Abstract
The cardiovascular effects of disease-modifying antirheumatic drugs and particularly of methotrexate (MTX) are complex and frequently incorrectly understood, which might lead to the unjustified discontinuation of this treatment. MTX, ‘the gold standard’ and first line treatment in rheumatoid arthritis, psoriatic arthritis, and other immune-mediated inflammatory diseases, has been proven to decrease inflammation, improve cardiovascular risk factors, and reduce mortality. This is supported by both the mechanism of action, as well as a body of clinical data evidence. MTX's cardiovascular effects, although incompletely understood, are explained by its antiproliferative, immunosuppressive, anti-inflammatory, and antiatherogenic effects. Several clinical trials have shown that MTX is associated with improved endothelial function, slower atherosclerosis progression, decreased risk of major cardiovascular adverse events, and benefits on survival. Given its systemic cardiovascular effects, MTX could be regarded as an important therapeutic agent not only to control disease activity in rheumatic diseases, but also to reduce cardiovascular risk and mortality.
Keywords: atherosclerosis, cardiovascular risk, inflammation, methotrexate, psoriatic arthritis, rheumatoid arthritis
1. Introduction
Immune-mediated rheumatic diseases in general, and rheumatoid arthritis (RA) and psoriatic arthritis (PsA) in particular, are important cardiovascular risk factors. This is the consequence of the characteristic inflammatory process involved in these diseases, which is a significant element in the pathogenesis of atherosclerosis. Both inherent and acquired immunity-related immunologic mechanisms play a key role in processes such as trans-endothelial migration and adhesion of inflammatory cells, smooth muscle migration, formation of fatty streaks, plaque progression and rupture, and thrombosis (1,2).
This pathophysiological background explains the high cardiovascular risk and clinical consequences [e.g., myocardial infarction (MI), stroke, cardiovascular mortality] associated with immune-mediated inflammatory diseases (1–4). Furthermore, significantly shorter life expectancy can be observed in these patients as compared with the general population, mainly due to cardiovascular mortality (5). The effects of antirheumatic drugs (aiming to reduce inflammation) on the cardiovascular system are extremely important, but frequently incorrectly understood and interpreted, which leads to the unjustified discontinuation of this treatment.
Methotrexate (MTX) still represents the gold standard and first line option in the treatment of RA, PsA, and other immune-mediated inflammatory diseases. Along leflunomide, sulfasalazine, cyclosporine, and hydroxychloroquine, MTX is included in the class of conventional synthetic disease-modifying antirheumatic drugs (DMARDs) (6). MTX has proven its efficacy in the treatment of RA and PsA when used as monotherapy or when combined with other synthetic or biological DMARDs. MTX leads to improvements in signs, symptoms, and physical function, slowed joint degradation, and increased life expectancy (7). It is indicated for long-term use in patients with mild, moderate, or active disease stages and is effective in approximately 60–70% of cases (8). MTX can also be used in RA complications (e.g., Felty syndrome, rheumatoid vasculitis) as well as in other inflammatory rheumatic diseases (e.g., PsA, adult-onset Still's disease, juvenile idiopathic arthritis, systemic lupus erythematosus, polymyositis, granulomatosis with polyangiitis, and Takayasu arteritis) (9). The therapeutic effects of MTX can be explained by several metabolic pathways, such as homocysteine metabolism, nucleic acid metabolism, and one-carbon metabolism, but there are other unknown mechanisms at play as well (10). The use of MTX is, however, limited due to the risk of potentially severe side effects, the most important of which are hepatic, hematologic, pulmonary, renal, muco-cutaneous, and infectious (11).
In this context, evaluating the cardiovascular effects of MTX is important and requires special attention, not only because of the pathogenic mechanisms responsible for these effects, but also with regard to clinical data.
