ABSTRACT
Toxoplasmosis causes substantial morbidity and mortality in the United States (US). Clinical manifestations to toxoplasmosis vary and there is limited information on incidence or treatment patterns in the US. Treatment pathways for pyrimethamine-based regimens and trimethoprim-sulfamethoxazole (TMP-SMX) for toxoplasmosis hospitalizations were investigated using the Vizient Health Systems inpatient and outpatient data. Between January 1st, 2011 and December 31st, 2017, 10,273 hospital visits from 4,736 unique patients received a primary or secondary ICD-9/ICD-10 diagnosis for toxoplasmosis. The projected annual hospital visits with a diagnosis of toxoplasmosis was 68,821, corresponding to a total annual incidence of 9,832 comprising ocular toxoplasmosis of 2,169, toxoplasmic encephalitis of 1,399, unspecified toxoplasmosis of 4,368, congenital toxoplasmosis of 381, multisystemic toxoplasmosis of 69 and other toxoplasmosis of 1,446. Only 16.3% of the study population received treatment with pyrimethamine-based regimens or TMP-SMX. Pyrimethamine-based regimens were used significantly more often than TMP-SMX in toxoplasmic encephalitis (88.7% vs 79.6%, p = 0.01), other toxoplasmosis (85.0% vs 79.2%, p = 0.04), and unspecified toxoplasmosis (87.6% vs 77.9%, p = 0.03) in hospitals with 300 beds or more. A significantly higher percentage of visits with TMP-SMX as first-line treatment switched to pyrimethamine-based regimens compared to visits initiated on pyrimethamine-based treatments (26.7% vs 4.1%, p < .001). Ocular toxoplasmosis patients receiving pyrimethamine-based therapy were more likely to be discharged home compared to TMP-SMC at rates of 72.4% and 55.2%, respectively. Our analysis of commercial insurance records suggest toxoplasmosis is undertreated. Overall, pyrimethamine-based regimens are favored over TMP-SMX, have higher rates of discharge home, and have lower switch rates.
KEYWORDS: Toxoplasma gondii, toxoplasmosis, incidence, toxoplasmosis encephalitis, cerebral toxoplasmosis, pyrimethamine, ICD-9 code, ICD-10 code, Charlson Comorbidity Index
Introduction
Toxoplasmosis is a disease resulting from infection with the parasite Toxoplasma gondii (T. gondii). There are three major clinical manifestations of the disease: 1) ocular toxoplasmosis in immunocompetent individuals is one of the most frequently identified etiologies of uveitis; 2) toxoplasmic encephalitis in immunocompromised patients as observed in patients with hematologic malignancies, organ transplant recipients, Acquired Immune Deficiency Syndrome (AIDS) and those receiving immunosuppressive therapy; and 3) congenital toxoplasmosis resulting from the acquisition of infection during pregnancy and vertical transmission that may lead to a variety of clinical presentations including chorioretinitis, blindness, psychomotor or mental retardation, encephalitis and hydrocephalus [1].
Toxoplasmosis is a neglected infection in the United States (US) [2] that has the potential to cause significant impact on health services. Seroprevalence was estimated to be 11.14% from 2011–2014 [3], and as many as 40 million people may be infected nationally [2]. Additionally, toxoplasmosis is the fourth leading cause of hospitalizations due to foodborne illnesses [4]. The incidence of toxoplasmosis was estimated to be 6,856 patients annually [5]. Annual case load estimates for the different clinical manifestations of toxoplasmosis in the US have been derived from older studies: 4,839 for symptomatic ocular toxoplasmosis [6], and 2,700 for acquired toxoplasmosis-associated chorioretinitis circa 2006 [7]; 2,985 for HIV-associated toxoplasmosis hospitalization and 600 for non-HIV associated toxoplasmosis hospitalizations in 2008 [8]; 1,515 hospital stays that included an HIV diagnosis and toxoplasmosis in 2013 [9]; and 400–4,400 for congenital toxoplasmosis from studies in the 1970s and 1980s [10,11] or 340 circa 2006 [7]. Between 2000 to 2010, 789 toxoplasmosis deaths were identified in the US, of which 271 deaths were caused by toxoplasmic encephalitis, 71 deaths were caused by congenital toxoplasmosis, <5 deaths were caused by ocular toxoplasmosis, 22 deaths were caused by pulmonary toxoplasmosis, 112 deaths were caused by other organ involvement, and the remaining deaths were unspecified [12].
Toxoplasmosis caused annually the most disability adjusted life years (DALYs; 32,700) out of seven leading foodborne pathogens in the US [7]. Annual direct healthcare costs for toxoplasmosis using commercial claims data from 2006 and 2007 were estimated to be 8,889 hospitalizations each year amounting to a mean total cost per hospitalization of $44,705 and total hospitalization cost of $397,386,567 [13]. From 1988 through 1997, toxoplasmic encephalitis accounted for 21,504 hospitalizations with a mean estimate cost for an encephalitis-associated hospitalization in 1997 of $28,151 [14]. Total productivity losses from all toxoplasmosis deaths during 2000–2010 were estimated to be $814.5 million [12].
Toxoplasmosis is an infection with high patient burden and significant individual healthcare cost consequences. Considering the high seroprevalence rates, and comparatively low clinical diagnosis rates spread across many of the disease’s manifestations, there is a high likelihood for misdiagnosis and under diagnosis. To inform clinical practice and health sector planning in this area, we evaluated diagnosis rates and treatment pathways using a large administrative dataset from representative hospitals in the US to help answer these questions.
