Abstract
Objectives
The current study investigated baseline and longitudinal relationships between memory complaints, depressive symptoms, and cognition in older adults.
Method
Using the sample from the Personal Reminder Information and Social Management trial, we generated path models predicting self-rated memory complaints measured by the Memory Functioning Questionnaire (MFQ).
Results
Our baseline models showed that more depressive symptoms were associated with reporting more frequent forgetting incidents and a greater decline in memory function. The baseline models also revealed that higher scores in a latent cognitive function were associated with reporting a greater decline in memory functioning and a greater use of mnemonics. However, cognitive predictors did not mediate the baseline associations between the MFQ measures and depressive symptoms. Further, these predictors were not able to directly predict the 12-month MFQ measures over and above the baseline effects. Including personality traits (neuroticism and conscientiousness) did not significantly affect the models.
Discussion
Our results suggest that memory complaints about frequency of forgetting can be the most reliable indicator of depression risk among the four factors in the MFQ. We discuss theoretical implications for longitudinal relationships between memory complaints, depressive symptoms, and cognitive function in older adults.
Keywords: Depression, Cognitive function, Longitudinal change, Subjective memory
The link between subjective memory complaints and depressive symptoms in older adults has intrigued aging researchers for decades. In their seminal work, Kahn, Zarit, Hilbert, and Niederehe (1975) found that subjective memory complaints were closely related, not to actual performance, but to depressive symptoms. Since then, the relationship between subjective memory complaints and depressive symptoms has been well documented as one of the candidate explanations for a weak relationship between self-assessment of memory and actual memory performance (e.g., Crane, Bogner, Brown, & Gallo, 2007; O’Shea, Dotson, Fieo, Tsapanou, Zahodne, & Stern, 2016; West, Boatwright & Schleser, 1984; Zelinski & Gilewski, 2004).
Most of the previous studies have relied on cross-sectional data rather than longitudinal data. To address the issue, Pearman, Hertzog, and Gerstorf (2014) recently examined a longitudinal relationship between subjective memory complaints and depressive symptoms in older adults from the Berlin Aging Study (BASE). Their results support the notion that changes in memory complaints over time may be affected by depressive symptoms rather than monitoring of actual age-related memory changes. Hülür, Hertzog, Pearman, Ram, and Gerstorf (2014) assessed the longitudinal relationships not only at the between-person level but also at the within-person level using data from the Health and Retirement Study (HRS). Their findings particularly support that at the within-person level, fluctuation in subjective memory complaints are linked to fluctuations in memory performance and depressive symptoms. The older adults who showed more depressive symptoms or worse memory performance tended to complain more about their memory.
However, evidence of the longitudinal relationship needs to be strengthened because those findings have mainly relied on brief, global assessments of subjective memory measured by participants’ responses on a limited number of items (e.g., one or four items). This measure may not be sensitive enough to detect subtle changes in memory (Hülür et al., 2014) and would simply reflect older adults’ general beliefs about their memory rather than actual changes in memory (Pearman et al., 2014). To address these limitations, the current study was designed to assess the longitudinal relationship between subjective memory complaints and depressive symptoms with a more extensive subjective memory complaint scale: the Memory Functioning Questionnaire (MFQ; Gilewski, Zelinski, & Schaie, 1990).
MFQ, Depressive Symptoms, and Associated Factors
Gilewski et al. (1990) developed the MFQ to measure self-rated everyday memory functioning based on four different factors: Frequency of Forgetting (FF), Seriousness of Forgetting (SF), Retrospective Functioning (RF), and Mnemonic Usage (MU). Previous studies demonstrated that the MFQ factors were not only independent of chronological age, education, or self-reported health status (Gilewski et al., 1990) but also of each other (Lane & Zelinski, 2003). In fact, several studies have shown relationships between the MFQ factors, memory performance, and depressive symptoms. In a study by Zelinski, Gilewski, and Anthony-Bergstone (1990), FF and SF were the two best factors, not only accounting for variance in MFQ scores, but also in predicting clinical memory tests. However, those two factors did not predict a longitudinal change in memory performance on laboratory tasks over and above individual differences in general cognitive ability measures (Zelinski, Gilewski, & Schaie, 1993). The negative relationship between FF and depressive symptoms was reported in Zelinski et al. (1990), which indicated that individuals with more depressive symptoms tended to report more frequent forgetting incidents. Zelinski and Gilewski (2004) showed that depressive symptoms significantly accounted for the short version of FF scale over and above other predictors, such as personality traits, memory performance, and demographic variables. Particularly, Gilewski et al. (1990) suggested that the investigation of specific patterns between the MFQ factors and depressive symptoms might allow us to differentiate individuals experiencing mild memory deficit from those with depressive symptoms. For instance, SF and RF scores might reflect negative cognition based on depressive symptoms, whereas MU scores might be more associated with minor impairment of memory function because it would reflect actual attempts to cope with the impairment (Gilewski et al., 1990).
Previous studies have also demonstrated that neuroticism and conscientiousness are associated with subjective memory complaints (e.g., Lane & Zelinski, 2003; Pearman & Storandt, 2004, 2005; Reid & MacLullich, 2006). Lane and Zelinski (2003) particularly examined the longitudinal relationship between the two personality traits and each MFQ measure using hierarchical linear models. They tested a hypothesis that negative affectivity, such as neuroticism and depression, would be associated with MFQ measures. It was also expected that individuals with high conscientiousness would report more frequent use of mnemonics because they tend to habitually engage in more preventive health behaviors (Ingledew & Brunning, 1999). In their models, neuroticism explained only intercepts but not slopes in FF and SF, which suggests a stable relationship between neuroticism and baseline FF and SF. However, inconsistent with their expectation, conscientiousness did not account for variances in MU. Recently, Pearman and colleagues (2014) found that subjective memory complaints in older adults were more strongly associated with neuroticism than with actual memory. In the same study, depressive symptoms still significantly predicted the memory complaints after controlling for neuroticism.