2. MTX mechanisms of action
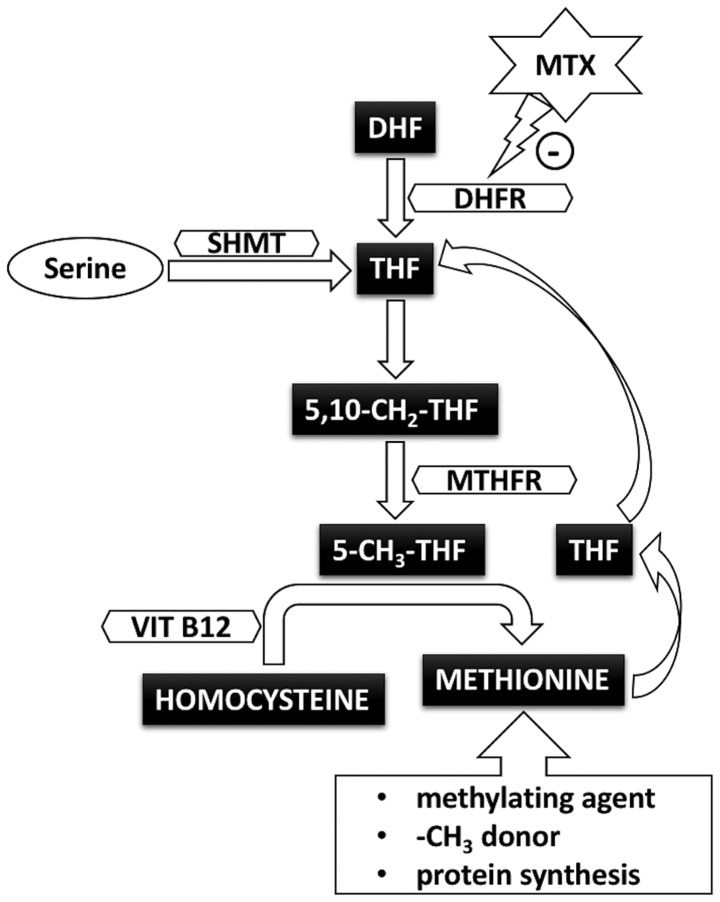
The main element in folate metabolism is tetrahydrofolate (THF), which is the central folate acceptor molecule obtained from dihydrofolate (DHF) in the presence of dihydrofolate reductase (DHFR) (Fig. 1) (12). THF participates in the transfer of several carbon-containing groups, such as methyl or methylene. The following step is the conversion of THF to 5,10-methylene-THF (5,10-CH2-THF). For this process, the 5,10-CH2 source is represented by serine, in the presence of pyridoxal phosphate (PLP)-dependent serine hydroxymethyltransferase (SHMT). Next, 5,10-CH2-THF is transformed into 5-methyl-THF (5-CH3-THF) by another enzyme, methylene tetrahydrofolate reductase (MTHFR).
Figure 1.
Folate pathway and methotrexate. 5-CH3-THF, 5-methyl-tetrahydrofolate; 5,10-CH2-THF, 5,10-methylene-tetrahydrofolate; DHF, dihydrofolate; DHFR, dihydrofolate reductase; MTHFR, methylene tetrahydrofolate reductase; MTX, methotrexate; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
The second metabolic loop that interacts with this pathway is one involving methionine. A methyl (-CH3) radical is removed from 5-CH3-THF, shifted to the vitamin B12 coenzyme, and then transferred to homocysteine in order to form methionine. Thus, homocysteine is formed by the cleavage of S-adenosyl-homocysteine, a product of transmethylation, and is then further salvaged to methionine (13). In this process, new molecules of THF are generated. Methionine has a key role not only in protein synthesis, but also as a -CH3 donor and in the formation of S-adenosylmethionine (SAM), the main methylating agent.
The folate pathway is involved in several important mechanisms such as amino acid metabolism, thymidylate, purine, and pyrimidine synthesis, methylation, protein production, cell proliferation. Any disruption of folate metabolism will alter all these physiological processes, with significant clinical consequences.
