Abstract
Background:
Endovascular treatment of acute ischemic stroke with large-vessel occlusion is the standard of care now. Initially restricted to 6 h after onset, the treatment can now be offered to selected patients up to 24 h based on clinical and imaging criteria.
Objective:
Perfusion imaging can help in identifying patients who may benefit from endovascular treatment in the extended time window. Manual analysis of perfusion images is time and skill intensive. Rapid processing of perfusion and diffusion (RAPID) is an automated image analysis system that analyzes perfusion maps. We report our initial experience of using this system in selection of patients for endovascular stroke treatment.
Methods:
All patients who presented with acute stroke underwent baseline imaging with computed tomography (CT) and CT angiogram or magnetic resonance imaging (MRI) and MR angiogram. Patients presenting between 6 and 24 h after onset underwent perfusion imaging, which was analyzed by RAPID. The results were used to select the patients who then underwent mechanical thrombectomy.
Results:
RAPID results identifying ischemic core and hypoperfused tissue were available within 5 min in each of the three cases. At 3 months, all patients showed improvement in the modified Rankin Scale.
Conclusion:
In extended time windows, RAPID provides a fast and reliable estimate of salvageable brain tissue to help select patients for endovascular treatment.
Keywords: Extended time window, ischemic stroke, perfusion, rapid processing of perfusion and diffusion, thrombectomy
INTRODUCTION
Stent retrievers revolutionized the way acute ischemic strokes with large-vessel occlusions were treated. After the landmark trials published in 2015, endovascular thrombectomy was included as first-line treatment of large-vessel strokes within 6 h of onset.[1] The quantification of the degree of neuron loss (more than 30,000 neurons are lost in each second that the brain does not get blood flow) corroborates the need for rapid treatment of stroke.[2] This rate of neuron loss depends on ischemic preconditioning and the status of collaterals. Persons with good collaterals may have viable brain tissue for a longer period. Perfusion imaging can help delineate the tissue that is irreversibly infarcted and that which is critically hypoperfused but still salvageable.
The Extending the Time for Thrombolysis in Emergency Neurological Deficits-Intra-Arterial (EXTEND-IA) and Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment (SWIFT PRIME) trials showed that patient selection based on perfusion imaging showing salvageable tissue led to better patient outcomes.[3,4] The next question was whether imaging-based selection could be used to select patients beyond 6 h. The diffusion-weighted imaging (DWI) or computerized tomography perfusion assessment with clinical mismatch in the triage of wake up and late presenting strokes undergoing neurointervention with trevo (DAWN) trial and the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) trials enrolled patients with target mismatch, who had stroke onset between 6–24 h and 6–16 h, respectively. Both the trials showed significant benefit in outcomes in the thrombectomy arms.[5,6] Following these results, the 2018 American Heart Association/American Stroke Association guidelines recommended thrombectomy in appropriately selected patients up to 24 h from stroke onset.[7]
While perfusion imaging can help identify patients with salvageable brain tissue, the time- and skill-intensive manual analysis of perfusion maps precludes its use in acute stroke situations. The rapid processing of perfusion and diffusion (RAPID) software (IschemaView, Menlo Park, CA) is an automated tool that analyzes perfusion maps and demonstrates the ischemic core and penumbra within minutes.[8] RAPID was used for patient selection in the EXTEND-IA, SWIFT PRIME, DAWN, and DEFUSE 3 trials. We report our initial experience using RAPID in extended window ischemic strokes. To the best of our knowledge, these are the first reported instances of patient selection using RAPID from India.
METHODS
At our institute, a written stroke protocol is in place. The stroke protocol is activated for all patients presenting with suspected stroke within 24 h of onset. The patient is evaluated by the neurology resident and the emergency physician simultaneously while shifting for imaging. For strokes presenting within 6 h from onset, computed tomography (CT) brain with CT angiogram is done, while magnetic resonance imaging (MRI) brain with MR angiogram is done for those presenting between 6 and 24 h and for suspected posterior circulation stroke. Eligible patients receive intravenous tissue plasminogen activator on the imaging table before angiogram. Patients presenting within 6 h with large-vessel occlusions are taken for mechanical thrombectomy. For those who present between 6 and 24 h and have large-vessel occlusion, perfusion imaging is done.