The Present Study
Based on Gilewski et al.’s (1990) assumption and previous findings, our principal interest was to assess separately the predictive value of depressive symptoms and cognitive functions for each MFQ measure, rather than to consider the four MFQ scales as a single factor of memory complaints. For this purpose, we generated path models with a latent trait of cognitive function using the sample from the Personalized Reminder Information and Social Management System (PRISM) trial (Czaja et al., 2015; Czaja, Boot, Charness, Rogers, Sharit, 2017). PRISM was a software application originally designed for older adults to support social connectivity, prospective memory, leisure activities, access to resources and knowledge about topics (Czaja et al., 2015). The PRISM trial was conducted to evaluate the impact of access to PRISM on primary and secondary outcomes including: social isolation, loneliness, social support, social network size, quality of life, and perceived vulnerability, as well as secondary outcomes such computer proficiency and attitudes towards technology (Trial Registration: NCT01497613).
Among the PRISM outcome measures, in order to create a latent variable of cognitive function, we specifically selected three cognitive measures which have been widely used as an index of general cognitive abilities: (a) cognitive status; Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), (b) simple reaction time; Shipley Institute of Living Scale (Shipley; Zachary, 1986), and (c) processing speed; Digit Symbol Substitution (DSST; Wechsler, 1981) scores. In addition to the latent cognitive function variable, the Fuld Object Memory Evaluation (FOME; Fuld, 1981) score was selected as another cognitive predictor, given that previous studies suggest that it can be a valid measure to evaluate memory function of older adults independent from other memory assessment tools (for a brief review, see Chung & Ho, 2009). In contrast to other memory assessment techniques that solely rely on auditory or visual modalities, it has been suggested that FOME allows older adults to use multiple sensory systems involving touch to encode information, which offsets age-related visual and/or auditory impairments in older adults (Shulman et al., 2006). FOME also has a lack of sensitivity to educational level and reading skills (Wall, Deshpande, Macneill, & Lichtenberg, 1998).
Using those outcome measures from the PRISM trial, the current study first investigated baseline relationships between depressive symptoms and each MFQ measure. In the baseline models, we also investigated how the latent cognitive function and FOME would mediate the potential relationships between depressive symptoms and MFQ measures. We then assessed longitudinal effects of the predictors on the 12-month MFQ measures. In the 12-month models, we were particularly interested in whether the baseline predictors would significantly explain the 12-month MFQ measures over and above the baseline relationships. For both the baseline and 12-month models, we additionally tested a hypothesis that the personality traits (i.e., neuroticism and conscientiousness) would account for MFQ measures after controlling for the other predictors, given the significant associations between the personality traits and MFQ measures described above.
Consistent with previous studies, we expected that depressive symptoms would be significantly related to lower levels of self-perceived memory functioning, namely, high levels of self-rated memory complaints. Based upon Gilewski et al.’s (1990) original hypotheses, we specifically anticipated that reporting more depressive symptoms would be associated with reporting more frequent and serious forgetting incidents (FF and SF), and/or greater decline in memory ability relative to earlier in life (RF). Given that MU score might reflect one’s attempt to cope with minor impairment of memory function (Gilewski et al.’s (1990), we expected that the latent cognitive function and/or FOME might only be associated with MU rather than the other three MFQ measures.
Methods
Study Design
The PRISM trial was designed as a multisite randomized controlled trial that was conducted at Miami, FL; Tallahassee, FL; and Atlanta, GA. Participants were selected and randomized into the PRISM condition or the Binder condition after a telephone screening for basic eligibility (e.g., age, computer/Internet experience, language, living arrangements) and a baseline assessment at home. The participants in the PRISM condition received a computer equipped with the PRISM software whereas the participants in the Binder condition received the notebook binder. For a complete description of design of PRISM software and the Binder condition, see Czaja et al. (2015). There were three outcome assessments during the 12 months of the intervention period: baseline, 6 months, and 12 months.
Participants
In the PRISM trial, the sample was selected based on criteria to identify older adults at risk for social isolation. The sample consisted of old adults (65 years old or above) living alone in independent housing. They were not employed or volunteering more than 5 hr per week and did not spend more than 10 hr per week at a Senior Center or formal organization. They were required to have at least 20/60 vision, with or without correction, to be able to read at the 6th grade level, and to be English-speaking. Participants were excluded if they had used the Internet “independently and regularly” over the past 3 months, were blind or deaf, had a terminal illness or severe motor impairment, or were cognitively impaired (Mungus corrected score of < 26 on the Mini-Mental Status Examination (MMSE); Folstein et al., 1975). However, the sample included 11 participants who scored between 23 and 25 on the MMSE because they denied having any memory complaints and met all other criteria. The Fuld Object Memory Evaluation (FULD) was additionally used to assess their cognitive impairment and a review by a board certified clinical neuropsychologist, who was part of the research team, also permitted their participation in the current study.
After the prescreening and baseline assessment, the final sample included 300 participants (150 in each condition) who met the eligibility criteria and were still interested in participation. Participants were mostly women (78%) and were ethnically diverse (54% White). The sample ranged in age from 64 (one participant turned 65 in the time window established for scheduling of the baseline assessment) to 98 years (M = 76.15, SD = 7.4). The other details of sample have been published in Czaja et al. (2015).
Procedures and Contact Schedule
A telephone screening assessing the eligibility criteria described above was given to interested participants who contacted the study coordinator at each site. Those who were eligible and still interested in participation were then scheduled a baseline assessment at home with the coordinator. During the baseline assessment participants completed the measurement battery after providing informed consent. Participants were then randomly assigned to either of the study conditions. The baseline assessment was administered by an assessor who was trained and certified, using a standardized protocol.
The follow-up assessments occurred at 6 and 12 months postrandomization, followed by a brief telephone interview at 18 months. Participants assigned to the PRISM condition received $25 monetary compensation for each assessment and were allowed to keep the computer. However, participants in the Binder condition received $25 for the baseline and 6-month assessments and $100 for the 12-month assessment with an opportunity to receive basic computer training following the 12-month assessment in order to provide more equal compensation.
A certified assessor was blinded to treatment condition and the assessor administered the primary outcome measures at 6 and 12 months via a telephone interview. The Institutional Review Boards at the three sites approved the study protocol and all sites applied equivalent procedures and standardized protocols for screening, tracking, training, and contacting participants.