Several mechanisms of action have been proposed to describe the effects of MTX on numerous metabolic and inflammatory pathways. First of all, MTX is a folic acid analogue. It is a prodrug which needs to be activated to MTX-polyglutamates (MTX-PG), a compound with prolonged tissue half-life. For this reason, unlike other antifolates, MTX can be administered once weekly. Quantification of individual MTX-PG is performed by different techniques (14).
MTX competitively inhibits DHFR, the enzyme which is essential in the reduction of DHF in THF (12). As described above, this reduced form of folic acid is an important single carbon donor in numerous reactions involved in the synthesis of purine and pyrimidine nucleotides.
By inhibiting DHFR, MTX prevents 5,10-CH2-THF conversion to 5-CH3-THF, decreasing the availability of reduced folates (15). The decreased THF levels inhibit thymidylate synthase, which is involved in pyrimidine synthesis. Subsequently, because THF is essential in further purine and pyrimidine synthesis, this decreases DNA and RNA production. Protein synthesis and cells multiplication are inhibited, explaining the antiproliferative and immunosuppressive effects of this drug (12,16).
3. MTX and homocysteine
Decreased THF concentrations have consequences in the metabolism of homocysteine. Once produced from of S-adenosyl-homocysteine, homocysteine is recovered to methionine (13). Since in most tissues this reaction is dependent on 5-CH3-THF, by interfering with folate metabolism, MTX leads to hyperhomocysteinemia (16). This could be one of the causes of MTX toxicity.
The adverse effects of hyperhomocysteinemia have been intensively studied and it has been proven to increase cardiovascular risk (17). There are several potential mechanisms that contribute to this effect: Vascular smooth muscle cell proliferation, decreased production of endothelial nitric oxide, alteration of oxidative stress leading to endothelial dysfunction, activation of the nuclear factor-κB (NF-κB), increased collagen production, and decreased arterial wall elasticity (18,19).
Homocysteine is involved in the atherogenic process by stimulating mRNA and C-reactive protein (CRP) expression in vascular smooth muscle cells, activating an inflammatory response (20). Higher serum homocysteine levels seem to be correlated with the severity of ischemic heart disease (21) and the effect of homocysteine on vascular endothelial integrity could increase blood pressure (22). Hyperhomocysteinemia has a higher risk of venous thrombosis because it is associated with increased concentrations of prothrombotic factors (particularly tissue plasminogen activator, β-thromboglobulin, and factor VIIc) and platelet adhesion to endothelial cells (13).
Vitamin B6 and B12 administration can reduce the concentration of homocysteine, but normalizing it has not been proven to improve endothelial function or decrease hypercoagulability (23). For this reason, classifying homocysteine as a cardiovascular risk factor is still under debate and recent guidelines have not included it in this category (24).
In RA patients, the effect of MTX on homocysteine metabolism is counterweighted by folic acid use. A small study on 113 RA patients treated with either MTX with folic/folinic acid or MTX without folic/folinic acid concluded that, although MTX increases plasma homocysteine levels, folate supplementation decreases homocysteine concentrations, subsequently protecting against potential cardiovascular risks (16).
4. MTX anti-inflammatory effects
Apart from hyperhomocystinemia and its antiproliferative effects, the mechanism of action of MTX is not entirely clear. An important aspect of its action is related to the anti-inflammatory and immunosuppressive effects in immune-mediated inflammatory diseases, which cannot be explained solely by the cytotoxic action on cells involved in inflammation and immune response.