Methods
Data source
This study was a retrospective analysis of de-identified inpatient and hospital-based outpatient data using Vizient Health System Data. The Vizient Health System Data includes administrative claims and detailed billing data for approximately 400 hospitals in the US. This all-payer database represents 41 states and includes more than 50 million annual visits from academic and non-academic facilities. Data are extracted from hospital billing systems approximately 30 days post discharge and are cleansed, de-identified, and uploaded to the analytic warehouse on a weekly basis. All data used in this analysis were statistically de-identified to conform with the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Population and cohort identification
The study population consisted of inpatient and outpatient visits discharged between 1 January 2011 and 31 December 2017 with a diagnosis of toxoplasmosis. Toxoplasmosis was identified from either primary or secondary International Classification of Disease (ICD) version 9 codes 130.x and version 10 codes B58.x and P37.1 (Supplemental Table 1). Types of toxoplasmosis were further defined using ICD codes except for congenital toxoplasmosis which was defined using either ICD-10 codes indicating congenital toxoplasmosis or patient age less than three years. Congenital toxoplasmosis was not specified in the ICD-9 coding system. Other toxoplasmosis included toxoplasmic myocarditis, toxoplasmic pneumonitis, toxoplasmic hepatitis, and toxoplasmosis of other specified sites. Following the transition from ICD-9 to ICD-10 diagnostic codes, the number of visits for unspecified toxoplasmosis, other toxoplasmosis and multisystemic toxoplasmosis dropped from 48.6% to 38.6%, 17.7% to 6.7% and 0.9% to 0.2%, respectively. Conversely, the number of visits for toxoplasmic encephalitis, ocular toxoplasmosis and congenital toxoplasmosis increased from 12.6% to 20.8%, 18.2% to 28.0% and 2.0% to 5.7% respectively. Visits were classified as multisystemic toxoplasmosis if they included the ICD-9 code for multisystemic or if they contained more than one type of toxoplasmosis (e.g. both ocular toxoplasmosis and toxoplasmic encephalitis). A person who was admitted to the hospital multiple times was counted each time as a separate discharge from the hospital.
The only two treatments considered for analysis were pyrimethamine based (pyrimethamine in combination with another treatment, e.g. pyrimethamine plus sulfadiazine) and trimethoprim-sulfamethoxazole (TMP-SMX). Treatments were identified using brand and generic names, e.g. Bactrim for TMP-SMX and Daraprim for pyrimethamine, as search criteria. Treatment cohorts were identified based on the first treatment administered during the visit. Visits with both treatments initiated simultaneously were excluded from all analyses comparing treatments (N = 416) as we were unable to determine if patients who received both treatments simultaneously were being treated for different indications such as infection.
Variable definitions
Patient comorbidities were assessed with the Charlson Comorbidity Index (CCI) in addition to history of transplant. The CCI uses patient comorbidities to predict short-term and long-term mortality.3 History of transplant was identified using ICD diagnostic codes indicating history of transplant as well as evaluation of ICD procedure codes for transplants occurring in prior visits. Payers were identified from detailed insurance plans and classified as Medicare, Medicaid, Commercial, Other, or Missing/Unknown. Other payers included insurance plans that did not fall into the remaining categories such as self-pay, Champus/Tricare, and specialty insurance. Insurance plans that were not provided or did not provide enough detail for classification were categorized as Missing/Unknown. Geographic regions were defined based on the US Census Bureau regions using the hospital state to map to regions. Toxoplasmosis treatment was defined as administration of either pyrimethamine-based or TMP-SMX regimens in the hospital setting. Monitoring for toxoplasmosis was defined as visits without treatment but having IgG, IgM, or IgA laboratory testing. Day of treatment initiation and days of treatment were identified based on billing service day. Days between visits and days between treatments were calculated using admission and discharge days. Re-treatment was defined as the number of days between pyrimethamine-based or TMP-SMX treatment, and a subsequent treatment, within 365 days. Switching was defined as starting a different treatment on the last day or day following the last day of an existing treatment. If treatments overlapped (e.g. both started on day 1 and one went to day 5 and one to day 7) they were considered to treat different indications.
Estimates of annual rates in the United States
Hospitals were classified into strata based on geographic region and number of beds in the Vizient Health System Database as well as for all community-based hospitals from the American Hospital Association Annual Survey Database™. Monthly projection weights were created for each hospital separately for inpatient and outpatient visits based on the proportion of national discharges compared to the Vizient data for each strata in a process similar to that described previously [9]. Hospital projection weights were applied to the study sample to generate national estimates.
Statistical analysis
Counts and percentages were used to report and compare categorical variables (e.g. counts by geographic region, HIV status, type of toxoplasmosis, switching) while mean, and standard deviation were used for continuous variables (e.g. patient age, CCI, days between visits, days between treatments, length of treatment). Chi-square tests were used to analyze differences between treatment cohorts for categorical variables with Fisher’s exact tests used for low cell count distributions. For continuous variables, t-tests were used to analyze differences between treatment cohorts with Mann-Whitney tests for non-normal distributions. All statistical tests were conducted using SAS version 9.4 (SAS Institute, Cary NC). Alpha was set at 0.05 for tests of significance.
Results
Study population and annualized hospitalization rates
The study population consisted of 10,273 hospital visits from 4,736 unique patients with either a primary or secondary ICD-9/ICD-10 diagnosis code for toxoplasmosis out of a total of 90 million unique patients and 389 million hospital visits. Toxoplasmosis was coded as a secondary diagnosis for 70% of visits in the study population. Visits were classified as 45.7% unspecified toxoplasmosis, 21.1% ocular toxoplasmosis, 15.0% toxoplasmic encephalitis, 14.5% other toxoplasmosis, 3.0% congenital toxoplasmosis, and 0.7% multisystemic toxoplasmosis. The projected national hospital visits with a diagnosis of toxoplasmosis was 68,821 visits over the study period, corresponding to an annual incidence of 9,832. Estimated annual incidences were: 2,169 visits for ocular toxoplasmosis; 1,399 visits for toxoplasmic encephalitis; 4,368 visits for unspecified toxoplasmosis; 381 visits for congenital toxoplasmosis; 69 visits for multisystemic toxoplasmosis; and 1,446 visits for other toxoplasmosis.
