Abstract
Background:
The aim of this study was to determine whether insulin resistance, beta-cell function, and their associations with alanine aminotransferase (ALT) are affected by the functional variants of paraoxonase-2 (PON2) as an intracellular antioxidant in patients with type 2 diabetes (T2D).
Materials and Methods:
Quantitative insulin sensitivity check index (QUICKI) and homeostasis model assessment for beta-cell function (HOMA-BCF) were assessed in T2D patients. Insulin levels were determined using ELISA. The variants PON2-A148G and PON2-S311C were genotyped using polymerase chain reaction-based restriction fragment length polymorphism.
Results:
According to the PON2-G148A variant, ALT was found to be significantly correlated with QUICKI (r = −0.616, P = 0.005) and HOMA-BCF (r = 0.573, P = 0.01) in the GA + GG group; however, the correlations were not statistically significant in the AA genotypes. Based on the genotypes of PON2-S311C, there was a significant correlation between ALT with QUICKI (r = −0.540, P = 0.031) and HOMA-BCF (r = 0.567, P = 0.022) in the SC + CC group. In the multiple adjusted logistic regression analyses, considering the variants PON2-G148A and PON2-C311S as independent variables and QUICKI and HOMA-BCF as the dependent variables, both variants were significantly associated with the QUICKI (P = 0.019 for PON2-G148A and P = 0.041 for PON2-C311S). Furthermore, PON2-C311S remained significantly associated with HOMA-BCF (P = 0.03).
Conclusion:
These data implicate a role for the functional variants of PON2 in insulin resistance and beta-cell function as well as underscore the effective role of these variants in the associations between them and ALT. Our data contribute to our understanding of the important physiologic functions of PON2 in glucose metabolism and its related metabolic diseases.
Keywords: Alanine aminotransferase, beta-cell, insulin resistance, paraoxonase-2, type 2 diabetes
INTRODUCTION
Approximately 90%–95% of patients with diabetes are classified as type 2 diabetes (T2D), which arises from progressive failure of pancreatic beta-cell function in the presence of chronic insulin resistance.[1,2] Insulin resistance is a complex process, and more investigations are needed for understanding the multifactorial etiology of this process in cellular and systemic levels.[1] A number of studies have demonstrated the role and importance of oxidative stress in insulin resistance, beta-cell function, and alanine aminotransferase (ALT) modulation.[3,4,5] Oxidative stress-mediated antioxidant response can protect cells from oxidative damage, and among the antioxidants, induction of antioxidant enzymes can increase cellular reactive oxygen species (ROS)-scavenging capacity.[5]
Paraoxonase-2 (PON2) is a member of paraoxonase family, which consists of three members: PON1, PON2, and PON3.[6] Unlike the other two members, PON2 is an intracellular enzyme localized in the mitochondria and in the endoplasmic reticulum, where it scavenges ROS.[7,8,9] The antioxidant and anti-inflammatory properties of PON2 along with ubiquitous expression in nearly every human tissue have caused PON2 to have an important physiological role in host defense.[10] The enzyme gene contains two common coding variants: serine (S) to cysteine (C) substitution at codon 311 (S311C; rs6954345) and alanine (A) to glycine (G) substitution at codon 148 (A148G; rs11545941).[9,11] According to studies, these variants are associated with diabetic nephropathy and cardiovascular complications in diabetes and could influence fasting plasma glucose.[12,13]
In this study, we focused on PON2 as an ignored antioxidant enzyme and analyzed cross-sectionally the effects of its functional variants on quantitative insulin sensitivity check index (QUICKI)-estimated insulin resistance and homeostasis model assessment for beta-cell function (HOMA-BCF)-estimated beta-cell function and evaluated whether these variants influence their interrelationships with ALT in patients with T2D. The importance of this issue becomes more apparent when one sees that QUICKI, HOMA-BCF, ALT, and their interrelationships are independent predictors of T2D.[14,15] In addition, it should be noted that ALT could be used as predictor of cardiovascular disease, which is the main cause of death among patients with T2D.[14,16]
MATERIALS AND METHODS
Study population
This cross-sectional study conducted on 40 Iranian patients with T2D aged 40–65 years from January 2016 to February 2017. The study was performed in Northern Province of Iran, Mazandaran. All patients were diagnosed according to the WHO criteria.[17] The participants underwent a physical examination, and data including medical history, medication, and personal habits were collected through a questionnaire. All the patients were taking metformin (1000–1500 mg/day). Some of the patients were receiving antihypertensive medication including losartan, an angiotensin-converting enzyme inhibitor, or a beta blocker. Type 1 diabetes, insulin therapy, chronic hepatic disease, renal failure, autoimmune diseases, and malignant diseases were exclusion criteria. The protocol study was performed in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethical Committee at Mazandaran University of Medical Sciences (ethnic number: 1394-4). All participants received written informed consent.