Measures
Czaja et al. (2015) provide a complete description of all outcome measures with an appropriate Cronbach’s α for each measure based on the baseline assessment. Among the outcome measures, the current study was only interested in measures described below. We did not include any measures from the 6-month assessment because our primary interest was to test baseline relationships between the measures and longitudinal effects on the 12-month MFQ scales. Further, most of the cognitive measures were not assessed at 6-months in the PRISM trial.
Subjective memory complaints
Subjective memory complaints were assessed with the MFQ (Gilewski et al., 1990), consisting of 64 items rated on 7-point Likert scale. Four factors comprise the MFQ: Frequency of Forgetting (FF), Seriousness of Forgetting (SF), Retrospective Functioning (RF), and Mnemonics Usage (MU). Lower scores reflect greater levels of memory complaints or more negatively perceived memory functioning, with more forgetting incidents, more serious forgetting incidents, decline in current memory ability compared to earlier in life, and more mnemonic usage. A factor analysis (Gilewski et al., 1990) and an evaluation of change patterns over 16–19 years (Lane & Zelinski, 2003) supported the assumption that the four factors are independent of each other. Particularly, the FF subscale has shown to account for the most variance in MFQ responses (Zelinski & Gilewski, 2004). There is also evidence supporting that MFQ factor scores reflect variance in ratings independent of chronological age, education, or self-reported health status (Gilewski et al., 1990).
Depressive symptoms
Depressive symptoms were assessed with the Center for Epidemiologic Studies Depression scale (CES-D; Radloff, 1977). The CES-D consists of 20 items assessing different feelings and emotional states of participants. The items were rated on a 3-point scale with higher scores indicating more depressive symptoms. The CES-D has generally been considered a good measure of depressive symptoms, although it cannot diagnose clinical depression on its own (Hülür et al., 2014).
Cognitive measures: latent cognitive function and FOME
We selected three general cognitive measures to generate a latent variable for cognitive functions: Mini-Mental State Examination (MMSE; Folstein, et al., 1975), Shipley Institute of Living Scale (Shipley; Zachary, 1986), and Digit Symbol Substitution (DSST; Wechsler, 1981) scores. The MMSE consists of a series of questions evaluating general cognitive functioning with a maximum score of 30, and has widely been used to screen individuals with cognitive impairment in research and clinical settings. The Shipley is composed of 40 items asking participants to indicate the one word out of four that is the same or nearly the same as a referent word. A higher score reflects greater verbal/vocabulary ability. The DSST consists of nine symbols matched with their corresponding numerical digits (1–9) and asks participants to match the symbols with their corresponding digits. The score is calculated based on the number of correct symbols within 90 s. A higher score indicates faster processing speed.
Fuld Object Memory Evaluation (FOME; Fuld, 1981) was developed as an assessment of episodic memory functions in older adults. In the FOME test, participants are asked to reach into a bag with 10 common objects and identify all of them by touch. Then, after participants are given a distraction task, such as saying words rapidly from a single category in 60 s, participants are asked to recall everything from the bag. Participants receive four more rounds to learn and recall the objects and are given a reminder about what they missed after each recall. In the PRISM trial, we used a slightly shorter version of the test (three learning and recall trials). The score is calculated based on the total number of correctly recalled objects across these trials.
Neuroticism and conscientiousness
Neuroticism and conscientiousness were assessed with the Ten-Item Personality Inventory (TIPI; Gosling, Rentfrow, & Swann, 2003). The TIPI consists of 10-items assessing Big 5 Personality Traits of extraversion, agreeableness, conscientiousness, openness, and neuroticism (Goldberg, 1992), and is widely used in research settings where personality is not the primary topic of interest.
Data Analysis and the Proposed Models
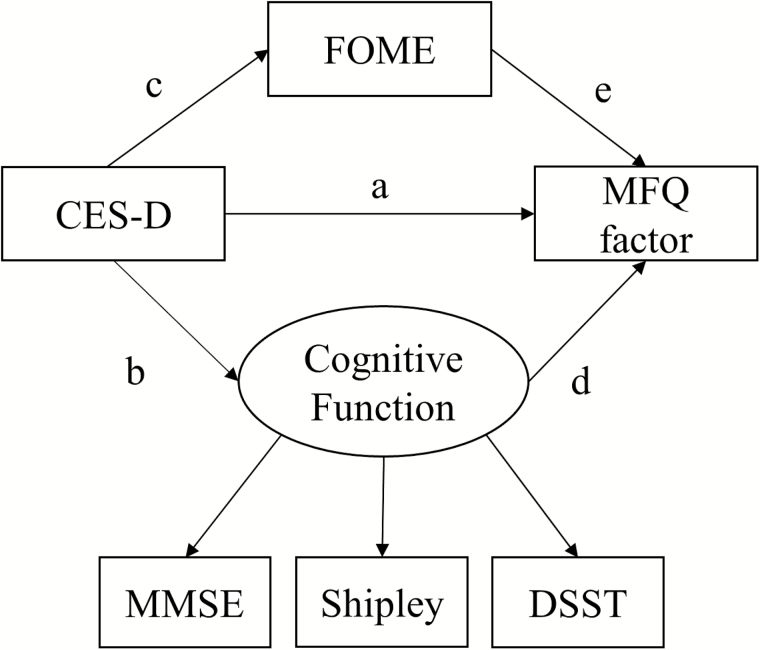
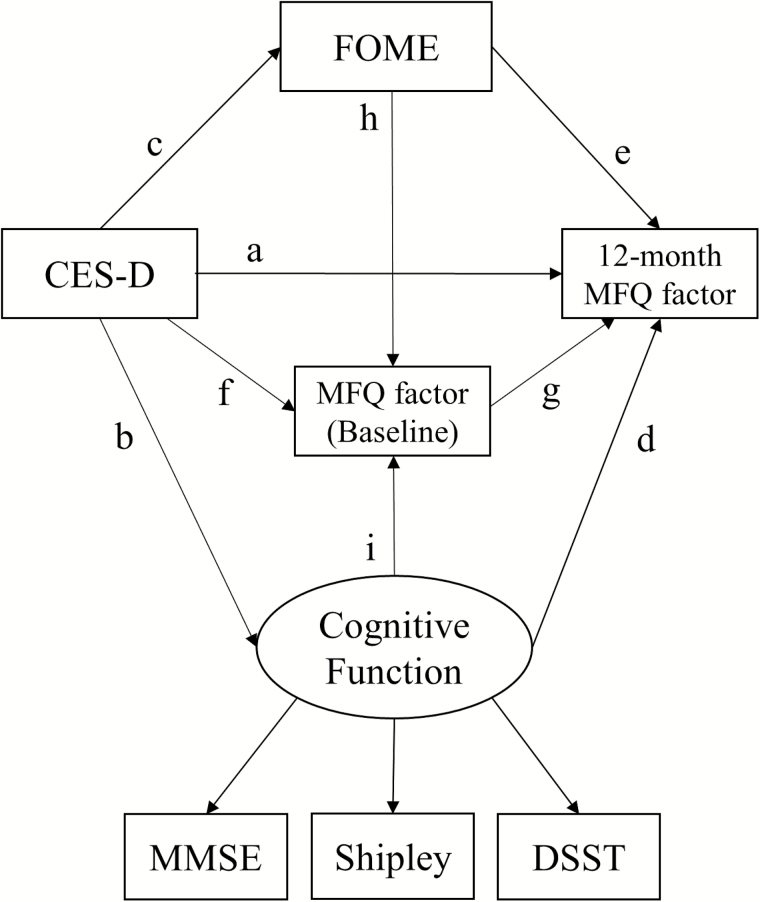