Patient demographics: age, gender and comorbidities
Table 1 compares patient demographics in the study population by type of toxoplasmosis. Patients in the study population ranged in age from newborns to over 90 years with a mean age of 46 years. Males tended to be more prevalent across different types of toxoplasmosis representing 54.3% of the study population. Thirty-nine percent of the study population were infected with human immunodeficiency virus (HIV). Rates of HIV were highest in toxoplasmic encephalitis (75.5%), other toxoplasmosis (69.6%), and multisystemic toxoplasmosis (52.9%). (Table 1) There was a history of transplantation in 18.0% of the study population: 34.9% in unspecified toxoplasmosis, 20.0% in multisystemic toxoplasmosis, 4.0% in toxoplasmic encephalitis, 3.9% in ocular toxoplasmosis, 3.6% in other toxoplasmosis, and 0% in congenital toxoplasmosis. The CCI varied in the different types of toxoplasmosis with the lowest scores in congenital toxoplasmosis (0.9 ± 2.0) and ocular toxoplasmosis (1.8 ± 3.0) and the highest scores in other toxoplasmosis (5.8 ± 3.5) and toxoplasmic encephalitis (5.9 ± 3.2). Other common comorbidities in the study population included diabetes (23.5%), chronic pulmonary disease (20.2%), renal disease (12.8%), malignancy (10.9%), and cerebrovascular disease (10.1%).
Table 1.
Patient demographics.
| Congenital |
Multisystemic |
Ocular |
Other |
Toxoplasmic Encephalitis |
Unspecified |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visits | % | Visits | % | Visits | % | Visits | % | Visits | % | Visits | % | |
| Gender | ||||||||||||
| Female | 207 | 66.1% | 29 | 41.4% | 1,079 | 49.8% | 611 | 41.0% | 540 | 35.1% | 2,217 | 47.2% |
| Male | 106 | 33.9% | 41 | 58.6% | 1,081 | 49.9% | 877 | 58.8% | 997 | 64.9% | 2,472 | 52.6% |
| Missing/Unknown | 0 | 0.0% | 0 | 0.0% | 5 | 0.2% | 3 | 0.2% | 0 | 0.0% | 8 | 0.2% |
| Age Group | ||||||||||||
| <18 years | 289 | 92.3% | 2 | 2.9% | 143 | 6.6% | 25 | 1.7% | 24 | 1.6% | 92 | 2.0% |
| 18–29 years | 8 | 2.6% | 8 | 11.4% | 287 | 13.3% | 128 | 8.6% | 107 | 7.0% | 576 | 12.3% |
| 30–39 years | 7 | 2.2% | 18 | 25.7% | 355 | 16.4% | 383 | 25.7% | 349 | 22.7% | 939 | 20.0% |
| 40–49 years | 3 | 1.0% | 7 | 10.0% | 263 | 12.1% | 437 | 29.3% | 562 | 36.6% | 906 | 19.3% |
| 50–59 years | 1 | 0.3% | 24 | 34.3% | 305 | 14.1% | 274 | 18.4% | 356 | 23.2% | 820 | 17.5% |
| 60–69 years | 1 | 0.3% | 3 | 4.3% | 355 | 16.4% | 143 | 9.6% | 90 | 5.9% | 911 | 19.4% |
| 70–79 years | 4 | 1.3% | 6 | 8.6% | 282 | 13.0% | 74 | 5.0% | 38 | 2.5% | 385 | 8.2% |
| 80–89 years | 0 | 0.0% | 1 | 1.4% | 159 | 7.3% | 23 | 1.5% | 8 | 0.5% | 52 | 1.1% |
| ≥90 years | 0 | 0.0% | 1 | 1.4% | 13 | 0.6% | 3 | 0.2% | 3 | 0.2% | 12 | 0.3% |
| Missing/Unknown | 0.0% | 3 | 0.1% | 1 | 0.1% | 4 | 0.1% | |||||
| Age in Years (Mean±SD) | 4.1 | 12.1 | 46.5 | 16.5 | 49.5 | 20.7 | 45.4 | 14.3 | 44.8 | 12.0 | 47.9 | 16.3 |
| Comorbidities | ||||||||||||
| History of transplant | 0 | 0.0% | 14 | 20.0% | 85 | 3.9% | 53 | 3.6% | 61 | 4.0% | 1,641 | 34.9% |
| Human Immunodeficiency Virus | 40 | 12.8% | 37 | 52.9% | 195 | 9.0% | 1,037 | 69.6% | 1,161 | 75.5% | 1,350 | 28.7% |
| Myocardial infarction | 0 | 0.0% | 6 | 8.6% | 96 | 4.4% | 35 | 2.3% | 16 | 1.0% | 196 | 4.2% |
| Congestive heart failure | 1 | 0.3% | 13 | 18.6% | 133 | 6.1% | 59 | 4.0% | 47 | 3.1% | 541 | 11.5% |
| Peripheral vascular disease | 0 | 0.0% | 3 | 4.3% | 68 | 3.1% | 25 | 1.7% | 8 | 0.5% | 230 | 4.9% |
| Cerebrovascular disease | 10 | 3.2% | 8 | 11.4% | 154 | 7.1% | 178 | 11.9% | 180 | 11.7% | 510 | 10.9% |
| Dementia | 0 | 0.0% | 0 | 0.0% | 12 | 0.6% | 10 | 0.7% | 10 | 0.7% | 38 | 0.8% |
| Chronic pulmonary disease | 5 | 1.6% | 11 | 15.7% | 293 | 13.5% | 249 | 16.7% | 164 | 10.7% | 1,358 | 28.9% |
| Rheumatic disease | 0 | 0.0% | 2 | 2.9% | 25 | 1.2% | 24 | 1.6% | 30 | 2.0% | 211 | 4.5% |
| Peptic ulcer disease | 0 | 0.0% | 3 | 4.3% | 45 | 2.1% | 39 | 2.6% | 17 | 1.1% | 150 | 3.2% |
| Mild liver disease | 0 | 0.0% | 0 | 0.0% | 9 | 0.4% | 20 | 1.3% | 8 | 0.5% | 66 | 1.4% |