MTX inhibits aminoimidazole carboxamide ribonucleotide transformylase (AICART), resulting in elevated intracellular AICAR levels (15). AICAR is an inhibitor of adenosine monophosphate (AMP) deaminase, so this inhibition will determine an increased level of intracellular AMP and adenosine (12). The high levels of AMP and adenosine are responsible for the anti-inflammatory effects of MTX. The drug has an agonist effect by binding to adenosine receptors (for example A2A) found on several cells involved in inflammation [e.g., natural killer cells, T cells, macrophages, monocytes, and neutrophils] (25). The consequences are suppression of T-cell activation and cell mediated cytotoxicity, inhibition of the neutrophil oxidative burst, and suppression of NF-κB (25). Stimulation of A2A prevents foam cell formation, induces synthesis of several proteins involved in cholesterol transport, and decreases adhesion molecule expression on endothelial cells, thus stalling atherosclerosis progression (26,27).
MTX also decreases production of pro-inflammatory cytokines (e.g., the tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6), with an extremely important role in atherosclerosis and immune-mediated inflammatory diseases as well (3,28). A recent study reaffirmed the complex effect of MTX on oxidative stress, as it not only of reduces it, but it also scavenges free radicals such as O2 and decreases the formation of malondialdehyde and acetaldehyde protein adducts, potentially favouring inflammatory cell apoptosis (29).
5. MTX and cardiovascular risk reduction
Recent studies have evaluated the effect of MTX on traditional cardiovascular risk factors. HDL function and HDL-associated proteins were investigated in a group of 550 RA patients included in the Treatment of Early Aggressive Rheumatoid Arthritis (TEAR) trial, given either MTX monotherapy or MTX combined with etanercept or other synthetic DMARDs for more than 2 years (30). The authors concluded that disease activity improvements obtained after MTX treatment in different combinations led to a correction in the HDL function. This could turn into a potential therapeutic objective for cardiovascular risk reduction in RA patients. Authors of a different study looking at the potential antiatherosclerotic effect on a cell model demonstrated that the adenosine A2A receptor can be activated by MTX, limiting foam cell formation and stimulating reverse cholesterol transport (27).
Similar data on serum lipoprotein(a) [Lp(a)] and endothelial adhesion molecule E-selectin were obtained in another study performed on 64 RA patients on MTX monotherapy or combined with a TNF inhibitor (31). In both treatment groups, there was a significant decrease in sLp(a) and E-selectin serum levels after 6 weeks and after 6 months (for sLp(a) mainly in the MTX group). This suggests that MTX might also reduce sLp(a), a proatherogenic factor, due to its anti-inflammatory effect.
Interesting data were provided by the Psoriatic arthritis, Ankylosing spondylitis, Rheumatoid Arthritis Study (PSARA), which included 114 patients treated with MTX alone, with a TNF inhibitor as monotherapy, or a TNF-inhibitor in combination with MTX (32). The study was conducted to assess the effect of these different treatment regimens on endothelial function for 6 months. Endothelial function was evaluated by reactive hyperemia peripheral arterial tonometry (RH-PAT), expressed by a parameter called Reactive Hyperemic Index (a ratio between the magnitude of the average post obstructive and pre-occlusion pulse wave amplitude). The study demonstrated that endothelial function improved after 6 months. Furthermore, the improvement was higher in patients receiving MTX as monotherapy, compared with those treated with a TNF blocker with or without MTX, and was not correlated with improvements in disease activity. These observations suggest that the effect of MTX on endothelial function might go beyond its well-known anti-inflammatory properties.
Measuring the carotid intima-media thickness (CIMT) or the femoral intima-media thickness (FIMT) is useful for detecting subclinical atherosclerosis. In a study performed on RA patients treated with different treatment regimens, CIMT, FIMT, and the prevalence of plaques were lower in patients treated with MTX (≥20 mg/week), cyclosporine, or biologics, than in patients treated with lower doses of MTX or other DMARDs (33). Similar results were found in a different study which measured CIMT in RA patients versus healthy subjects, finding overall higher CIMT in RA patients and significantly lower CIMT in RA patients with MTX versus RA patients without MTX (34).