We used Amos (Version 23.0.0) to test the baseline and 12-month structural equation models. In the baseline models, we assessed the relationship between CES-D and MFQ measures and how this potential relationship is mediated by general cognitive abilities. For that purpose, as shown in Figure 1, the baseline models tested direct effects of the predictors (i.e., CES-D, the latent cognitive function, and FOME: path a, d, and e) as well as indirect effects of CES-D on each MFQ measure through the latent cognitive function (path b–d) and FOME (path c–e). Then, our principal interest in the 12-month models was to test longitudinal effects of the baseline predictors on each 12-month MFQ measure after controlling for the baseline relationships. For this purpose, as shown in Figure 2, the 12-month models tested direct effects of the baseline predictors (path a, d, e, and g) and indirect effects of CES-D on each 12-month MFQ via the latent cognitive function (path b–d), FOME (path c–e), and the corresponding baseline MFQ measure (path f–g). The indirect effects of the latent cognitive function and FOME via the baseline MFQ (path i–g and h–g) were additionally assessed. However, since we found little or no change in the predictors between baseline and at 12-months, we did not assess the effects of changes in the predictors on MFQ measures in the 12-month models.
Figure 1.
Baseline model used for predicting each MFQ factor from CES-D, the latent cognitive function variable, and FOME. CES-D = Center for Epidemiologic Studies Depression scale; DSST = Digit Symbol Substitution; FOME = Fuld Object Memory Evaluation; MFQ = Memory Functioning Questionnaire; MMSE = Mini-Mental State Examination.
Figure 2.
Twelve-month model used for predicting each Memory Functioning Questionnaire factor from CES-D, the latent cognitive function variable, and FOME after controlling for the baseline relationships. CES-D = Center for Epidemiologic Studies Depression scale; DSST = Digit Symbol Substitution; FOME = Fuld Object Memory Evaluation; MFQ = Memory Functioning Questionnaire; MMSE = Mini-Mental State Examination.
To evaluate the goodness of fit of the models, we used three indices: chi-square, the Comparative Fit Index (CFI), and the Root-Mean-Square Error of Approximation (RMSEA). In general, a good model fit is indicated by a non-significant chi-square value, although the chi-square value is more affected by large sample size (Bentler & Bonett, 1980). We also used a value of CFI ≥ .95 and a value of RMSEA ≤ .06 as criteria for good model fit (Hu & Bentler, 1999), although a cutoff for acceptable fit has been .09 and .08, respectively. We tested the proposed models separately for each MFQ factor. Means, SD, and correlations of variables are reported in Table 1.
Table 1.
Means, SD, and Correlations of Variables
| Correlations | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Mean (SD) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 1 CES-D | 11.11 (9.03) | — | ||||||||||||
| 2 MMSE | 28.09 (1.43) | −.12* | — | |||||||||||
| 3 SHIPLEY | 29.82 (6.31) | −.07 | .35** | — | ||||||||||
| 4 DSST | 34.95 (11.31) | −.00 | .32** | .35** | — | |||||||||
| 5 FOMEa | 22.76 (3.12) | .10 | .06 | −.10 | .06 | — | ||||||||
| 6 FF baseline | 4.98 (0.83) | −.33* | .16** | .17** | .06 | −.01 | — | |||||||
| 7 SF baseline | 4.61 (1.28) | −.09 | .10 | .13* | −.03 | −.09 | .37** | — | ||||||
| 8 RF baseline | 3.53 (1.12) | −.18** | −.06 | −.20** | −.13* | −.02 | .34** | .23** | — | |||||
| 9 MU baseline | 3.30 (1.27) | −.01 | −.09 | −.24** | −.27** | .03 | .01 | .05 | .01 | — | ||||
| 10 FF 12-month | 4.96 (0.89) | −.31** | .15* | .08 | .10 | .09 | .68** | .32** | .28** | −.02 | — | |||
| 11 SF 12-month | 4.53 (1.24) | −.12 | .02 | .05 | .01 | .10 | .24** | .32** | .09 | .00 | .36** | — | ||
| 12 RF 12-month | 3.36 (1.09) | −.04 | .03 | −.10 | .01 | −.00 | .15* | .15* | .36** | −.03 | .32** | .17* | — | |
| 13 MU 12-month | 3.05 (1.22) | .12 | −.09 | −.15* | −.18** | −.04 | −.07 | .01 | .02 | .59** | −.05 | .05 | −.02 | — |
Note: CES-D = Center for Epidemiologic Studies Depression scale; DSST = Digit Symbol Substitution; FF = Frequency of Forgetting; FOME = Fuld Object Memory Evaluation; MMSE = Mini-Mental State Examination; MU = Mnemonic Usage; RF = Retrospective Functioning; SF = Seriousness of Forgetting.
*p < .05. **p < .01. aBased on the total number of correctly recalled objects across three trials.