| Diabetes without chronic complication | 1 | 0.3% | 6 | 8.6% | 284 | 13.1% | 231 | 15.5% | 140 | 9.1% | 1,505 | 32.0% |
| Diabetes with chronic complication | 0 | 0.0% | 1 | 1.4% | 106 | 4.9% | 32 | 2.1% | 8 | 0.5% | 100 | 2.1% |
| Hemiplegia or paraplegia | 4 | 1.3% | 5 | 7.1% | 64 | 3.0% | 163 | 10.9% | 168 | 10.9% | 175 | 3.7% |
| Renal disease | 3 | 1.0% | 15 | 21.4% | 193 | 8.9% | 204 | 13.7% | 170 | 11.1% | 727 | 15.5% |
| Any malignancy | 4 | 1.3% | 18 | 25.7% | 207 | 9.6% | 197 | 13.2% | 209 | 13.6% | 488 | 10.4% |
| Moderate or severe liver disease | 0 | 0.0% | 0 | 0.0% | 5 | 0.2% | 9 | 0.6% | 6 | 0.4% | 43 | 0.9% |
| Metastatic solid tumor | 0 | 0.0% | 2 | 2.9% | 41 | 1.9% | 24 | 1.6% | 24 | 1.6% | 105 | 2.2% |
| Charlson Comorbidity Index (Mean±SD) | 0.9 | 2.0 | 5.3 | 3.2 | 1.8 | 3.0 | 5.8 | 3.5 | 5.9 | 3.2 | 3.9 | 3.4 |
| Discharge Destination | ||||||||||||
| Home | 189 | 60.4% | 44 | 62.9% | 1,757 | 81.2% | 981 | 65.8% | 1,150 | 74.8% | 4,094 | 87.2% |
| Home Health | 5 | 1.6% | 2 | 2.9% | 63 | 2.9% | 88 | 5.9% | 84 | 5.5% | 46 | 1.0% |
| Intermediate care/Skilled nursing/Long term care facility | 0 | 0.0% | 5 | 7.1% | 46 | 2.1% | 110 | 7.4% | 81 | 5.3% | 78 | 1.7% |
| Transfer to another facility | 5 | 1.6% | 1 | 1.4% | 11 | 0.5% | 22 | 1.5% | 18 | 1.2% | 31 | 0.7% |
| Expired | 0 | 0.0% | 2 | 2.9% | 9 | 0.4% | 45 | 3.0% | 32 | 2.1% | 19 | 0.4% |
| Rehabilitation | 1 | 0.3% | 1 | 1.4% | 2 | 0.1% | 27 | 1.8% | 23 | 1.5% | 12 | 0.3% |
| Left against medical advice | 0 | 0.0% | 0 | 0.0% | 7 | 0.3% | 28 | 1.9% | 9 | 0.6% | 18 | 0.4% |
| Other | 0 | 0.0% | 0 | 0.0% | 11 | 0.5% | 7 | 0.5% | 14 | 0.9% | 59 | 1.3% |
| Transfer to other type of facility | 5 | 1.6% | 2 | 2.9% | 5 | 0.2% | 13 | 0.9% | 9 | 0.6% | 10 | 0.2% |
| Hospice | 0 | 0.0% | 2 | 2.9% | 8 | 0.4% | 49 | 3.3% | 29 | 1.9% | 13 | 0.3% |
| Missing/Unknown | 108 | 34.5% | 11 | 15.7% | 246 | 11.4% | 121 | 8.1% | 88 | 5.7% | 317 | 6.7% |
| Payer | ||||||||||||
| Commercial | 73 | 23.3% | 11 | 15.7% | 615 | 28.4% | 215 | 14.4% | 242 | 15.7% | 815 | 17.4% |
| Medicaid | 142 | 45.4% | 19 | 27.1% | 224 | 10.3% | 412 | 27.6% | 441 | 28.7% | 701 | 14.9% |
| Medicare | 8 | 2.6% | 25 | 35.7% | 806 | 37.2% | 370 | 24.8% | 301 | 19.6% | 1,364 | 29.0% |
| Other | 8 | 2.6% | 4 | 5.7% | 260 | 12.0% | 153 | 10.3% | 197 | 12.8% | 348 | 7.4% |
| Unknown | 82 | 26.2% | 11 | 15.7% | 260 | 12.0% | 341 | 22.9% | 356 | 23.2% | 1,469 | 31.3% |
| Hospital Bed Size | ||||||||||||
| <100 Beds | 43 | 13.7% | 0 | 0.0% | 61 | 2.8% | 52 | 3.5% | 24 | 1.6% | 96 | 2.0% |
| 100–199 Beds | 17 | 5.4% | 4 | 5.7% | 212 | 9.8% | 119 | 8.0% | 78 | 5.1% | 285 | 6.1% |
| 200–299 Beds | 22 | 7.0% | 1 | 1.4% | 139 | 6.4% | 88 | 5.9% | 47 | 3.1% | 193 | 4.1% |
| 300–499 Beds | 63 | 20.1% | 18 | 25.7% | 680 | 31.4% | 517 | 34.7% | 376 | 24.5% | 965 | 20.5% |
| ≥500 Beds | 168 | 53.7% | 47 | 67.1% | 1,073 | 49.6% | 715 | 48.0% | 1,012 | 65.8% | 3,158 | 67.2% |
| Hospital Teaching Status | ||||||||||||
| Non-teaching | 79 | 25.2% | 10 | 14.3% | 427 | 19.7% | 401 | 26.9% | 218 | 14.2% | 667 | 14.2% |
| Teaching | 224 | 71.6% | 60 | 85.7% | 1,738 | 80.3% | 1,090 | 73.1% | 1,319 | 85.8% | 4,030 | 85.8% |
Hospital characteristics: size, type, geographic distribution
Toxoplasmosis visits in the study population were more prevalent in large, teaching hospitals regardless of the type of toxoplasmosis: 85.7% in facilities with 300 beds or more and 82.4% in teaching facilities (Table 1). Notably, there appeared to be more visits with congenital toxoplasmosis in smaller hospitals (<100 beds).
A comparison of the geographic distribution by US census region for the study population and the geographic distribution of the data source is shown in Figure 1. Toxoplasmosis visits were more prevalent in the South Atlantic and West South-Central regions. Comparison of the study population distribution to the distribution of the source data shows that the skew to these regions is driven by toxoplasmosis rather than by the hospitals that are included in the data source.
Figure 1.
