Abstract
Introduction:
Acute organophosphate (OP) poisoning is one of the most common poisoning causing significant morbidity and mortality in developing countries. Acute cholinergic manifestations predominate with many patients requiring intensive care management and ventilator support. Nerve conduction studies including repetitive nerve stimulation can evaluate the altered neuromuscular transmission and peripheral nerve function by OPs.
Objective:
To evaluate the electrophysiological abnormalities in patients with acute OP poisoning and correlate with clinical status.
Materials and Methods:
Patients with acute OP poisoning admitted from August 2016 to August 2017 were prospectively studied. Nerve conduction studies including phrenic nerve conduction were performed within 24 h of admission. Repetitive nerve stimulation was performed at 3 and 30 Hz. Nerve conduction findings were compared with data from age-matched healthy controls.
Results:
Thirty patients were included (18 men and 12 women) in the study. Their age ranged from 16 to 47 years (30 ± 9.2). The first assessment revealed a mild reduction of compound muscle action potential (CMAP) amplitude and reduced F-wave persistence. Eleven patients had repetitive CMAPs suggesting cholinergic excess. Seven among the 11 patients requiring mechanical ventilation had decrement–increment response with 30 Hz stimulation and reduced diaphragmatic CMAP amplitude (P = 0.02).
Conclusion:
The presence of repetitive CMAPs, decrement–increment response to tetanic stimulation and reduced diaphragmatic CMAP amplitude in OP poisoning patients correlate with neuromuscular paralysis and need for mechanical ventilation.
Keywords: Mechanical ventilation, nerve conduction study, neuromuscular paralysis, organophosphate poisoning, repetitive nerve stimulation
INTRODUCTION
Organophosphate (OP) compounds are the most widely used pesticides throughout the world.[1] Acute OP poisoning is a significant cause of morbidity and mortality in developing countries including India. It is the most common poisoning in rural India and constitutes nearly half of the emergency admissions due to toxic exposure.[2] According to the World Health Organization, 1 million serious accidental and two million suicidal poisonings from insecticide use occur worldwide every year. Among them, about 200,000 die and most of these deaths occur in the developing countries.[2] OP poisoning leads to four well-defined clinical syndromes: initial acute cholinergic syndrome, intermediate syndrome, OP-induced delayed polyneuropathy, and chronic OP-induced neuropsychiatric disorder.[1,3]
Electrophysiological evaluation of patients with acute OP poisoning provides insights into the pathophysiology of neuromuscular paralysis.[4] Reduction of compound muscle action potential (CMAP) amplitude was demonstrated in acute OP poisoning patients.[5,6] Repetitive nerve stimulation at slow rates revealed decrement[5,7] while tetanic stimulation revealed decrement–increment response.[2,5,8] Patients with acute OP poisoning can have repetitive CMAPs with single stimulation due to cholinergic excess at the neuromuscular junction.[5,9] This study was conducted to evaluate the electrophysiological abnormalities and correlate with the neurological findings of patients with acute OP poisoning.
MATERIALS AND METHODS
This prospective study was conducted on patients admitted with acute OP poisoning to Intensive Care Unit of the university teaching hospital in South India from August 2016 and August 2017 after obtaining the Institutional Ethical Clearance. Patients admitted to the hospital with a diagnosis of acute OP poisoning during the study and satisfying the inclusion and exclusion criteria were taken for the study after informed consent from caregivers. Patients with and without neuromuscular paralysis were included in the study. Patients with diabetes mellitus, alcohol consumption, history of neuropathy, and exposure to drugs causing neuropathy were excluded from the study. Patients who required neuromuscular paralyzing agents before the electrophysiological studies were also excluded from the study. Detailed demographic, clinical, and laboratory data were obtained. Serum pseudocholinesterase levels were estimated in all patients along with biochemical parameters. Nerve conduction studies were performed within 24 h of admission in the Intensive Care Unit. Testing was done in ambient room temperature using Nihon Kohden MEB 9400 system with 10 mm diameter silver-silver chloride surface electrodes and standard filter settings. The second nerve conduction study was performed at the time of discharge or 1 week whichever was earlier. Motor conductions and F-wave elicitation were done on median, ulnar, and common peroneal nerves. Repetitive CMAPs to single supramaximal stimulation were also looked for. In addition, phrenic nerve motor conduction was performed on patients who could cooperate for the procedure using MacLean's method.[10] Orthodromic sensory nerve conductions were performed on median and ulnar; antidromic sensory conductions on superficial peroneal and sural nerves. Normative data were obtained from age-matched healthy controls. Repetitive nerve stimulation at 3 Hz was performed on median and spinal accessory nerves with recording from abductor pollicis brevis and trapezius muscles, respectively. Decrement exceeding 10% was considered as abnormal. Tetanic stimulation at 30 Hz was performed on the median nerve at wrist.
