kokyuki Gakkai Zasshi. 2011;49:663–666. (In Japanese) [PubMed] [Google Scholar]
- 11.Hakeem AH, Aziz SA, lone AR, Bhat GM, Wani B, Hussain I. Erlotinib induced vasculitis. JMSCR. 2015;3:3890–3895. [Google Scholar]
- 12.Su BA, Shen WL, Chang ST, Feng LY, Wu CJ, Feng YH. Successful rechallenge with reduced dose of erlotinib in a patient with lung adenocarcinoma who developed erlotinib-associated leukocytoclastic vasculitis: A case report. Oncol Lett. 2012;3:1280–1282. doi: 10.3892/ol.2012.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuba T, Nagata K, Shiotsu S, Okano A, Hatsuse M, Murakami S, Morihara K, Shimazaki C. Henoch-Schönlein purpura induced by erlotinib (Tarceva): A case report. Nihon Kokyuki Gakkai Zasshi. 2010;48:81–85. (In Japanese) [PubMed] [Google Scholar]
- 14.Jin F, Zhu H, Kong L, Yu J. A spectrum of cutaneous toxicities from erlotinib may be a robust clinical marker for non-small-cell lung therapy: A case report and literature review. Onco Targets Ther. 2015;8:943–946. doi: 10.2147/OTT.S83888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan DM, Song Y. Skin rash could predict the response to EGFR tyrosine kinase inhibitor and the prognosis for patients with non-small cell lung cancer: A systematic review and meta-analysis. PLoS One. 2013;8:e55128. doi: 10.1371/journal.pone.0055128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brănișteanu DE, Ianoşi SL, Dimitriu A, Stoleriu G, Oanţǎ A, Brănișteanu DC. Drug-induced Rowell syndrome, a rare and difficult to manage disease: A case report. Exp Ther Med. 2018;15:785–788. doi: 10.3892/etm.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gheorghe I, Tatu AL, Lupu I, Thamer O, Cotar AI, Pircalabioru GG, Popa M, Cristea VC, Lazar V, Chifiriuc MC. Molecular characterization of virulence and resistance features in Staphylococcus aureus clinical strains isolated from cutaneous lesions in patients with drug adverse reactions. Rom Biotechnol Lett. 2017;22:12321–12327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