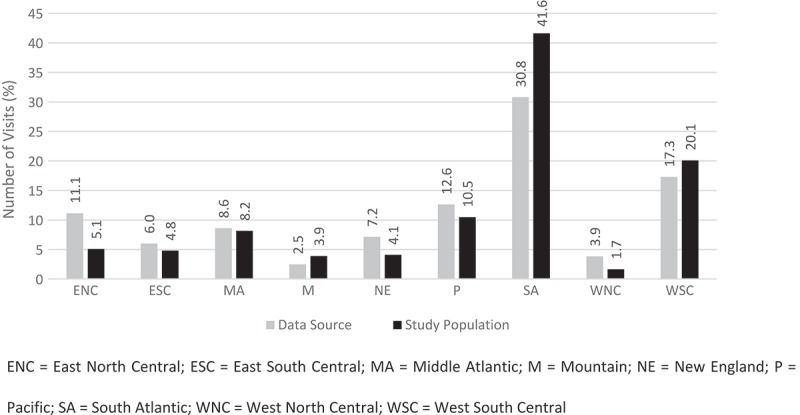
Geographic distribution of toxoplasmosis in the United States.
Hospital utilization: frequency of visits, discharge destination, and payer distribution
Two thirds (66.9%) of the study population had a single hospital visit during the study period. Additionally, 13.2% had two visits, 6.0% had three visits, 11.6% had four to ten visits, and 2.3% had more than 10 visits. Figure 2 compares the number of hospital visits by type of toxoplasmosis for patients with and without HIV. Patients with HIV infection had comparable number of visits to non-HIV patients for all types of toxoplasmosis except for congenital toxoplasmosis and multisystemic toxoplasmosis. In the study population, the mean duration between hospital visits was 124.9 ± 193.5 days and the mean duration between treatment visits was 130.7 ± 229.2 days. Figure 3 shows the mean duration between hospital visits and between treatments by type of toxoplasmosis. Discharge destinations and payers for the different types of toxoplasmosis are shown in Table 1. In each type of toxoplasmosis patients were primarily discharged to home.
Figure 2.
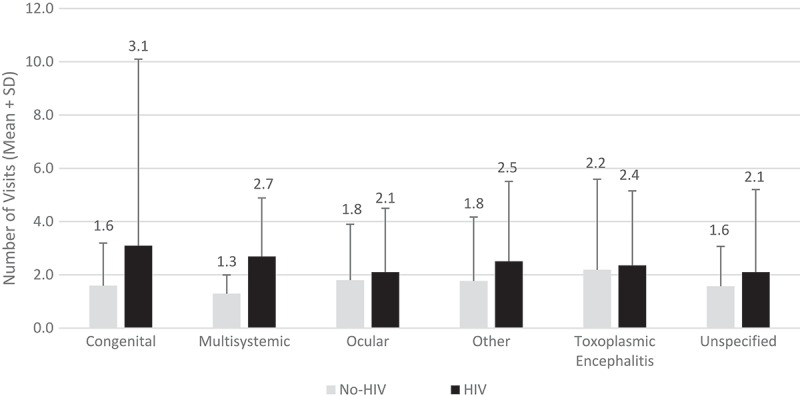
Inpatient and hospital-based outpatient visits by type of toxoplasmosis and HIV status.
Figure 3.
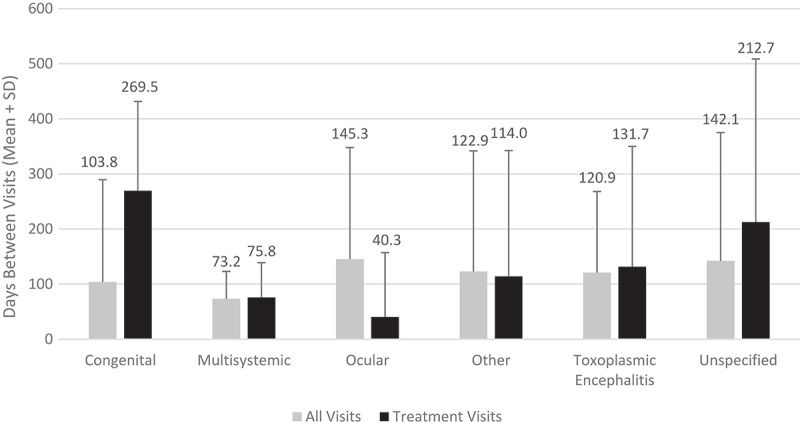
Days between inpatient and hospital-based outpatient visits by type of toxoplasmosis.
Treatment pathway for toxoplasmosis
The treatment population consisted of 1,671 hospital visits for 1,113 unique patients receiving pyrimethamine-based or TMP-SMX treatments. Of the total study population 83.7% did not receive either treatment. Treatment occurred in 51.5% of visits with a diagnosis of other toxoplasmosis, 37.5% of visits with multisystemic toxoplasmosis, 28.2% of visits with toxoplasmic encephalitis, 6.1% of visits with ocular toxoplasmosis, and 1.4% of visits with congenital toxoplasmosis (Figure 4). Monitoring-only visits were most prevalent in congenital toxoplasmosis and transplant associated toxoplasmosis (Figure 4). No treatment or monitoring occurred most often in ocular toxoplasmosis (Figure 4).
Figure 4.
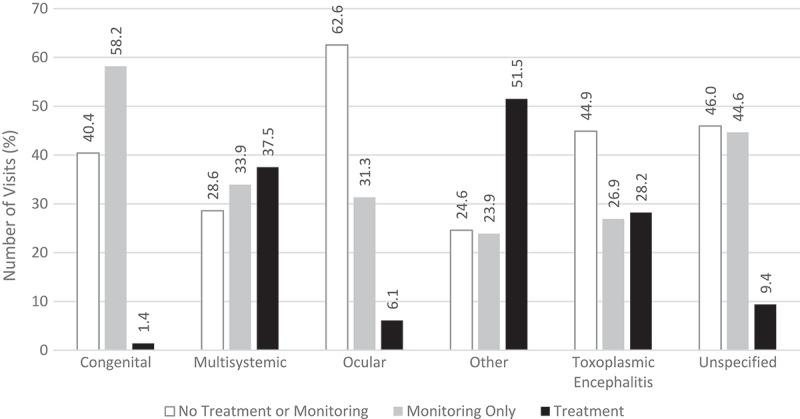
Treatment patterns in inpatient and hospital-based outpatient visits by type of toxoplasmosis.