The compiled data were analyzed using SPSS version 22 (IBM SPSS Statistics for Windows, Version 22.0. IBM Corp., Armonk, NY, USA). Student's t- test and Pearson's coefficient test were used. Significance was assessed at 5% level of significance, and P < 0.05 was considered to be statistically significant.
RESULTS
Thirty-nine patients of OP poisoning were admitted during the study. Nine patients were excluded from the study (three succumbed before the first assessment, two were on skeletal muscle relaxants, two had diabetes mellitus, and two were on medications that could cause neuropathy). The final study group consisted of 30 patients (18 men and 12 women). The OP compounds identified were chlorpyrifos in nine, dichlorvos in three, dimethoate in two, and monocrotophos and ethion one patient each. The specific compound could not be identified in 14 patients. Their age ranged from 16 to 47 years (30 ± 9.2 years) without gender difference. The interval from consumption of OP compound to the first assessment was 11–30 hours (16.61 ± 5.5). Glasgow coma score ranged from 2 to 15 (10 ± 4.1) with fifteen patients being in altered sensorium. Eleven patients required mechanical ventilation and nine among them had neuromuscular paralysis. Two required mechanical ventilation due to depressed consciousness at admission and poor respiratory drive. Eleven patients had fasciculations. Three patients had generalized seizures at presentation. The median cholinesterase was 255.5U/L, ranging from 63 to 10758 U/L (reference value: 3167–6333 U/L).
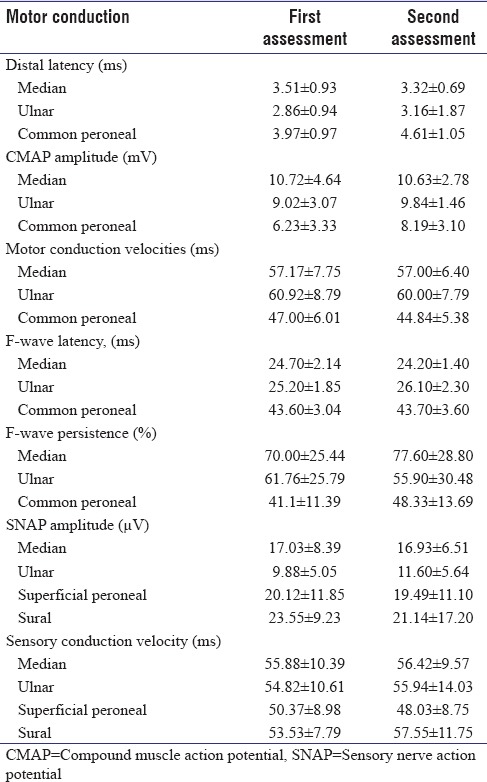
Nerve conduction study at admission revealed prolongation of distal motor latencies, reduced motor conduction velocities, and reduced F-wave persistence. Mean amplitude of CMAP was mildly reduced in the patients with absolute amplitude reduction in four patients. The SNAP amplitude, sensory conduction velocities, and F-wave latencies were normal [Table 1]. Nerve conduction study could be repeated in 14 patients of whom seven had completely recovered, and the remaining seven had no change from the initial clinical status [Table 2].
Table 1.
The nerve conduction parameters at admission compared with healthy controls
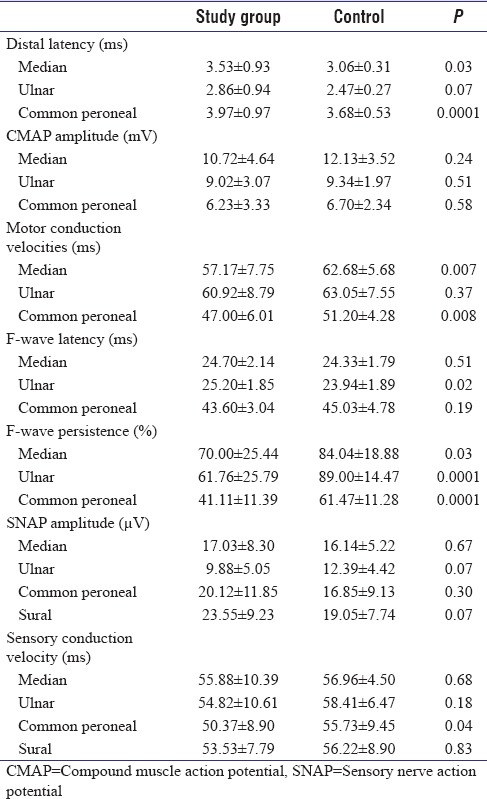
Table 2.
