Abstract
MicroRNA (miRNA)-373 has been demonstrated to be involved in several types of cancer, whereas its involvement in urinary bladder cancer and the mechanism of its function remains poorly understood. The present study aimed to investigate the functionality of miRNA-373 in urinary bladder cancer. Tumor tissues and adjacent healthy tissues were collected from patients with urinary bladder cancer (n=55), and blood samples were collected from patients with urinary bladder cancer and healthy controls (n=45). The expression of miRNA-373 in these tissues was detected by reverse transcription quantitative polymerase chain reaction. The diagnostic value of serum miRNA-373 for urinary bladder cancer was investigated by receiver operating characteristic curve analysis and survival curve analysis, respectively. miRNA-373 mimics were transfected into urinary bladder cancer cells, and the effects on cancer cell proliferation, migration and invasion, and on epidermal growth factor receptor (EGFR) expression was assessed by Cell Counting kit-8 assay, Transwell migration and invasion assays, and western blot analysis. It was identified that the miRNA-373 expression level was increased in tumor tissues compared with adjacent healthy tissues. The serum level of miRNA-373 was increased in patients with cancer compared with the healthy controls. Serum miRNA-373 may be used to accurately predict urinary bladder cancer. miRNA-373 overexpression promoted tumor cell proliferation, migration and invasion, and resulted in upregulated EGFR expression in urinary bladder cancer cells. It was concluded that miRNA-373 overexpression may promote urinary bladder cancer cell proliferation, migration and invasion by upregulating EGFR.
Keywords: urinary bladder cancer, microRNA-373, epidermal growth factor receptor
Introduction
As the second most common malignancy of the genitourinary tract, urinary bladder cancer affects more than 2 million people worldwide (1). The incidence of urinary bladder cancer is increased in developed countries compared with less developed regions. It has been demonstrated that urinary bladder cancer accounts for >5% of newly diagnosed tumors in European countries (2). With the growth of the aging population and changes in exposure to risk factors, the incidence of urinary bladder cancer demonstrates an increasing trend and the age of onset is decreasing (1). With the efforts made in the prevention and treatment of urinary bladder cancer, the 5-year survival rate of patients with this disease is 60% (3). However, the prognosis of patients with metastatic urinary bladder cancer is usually poor (4). Therefore, early diagnosis and treatment remains critical for the survival of patients with urinary bladder cancer.
In addition to messenger RNAs (mRNAs) that encode protein products, the human genome also transcribes a large set of non-coding RNAs that serve pivotal roles in normal physiological and pathological processes (5). The comparison of non-coding RNA expression under physiological and pathological conditions provides references for the diagnosis and prognosis of human diseases (6). MicroRNA (miRNA) is a subgroup of non-coding RNA molecules, each measuring ~22 nucleotides (7). Studies from previous decades have indicated that miRNAs are involved in almost all aspects of critical biological processes in the human body (8). miRNA-373 serves different roles in different types of cancer (9,10). miRNA-373 is likely an oncogene in testicular germ cell tumors, and the overexpression of miRNA-373 in tumor tissues promotes tumor progression (9). By contrast, miRNA-373 serves a tumor suppression role in estrogen receptor negative breast cancer by targeting nuclear factor kappa-light-chain-enhancer of activated B cells and transforming growth factor-β signaling pathways (10). The contradictory functions of miRNA-373 reveal the complexity of its regulatory role in cancer biology. In the present study, it was identified that miRNA-373 promoted the proliferation, migration and invasion of urinary bladder cancer by upregulating epidermal growth factor receptor (EGFR). The present study provided novel insights for the diagnosis and treatment of urinary bladder cancer.
Patients and methods
Patients
From January 2015 to January 2018, a total of 55 patients who were pathologically diagnosed with urinary bladder cancer and treated in Shengjing Hospital of China Medical University (Shenyang, China) were included. Those patients included 40 males and 15 females, and the ages ranged from 28–69 years, with a mean age of 45±10.1 years. Concurrently, a total of 45 healthy people were also included to serve as control group. The control group included 37 males and 8 females, and the ages ranged from 27–70 years, with a mean age of 44±10.9 years. No significant differences in age and sex were identified between the two groups. The present study was approved by the Ethics Committee of Shengjing Hospital of China Medical University. All patients signed informed consent.