Biochemical analyses
Levels of fasting glucose, ALT, total cholesterol (TC), triglycerides (TGs), and high-density lipoprotein-cholesterol (HDL-C) were assessed on the basis of standard enzymatic methods using an automated analyzer (Prestige, Japan). The low-density lipoprotein-cholesterol (LDL-C) levels were calculated according to the Friedewald formula. The hemoglobin A1c (HbA1c) levels were determined by a NycoCard HbA1c kit (Oslo, Norway). Insulin concentrations were measured by ELISA kit (Demeditec Diagnostics GmbH, Lise-Meitner-Straée, Germany). Intra-assay coefficient of variation (CV) was 1.8%, and inter-assay CV was 6.0%. Fasting glucose and insulin levels were used to calculate the indices QUICKI and HOMA-BCF using the following equations:[18,19,20]
QUICKI = 1/[log fasting insulin (μU/mL) + log fasting glucose (mg/dL)].
HOMA-BCF = 20 × Fasting insulin (μU/mL)/[Fasting glucose (mmol/L) −3.5].
Genetic analyses
The study variants were genotyped using polymerase chain reaction (PCR)-based restriction fragment length polymorphism (RFLP) analysis. The forward primer 5’ AATCAGTGTGTCATTGTGG 3’ and reverse primer 5’ GAGGCTACAGAACTTCC 3’ amplified a 190-bp product containing the variant PON2-S311C.[21] PCR products were quantified by 1.5% gel electrophoresis and were visualized by green viewer staining. Amplification products were digested using the restriction enzyme DdeI (Thermo Scientific, Lithuania) and resulted in 121- and 69-bp fragments, which were subjected to electrophoresis on a 2.5% agarose gel. Considering that the variant PON2-A148G did not alter a restriction site, a mismatch PCR/RFLP was used for its genotyping (GCA → GGA substitution), as previously described.[22] For this, work forward primer 5’ AGTGGAAATTTTTAAATTTGAAGCAG 3’ and reverse primer 5’ TTGTTTGCAAATGCTGGGGAT 3’ were used.[21] PCR and cycling were the same as above except that the annealing step was made at 53°C. The PCR products (130 bp) were digested with the restriction enzyme SatI (Thermo Scientific, Lithuania) and resulted in 106- and 24-bp fragments, which were subjected to electrophoresis on a 4% agarose gel.
Statistical analyses
The differences of the parametric variables were analyzed using t-test, and the nonparametric variables were tested by Mann–Whitney test. The study variants were tested for deviations from Hardy–Weinberg equilibrium (HWE) expectations by Chi-square test. Spearman's correlation coefficient was used to analyze the association between the study parameters. We used linear regression analyses to estimate the independent association of the study variants with QUICKI and HOMA-BCF. Two-tailed P < 0.05 was considered to be statistically significant. Statistical analyses were performed by the software SPSS (version 16.0, SPSS, Chicago, IL, USA) and R (version 3.0.1).
RESULTS
Genotype distribution and allele frequency of PON2 variants
The genotypes frequencies were 50.0% AA, 42.5% AG, and 7.5% GG for PON2-A148G variant and 57.5% SS, 35.0% SC, and 7.5% CC for PON2-S311C in the study population. The frequencies of the A and G allele were found to be 0.71 and 0.29, respectively, and for the S and C allele were 0.75 and 0.25, respectively. There was no deviation from HWE for the study variants.