The repeat nerve conductions in 14 patients compared with their data at admission showing no significant differences between the two studies
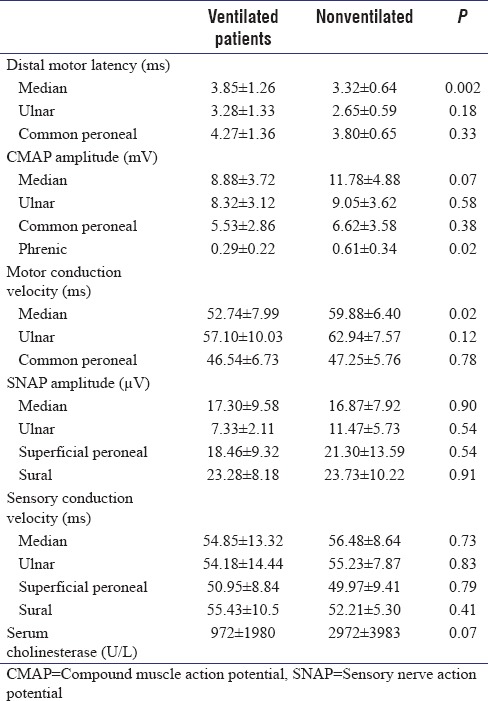
The median CMAP amplitude and motor conduction velocity were lower in patients who were ventilated in comparison with those who did not require ventilation. Diaphragmatic CMAP amplitude was significantly lower in patients who required ventilation [Table 3]. Serum cholinesterase level was lower in the patients who required mechanical ventilation.
Table 3.
The nerve conduction parameters and serum cholinesterase levels of the organophosphate poisoning patients in relation to the ventilation status during the hospital stay
Repetitive CMAPs to single supramaximal stimulus were seen in 11 patients, eight of whom were on mechanical ventilation. Among the 19 patients who did not have repetitive CMAPs, only three patients required ventilation (P < 0.0001) [Figure 1]. These repetitive CMAPs disappeared during tetanic stimulation.
Figure 1.
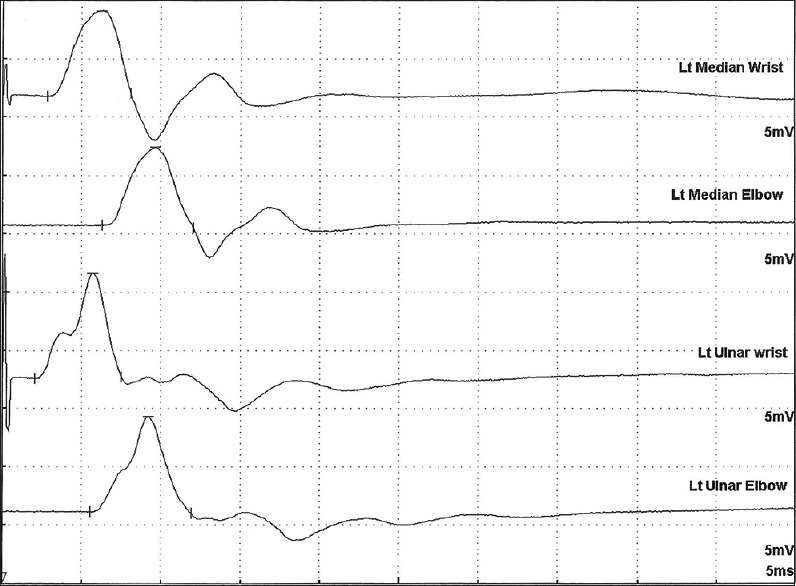
Repetitive compound muscle action potential following single supramaximal stimulus in the median and ulnar nerves stimulated at wrist and elbow
Repetitive nerve stimulation at 3 Hz revealed decremental response in three patients. Decrement–increment response during 30 Hz stimulation was seen in seven patients and all required mechanical ventilation among whom six had neuromuscular paralysis [Figure 2a]. Only two of the patients who had no change or increment in CMAP amplitude required mechanical ventilation. On review of the individual CMAP waveforms to 30 Hz stimulation, marked decrement was observed after the second or third response with the disappearance of repetitive CMAP and subsequent partial recovery of the amplitude [Figure 2b].
Figure 2.