Tissue collection and processing
Tumor tissues and adjacent healthy tissues within 2 cm around the tumors were collected from all patients during surgical resection. Blood (10 ml) was extracted from the elbow vein of the patients with urinary bladder cancer and healthy controls. Blood was maintained at room temperature for 2 h, followed by centrifugation of blood samples for 10 min at 1,875 × g at room temperature to collect serum. All tissues were stored in liquid nitrogen prior to use.
Cell lines and cell culture
Urinary bladder cancer HT-1376 (ATCC® CRL-1472™) and HT-1197 (ATCC® CRL-1473™) cell lines were purchased from the American Type Culture Collection (ATCC). Cells were cultured with Eagle's Minimum Essential Medium (cat. no. 30-2003; ATCC) containing 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C with 5% CO2.
Cell transfection
miR-373 mimic hsa-miR-373* (HMI0531) and negative control 1 miRNA (GGUUCGUACGUACACUGUUCA; HMC0002) were purchased from Sigma-Aldrich; Merck KGaA. Prior to transfection, cells were cultured at 37°C with 5% CO2 overnight to reach 80–90% confluence. Lipofectamine 2000® reagent (11668-019; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to transfected 50 nM miRNA into 5×105 cells. Cells without transfection were control cells (C). Cells transfected with negative control miRNA were negative control cells (NC). Subsequent experiments were performed at 24 h after transfection.
Cell proliferation assay
A Cell Counting kit-8 assay kit (Sigma-Aldrich; Merck KGaA) was used to evaluate cell proliferation ability. Briefly, cells were collected and centrifuged at 600 × g for 5 min at room temperature and were used to prepare a cell suspension with a density of 5×104 cells per well, and 100 µl cell suspension containing 5×103 was added into each well of 96-well plates. Cells were cultured in an incubator at 37°C with 5% CO2, and 10 µl CCK-8 solution was added at 12, 24, 48, 72 and 96 h. Subsequent to incubation at 37°C for an additional 4 h, optical density values at 450 nm were measured using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell cell migration and invasion assay
A Transwell cell migration assay kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to evaluate cell migration ability. Briefly, cells were collected and centrifuged at 600 × g for 5 min at room temperature and were used to prepare a cell suspension with a density of 5×104 cells per well, and 100 µl cell serum-free suspension containing 5×103 cells was added to the upper chamber. Then, RPMI-1640 medium (Thermo Fisher Scientific, Inc.) containing 20% fetal calf serum (Sigma-Aldrich; Merck KGaA) was added into the lower chamber. Following incubation at 37°C with 5% CO2 for 24 h, membranes were collected and stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) at room temperature for 15 min. Stained cells were counted under an optical microscope (Olympus Corporation, Tokyo, Japan) at magnification, ×20. A cell invasion assay was performed using the same protocol, but the upper chamber was pre-coated with Matrigel (EMD Millipore, Billerica, MA, USA) at 4°C for 4 h.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
A miRNeasy kit (Qiagen GmbH, Hilden, Germany) was used for all miRNA extraction from both cells and tissues. cDNA was prepared using the miScript II RT kit (Qiagen GmbH) under the following conditions: 25°C for 5 min, 50°C for 25 min and 75°C for 10 min. All protocols were performed according to the manufacturer's instructions. An miScript SYBR-Green PCR kit (Qiagen GmbH) was used to assay miRNA-373 expression using RNU6 (miRNA) as an endogenous control. PCR reaction conditions were: 95°C for 1 min, then 95°C for 15 sec and 59°C for 40 sec for 40 cycles. All primers were purchased directly from Beyotime Institute of Biotechnology (Jiangsu, China). Data were analyzed using the 2−ΔΔCt method (11).