The perfusion images are pushed into the RAPID system directly from the scanner machine. The results are available within 5 min and are sent back to the Picture Archiving and Communication System (PACS) as images. On CT, the ischemic core is defined on the cerebral blood flow (CBF) map as CBF reduction >70% and is marked pink in color. The critically hypoperfused area is defined as tissue with time to maximum (Tmax) of >6 s and is marked green in color. The difference between these two (mismatch volume) is the ischemic penumbra. The mismatch ratio is also calculated and displayed on the images. On MRI, the ischemic core is defined by the area with apparent diffusion coefficient <620 and the critically hypoperfused tissue as Tmax >6 s.
In the extended window period (6–24 h), patient selection for endovascular treatment was done based on the DAWN and DEFUSE 3 trial criteria. Between 6 and 16 h from onset, either DAWN or DEFUSE 3 criteria were used. For patients presenting between 16 and 24 h from onset, DAWN criteria were used. Written informed consent was taken from all patients or their legally authorized representative if the patient was unable to consent. The data were retrieved from a prospectively maintained institutional stroke registry. Ethical clearance was obtained from the Institutional Ethics Committee.
RESULTS
Case 1
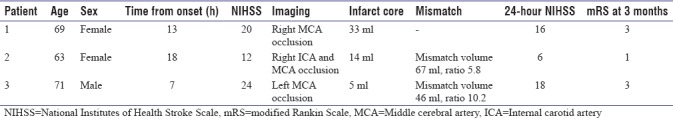
A 69-year-old female, a known hypertensive, presented with left-sided weakness for 13 h. The National Institutes of Health Stroke Scale (NIHSS) score on admission was 20. MRI brain with angiogram showed right middle cerebral artery (MCA) territory infarct with right proximal MCA occlusion. The magnetic resonance–DWI Alberta stroke program early CT score (MR-DWI ASPECTS) was 8. Perfusion imaging with RAPID analysis showed a core volume of 33 ml. As the patient met DAWN criteria, mechanical thrombectomy was done. CT brain done after 24 h showed a right MCA territory infarct corresponding to the RAPID core volume [Figure 1]. The patient did well and was discharged home after a few days. The modified Rankin Scale (mRS) score at 90 days was 3.
Figure 1.
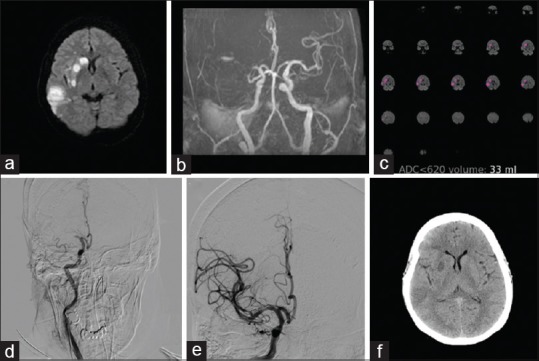
A 69-year-old female with right middle cerebral artery territory stroke. (a) Diffusion-weighted images show the right middle cerebral artery territory infarcts. (b) Time-of-flight magnetic resonance angiogram demonstrates right middle cerebral artery occlusion. (c) Rapid processing of perfusion and diffusion images indicating ischemic core (apparent diffusion coefficient <620) of 33 ml. (d) Preprocedure digital subtraction angiogram showing the right middle cerebral artery occlusion. (e) Postthrombectomy digital subtraction angiogram image showing recanalization of the middle cerebral artery branches. (f) Computed tomography brain done after 24 h shows small infarct corresponding to the core volume
Case 2
A 63-year-old female, a known hypertensive and diabetic, presented with left-sided weakness for 18 h. NIHSS score on admission was 12. MRI brain with angiogram showed right MCA territory infarct with right internal carotid artery (ICA) and MCA occlusion. MR-DWI ASPECTS was 8. Perfusion imaging with RAPID analysis showed a core volume of 14 ml and a penumbra of 81 ml (mismatch volume 67 ml and mismatch ratio 5.8). DAWN criteria were met; so, she underwent mechanical thrombectomy followed by emergency stenting of severe right cavernous ICA stenosis [Figure 2]. Twenty-four-hour NIHSS score was 6 and the mRS score on follow-up was 1.
Figure 2.