Results
Baseline Assessment
Frequency of forgetting
For the model predicting FF, the chi-square test was not statistically significant, χ2 (7) = 12.58, p = .08. The other two indices supported a good model fit (CFI = .958, RMSEA = .052). As reported in Table 2, the model showed FF was significantly linked to both CES-D (β = −.31, p < .001) and the latent cognitive function (β = .20, p < .01). The negative path coefficient between CES-D and FF indicated that the participants with higher CES-D scores reported more forgetting incidents (i.e., lower scores in FF). The positive path coefficient between the latent cognitive function and FF showed that the participants with lower cognitive function scores reported more forgetting incidents (i.e., higher scores in FF). The indirect path from CES-D to the latent cognitive function to FF was not statistically significant (p = .18), which suggests that the latent cognitive function did not mediate the relationship between CES-D and FF. FOME score did not significantly predict FF (p = .66). The indirect path from CES-D to FOME to FF was also not statistically significant (p = .68), suggesting that FOME did not mediate the relationship between CES-D and FF. The predictors accounted for 15% of variance in FF at baseline. When neuroticism and conscientiousness were added in the model, neither of them significantly accounted for FF (p = .45, .32, respectively).
Table 2.
Standardized Path Coefficients of the Main Predictors for Baseline MFQ Factors
| Predictor | Path (path in Figure 1) | Baseline MFQ factors | |||
|---|---|---|---|---|---|
| FF | SF | RF | MU | ||
| CES-D | Direct (a) | −.31** | −.07 | −.20** | .05 |
| via Cognitive function (b - d) | −.02 | .01 | .03 | .04 | |
| via FOME (c - e) | −.002 | .01 | −.0003 | .003 | |
| Cognitive function | Direct (d) | .20** | .12 | −.25** | −.36** |
| FOME | Direct (e) | .02 | −.08 | −.003 | .03 |
Note: CES-D = Center for Epidemiologic Studies Depression scale; FF = Frequency of Forgetting; FOME = Fuld Object Memory Evaluation; MFQ = Memory Functioning Questionnaire; MU = Mnemonic Usage; RF = Retrospective Functioning; SF = Seriousness of Forgetting.
*p < .05. **p < .01.
Seriousness of forgetting
For the model predicting SF, the chi-square test was statistically significant, χ2(7) = 16.30, p = .02. But the other two indices supported an acceptable model fit (CFI = .908, RMSEA = .067). The model showed that neither CES-D (p = .26) nor the latent cognitive function (p = .11) was significantly linked to SF (see Table 2). FOME score also did not significantly predict SF (p = .15). The model only explained 3% of variance in SF at baseline. When the personality traits were added in the model, neither neuroticism nor conscientiousness significantly explained variance in SF (p = .07, .81, respectively).
Retrospective functioning
For the model predicting RF, the chi-square test was marginally significant, χ2(7) = 13.80, p = .06. The other two indices supported an acceptable model fit (CFI = .94, RMSEA = .057). Table 2 shows that RF was significantly linked to both CES-D (β = −.20, p < .001) and the latent cognitive function (β = −.25, p = .001). Similar to the effects of CES-D on FF, the participants with higher CES-D scores reported greater decline in memory ability relative to earlier in life (i.e., lower scores in RF). However, contrary to the effects of latent cognitive function on FF, the participants with higher cognitive function scores reported worse decline in memory ability. The indirect path from CES-D to the latent cognitive function to RF was not significant (p = .17). FOME score did not significantly predict RF (p = .95). The model accounted for 9.5% of variance in RF at baseline. When the personality traits were added in the model, the path between conscientiousness and MU was marginally significant (β = .11, p = .06). But neuroticism did not significantly explain RF (p = .45).
Mnemonic usage
For the model predicting MU, the chi-square test was statistically significant, χ2(7) = 18.32, p = .01. But, the other two indices supported an acceptable model fit (CFI = .906, RMSEA = .074). The path analysis indicated that only the latent cognitive function significantly predicted MU (β = −.36, p < .001) (see Table 2). The negative path coefficient between the latent cognitive function and MU indicated that the participants with higher cognitive function scores reported more use of mnemonics. CES-D did not significantly predict MU (p = .35) and neither did FOME (p = .54). The model accounted for 13% of variance in MU at baseline. When the personality traits were added in the model, only the path between conscientiousness and MU was marginally significant (β = −.11, p = .06). However, neuroticism did not significantly account for MU (p = .85).
Predicting 12-Month MFQ Scales From Baseline Measures (CES-D, FOME, and the Latent Cognitive Function) Controlling for Baseline MFQ Scales
Frequency of Forgetting
For the model, the chi-square test was not statistically significant, χ2(9) = 13.93, p = .13. The other two indices supported a good model fit (CFI = .983, RMSEA = .043). As expected (see Table 3), a significant direct effect of the baseline FF on the 12-month FF was observed (β = .65, p < .001). Whereas there were significant direct effects of CES-D and the latent cognitive function on the baseline FF, only the direct effect of CES-D on the 12-month FF was marginally significant (β = −.09, p = .052). The latent cognitive function did not directly predict the 12-month FF after controlling for the baseline relationships (p = .44). The indirect path from CES-D to the baseline FF to the 12-month FF (β = −.20, p < .01) was significant, as well as the indirect path from the latent cognitive function to the baseline FF to 12-month FF (β = .13, p < .01). Neither of the indirect paths via the latent cognitive function and FOME to the 12-month FF was statistically significant (p = .48, .13, respectively). Consistent with the baseline model, the result suggests that both the latent cognitive function and FOME did not mediate the relationship between CES-D and the 12-month FF.
Table 3.