Western blot analysis
Total protein extraction from HT-1376 and HT-1197 cell lines was performed using a radioimmunoprecipitation assay lysis solution (Thermo Fisher Scientific, Inc.), and BCA assay was used for protein quantification. Subsequently, 20 µg protein from each sample was subjected to 10% SDS-PAGE gel electrophoresis, followed by gel transfer to polyvinylidene fluoride membranes. Membranes were blocked with 5% skimmed milk at room temperature for 2 h, followed by washing with PBS and incubation with primary antibodies including rabbit anti-human EGFR (1:2,000; cat. no. ab131498; Abcam, Cambridge, UK) and rabbit anti-human GAPDH (1:1,000; cat. no. ab8245; Abcam) overnight at 4°C. Following washing with PBS, the membranes were incubated with an anti-rabbit IgG-horseradish peroxidase secondary antibody (1:1,000; cat. no. MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room temperature for 1 h. Then, enhanced chemiluminescent reagents (Sigma-Aldrich; Merck KGaA) were added to detect the signals. Membranes were scanned using MYECL™ Imager (Thermo Fisher Scientific, Inc.), and ImageJ v1.46 software (National Institutes of Health, Bethesda, MD, USA) was used to normalize the relative expression level of EGFR to the endogenous control GAPDH.
Statistical analysis
All experiments were performed in triplicate. Data were processed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Count data are expressed as rate and were compared using a χ2 test. Measurement data were expressed as mean ± standard deviation, and comparisons among multiple groups were performed using a one-way analysis of variance followed by Tukey's test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of serum miRNA-373 for urinary bladder cancer. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miRNA-373 in tumor tissues and adjacent healthy tissues of patients with urinary bladder cancer
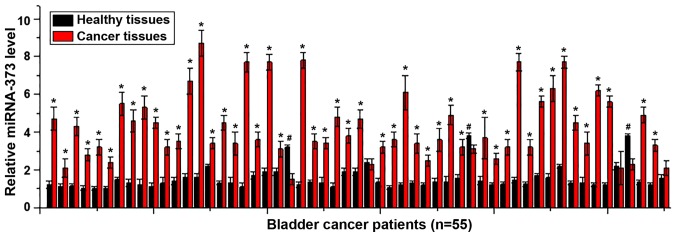
Analysis of the expression of miRNA-373 in tumor tissues and adjacent healthy tissues of 55 patients with urinary bladder cancer using RT-qPCR, which indicated that the expression of miRNA-373 was significantly upregulated in tumor tissues compared with adjacent healthy tissues in 49 out of 55 patients (P<0.05; Fig. 1), accounting for 89.0% of this cohort. By contrast, the expression of miRNA-373 was significantly downregulated in tumor tissues compared with adjacent healthy tissues in 3 patients (P<0.05; Fig. 1), accounting for 5.5%. No significant differences were identified in the remaining 3 cases, accounting for 5.5%. Those data suggest that the upregulation of miRNA-373 is likely to be involved in the pathogenesis of urinary bladder cancer.
Figure 1.
Expression of miRNA-373 in tumor tissues and adjacent healthy tissues of patients with urinary bladder cancer. This experiment was performed in triplicate and all data are expressed as mean ± standard deviation. *P<0.05, expression level of miRNA-373 was significantly higher in tumor tissues compared with adjacent healthy tissues; #P<0.05, expression level of miRNA-373 was significantly lower in tumor tissues compared with adjacent healthy tissues. miRNA, microRNA.
Comparison of serum levels of miRNA-373 and the diagnostic values
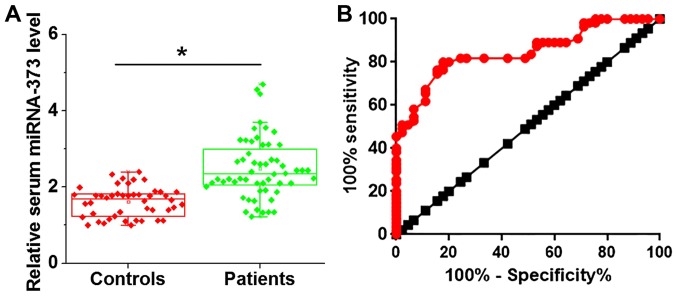
As demonstrated in Fig. 2A, the serum levels of miRNA-373 were significantly increased in patients with urinary bladder cancer compared with the healthy controls. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic value of serum miRNA-373 for urinary bladder cancer. As indicated in Fig. 2B, the area under the curve was 0.8473, with a 95% confidence interval of 0.7722–0.9223 (P<0.001). These data suggest that the upregulation of serum miRNA-373 may serve as a potential diagnostic marker for urinary bladder cancer.