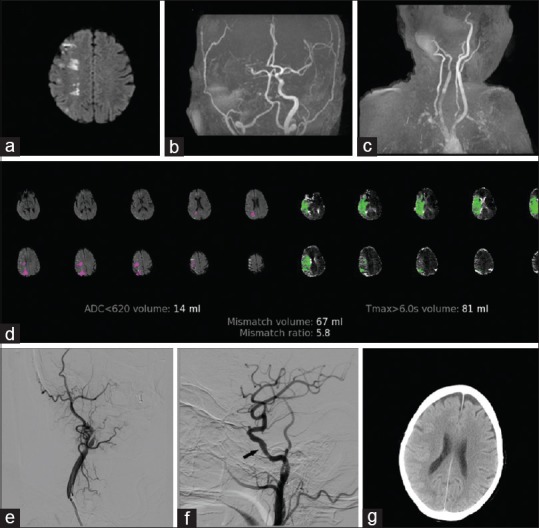
A 63-year-old female with the right middle cerebral artery territory stroke. (a) Diffusion-weighted images show the right middle cerebral artery watershed infarcts. (b) Time-of-flight magnetic resonance angiogram demonstrates right middle cerebral artery and intracranial internal carotid artery occlusion. (c) Time-of-flight magnetic resonance angiogram shows the right internal carotid artery occlusion. (d) Rapid processing of perfusion and diffusion images indicating ischemic core (apparent diffusion coefficient <620) of 14 ml, hypoperfused area (Tmax >6 s) volume of 81 ml, mismatch volume, and mismatch ratio. (e) Preprocedure digital subtraction angiogram showing the right internal carotid artery occlusion. (f) Postprocedure digital subtraction angiogram image showing recanalization of the middle cerebral artery branches with stent in situ (arrow). (g) Computed tomography brain done after 24 h shows no infarct
Case 3
A 61-year-old male, a known hypertensive and diabetic, presented with right-sided weakness and loss of speech for 7 h. He had a history of ischemic heart disease (low ejection fraction with left ventricular apical clot) and prior stroke 3 years back. He had stopped medications for the past 4 days. NIHSS score on admission was 24. CT brain with angiogram showed left gangliocapsular infarct with left proximal MCA occlusion. ASPECTS score was 7. Perfusion imaging with RAPID analysis showed a core volume of 5 ml and a penumbra of 51 ml (mismatch volume 46 ml and mismatch ratio 10.2) [Figure 3]. As he satisfied DEFUSE 3 criteria, he underwent mechanical thrombectomy. Twenty-four-hour NIHSS score was 18. He underwent further treatment at another hospital. At 3-month follow-up, he was able to walk with minimal support (mRS 3).
Figure 3.
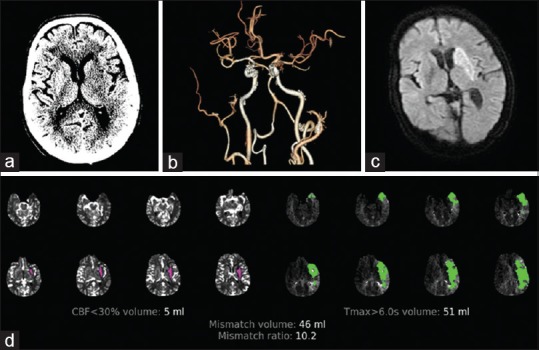
A 61-year-old male with left middle cerebral artery territory stroke. (a) Computed tomography brain shows hypoattenuation in the left basal ganglia. (b) Reconstructed computed tomography angiogram showing left middle cerebral artery occlusion. (c) Diffusion-weighted imaging after 24 h shows infarct corresponding to the ischemic core. (d) Rapid processing of perfusion and diffusion images showing a small core and large penumbra
RAPID results were available in the hospital PACS within 5 min in all three cases. Table 1 summarizes the cases.
Table 1.
Summary of patients treated with mechanical thrombectomy based on the rapid processing of perfusion and diffusion
DISCUSSION
That “time is brain” is well known.[2] Stroke outcomes are better when definitive treatment (either intravenous thrombolysis or endovascular clot retrieval) is done as early as possible. Based on this, a period of 4.5 h from onset has been recommended for intravenous thrombolysis. Earlier, mechanical thrombectomy too was restricted till 6 h from the onset of stroke. However, there is a subset of patients who have salvageable tissue well beyond these time limits.