Standardized Path Coefficients of the Main Predictors for the 12-month MFQ Factors
| The 12-month MFQ factors | |||||
|---|---|---|---|---|---|
| Predictor | Path (path in Figure 2) | FF | SF | RF | MU |
| The corresponding | Direct (g) | .65** | .33** | .37** | .58** |
| baseline MFQ | |||||
| CES-D | Direct (a) | −.09 | −.10 | .03 | .10 |
| via Cognitive function (b–d) | −.01 | .001 | −.01 | .004 | |
| via FOME (c–e) | .01 | .01 | .002 | −.01 | |
| via The baseline MFQ (f – g) | −.20** | .02 | −.07** | −.03 | |
| Cognitive function | Direct (d) | .05 | −.01 | .05 | −.04 |
| via The baseline MFQ (i – g) | .13** | .04 | −.09** | −.21** | |
| FOME | Direct (e) | .14** | .14* | .02 | −.08 |
| via The baseline MFQ (h – g) | .01 | −.03 | −.001 | .02 | |
Note: CES-D = Center for Epidemiologic Studies Depression scale; FF = Frequency of Forgetting; FOME = Fuld Object Memory Evaluation; MFQ = Memory Functioning Questionnaire; MU = Mnemonic Usage; RF = Retrospective Functioning; SF = Seriousness of Forgetting.
*p < .05. **p < .01.
However, the direct path between FOME score and the 12-month FF was significant (β = .14, p = .002), although it did not directly predict the baseline FF. This indicates that the participants with lower FOME score at baseline reported more forgetting incidents at 12 months. The predictors accounted for 50% of variance in the 12-month FF. When the two personality traits were added in the model, the direct effect of conscientiousness on the 12-month FF was significant (β = .10, p = .04). The path between neuroticism and the 12-month FF was not significant (p = .31).
Seriousness of forgetting
For the model, the chi-square test was not statistically significant, χ2(9) = 16.61, p = .06. The other two indices supported an acceptable model fit (CFI = .939, RMSEA = .053). As reported in Table 3, a significant direct effect of the baseline SF on the 12-month SF was observed (β = .33, p < .001). But, neither CES-D (p = .11) nor the latent cognitive function (p = .94) directly explained the 12-month SF. Interestingly, FOME score directly predicted the 12-month SF (β = .14, p = .02), although it did not predict the baseline SF. None of the indirect paths was significant in this model. The model accounted for 13% of variance in the 12-month SF. When the personality traits were added, neither neuroticism nor conscientiousness significantly accounted for the 12-month SF (p = .77, .33, respectively).
Retrospective functioning
For the model, the chi-square test was not statistically significant, χ2(9) = 16.37, p = .06. The other two indices supported a satisfactory model fit (CFI = .948, RMSEA = .052). A significant direct effect of the baseline RF on the 12-month RF (β = .37, p < .001) was observed (see Table 3). However, in spite of significant direct effects of CES-D and the latent cognitive function in the baseline model for predicting RF, neither of them directly predicted the 12-month RF after controlling for the baseline RF (p = .63, .55, respectively). Both indirect paths from CES-D and the latent cognitive function to the baseline RF to the 12-month RF were significant (β = −.07, −.09, p < .01, .01, respectively). None of the indirect paths via FOME or the latent cognitive function was significant. FOME score also did not significantly predict the 12-month RF (p = .77). The model explained 12% of variance in the 12-month RF. When the personality traits were added in the model, neither neuroticism nor conscientiousness significantly accounted for the 12-moth RF (p = .60, .61, respectively).
Mnemonic usage
For the model, the chi-square test was statistically significant, χ2(9) = 18.46, p = .03 but the other two indices supported a satisfactory model fit (CFI = .957, RMSEA = .059). A significant direct effect of the baseline MU on the 12-month MU was observed (β = .58, p < .001) (see Table 3). However, the direct effect of CES-D on the 12-month MU was marginally significant (β = .10, p = .054) and the latent cognitive functions did not directly predict the 12-month MU after controlling for the baseline MU (p = .58). FOME score also did not significantly predict the 12-month MU (p = .13). Among the indirect paths, only the path from the latent cognitive function to the baseline MU to the 12-month MU (β = −.21, p < .01) was significant. The model explained 37% of variance in the 12-month MU. When the personality traits were added, neither neuroticism nor conscientiousness significantly accounted for the 12-moth MU (p = .29, .30, respectively), although the direct effect of conscientiousness on MU was marginally significant in the baseline model.
Discussion
In line with previous studies on depressive symptoms and memory complaints, our results support that older adults with more depressive symptoms show more negative perception of their memory functioning compared to those with fewer depressive symptoms (Zelinski et al., 1990; Zelinski & Gilewski, 2004). Particularly, the baseline models suggest that negative perception about memory can be identified by reporting more frequent forgetting incidents (FF) or greater decline in memory function (RF) in MFQ measures. The nonsignificant indirect path from CES-D to the two cognitive predictors (i.e., the latent cognitive function and FOME) to FF or RF further supports that the association between depressive symptoms and subjective memory complaints tends to be independent from the effects of level of general cognitive abilities.
Our baseline models also support the view that memory complaints can be directly tied to level of cognitive functioning, although the explanatory power (variance accounted for) of objective cognitive functioning was relatively weak. More interestingly, the pattern of relationships between cognitive functioning and memory complaints varied with the type of MFQ measure. The positive path coefficient between FF and the latent cognitive function indicates that older adults with higher cognitive functioning scores complain less about their memory performance. On the contrary, the negative path coefficient between RF and the latent cognitive function, or between MU and the latent cognitive function, suggests that higher cognitive functioning would be associated with negative judgment on memory functioning and more frequent use of mnemonics.
In the 12-month models, the baseline predictors were not able to directly predict the 12-month MFQ measures over and above the baseline relationships described above. Consistent with the baseline model, neither of the cognitive predictors significantly mediated the relationship between CES-D and the MFQ measures. The indirect paths from the depressive symptoms and the latent cognitive function via the baseline MFQ measures to the 12-month MFQ measures were significant only if the corresponding baseline relationship was significant. The results support that longitudinal relationships between the predictors and the MFQ measures were essentially established by the time of measurement of the baseline effects, or that a 1-year interval was too short to reveal differing relationships. Overall, our findings do not support Gilewski et al.’s (1990) hypotheses that (a) ratings of SF or RF might be an indicator of depressive symptoms and (b) rating of MU might reflect an attempt to cope with minor impairment of memory function. Rather, only FF scale was consistently related to depressive symptoms and to the latent cognitive function at both baseline and longitudinally (although the longitudinal effects was indirect), and particularly, depressive symptoms were the stronger predictor for FF. The pattern of the relationship between MU and cognitive functioning revealed in the current study was also a reversal of the pattern originally expected in Gilewski et al. (1990).