In a repeat cross-sectional study, pulse wave velocity and blood pressure were measured in RA patients treated with MTX or different synthetic or biological DMARDs at baseline and after eight months (35). The results showed that MTX was able to control arterial stiffening and to prevent blood pressure (mainly systolic) elevation. These effects were not observed in patients on different DMARDs.
Results of a systematic review and meta-analysis of clinical trials and observational studies (prospective, retrospective, or case-control studies) investigating patients with immune-mediated inflammatory diseases (mainly RA) taking MTX, to assess the incidence of major adverse cardiac events (e.g., stroke, MI, coronary artery disease, and sudden cardiac death), showed a 21% overall lower cardiovascular risk and an 18% lower MI risk in patients on MTX, with a comparable inverse relationship with stroke as well (36).
There are data showing that in patients with immunemediated inflammatory diseases (mainly RA, PsA, and psoriasis) MTX has a limited influence on platelet function, insulin resistance, and lipid levels, but that it significantly changes inflammatory biomarkers such as CRP, TNF-α, or IL-6 (37–39). MTX's protective effect on cardiovascular events is thought to be mainly due to its anti-inflammatory mechanism. Moreover, this data highlights the important role of inflammation in atherosclerosis. Anti-inflammatory drugs might be a useful therapeutic tool in patients with immune-mediated inflammatory diseases in order to decrease cardiovascular risk.
A meta-analysis of 7 studies on patients with RA treated with MTX was performed to assess the potential association of chronic MTX use with risk for any major acute cardiovascular event (40). It concluded that MTX use was associated with lower cardiovascular risk due to a different mechanism than that of antiplatelet drugs or statins.
As revealed in the larger context of meta-analyses and systematic literature reviews (41), MTX and TNF blockers demonstrated the capacity to reduce the risk of all cardiovascular events, including MI. On the other hand, corticosteroid and NSAID use have a significantly higher overall cardiovascular risk. MTX has been associated with significant survival improvements as well (up to 70%), primarily by decreasing cardiovascular mortality (42,43).
Nevertheless, despite their anti-inflammatory effects and their ability to improve disease activity, other synthetic DMARDs (like leflunomide or sulfasalazine) have not shown a similar effect on cardiovascular risk or the capacity to influence mortality in RA patients (44).
These positive effects of MTX on cardiovascular risk has also caught the attention of cardiologists. Since the role of inflammation in atherosclerosis is well established, it will be of great interest to find whether decreasing inflammation with anti-inflammatory drugs can also reduce cardiovascular events in patients with other types of conditions. A first trial in this regard is The Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS) (45). Canakinumab is a monoclonal IL-1β antibody, an important pro-inflammatory cytokine, involved not only in several immune-mediated inflammatory diseases, but also in atherosclerosis. This double-blind, randomized trial included 10,061 patients with a history of MI and high CRP levels and treated them with either canakinumab or placebo. Nonfatal stroke, MI, or cardiovascular death were the primary efficacy endpoints, and trial results showed that canakinumab led to significantly lower rates of recurrent cardiovascular events than placebo. Since canakinumab has no lipid-level lowering effects, this effect is due to IL-1β blockade and the anti-inflammatory consequences of this inhibition.
A potent anti-inflammatory and less expensive drug such as MTX was also taken into account with respect to its ability to reduce cardiovascular risk. As discussed before, although there are many trials evaluating the effect of MTX on cardiovascular risk in patients with immune-mediated rheumatic diseases, most clinical data supporting a cardiovascular benefit come from interventional or observational studies that have many limitations (e.g., small sample sizes, limited control of bias, absence of a control arm).