Age and gender were similar for those receiving pyrimethamine-based regimens or TMP-SMX as first-line treatment in the different types of toxoplasmosis except for the unspecified group where patients with TMP-SMX were older and more likely to be male (Table 2). Treatment with pyrimethamine-based regimens were used significantly more often than TMP-SMX in other toxoplasmosis (85.0% vs 79.2%, p = 0.0401), toxoplasmic encephalitis (88.7% vs 79.6%, p = 0.0105) and unspecified toxoplasmosis (87.6% vs 77.9%, p = 0.0308) in hospitals with 300 beds or more, and to treat toxoplasmic encephalitis (80.4% vs 64.9%, p = 0.0004) and unspecified toxoplasmosis (77.5% vs 65.5%, p = 0.0263) in teaching hospitals.
Table 2.
Treatment regimens.
| Ocular |
Other |
TE |
Unspecified |
|||||
|---|---|---|---|---|---|---|---|---|
| TMP-SMX |
Pyrimethamine-based |
TMP-SMX |
Pyrimethamine-based |
TMP-SMX |
Pyrimethamine-based |
TMP-SMX |
Pyrimethamine-based |
|
| (N = 62) | (N = 58) | (N = 283) | (N = 454) | (N = 191) | (N = 229) | (N = 113) | (N = 169) | |
| Demographics | ||||||||
| Male | 64.5% | 63.8% | 63.6% | 63.4% | 63.9% | 61.1% | 62.0% | 50.9% |
| Age (Mean±SD) | 49.3 (17.0) | 48.3 (17.6) | 44.0 (12.8) | 44.0 (12.3) | 44.1 (11.6) | 44.7 (12.7) | 47.3 (13.9) | 43.0 (12.3) ** |
| Discharged home | 55.2% | 72.4% * | 57.6% | 55.5% | 50.3% | 47.6% | 63.7% | 65.1% |
| Payer | ||||||||
| Commercial | 14.5% | 8.6% | 15.6% | 11.0% | 18.9% | 14.0% | 18.6% | 17.8% |
| Medicaid | 22.6% | 27.6% | 30.4% | 38.9% | 36.1% | 33.2% | 25.7% | 27.8% |
| Medicare | 37.1% | 31.0% | 18.7% | 18.9% | 14.1% | 17.0% | 31.0% | 12.4% |
| Other | 6.5% | 13.8% | 8.1% | 8.8% | 11.0% | 12.2% | 7.1% | 14.8% |
| Missing/Unknown | 19.4% | 19.0% | 27.2% | 32.4% | 19.9% | 23.6% | 17.7% | 27.2% ** |
| Hospital Characteristics | ||||||||
| 300 beds or more | 85.5% | 86.2% | 79.2% | 85.0% * | 79.6% | 88.7% * | 77.9% | 87.6% * |
| Teaching facility | 72.6% | 72.4% | 70.7% | 70.9% | 64.9% | 80.4% *** | 65.5% | 77.5% * |
| 340B Status | 69.4% | 77.6% | 81.6% | 83.9% | 74.4% | 84.7% ** | 79.7% | 82.3% |
| Treatment | ||||||||
| Length of treatment (in-hospital) (Mean±SD) | 9.4 (15.7) | 6.4 (6.8) | 10.1 (14.6) | 8.5 (11.8) | 11.1 (11.7) | 11.0 (10.9) | 6.6 (8.1) | 5.9 (6.9) |
| Time to treatment initiation (days; Mean±SD) | 2.1 (3.0) | 3.0 (4.1) | 2.5 (3.8) | 2.5 (2.8) | 3.9 (7.5) | 2.9 (3.9) | 2.7 (3.0) | 2.5 (3.9) |
| Switch rates | 19.4% | 5.2% * | 39.2% | 2.2% *** | 23.6% | 6.1% *** | 18.6% | 6.5% ** |
| Days to re-treatment◊ (Mean±SD) | 36.8 (46.9) | 59.2 (44.9) | 28.0 (29.3) | 59.2 (58.3) * | 66.6 (64.1) | 73.5 (75.2) | 45.1 (67.4) | 84.9 (79.7) |
Amongst the population receiving treatment a significantly higher percentage of visits treated with TMP-SMX as first-line treatment (26.7%) switched to pyrimethamine-based regimens compared to visits initiated on pyrimethamine-based regimens (4.1%). This difference was consistent across the different types of toxoplasmosis: ocular toxoplasmosis, other toxoplasmosis, toxoplasmic encephalitis and unspecified toxoplasmosis (Table 2). Although duration of treatment with pyrimethamine-based regimens tended to be shorter than with TMP-SMX in all types toxoplasmosis these differences were not statistically significant (Table 2). No difference was seen in time to treatment initiation between treatments. Notably, 72.4% of patients with ocular toxoplasmosis were discharged home when treated with pyrimethamine compared to 55.2% treated with TMP-SMX (Table 2). Within a year, 24.2% of visits in the treatment population returned to hospital to receive additional treatment. The mean duration until re-treatment with pyrimethamine-based regimens was 22.4 days, 31.2 days, 6.9 days and 39.8 days longer compared to TMP-SMX for ocular toxoplasmosis, other toxoplasmosis, toxoplasmic encephalitis and unspecified toxoplasmosis respectively.
Discussion
Toxoplasmosis is a treatable condition, but an inability to appreciate the magnitude of the likely problem in the US presents a barrier to appropriate management of the disease and its manifestations [5]. This analysis using a large, all-payer national hospital administrative database offers insight into the prevalence of diagnosed toxoplasmosis, and its treatment, in the inpatient and hospital-based outpatient setting.
Annual incidences of toxoplasmosis in the US derived from this study population are of a similar magnitude to those from previous studies [5–8,10,11].Toxoplasmosis can present with a wide spectrum of clinical manifestations. Almost half of identified patients had diagnostic codes indicating unspecified toxoplasmosis making it impossible to accurately quantify disease manifestations. The prevalence of toxoplasmosis, specifically ocular toxoplasmosis, toxoplasmic encephalitis, unspecified toxoplasmosis and other toxoplasmosis remain high, especially in HIV patients, compared to other manifestations of the disease. However, any variations in magnitude from other studies might arise from different data sources: inclusion of patients without insurance or with non-traditional insurance services in this study that were not included in the Lykins et al (2016) analysis [5]; estimates for hospital visits with a specified diagnosis of toxoplasmosis in this study contrasts with the data analyzed in Jones [6] from patients surveyed in the National Health and Nutrition Examination Survey (NHANES 2018), and in Scallan et al [7] from public health surveillance; and that persons with toxoplasmosis could seek treatment at other types of facilities besides the hospital setting. The comparatively high frequency of eye disease relative to other types of toxoplasmosis may indicate a higher diagnosis rate of eye disease due to T. gondii or a tendency for patients to seek more care for ocular toxoplasmosis than for the other types. Also similar to other studies, toxoplasmosis was more common in males than females [8,14] more prevalent in the middle-age [8], distribution varied geographically [6,14], and associated with a variety of comorbidities [1,12,15–17].