Changes in the study parameters according to the genotypes of PON2-A148G and PON2-S311C
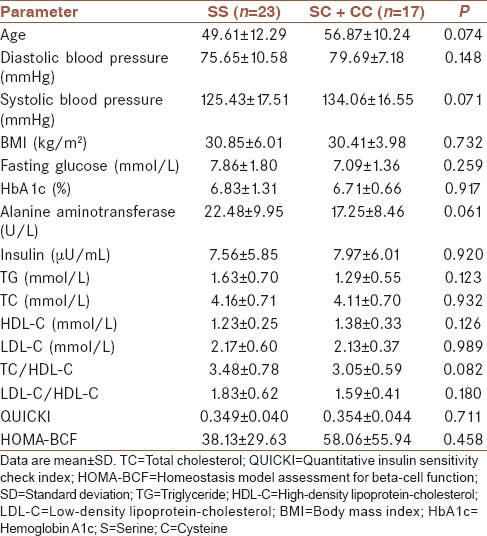
As shown in Tables 1 and 2, there were no significant differences between the genotypes of PON2-A148G (AA and AG + GG group) and PON2-S311C (SS and SC + CC group) with respect to the most studied parameters including age, systolic and diastolic blood pressure, TG, TC, LDL-C, HDL-C, fasting glucose, HbA1c, insulin, HOMA-BCF, and QUICKI levels.
Table 1.
Changes in the study parameters according to the genotypes of PON2-A148G
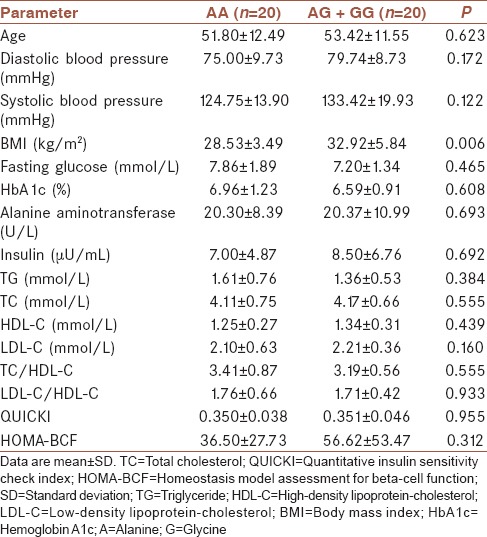
Table 2.
Changes in the study parameters according to the genotypes of PON2-S311C
Correlations between quantitative insulin sensitivity check index and homeostasis model assessment for beta-cell function with alanine aminotransferase in total data and according to good and poor glycemic control
Our findings showed that ALT was significantly and negatively correlated with QUICKI (r = −0.338, P = 0.033) and positively correlated with HOMA-BCF (r = 0.245, P = 0.127) in total data. In both good glycemic control and poor glycemic control,[23] ALT was negatively correlated with QUICKI, but the correlation was stronger in patients with poor glycemic control (r = −0.551, P = 0.064) compared with good glycemic controls (r = −0.272, P = 0.17). In addition, in both good glycemic control and poor glycemic control, ALT was positively correlated with HOMA-BCF, but the correlation was stronger in patients with poor glycemic control (r = 0.451, P = 0.141) in comparison with good glycemic control (r = 0.144, P = 0.475).
Correlations between quantitative insulin sensitivity check index and homeostasis model assessment for beta-cell function with alanine aminotransferase according to the genotypes of PON2-A148G and PON2-S311C
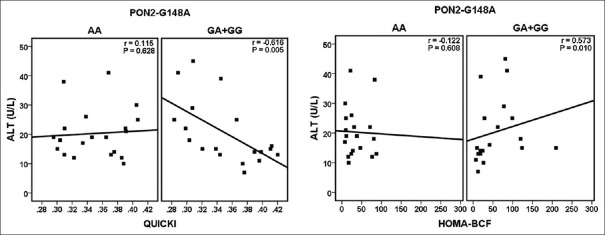
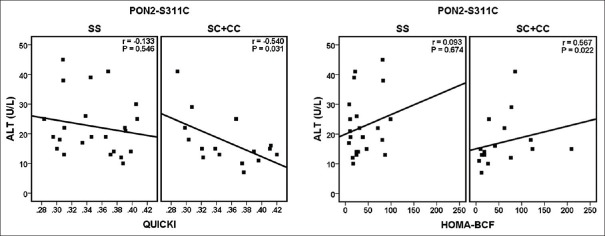
As shown in Figure 1, based on the genotypes of PON2-A148G, ALT was found to be significantly and negatively correlated with QUICKI (r = −0.616, P = 0.005) in the GA + GG group; however, the correlations were not statistically significant in the AA genotypes (r = 0.115, P = 0.628). Furthermore, only in the GA + GG group, there was statistically significant correlation between ALT and HOMA-BCF (r = 0.573, P = 0.01). Based on the genotypes of PON2-S311C, there was a negative and significant correlation between ALT and QUICKI (r = −0.540, P = 0.031) in the SC + CC group. In people with SS genotypes, the correlations were still negative although were not significant (r = −0.133, P = 0.546) [Figure 2]. In addition, ALT was found to be positively correlated with HOMA-BCF in the two groups; however, only the correlation in the SC + CC group was statistically significant (r = 0.567, P = 0.022).