The Cardiovascular Inflammation Reduction Trial (CIRT) (46) was designed to shed more evidence on this topic, since until recently, there were few data regarding the consequences of reducing inflammation on cardiovascular risk. In this clinical trial, approximately 7,000 patients will be randomized to receive either MTX (target dose 15–20 mg/week, the same as in most immune-mediated inflammatory diseases) or placebo over a 3–5 year follow-up period (41). All the participants in this trial have increased cardiovascular risk: Either metabolic syndrome or type 2 diabetes and past MI. The primary endpoint established in the CIRT trial is a combination of nonfatal stroke or MI and cardiovascular death, whereas its secondary endpoints are notably coronary revascularization, hospitalization for congestive heart failure mortality, and all-cause mortality. MTX was the perfect choice for this trial due to its noteworthy systemic anti-inflammatory effects, bearing no important blood pressure or lipid-related influence. The results from this trial are expected in the following years and, provided its endpoints are reached, CIRT would reinforce the inflammatory hypothesis of atherosclerosis and promote the development of new therapeutic agents specifically targeting vascular inflammation.
6. Conclusion
The global effects of MTX in patients with immune-mediated inflammatory diseases (e.g., RA and PsA) are associated with a decreased cardiovascular risk. This effect has been noticed despite the induction of hyperhomocysteinemia and the lack of complete understanding of MTX's mechanisms of action. Given its systemic anti-inflammatory effect and its ability to slow the progression of atherosclerosis, MTX should be regarded as an important therapeutic agent not only to control rheumatic diseases, but also to reduce cardiovascular risk and mortality.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ARB, VCB, MB, TD and SMB contributed to writing and preparing the manuscript, and collecting the relevant literature. All authors read and approved the final version of manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford) 2014;53:2143–2154. doi: 10.1093/rheumatology/keu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.del R, incón I, Polak JF, O'Leary DH, Battafarano DF, Erikson JM, Restrepo JF, Molina E, Escalante A. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis. 2015;74:1118–1123. doi: 10.1136/annrheumdis-2013-205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Breukelen-van der Stoep DF, Klop B, van Zeben D, Hazes JM, Castro Cabezas M. Cardiovascular risk in rheumatoid arthritis: How to lower the risk? Atherosclerosis. 2013;231:163–172. doi: 10.1016/j.atherosclerosis.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Soubrier M, Barber Chamoux N, Tatar Z, Couderc M, Dubost JJ, Mathieu S. Cardiovascular risk in rheumatoid arthritis. Joint Bone Spine. 2014;81:298–302. doi: 10.1016/j.jbspin.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Aviña-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008;59:1690–1697. doi: 10.1002/art.24092. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewé R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. 2014;73:3–5. doi: 10.1136/annrheumdis-2013-204317. [DOI] [PubMed] [Google Scholar]
- 7.Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: A systematic review of the literature. Ann Rheum Dis. 2009;68:1094–1099. doi: 10.1136/ard.2008.092668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Lin CS. Plasma levels of D-dimer in a 5-year-old girl with systemic juvenile idiopathic arthritis: A case report and literature review. Exp Ther Med. 2016;11:1027–1030. doi: 10.3892/etm.2016.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Chen Z, Yang S, Wang Y, Yu L, Zhang B, Rao Z, Gao J, Tu S. (1)H NMR-based metabolomic analysis for identifying serum biomarkers to evaluate methotrexate treatment in patients with early rheumatoid arthritis. Exp Ther Med. 2012;4:165–171. doi: 10.3892/etm.2012.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Negrei C, Bojinca V, Balanescu A, Bojinca M, Baconi D, Spandidos DA, Tsatsakis AM, Stan M. Management of rheumatoid arthritis: Impact and risks of various therapeutic approaches. Exp Ther Med. 2016;11:1177–1183. doi: 10.3892/etm.2016.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochberg M, Silman AJ, Smolen J, Weinblatt M, Weisman M. Rheumatology. 6th. Vol. 1. Elsevier; 2015. Methotrexate; pp. 442–450. [Google Scholar]