Figure 2.
Comparison of serum levels of miRNA-373 and the diagnostic values. (A) Comparison of serum levels of miRNA-373 between patients with urinary bladder cancer and healthy controls. (B) Receiver operative characteristic curve of the diagnosis of urinary bladder cancer using serum miRNA-373. Red indicates the diagnostic line and black indicates the line of identity. miRNA, microRNA. *P<0.05.
Association between serum levels of miRNA-373 and clinicopathological data of patients with urinary bladder cancer
Patients were divided into high expression (n=28) and low expression groups (n=27) according to the median serum level of miRNA-373 (> or <2.56, respectively). The associations between serum levels of miRNA-373 and clinicopathological data of patients with urinary bladder cancer were analyzed by χ2 test. As summarized in Table I, serum levels of miRNA-373 were not significantly associated with the sex, age, or drinking and smoking habits (P>0.05), but were significantly associated with the diameter of primary tumors and distant metastasis (P<0.05).
Table I.
Association between serum levels of miRNA-373 and clinicopathological data of patients with urinary bladder cancer.
| miRNA-373 expression groups | ||||||
|---|---|---|---|---|---|---|
| Patient characteristics | Groups | Cases, n | High | Low | χ2 | P-value |
| Sex | Male | 40 | 19 | 21 | 0.68 | 0.41 |
| Female | 15 | 9 | 6 | |||
| Age, years | ≥45 | 27 | 15 | 12 | 0.46 | 0.50 |
| <45 | 28 | 13 | 15 | |||
| Primary tumor diameter, cm | ≥2 | 32 | 20 | 12 | 4.11 | 0.04 |
| <2 | 23 | 8 | 15 | |||
| Distant metastasis | Yes | 26 | 18 | 8 | 6.62 | 0.01 |
| No | 29 | 10 | 19 | |||
| Smoking | Yes | 31 | 15 | 16 | 0.18 | 0.67 |
| No | 24 | 13 | 11 | |||
| Drinking | Yes | 38 | 20 | 18 | 0.15 | 0.70 |
| No | 17 | 8 | 9 | |||
miRNA, microRNA.
Effects of miRNA-373 overexpression of urinary bladder cancer cell proliferation, migration and invasion
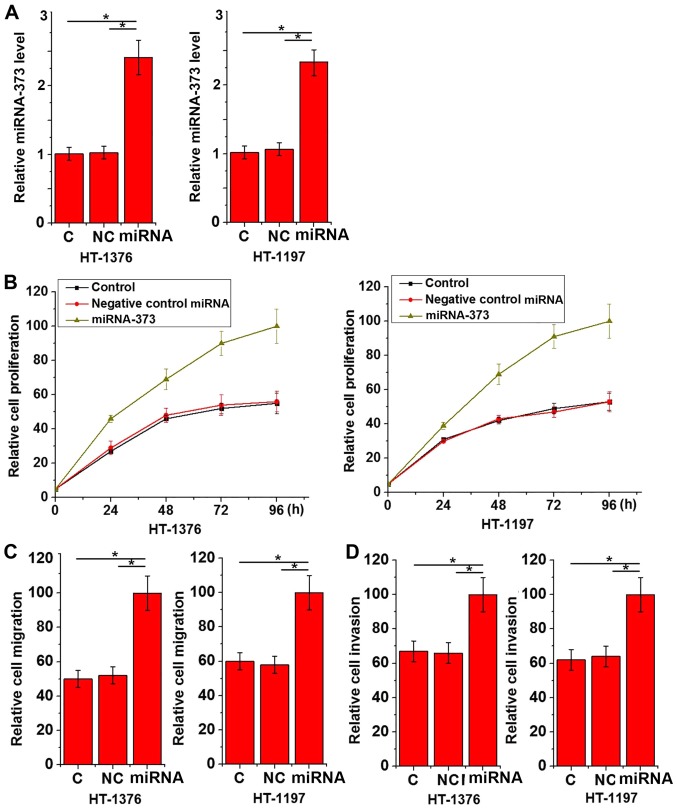
The aforementioned data suggested that upregulation of miRNA-373 may be associated with tumor growth and distant metastasis of urinary bladder cancer. To additionally investigate the role of miRNA-373 in urinary bladder cancer proliferation and migration, miRNA-373 mimics were transfected into urinary bladder cancer HT-1376 and HT-1197 cell lines. The overexpression of miRNA-373 was confirmed by RT-qPCR (Fig. 3A). As indicated in Fig. 3, miRNA-373 mimic transfection significantly promoted the proliferation (Fig. 3B), migration (Fig. 3C) and invasion (Fig. 3D) of the two cell lines.