Figure 1.
Associations between quantitative insulin sensitivity check index (QUICKI) and homeostasis model assessment for beta-cell function (HOMA-BCF) with alanine aminotransferase according to the genotypes of PON2-A148G
Figure 2.
Associations between quantitative insulin sensitivity check index (QUICKI) and homeostasis model assessment for beta-cell function (HOMA-BCF) with alanine aminotransferase according to the genotypes of PON2-S311C
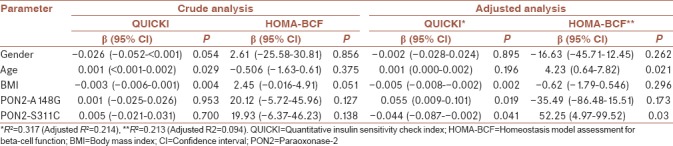
The effects of PON2 functional variants on quantitative insulin sensitivity check index and homeostasis model assessment for beta-cell function using regression analyses
First, crude analysis by simple logistic regression did not show a significant association between the study variants with QUICKI and HOMA-BCF. However, the adjusted analysis (for age, sex, and body mass index) was significantly different from the crude analysis. In the multiple analysis, considering QUICKI and HOMA-BCF as the dependent variables and the variants PON2-G148A and PON2-C311S as independent variables, both variants were significantly associated with the QUICKI (P = 0.019 for PON2-G148A and P = 0.041 for PON2-C311S). Furthermore, PON2-C311S remained significantly associated with HOMA-BCF (P = 0.03) [Table 3]. It should be noted that the interaction analysis of study two variants was not statistically significant (results not shown).
Table 3.
Simple and multiple linear regression analyses for associations of the study variants with quantitative insulin sensitivity check index and homeostasis model assessment for beta-cell function
DISCUSSION
In the present study, as expected, insulin resistance in accordance with the previous investigations was significantly correlated with ALT.[24] When we analyzed the associations according to glycemic control, our findings showed that the correlations of both indices QUICKI and HOMA-BCF with ALT become stronger in patients with poor glycemic control. Although the association of ALT and these two indices has not been studied in previous investigations (to our knowledge), generally, in accordance with previous studies, our results confirm that the association of ALT and insulin resistance was potentiated in poorer glycemic control.[25]
When the analyses were performed according to PON2-G148A variant, our findings indicated that there were significant correlations between QUICKI and HOMA-BCF with ALT only in the GA + GG group but not in the AA genotypes. On the other hand, in the G allele-carrying genotypes, insulin resistance and beta-cell function significantly correlated with the ALT in patients with T2D. Our analyses according to the PON2-S311C variant revealed that significant correlations between QUICKI and HOMA-BCF with ALT exist only in the SC + CC group but not in the SS genotypes. These findings indicate that both variants may potentially influence these interrelationships. ALT is a biochemical marker of liver injury, the organ which is a major site for insulin-mediated glucose uptake, production, and storage.[26] PON2 contributes to reducing oxidative stress and inflammatory responses,[10] the important features that can markedly influence liver. It should be noted that the role of this organ in glucose metabolism is strongly affected by oxidative and inflammatory stimuli, which in turn would affect whole-body insulin responsiveness.[26]
We further analyzed the strength of the relationship between each variant with QUICKI and HOMA-BCF according to regression analyses to clarify whether these variants independently associate with insulin resistance and beta-cell function in T2D. Our findings showed that PON2-G148A and PON2-S311C, as functional variants in the PON2 gene, were independently associated with these factors in the multiple regression analyses. There were no similar studies to our knowledge in the literature to compare the association of PON2 variants with insulin resistance and beta-cell function. However, our findings are generally supported by the results obtained by Bourquard et al.[26] that investigated the role of PON2 in the mechanistic etiology of insulin resistance. The researchers demonstrated that in the livers of PON2-deficient mice, the modifications in insulin-mediated phosphorylation of insulin receptor substrate-1 could result in the impairment of the hepatic insulin signaling and they concluded that increased susceptibility against atherosclerosis in mice is associated with impairment in this signaling. Furthermore, they showed that macrophages from PON2-deficient mice directly affect insulin action in hepatocytes.