Studies showed that patients with salvageable tissue had better outcomes irrespective of time to reperfusion.[9,10,11] The identification of these patients was done based on perfusion imaging which showed “target mismatch,” that is, the difference between infarcted tissue and hypoperfused tissue. The EXTEND-IA and SWIFT PRIME studies showed that patients selected with perfusion mismatch had better reperfusion rates.[3,4] The final infarct volume, an independent predictor of outcome, was dependent on the ischemic core and hypoperfusion volumes.[12] Other studies showed similar results in patients presenting beyond 6 h.[11,13] The DAWN and DEFUSE 3 trials definitively showed that appropriately selected patients have significantly better outcomes even in the extended time window.[5,6] Benefits of endovascular therapy were similar in both the CT and MRI perfusion imagings.[14]
The problem with using perfusion imaging was the need for manual interpretation of data that required time and specialized skill. This was a hurdle in acute stroke where treatment decisions needed to be made rapidly. The RAPID software automatically calculates ischemic core and hypoperfused tissue volumes and gives information of target mismatch and mismatch ratios. The results are available in the PACS within minutes. The software has been extensively tested and validated with high sensitivity and specificity.[3,4,5,6,8] It has a standardized set of parameters for image analysis. Comparison of different perfusion analysis software revealed that RAPID has the best accuracy and approximation of the final infarct volume.[15]
Despite its utility, the RAPID software is not without limitations. Patient motion artifacts, especially in a restless stroke patient, can compromise perfusion imaging data and give falsely elevated hypoperfusion volumes. The DEFUSE 3 trial specified alternative imaging criteria to compensate for inadequate perfusion mapping, while the DAWN trial used only the core infarct size. Second, the software itself is not widely available, making patient selection in the extended time window based on the current criteria difficult. Third, additional imaging sequences add to the cost of treatment for the patients.
In our series, all patients had significant improvement over their baseline status. While the worldwide data are still small, further studies will help refine selection thresholds and provide long-term efficacy data. This will make definitive stroke treatment available to a larger number of patients and help reduce the morbidity associated with stroke.
CONCLUSION
This series is the first reported use of RAPID from India in selecting patients for mechanical thrombectomy in the extended time window. Our patients, selected using RAPID data, DAWN, and DEFUSE 3 criteria, had a significant neurological recovery at 90 days. Thus, the software provides a fast and reliable tool for decision-making in acute stroke situations.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:3020–35. doi: 10.1161/STR.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL. Time is brain – Quantified. Stroke. 2006;37:263–6. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. T-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 6.Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3) Int J Stroke. 2017;12:896–905. doi: 10.1177/1747493017701147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 8.Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging. 2010;32:1024–37. doi: 10.1002/jmri.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansberg MG, Cereda CW, Mlynash M, Mishra NK, Inoue M, Kemp S, et al. Response to endovascular reperfusion is not time-dependent in patients with salvageable tissue. Neurology. 2015;85:708–14. doi: 10.1212/WNL.0000000000001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lansberg MG, Christensen S, Kemp S, Mlynash M, Mishra N, Federau C, et al. Computed tomographic perfusion to predict response to recanalization in ischemic stroke. Ann Neurol. 2017;81:849–56. doi: 10.1002/ana.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouslama M, Haussen DC, Grossberg JA, Dehkharghani S, Bowen MT, Rebello LC, et al. Computed tomographic perfusion selection and clinical outcomes after endovascular therapy in large vessel occlusion stroke. Stroke. 2017;48:1271–7. doi: 10.1161/STROKEAHA.116.015636. [DOI] [PubMed] [Google Scholar]
- 12.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol. 2016;79:76–89. doi: 10.1002/ana.24543. [DOI] [PubMed] [Google Scholar]
- 13.Wouters A, Lemmens R, Christensen S, Wilms G, Dupont P, Mlynash M, et al. Magnetic resonance imaging-based endovascular versus medical stroke treatment for symptom onset up to 12 h. Int J Stroke. 2016;11:127–33. doi: 10.1177/1747493015607503. [DOI] [PubMed] [Google Scholar]
- 14.Menjot de Champfleur N, Saver JL, Goyal M, Jahan R, Diener HC, Bonafe A, et al. Efficacy of stent-retriever thrombectomy in magnetic resonance imaging versus computed tomographic perfusion-selected patients in SWIFT PRIME trial (Solitaire FR with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke) Stroke. 2017;48:1560–6. doi: 10.1161/STROKEAHA.117.016669. [DOI] [PubMed] [Google Scholar]
- 15.Austein F, Riedel C, Kerby T, Meyne J, Binder A, Lindner T, et al. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke. 2016;47:2311–7. doi: 10.1161/STROKEAHA.116.013147. [DOI] [PubMed] [Google Scholar]