Figure 3.
Effects of miRNA-373 overexpression on the proliferative, migratory and invasive abilities of urinary bladder cancer cells. (A) The expression of miRNA-373 in HT-1376 and HT-1197 cells, and (B) cell proliferation, (C) migration and (D) invasion of those cells following miRNA-373 overexpression. This experiment was performed in triplicate and all data are expressed as mean ± standard deviation. *P<0.05. siRNA, small interfering RNA; miRNA, microRNA; C, control cells without transfection; NC, negative control cells transfected with negative control miRNA; Mimic, cells transfected with miRNA-373 mimic.
Effects of miRNA-373 overexpression on EGFR expression
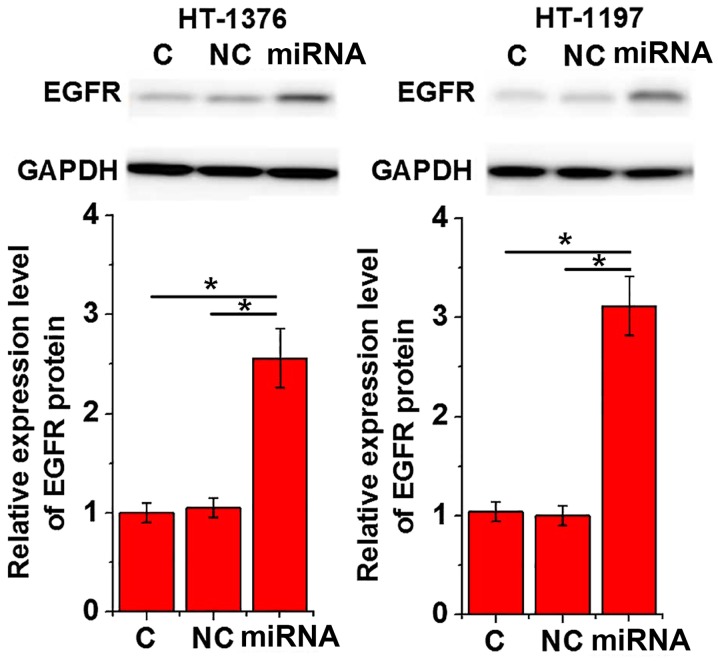
The overexpression of EGFR stimulates cell proliferation, migration and invasion in certain types of cancer cells (12). Therefore, the effects of miRNA-373 overexpression on EGFR expression were investigated. As indicated in Fig. 4, transfection of miRNA-373 mimics significantly promoted the expression of EGFR in cells of the two urinary bladder cancer cell lines (P<0.05).
Figure 4.
Effects of miRNA-373 overexpression on EGFR expression. The upper panel demonstrates representative western blot analysis results. The lower panel represents EGFR expression normalized to endogenous control GAPDH. This experiment was performed in triplicate and all data are expressed as mean ± standard deviation. *P<0.05. miRNA, microRNA; siRNA, small interfering RNA; EGFR, epidermal growth factor receptor; C, control cells without transfection; NC, negative control cells transfected with negative control miRNA; Mimic, cells transfected with microRNA-373 mimic.