In addition, it appears that one of the reasons for contribution of PON2 in insulin resistance and beta-cell function and their associations with ALT lies in the fact that PON2 is necessary for the proper functioning of the mitochondria so that PON2 deficiency may lead to mitochondrial dysfunction, something that would affect insulin resistance and ALT.[9] Furthermore, it should be noted that PON2 affects electron transport chain function in the inner mitochondrial membrane and thus produces ATP, which is the main coupling messenger in insulin secretion.[26,27] In addition, stress in the endoplasmic reticulum, which is another cellular site for PON2, can affect the development of insulin resistance.[28]
PON2 is an antiatherogenic enzyme, and the mechanisms for this important property include its ability to protect against the oxidation of lipoproteins, particularly LDL, to regulate monocyte chemotaxis, and to reduce inflammatory response and intracellular oxidative stress.[10] Thus, preservation of PON2 status (activity and gene expression) can be useful in the improvement of cardiovascular complications, which is the main cause of mortality in patients with T2D.[16] In this context, natural compounds can be important. Accordingly, Yehuda et al.[29] demonstrated that glabridin, which is a licorice root-derived isoflavan, preserves the antiatherogenic capacities of PON2 through maintaining its concentrations, in vivo. The researchers also found that this isoflavan can protect PON2 activity under normal and high glucose conditions.[29] Hence, the consumption of antioxidants such as glabridin can have useful effects in the improvement of diabetes and in decreasing its complications.
The small sample size should be considered as a limitation of the current study, and thus, a larger sample size would have allowed to clarify and confirm these findings. Association study of two functional variants in the coding region of PON2 as an ignored antioxidant enzyme with clinical parameters in T2D can be considered as the strong point of this study.
CONCLUSION
Results from the present study implicate a role for the functional variants of PON2 in insulin resistance and beta-cell function as well as underscore the effective role of these variants in the associations between them and ALT. Our data contribute to our understanding of the important physiologic functions of PON2 in glucose metabolism and its related metabolic diseases.
Financial support and sponsorship
This study was funded by Mazandaran University of Medical Sciences under the Grant No. 1394.4.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 3.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–75. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, et al. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189:198–205. doi: 10.1016/j.atherosclerosis.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahrooz A. Pharmacological interactions of paraoxonase 1 (PON1): A HDL-bound antiatherogenic enzyme. Curr Clin Pharmacol. 2016;11:259–64. doi: 10.2174/1574884711666160915153433. [DOI] [PubMed] [Google Scholar]
- 7.Furlong CE, Marsillach J, Jarvik GP, Costa LG. Paraoxonases-1, -2 and -3: What are their functions? Chem Biol Interact. 2016;259:51–62. doi: 10.1016/j.cbi.2016.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li HL, Liu DP, Liang CC. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J Mol Med (Berl) 2003;81:766–79. doi: 10.1007/s00109-003-0481-4. [DOI] [PubMed] [Google Scholar]
- 9.Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, et al. Developmental expression of paraoxonase 2. Chem Biol Interact. 2016;259:168–74. doi: 10.1016/j.cbi.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng CJ, Bourquard N, Grijalva V, Hama S, Shih DM, Navab M, et al. Paraoxonase-2 deficiency aggravates atherosclerosis in mice despite lower apolipoprotein-B-containing lipoproteins: Anti-atherogenic role for paraoxonase-2. J Biol Chem. 2006;281:29491–500. doi: 10.1074/jbc.M605379200. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta S, Demirci FY, Dressen AS, Kao AH, Rhew EY, Ramsey-Goldman R, et al. Association analysis of PON2 genetic variants with serum paraoxonase activity and systemic lupus erythematosus. BMC Med Genet. 2011;12:7. doi: 10.1186/1471-2350-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calle R, McCarthy MI, Banerjee P, Zeggini E, Cull CA, Thorne KI, et al. Paraoxonase 2 (PON2) polymorphisms and development of renal dysfunction in type 2 diabetes: UKPDS 76. Diabetologia. 2006;49:2892–9. doi: 10.1007/s00125-006-0436-8. [DOI] [PubMed] [Google Scholar]