It should be noted that we found no significant correlation between any of cognitive function measures and the FOME scores at baseline. We confirmed that there were no ceiling or floor effects that might constrain the relationships between them. A further inspection of residuals did not show any significant unaccounted for relationships between FOME and the cognitive function variables. FOME has been regarded as a unique index to capture memory function of older adults because the test allows participants to use multiple sensory system. During the test, participants are asked to identify the objects by touch, then verify them by vision and name the objects (Chung & Ko, 2009). Conceivably, our cognitive function measures were more likely to reflect general aspects of cognitive ability (e.g., processing speed and verbal ability) rather than the unique aspect of the memory construct reflected in FOME, namely, a multimodal channel of encoding processes (Shulman et al., 2006). In fact, a lack of strong association between FOME and a general intelligence index has been reported in previous literature (Anderson-Hanley, Miele, & Dunnam, 2013). Therefore, it is still interesting to note that the baseline FOME scores significantly predicted FF and SF at 12 months after controlling for the other predictors. The result indicates that FOME might be a unique indicator of longitudinal change in FF and SF. Given the unique aspects of FOME, future studies involving FOME may also provide insight into the mechanism of association between age-related memory decline and memory complaints. Other studies will be also needed to see if the associations between FOME and memory complaints are replicated.
Additionally, inconsistent with previous studies demonstrating that neuroticism and conscientiousness can be significant predictors for memory complaints (e.g., Lane & Zelinski, 2003, Pearman & Storandt, 2004, 2005; Reid & MacLullich, 2006), our study failed to replicate the effects of personality traits on MFQ measures. Only the effects of conscientiousness on some MFQ measures were marginally significant. One possible explanation is sample differences. The sample of the PRISM trial was based on older adults at risk for social isolation and they may not be representative of other samples used in previous studies.
We believe that our paper is the first to investigate the associations between depressive symptoms and subjective memory complaints with a longer and context-specific memory complaints scale (i.e., MFQ). Previous findings relying on brief assessment of memory complaints might reflect older adults’ general beliefs about age-related memory decline rather than a specific incidents of memory failure and thus it might affect the relationship between memory complaints and the non-cognitive variables (e.g., depressive symptoms and personality trait: Pearman et al., 2014). Our results suggest that investigators should carefully examine if and how the associations between memory complaints, depressive symptoms, and cognitive factors vary with the specific type of memory complaints. However, our study was still not successful in demonstrating the associations between depressive symptoms and subjective memory complaints at within-person level. In other words, these findings do not necessarily indicate that changes in MFQ measures would be coupled with changes in depressive symptoms or cognitive abilities as Hülür et al. (2014) pointed out the limitation of between-person approach to the study of subjective memory and depressive symptoms. Future studies with a context-specific memory complaints scale are needed to assess within-person level associations of subjective memory and depressive symptoms with a longer interval from baseline than 1 year.
A caveat from our study is the particular sample that we recruited, one selected to be at risk for social isolation. Participants may have had greater depressive affect and hence, more memory complaints. Similarly, as is typical for an older sample, ours was skewed female compared to male, and females are more likely than males to have significant depressive affect. Sample characteristics may have amplified path coefficients related to depressive affect relative to representative samples in that age range.
Taken together, consistent with prior studies suggesting that memory complaints might be an indicator of depression (e.g., Geriatric Depression Scale; Yesavage et al., 1982), our results suggest that clinicians screen for depression when older clients present with complaints about frequent forgetting. Difficulties in disengaging from or an increased elaboration of negative material might be the underlying mechanism between depressive symptoms and subjective memory complaints (e.g., Gotlib & Joormann, 2010). Depressive symptoms might lead individuals to interpret a common, everyday memory problem, such as a forgetting incident, more seriously because it would increase concern for the negative information (i.e., memory problem). It is also possible that older adults with depressive symptoms might be more susceptible to everyday memory problems, perceiving them as an indicator of more serious age-related cognitive decline (Hülür et al., 2014). Similarly, those with better cognitive functioning might be more likely to notice declines in memory functioning and undertake compensating activities (e.g., use of mnemonics) to cope with the perceived memory problems. In order to probe the hypothesis, we conducted an additional post-hoc analysis after dividing the sample into high and low cognitive function groups. We used a median split of the three cognitive measures (MMSE, Shipley, and DSST). Consistent with the hypothesis, older adults with high cognitive function showed a more negative perception of memory function (RF) and more frequent mnemonic usage (MU), t(212) = 2.29, 2.87, p = .023, 004, d = .32, .39, respectively. However, in spite of possible candidate explanations, it still remains unclear which specific mechanisms explain the association between depressive symptoms and subjective memory complaints. Future studies could benefit from exploring activated neural pathways underlying the association between depressive symptoms, cognition, and memory complaints as well as the effect of aging on them.
Funding
The National Institute on Aging/National Institutes of Health supported this work (NIA 3 PO1 AG017211, Project CREATE III –Center for Research and Education on Aging and Technology Enhancement).
Conflict of Interest
The authors report no conflicts of interest.
Acknowledgments
The authors would like to thank Dr Rick Wagner for his invaluable advice on the path analyses. Trial Registration #: NCT01497613.