Guidelines for the treatment of the three major manifestations of toxoplasmosis – toxoplasmic encephalitis, ocular toxoplasmosis and congenital toxoplasmosis – have been published [1,18–23]. The recommended first-line treatment is pyrimethamine plus sulfadiazine plus leucovorin, whereas TMP-SMX is considered an alternative regimen. In our analysis, toxoplasmosis appears to be undertreated with only 16.3% of the study population treated with either pyrimethamine-based regimens or TMP-SMX. Furthermore, treatment rates varied by type of toxoplasmosis. Pyrimethamine-based regimens were favored over TMP-SMX in toxoplasmic encephalitis, other toxoplasmosis, and unspecified toxoplasmosis, particularly in larger, teaching hospitals. The recommended length of acute treatment for toxoplasmosis is 4–6 weeks [1,18,21]. Although duration of treatment while in hospital in this study ranged from 5.9 days to 11.1 days, the duration of treatment for pyrimethamine-based regimens tended to be shorter than with TMP-SMX, and 72.4% of patients with ocular toxoplasmosis were discharged home when treated with pyrimethamine-based regimens compared to 55.2% treated with TMP-SMX.
Despite treatment efforts, a percentage of patients receiving therapy for toxoplasmic encephalitis and ocular toxoplasmosis will experience relapse. The rate of relapse associated with pyrimethamine-based maintenance therapy in patients with toxoplasmic encephalitis and HIV has been found to be approximately 11.1% in the post – highly active antiretroviral therapy (HAART) era [24]. By comparison, the relapse rate for TMP-SMX in the treatment of toxoplasmic encephalitis in HIV-infected adults was 16.4% when pre-HAART and post-HAART studies were combined [25]. The limited TMP-SMX studies and heterogeneity in trial data in the post-HAART era preclude presenting the post-HAART data independently. Furthermore, while this study did not directly measure relapse, days to re-treatment, an indirect measure of relapse, was longer with pyrimethamine-based regimens compared to TMP-SMX. Additionally, rates of switching treatments during the stay were significantly less for pyrimethamine-based regimens than for TMP-SMX in both toxoplasmic encephalitis and ocular toxoplasmosis. Similarly, switch rates in other toxoplasmosis and unspecified toxoplasmosis were less for pyrimethamine-based treatments than for TMP-SMX. Switching amongst treatments may result from several causes including treatment efficacy, patient tolerability, product availability, and treatment adherence. As described in this study, pyrimethamine-based regimens tended to result in a shorter duration of treatment, higher rates of discharge to the home, lower switch rates, and longer days to re-treatment which suggests a more favorable profile with pyrimethamine compared to TMP-SMX.
Multiple challenges exist when analyzing toxoplasmosis in an administrative database. ICD coding for toxoplasmosis is primarily for reimbursement purposes. Coding guidelines suggest that the primary diagnosis for a visit should be the most serious and/or resource-intensive condition while secondary diagnoses include all conditions that co-exist at the time of admission or develop subsequently that affect patient care [26]. However, in the study population, toxoplasmosis was coded as a secondary diagnosis in more than 70% of all visits. Since T. gondii infection is asymptomatic in the majority of immunocompetent patients [1], toxoplasmosis may be coded as a secondary diagnosis when there is no evidence of clinical or pathologic disease. Immunocompromised patients, such as those with HIV, with low CD4 counts (T-cell count) may receive prophylactic treatment for toxoplasmosis and may be maintained on treatment until the CD4 count is greater than 200 cells/mm3 [21]. Also, the classification of toxoplasmosis types for reimbursement may differ from clinical classification. There are multiple ICD diagnosis codes for toxoplasmosis including codes for generic terms such as ‘other toxoplasmosis’ and ‘unspecified toxoplasmosis’. Similar to the Lykins et al. [5] analysis more than 50% of visits were coded to these two generic classifications of toxoplasmosis rather than to codes identifying specific organ system manifestations. The transition from ICD version 9 to version 10 also introduced challenges for specifying type of toxoplasmosis as the categories changed between versions. However, the decrease in visits for unspecified and other toxoplasmosis following the transition from ICD-9 to ICD-10 coding suggest a closer reconciliation of reimbursement codes and clinical diagnosis.
There are several limitations of this study that should be noted. Administrative data like the Vizient health system database used for this analysis are primarily used to support the hospital billing process and do not contain clinical information such as lab results and patient vital signs. As such we were not able to discern active versus latent toxoplasmosis. A considerable number of patients coded to ‘unspecified toxoplasmosis’ indicates a potential lack of appropriate coding and offers no information about the symptoms and types of toxoplasmosis. Settings of care captured in this analysis are limited to inpatient hospitalizations and hospital-based outpatient visits and do not account for all settings of care required for patient management such as physician clinics. Patients may elect to seek treatment in another setting or at another provider not included in the dataset which may affect estimates as well as evaluations of visits across time. It is likely that our analysis underestimates prevalence of toxoplasmosis as well as its treatment. Despite these limitations, this is one of the first large scale studies to examine real-world utilization among patients diagnosed with toxoplasmosis in the hospital setting. The only other study of its kind in toxoplasmosis was that of Lykins et al. [5]. Both studies likely underestimated prevalence of toxoplasmosis due to limitations in source data. Increased awareness and reporting of the disease as well as its manifestations may provide more accurate assessment. As a treatable condition, better identification of the disease, better understanding of available treatments and creation of standard therapeutic and prophylactic treatment protocols will ultimately lead to a reduction in the serious morbidity and mortality associated with this disease.
Funding Statement
This work was supported by the Vyera Pharmaceuticals [2017].