- 13.Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Negrei C, Căruntu C, Ginghină O, Burcea Dragomiroiu G, Toderescu C, Boda D. Qualitative and quantitative determination of methotrexate polyglutamates in erythrocytes by high performance liquid chromatography. Rev Chim. 2015;66:607–610. [Google Scholar]
- 15.Chan ES, Cronstein BN. Methotrexate-how does it really work? Nat Rev Rheumatol. 2010;6:175–178. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 16.van Ede AE, Laan RF, Blom HJ, Boers GH, Haagsma CJ, Thomas CM, De Boo TM, van de Putte LB. Homocysteine and folate status in methotrexate-treated patients with rheumatoid arthritis. Rheumatology (Oxford) 2002;41:658–665. doi: 10.1093/rheumatology/41.6.658. [DOI] [PubMed] [Google Scholar]
- 17.Peng HY, Man CF, Xu J, Fan Y. Elevated homocysteine levels and risk of cardiovascular and all-cause mortality: A meta-analysis of prospective studies. J Zhejiang Univ Sci B. 2015;16:78–86. doi: 10.1631/jzus.B1400183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Bai YY, Luo LM, Xiao WK, Wu HM, Ye P. Association between serum homocysteine and arterial stiffness in elderly: A community-based study. J Geriatr Cardiol. 2014;11:32–38. doi: 10.3969/j.issn.1671-5411.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu A, Jenkins AJ, Stoner JA, Thorpe SR, Klein RL, Lopes-Virella MF, Garvey WT, Lyons TJ. DCCT/ EDIC Research Group: Plasma total homocysteine and carotid intima-media thickness in type 1 diabetes: A prospective study. Atherosclerosis. 2014;236:188–195. doi: 10.1016/j.atherosclerosis.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang X, Liu J, Zhao J, Mao J, Zhang X, Feng L, Han C, Li M, Wang S, Wu D. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-κB signal pathway in rat vascular smooth muscle cells. Atherosclerosis. 2014;236:73–81. doi: 10.1016/j.atherosclerosis.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem. 2014;29:339–344. doi: 10.1007/s12291-013-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002;156:1105–1113. doi: 10.1093/aje/kwf157. [DOI] [PubMed] [Google Scholar]
- 23.Peeters AC, van der Molen EF, Blom HJ, den Heijer M. The effect of homocysteine reduction by B-vitamin supplementation on markers of endothelial dysfunction. Thromb Haemost. 2004;92:1086–1091. doi: 10.1160/TH04-05-0284. [DOI] [PubMed] [Google Scholar]
- 24.Mangge H, Becker K, Fuchs D, Gostner JM. Antioxidants, inflammation and cardiovascular disease. World J Cardiol. 2014;6:462–477. doi: 10.4330/wjc.v6.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cronstein B. How does methotrexate suppress inflammation? Clin Exp Rheumatol. 2010;28(Suppl 61):S21–S23. [PubMed] [Google Scholar]
- 26.Reiss AB, Rahman MM, Chan ES, Montesinos MC, Awadallah NW, Cronstein BN. Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol. 2004;76:727–734. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 27.Reiss AB, Carsons SE, Anwar K, Rao S, Edelman SD, Zhang H, Fernandez P, Cronstein BN, Chan ES. Atheroprotective effects of methotrexate on reverse cholesterol transport proteins and foam cell transformation in human THP-1 monocyte/macrophages. Arthritis Rheum. 2008;58:3675–3683. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman MC, Clemens DL, Duryee MJ, Sarmiento C, Chiou A, Hunter CD, Tian J, Klassen LW, O'Dell JR, Thiele GM, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. 2017;13:588–593. doi: 10.1016/j.redox.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charles-Schoeman C, Yin Lee Y, Shahbazian A, Wang X, Elashoff D, Curtis JR, Navarro-Millán I, Yang S, Chen L, Cofield SS, et al. Improvement of high-density lipoprotein function in patients with early rheumatoid arthritis treated with methotrexate monotherapy or combination therapies in a randomized controlled trial. Arthritis Rheumatol. 2017;69:46–57. doi: 10.1002/art.39833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hjeltnes G, Hollan I, Førre O, Wiik A, Lyberg T, Mikkelsen K, Agewall S. Serum levels of lipoprotein(a) and E-selectin are reduced in rheumatoid arthritis patients treated with methotrexate or methotrexate in combination with TNF-α-inhibitor. Clin Exp Rheumatol. 2013;31:415–421. [PubMed] [Google Scholar]