Discussion
miRNA-373, as a human embryonic stem cell-specific miRNA, has been demonstrated to be involved the regulation of cell proliferation, senescence, apoptosis, migration and invasion (13). In addition, miRNA-373 also serves a pivotal role in DNA damage repair following hypoxia stress (14). The altered expression of miR-373 has been observed in several types of human cancer, indicating the role of miR-373 as an oncogene or tumor suppression gene in those diseases. In the study of testicular germ cell tumors, Voorhoeve et al (9) suggested that miRNA-373 was upregulated in tumors and is likely to serve an oncogenic role. However, a contrasting study identified that miRNA-373 was downregulated in estrogen receptor negative breast cancer, suggesting its role as a tumor suppression gene in this disease (10). In the present study, the upregulation of miRNA-373 expression in tumor tissues compared with adjacent healthy tissues was observed in the majority of patients with urinary bladder cancer. In addition, serum levels of miRNA-373 were also increased in patients with urinary bladder cancer compared with the healthy controls. These data suggest that upregulation of miRNA-373 is likely involved in the pathogenesis of urinary bladder cancer.
Due to the poor prognosis of patients with advanced urinary bladder cancer (3), early diagnosis and treatment of this disease remains critical. Development of human diseases is usually accompanied by changes in certain factors in the blood circulation system and comparison of those factors under physiological and pathological conditions may provide valuable references for the diagnosis of diseases (15). In the present study, ROC curve analysis revealed that serum miRNA-373 may be an accurate biomarker for urinary bladder cancer. It has been established previously that the expression pattern of certain miRNAs may be altered by smoking (16), alcohol consumption (17) and aging (18), which may affect the reliability of miRNAs in the diagnosis of diseases. In the present study, no significant associations between serum levels of miRNA-373 with patient age, sex or behavioral habits including smoking and drinking were observed. These data suggest that miRNA-373 is a reliable and effective biomarker for urinary bladder cancer. However, it is worth noting that an altered expression of miRNA-373 has been observed in different human diseases (9,10). Therefore, multiple biomarkers should be combined to improve the diagnostic accuracy.
The present study also indicated that serum levels of miRNA-373 were closely associated with tumor size and distant tumor metastasis. Previous studies have confirmed that miRNA-373 is involved in the regulation of cell proliferation, migration and invasion of several types of human cancer (19–21). In the present study, transfection with miRNA-373 significantly promoted cell proliferative, migratory and invasive abilities of two urinary bladder cancer cell lines, indicating that the role of miRNA-373 was a promotor of urinary bladder cancer growth and metastasis. The overexpression of EGFR stimulates cell proliferation, migration and invasion of certain types of cancer cells, and EGFR is considered a target for the treatment of several malignancies, including urinary bladder cancer (12,22). In the present study, transfection with miRNA-373 significantly promoted the expression of EGFR in two urinary bladder cancer cell lines. These data suggest that miRNA-373 may promote the growth and metastasis of urinary bladder cancer, and that this function may be associated with the upregulation of EGFR.
Notably, Zhang et al (23) revealed an expression pattern and functionality of miRNA-373 in bladder cancer contradictory to that described in the present study. The discordance in these observations may be due to the different cell lines used in the present study and individual and/or regional differences across patients. In addition, the present study only examined miRNA-373-EGFR signaling in bladder cancer. The data obtained did not provide any evidence on whether this interaction was direct or indirect. Future studies should aim to reveal more information regarding the signaling pathway. An additional limitation of the present study was the small sample size. Future studies with bigger sample sizes are required to additionally confirm the results. In addition, more cells lines of different subtypes should be used to investigate the role of miRNA-373 in bladder cancer.