- 13.Hegele RA, Connelly PW, Scherer SW, Hanley AJ, Harris SB, Tsui LC, et al. Paraoxonase-2 gene (PON2) G148 variant associated with elevated fasting plasma glucose in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:3373–7. doi: 10.1210/jcem.82.10.4289. [DOI] [PubMed] [Google Scholar]
- 14.Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–95. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 15.Cho NH, Jang HC, Choi SH, Kim HR, Lee HK, Chan JC, et al. Abnormal liver function test predicts type 2 diabetes: A community-based prospective study. Diabetes Care. 2007;30:2566–8. doi: 10.2337/dc07-0106. [DOI] [PubMed] [Google Scholar]
- 16.Farias JM, Tinetti M, Khoury M, Umpierrez GE. Low testosterone concentration and atherosclerotic disease markers in male patients with type 2 diabetes. J Clin Endocrinol Metab. 2014;99:4698–703. doi: 10.1210/jc.2014-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahrooz A, Alizadeh A, Hashemi-Soteh MB, Ghaffari-Cherati M, Hosseyni-Talei SR. Polymorphic variants rs3088442 and rs2292334 in the organic cation transporter 3 (OCT3) gene and susceptibility against type 2 diabetes: Role of their interaction. Arch Med Res. 2017;48:162–8. doi: 10.1016/j.arcmed.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 19.Kashi Z, Masoumi P, Mahrooz A, Hashemi-Soteh MB, Bahar A, Alizadeh A. The variant organic cation transporter 2 (OCT2)-T201M contribute to changes in insulin resistance in patients with type 2 diabetes treated with metformin. Diabetes Res Clin Pract. 2015;108:78–83. doi: 10.1016/j.diabres.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Bullą M, Moreno-Navarrete JM, Fernández-Real JM, Salas-Salvadą J. Total and undercarboxylated osteocalcin predict changes in insulin sensitivity and β cell function in elderly men at high cardiovascular risk. Am J Clin Nutr. 2012;95:249–55. doi: 10.3945/ajcn.111.016642. [DOI] [PubMed] [Google Scholar]
- 21.Mahrooz A, Hashemi-Soteh MB, Heydari M, Boorank R, Ramazani F, Mahmoudi A, et al. Paraoxonase 1 (PON1)-L55M among common variants in the coding region of the paraoxonase gene family may contribute to the glycemic control in type 2 diabetes. Clin Chim Acta. 2018;484:40–6. doi: 10.1016/j.cca.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Mochizuki H, Scherer SW, Xi T, Nickle DC, Majer M, Huizenga JJ, et al. Human PON2 gene at 7q21.3: Cloning, multiple mRNA forms, and missense polymorphisms in the coding sequence. Gene. 1998;213:149–57. doi: 10.1016/s0378-1119(98)00193-0. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T, et al. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95:4338–44. doi: 10.1210/jc.2010-0135. [DOI] [PubMed] [Google Scholar]
- 24.Schindhelm RK, Diamant M, Bakker SJ, van Dijk RA, Scheffer PG, Teerlink T, et al. Liver alanine aminotransferase, insulin resistance and endothelial dysfunction in normotriglyceridaemic subjects with type 2 diabetes mellitus. Eur J Clin Invest. 2005;35:369–74. doi: 10.1111/j.1365-2362.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 25.Williams KH, Sullivan DR, Veillard AS, O’Brien R, George J, Jenkins AJ, et al. Low alanine aminotransferase levels and higher number of cardiovascular events in people with type 2 diabetes: Analysis of the fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabet Med. 2016;33:356–64. doi: 10.1111/dme.12972. [DOI] [PubMed] [Google Scholar]
- 26.Bourquard N, Ng CJ, Reddy ST. Impaired hepatic insulin signalling in PON2-deficient mice: A novel role for the PON2/ApoE axis on the macrophage inflammatory response. Biochem J. 2011;436:91–100. doi: 10.1042/BJ20101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newsholme P, Brennan L, Bender K. Amino acid metabolism, β-cell function, and diabetes. Diabetes. 2006;55:S39–47. [Google Scholar]
- 28.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 29.Yehuda I, Madar Z, Leikin-Frenkel A, Szuchman-Sapir A, Magzal F, Markman G, et al. Glabridin, an isoflavan from licorice root, upregulates paraoxonase 2 expression under hyperglycemia and protects it from oxidation. Mol Nutr Food Res. 2016;60:287–99. doi: 10.1002/mnfr.201500441. [DOI] [PubMed] [Google Scholar]