- 32.Deyab G, Hokstad I, Whist JE, Smastuen MC, Agewall S, Lyberg T, Ronda N, Mikkelsen K, Hjeltnes G, Hollan I. Methotrexate and anti-tumor necrosis factor treatment improves endothelial function in patients with inflammatory arthritis. Arthritis Res Ther. 2017;19:232. doi: 10.1186/s13075-017-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kisiel B, Kruszewski R, Juszkiewicz A, Raczkiewicz A, Bachta A, Tłustochowicz M, Staniszewska-Varga J, Kłos K, Duda K, Bogusławska-Walecka R, et al. Methotrexate, cyclosporine A, and biologics protect against atherosclerosis in rheumatoid arthritis. J Immunol Res. 2015;2015:759610. doi: 10.1155/2015/759610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HJ, Kim MJ, Lee CK, Hong YH. Effects of methotrexate on carotid intima-media thickness in patients with rheumatoid arthritis. J Korean Med Sci. 2015;30:1589–1596. doi: 10.3346/jkms.2015.30.11.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodman RJ, Baghdadi LR, Shanahan ME, Mangoni AA. The temporal relationship between arterial stiffening and blood pressure is modified by methotrexate treatment in patients with rheumatoid arthritis. Front Physiol. 2017;8:593. doi: 10.3389/fphys.2017.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micha R, Imamura F, Wyler von Ballmoos M, Solomon DH, Hernán MA, Ridker PM, Mozaffarian D. Systematic review and meta-analysis of methotrexate use and risk of cardiovascular disease. Am J Cardiol. 2011;108:1362–1370. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westlake SL, Colebatch AN, Baird J, Kiely P, Quinn M, Choy E, Ostor AJ, Edwards CJ. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: A systematic literature review. Rheumatology (Oxford) 2010;49:295–307. doi: 10.1093/rheumatology/keq736. [DOI] [PubMed] [Google Scholar]
- 38.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S. Patterns of cardiovascular risk in rheumatoid arthritis. Ann Rheum Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation. 2007;116:2346–2355. doi: 10.1161/CIRCULATIONAHA.106.678334. [DOI] [PubMed] [Google Scholar]
- 40.De Vecchis R, Baldi C, Palmisani L. Protective effects of methotrexate against ischemic cardiovascular disorders in patients treated for rheumatoid arthritis or psoriasis: Novel therapeutic insights coming from a meta-analysis of the literature data. Anatol J Cardiol. 2016;16:2–9. doi: 10.5152/akd.2015.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wasko MC, Dasgupta A, Hubert H, Fries JF, Ward MM. Propensity-adjusted association of methotrexate with overall survival in rheumatoid arthritis. Arthritis Rheum. 2013;65:334–342. doi: 10.1002/art.37723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: A prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 44.Tabit CE, Holbrook M, Shenouda SM, Dohadwala MM, Widlansky ME, Frame AA, Kim BH, Duess MA, Kluge MA, Levit A, et al. Effect of sulfasalazine on inflammation and endothelial function in patients with established coronary artery disease. Vasc Med. 2012;17:101–107. doi: 10.1177/1358863X12440117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. CANTOS Trial Group: Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 46.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, et al. Rationale and design of the Cardiovascular Inflammation Reduction Trial: A test of the inflammatory hypothesis of atherothrombosis. Am Heart J. 2013;166:199–207.e15. doi: 10.1016/j.ahj.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.