References
- Anderson-Hanley C., Miele A. S., Dunnam M. (2013). The fuld object-memory evaluation: Development and validation of an alternate form. Applied Neuropsychology. Adult, 20, 1–6. doi:10.1080/09084282.2012.670156 [DOI] [PubMed] [Google Scholar]
- Bentler P. M., & Bonett D. G. (1980). Significance tests and goodness of fit in the analysis of covariance structures. Psychological Bulletin, 88, 588. doi:10.1037/0033-2909.88.3.588 [Google Scholar]
- Chung J. C., S K Ho W. (2009). Validation of Fuld object memory evaluation for the detection of dementia in nursing home residents. Aging & Mental Health, 13, 274–279. doi:10.1080/13607860802667649 [DOI] [PubMed] [Google Scholar]
- Crane M. K., Bogner H. R., Brown G. K., Gallo J. J. (2007). The link between depressive symptoms, negative cognitive bias and memory complaints in older adults. Aging & Mental Health, 11, 708–715. doi:10.1080/13607860701368497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja S. J., Boot W. R., Charness N., Rogers W. A., Sharit J. (2017). Improving social support for older adults through technology: Findings from the PRISM randomized controlled trial. The Gerontologist. doi:10.1093/geront/gnw249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja, S. J., Boot, W. R., Charness, N., Rogers, W. A., Sharit, J., Fisk, A. D., ... & Nair, S. N. (2015). The personalized reminder information and social management system (PRISM) trial: rationale, methods and baseline characteristics. Contemporary Clinical Trials, 40, 35–46. doi:10.1016/j.cct.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fuld P.A. (1981). Fuld object memory evaluation instruction manual. Wood Dale, IL: Stoelting. [Google Scholar]
- Gilewski M. J., Zelinski E. M., Schaie K. W. (1990). The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychology and Aging, 5, 482–490. doi:10.1037/0882-7974.5.4.482 [DOI] [PubMed] [Google Scholar]
- Goldberg L. R. (1992). The development of markers for the Big-Five factor structure. Psychological Assessment, 4, 26–42. doi:10.1037/1040-3590.4.1.26 [Google Scholar]
- Gosling S. D. Rentfrow P. J., & Swann W. B. (2003). A very brief measure of the Big-Five personality domains. Journal of Research in Personality, 37, 504–528. doi:10.1016/S0092-6566(03)00046-1 [Google Scholar]
- Gotlib I. H., Joormann J. (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. doi:10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L. T., & Bentler P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6, 1–55. doi:10.1080/10705519909540118 [Google Scholar]
- Hülür G., Hertzog C., Pearman A., Ram N., Gerstorf D. (2014). Longitudinal associations of subjective memory with memory performance and depressive symptoms: Between-person and within-person perspectives. Psychology and Aging, 29, 814–827. doi:10.1037/a0037619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew D. K., Brunning S. (1999). Personality, preventive health behaviour and comparative optimism about health problems. Journal of Health Psychology, 4, 193–208. doi:10.1177/135910539900400213 [DOI] [PubMed] [Google Scholar]
- Kahn R. L., Zarit S. H., Hilbert N. M., Niederehe G. (1975). Memory complaint and impairment in the aged. The effect of depression and altered brain function. Archives of General Psychiatry, 32, 1569–1573. doi:10.1001/archpsyc.1975.01760300107009 [DOI] [PubMed] [Google Scholar]
- Lane C. J., Zelinski E. M. (2003). Longitudinal hierarchical linear models of the memory functioning questionnaire. Psychology and Aging, 18, 38–53. doi:10.1037/0882-7974.18.1.38 [DOI] [PubMed] [Google Scholar]
- O’Shea D. M., Dotson V. M., Fieo R. A., Tsapanou A., Zahodne L., Stern Y. (2016). Older adults with poor self-rated memory have less depressive symptoms and better memory performance when perceived self-efficacy is high. International Journal of Geriatric Psychiatry, 31, 783–790. doi:10.1002/gps.4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearman A., Hertzog C., Gerstorf D. (2014). Little evidence for links between memory complaints and memory performance in very old age: Longitudinal analyses from the Berlin Aging Study. Psychology and Aging, 29, 828–842. doi:10.1037/a0037141 [DOI] [PubMed] [Google Scholar]
- Pearman A., Storandt M. (2004). Predictors of subjective memory in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 59, P4–P6. doi:10.1093/geronb/59.1.P4 [DOI] [PubMed] [Google Scholar]
- Pearman A., Storandt M. (2005). Self-discipline and self-consciousness predict subjective memory in older adults. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, P153–P157. doi:10.1093/geronb/60.3.P153 [DOI] [PubMed] [Google Scholar]
- Radloff L. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measures, 1, 385–401. doi:10.1177/014662167700100306 [Google Scholar]
- Reid L. M., Maclullich A. M. (2006). Subjective memory complaints and cognitive impairment in older people. Dementia and Geriatric Cognitive Disorders, 22, 471–485. doi:10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- Shulman K. I., Herrmann N., Brodaty H., Chiu H., Lawlor B., Ritchie K., Scanlan J. M. (2006). IPA survey of brief cognitive screening instruments. International Psychogeriatrics, 18, 281–294. doi:10.1017/S1041610205002693 [DOI] [PubMed] [Google Scholar]
- Wall J. R. Deshpande S. A. Macneill S. E., & Lichtenberg P. A. (1998). The Fuld Object Memory Evaluation, a useful tool in the assessment of urban geriatric patients. Clinical Gerontologist, 19, 39–49. doi:10.1300/J018v19n01_04 [Google Scholar]
- Wechsler D. (1981). Manual for Wechsler Memory Scale Revised. New York: The Psychological Corp. [Google Scholar]
- West R. L., Boatwright L. K., Schleser R. (1984). The link between memory performance, self-assessment, and affective status. Experimental Aging Research, 10, 197–200. doi:10.1080/03610738408258464 [DOI] [PubMed] [Google Scholar]
- Yesavage J. A., Brink T. L., Rose T. L., Lum O., Huang V., Adey M., Leirer V. O. (1982). Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Zachary R. A. (1986). Shipley institute of living scale: Revised manual. Los Angeles: Western Psychological Services. [Google Scholar]
- Zelinski E. M., Gilewski M. J. (2004). A 10-item Rasch modeled memory self-efficacy scale. Aging & Mental Health, 8, 293–306. doi:10.1080/13607860410001709665 [DOI] [PubMed] [Google Scholar]
- Zelinski E. M., Gilewski M. J., Anthony-Bergstone C. R. (1990). Memory Functioning Questionnaire: Concurrent validity with memory performance and self-reported memory failures. Psychology and Aging, 5, 388–399. doi:10.1037/0882-7974.5.3.388 [DOI] [PubMed] [Google Scholar]
- Zelinski E. M., Gilewski M. J., Schaie K. W. (1993). Individual differences in cross-sectional and 3-year longitudinal memory performance across the adult life span. Psychology and Aging, 8, 176–186. doi:10.1037/0882-7974.8.2.176 [DOI] [PubMed] [Google Scholar]