Disclosure statement
The author RRBH works for Vyera which manufactures treatments for toxoplasmosis.
Supplementary material
Supplementary data can be accessed here.
References
- [1].Montoya JG, Boothroyd JC, Kovacs JA.. Toxoplasma gondii in Mandell Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed., Mandell GL, Bennett JE, Dolin R, editors. Churchill Philadelphia: Livingstone Elsevier; 2015.
- [2].Parasites - Toxoplasmosis (Toxoplasma infection): centers for Disease Control and Prevention (CDC). 2018. [cited 2018 August28]. Available from: https://www.cdc.gov/parasites/toxoplasmosis/index.html.
- [3].Jones JL, Kruszon-Moran D, Elder S, et al. Toxoplasma gondii infection in the United States, 2011–2014. Am J Trop Med Hyg. 2018February;98(2):551–557. PubMed PMID: 29260660; PubMed Central PMCID: PMCPMC5929212. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States–major pathogens. Emerg Infect Dis. 2011January;17(1):7–15. PubMed PMID: 21192848; PubMed Central PMCID: PMCPMC3375761. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lykins J, Wang K, Wheeler K, et al. Understanding toxoplasmosis in the United States through “Large Data” analyses. Clin Infect Dis. 2016;63(4):468–475. 01/11/received 04/16/accepted PubMed PMID: PMC4967610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jones JL, Holland GN.. Annual burden of ocular toxoplasmosis in the United States. Am J Trop Med Hyg. 2010;82(3):464–465. 11/05/received11/29/accepted PubMed PMID: PMC2829910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Scallan E, Hoekstra RM, Mahon BE, et al. An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect. 2015October;143(13):2795–2804. PubMed PMID: 25633631; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jones JL, Roberts JM. Toxoplasmosis hospitalizations in the United States, 2008, and trends, 1993–2008. Clin Infect Dis. 2012April;54(7):e58–61. PubMed PMID: 22267718; eng. [DOI] [PubMed] [Google Scholar]
- [9].Heslin KC, Elixhauser A. HIV hospital stays in the United States, 2006–2013; 2016. (Healthcare Cost and Utilization Project (HCUP) Statistical Briefs #206).
- [10].Stillwaggon E, Carrier CS, Sautter M, et al. Maternal serologic screening to prevent congenital toxoplasmosis: a decision-analytic economic model. PLoS Negl Trop Dis. 2011September;5(9):e1333 PubMed PMID: 21980546; PubMed Central PMCID: PMCPMC3181241. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Maldonado YA, Read JS. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics. 2017February;139(2). PubMed PMID: 28138010; eng. [DOI] [PubMed] [Google Scholar]
- [12].Cummings PL, Kuo T, Javanbakht M, et al. Trends, productivity losses, and associated medical conditions among toxoplasmosis deaths in the United States, 2000–2010. Am J Trop Med Hyg. 2014;91(5):959–964. 05/07/received 07/25/accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Collier SA, Stockman LJ, Hicks LA, et al. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012November;140(11):2003–2013. PubMed PMID: 22233584; PubMed Central PMCID: PMCPMC4629238. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Khetsuriani N, Holman RC, Anderson LJ. Burden of encephalitis-associated hospitalizations in the United States, 1988-1997. Clin Infect Dis. 2002July15;35(2):175–182. PubMed PMID: 12087524; eng. [DOI] [PubMed] [Google Scholar]
- [15].Del Grande C, Galli L, Schiavi E, et al. Is toxoplasma gondii a trigger of bipolar disorder? Pathogens. 2017January10;6(1). pii: E3. PubMed PMID: 28075410; PubMed Central PMCID: PMCPMC5371891. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Flegr J, Prandota J, Sovickova M, et al. Toxoplasmosis–a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PloS one. 2014;9(3):e90203 PubMed PMID: 24662942; PubMed Central PMCID: PMCPMC3963851. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bolduc P, Roder N, Colgate E, et al. Care of patients with HIV infection: medical complications and comorbidities. FP Essent. 2016April;443:16–22. PubMed PMID: 27092563; eng. [PubMed] [Google Scholar]
- [18].Parasites - Toxoplasmosis (Toxoplasma infection): Centers for Disease Control and Prevention: Resources for Health Professionals 2018. [cited2018October26]. Available from: https://www.cdc.gov/parasites/toxoplasmosis/health_professionals/index.html
- [19].de-la-Torre A, Stanford M, Curi A, et al. Therapy for ocular toxoplasmosis. Ocul Immunol Inflamm. 2011October;19(5):314–320. PubMed PMID: 21970662; eng. [DOI] [PubMed] [Google Scholar]
- [20].Montoya JG, Remington JS. Management of toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008August15;47(4):554–566. PubMed PMID: 18624630; eng. [DOI] [PubMed] [Google Scholar]
- [21].Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America 2018. [cited2018November30]. Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf
- [22].Dedicoat M, Livesley N. Management of toxoplasmic encephalitis in HIV-infected adults (with an emphasis on resource-poor settings). Cochrane Database Syst Rev. 2006July;19(3):Cd005420 PubMed PMID: 16856096; eng. [DOI] [PubMed] [Google Scholar]
- [23].Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004June12;363(9425):1965–1976. PubMed PMID: 15194258; eng. [DOI] [PubMed] [Google Scholar]
- [24].Connolly MP, Goodwin E, Schey C, et al. Toxoplasmic encephalitis relapse rates with pyrimethamine-based therapy: systematic review and meta-analysis. Pathog Glob Health. 2017February;111(1):31–44. PubMed PMID: 28090819; PubMed Central PMCID: PMCPMC5375610. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Connolly MP, Haitsma G, Hernandez AV, et al. Systematic review and meta-analysis of secondary prophylaxis for prevention of HIV-related toxoplasmic encephalitis relapse using trimethoprim-sulfamethoxazole. Pathog Glob Health. 2017September;111(6):327–331. PubMed PMID: 29052492; PubMed Central PMCID: PMCPMC5694860. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stati NCH, Medicaid CM. S DHH. ICD-10-CM official guidelines for coding and reporting - Fy 2018 (October 1, 2017 - September 30, 2018); 2017. (Section II).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