In conclusion, miRNA-373 expression was upregulated in urinary bladder cancer. Serum miRNA-373 may serve as an effective and reliable diagnostic marker for urinary bladder cancer. miRNA-373 overexpression promoted tumor cell proliferation, migration and invasion, and EGFR expression. The present study concluded that miRNA-373 overexpression may promote urinary bladder cancer cell proliferation, migration and invasion by upregulating EGFR. However, the mechanism of miRNA-373-mediated upregulation of EGFR remains unknown. Additional studies are required.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YW and XW designed experiments. YW and ZX performed experiments and collected data. YW and XW analyzed and interpreted data. XW drafted the manuscript. All authors read and approved the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Shengjing Hospital of China Medical University and all participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volanis D, Kadiyska T, Galanis A, Delakas D, Logotheti S, Zoumpourlis V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol Lett. 2010;193:131–137. doi: 10.1016/j.toxlet.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Marcos-Gragera R, Mallone S, Kiemeney LA, Vilardell L, Malats N, Allory Y, Sant M. EUROCARE-5 Working Group: Urinary tract cancer survival in Europe 1999–2007: Results of the population-based study EUROCARE-5. Eur J Cancer. 2015;51:2217–2230. doi: 10.1016/j.ejca.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoud-Ahmed AS, Suh JH, Kupelian PA, Klein EA, Peereboom DM, Dreicer R, Barnett GH. Brain metastases from bladder carcinoma: Presentation, treatment and survival. J Urol. 2002;167:2419–2422. doi: 10.1016/S0022-5347(05)64996-8. [DOI] [PubMed] [Google Scholar]
- 5.Mattick JS, Makunin IV. Non-coding RNA. Human Mol Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 6.Martens-Uzunova ES, Jalava SE, Dits NF, van Leenders GJ, Møller S, Trapman J, Bangma CH, Litman T, Visakorpi T, Jenster G. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene. 2012;31:978–991. doi: 10.1038/onc.2011.304. [DOI] [PubMed] [Google Scholar]
- 7.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Voorhoeve PM, Le Sage C, Schrier M, Gillis AJ, Stoop H, Nagel R, Liu YP, van Duijse J, Drost J, Griekspoor A, et al. A genetic screen implicates miRNA-372 and miRNA-373 as oncogenes in testicular germ cell tumors. Cell. 2006;124:1169–1181. doi: 10.1016/j.cell.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 10.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, Allgayer H, Gückel B, Fehm T, Schneeweiss A, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Andl CD, Mizushima T, Nakagawa H, Oyama K, Harada H, Chruma K, Herlyn M, Rustgi AK. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J Biol Chem. 2003;278:1824–1830. doi: 10.1074/jbc.M209148200. [DOI] [PubMed] [Google Scholar]
- 13.Wei F, Cao C, Xu X, Wang J. Diverse functions of miR-373 in cancer. J Transl Med. 2015;13:162. doi: 10.1186/s12967-015-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69:1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borovecki F, Lovrecic L, Zhou J, Jeong H, Then F, Rosas HD, Hersch SM, Hogarth P, Bouzou B, Jensen RV, Krainc D. Genome-wide expression profiling of human blood reveals biomarkers for Huntington's disease. Proc Natl Acad Sci USA. 2005;102:11023–11028. doi: 10.1073/pnas.0504921102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenet. 2012;7:432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- 17.Mamdani M, Williamson V, McMichael GO, Blevins T, Aliev F, Adkins A, Hack L, Bigdeli T, van der Vaart AD, Web BT, et al. Integrating mRNA and miRNA weighted gene co-expression networks with eQTLs in the nucleus accumbens of subjects with alcohol dependence. PLoS One. 2015;10:e0137671. doi: 10.1371/journal.pone.0137671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivieri F, Capri M, Bonafè M, Morsiani C, Jung HJ, Spazzafumo L, Viña J, Suh Y. Circulating miRNAs and miRNA shuttles as biomarkers: Perspective trajectories of healthy and unhealthy aging. Mech Ageing Dev. 2017;165:162–170. doi: 10.1016/j.mad.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, et al. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat Cell Biol. 2008;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- 21.Lee KH, Goan YG, Hsiao M, Lee CH, Jian SH, Lin JT, Chen YL, Lu PJ. MicroRNA-373 (miR-373) post-transcriptionally regulates large tumor suppressor, homolog 2 (LATS2) and stimulates proliferation in human esophageal cancer. Exp Cell Res. 2009;315:2529–2538. doi: 10.1016/j.yexcr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Weintraub MD, Vourganti S, Li Q, Apolo AB, Metwalli AR, Agarwal PK. Targeting the epidermal growth factor receptor in bladder cancer. J Carcinog Mutag. 2013;4:10–30. [Google Scholar]
- 23.Zhang Q, Wang C, Miao S, Li C, Chen Z, Li F. Enhancing E-cadherin expression via promoter-targeted miR-373 suppresses bladder cancer cells growth and metastasis. Oncotarget. 2017;8:93969–93983. doi: 10.18632/oncotarget.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.