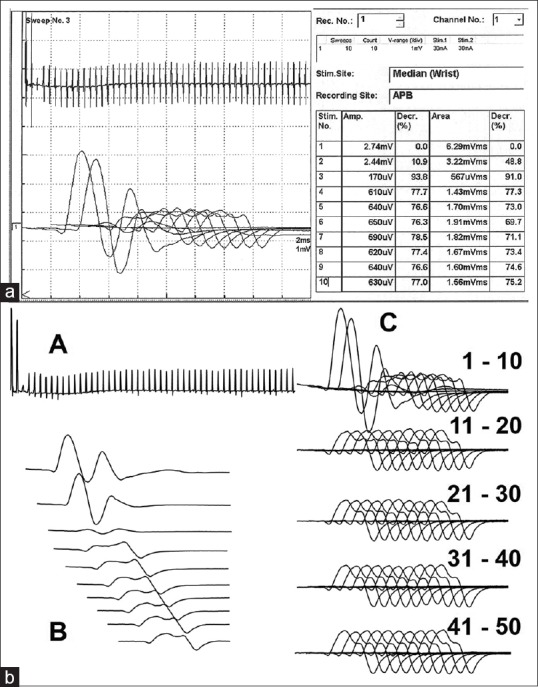
Decrement–increment phenomenon at 30Hz stimulation. (a) Marked decrement of the compound muscle action potential amplitude and (b) Amplitude graph of 50 stimuli (A), Individual 1st ten stimuli (B), and set of ten responses from first to the fiftieth stimulus (C)
DISCUSSION
Acute organophosphorus poisoning produces three different types of manifestations: acute cholinergic syndrome, intermediate syndrome, and delayed OP-induced polyneuropathy.[1] The acute cholinergic syndrome occurs due to excessive stimulation of muscarinic and nicotinic receptors peripherally and in the central nervous system. Muscarinic effects produce increased respiratory and gastrointestinal secretions, diarrhea, fecal incontinence, bradycardia, hypotension, arrhythmias, ventricular fibrillation, urinary incontinence, miosis, and blurred vision. Nicotinic manifestations include restlessness, drowsiness, confusion, coma, seizures, and depression of respiratory and circulatory centers. OP intoxication leads to typical endplate abnormalities that reflect the degree of acetylcholinesterase inhibition.[4] Nicotinic effects of these compounds produce initial excitation characterized by fasciculations, and at higher concentrations, there is a reduction of MEPP producing neuromuscular paralysis.[11] The sustained postsynaptic depolarization is responsible for the weakness of muscles. Single channel patch-clamp studies revealed biphasic effect with potentiation of carbachol-induced currents at low concentrations and suppression of channel activation at higher concentrations.[12] Cholinergic excess produces repetitive muscle action potential following single nerve stimulation due to the persistence of acetylcholine (ACh) in the neuromuscular junction. Repetitive CMAPs occur due to backfiring of nerve action potentials following stimulation of presynaptic ACh receptors.[4,13,14] There is excessive ACh in the synaptic cleft, increased contact period of the transmitter with the postsynaptic receptor leading to prolonged channel open time and prolonged end-plate potentials.[15] This results in repetitive muscle membrane excitation with repetitive muscle action potentials. There could be also direct action of the OPs on the nicotinic ACh receptors channels and increase the opening time.[12] In the present study, we found that patients who had more severe OP poisoning characterized by neuromuscular paralysis and need for mechanical ventilation had repetitive CMAPs. Repetitive CMAPs can also occur in cholinergic crisis in myasthenia gravis and congenital myasthenic syndromes (slow channel syndromes and end-plate cholinesterase deficiency).
Reported findings of nerve conductions in OP poisoning demonstrated reduced CMAP of the peripheral nerves,[5,16] while distal latencies and conduction velocities were normal in most of the patients. However, similar observations were not uniformly encountered in another study which reported normal CMAP amplitudes even in severe stages of the disease.[13] In our patients, the reduction in the amplitudes of CMAP was not statistically significant. The reduced motor conduction velocities and increased distal latencies could partially be due to lower ambient temperature in the Intensive Care Unit where these patients were being treated. Correction for reduced skin temperature could not be done as the skin temperature was not measured. The basis of the reduced amplitudes was due to transmission failure in the motor endplates.[4]
Diaphragmatic weakness significantly contributes to respiratory failure. Phrenic nerve conduction study with recording of the CMAP from diaphragm revealed the correlation of reduced CMAP and need for mechanical ventilation.[17] In our patients, similar findings with lower amplitude of diaphragmatic CMAP were seen in patients who required mechanical ventilation.
The evaluation of neuromuscular transmission using repetitive nerve stimulation at 3 Hz showed a decremental response in few patients, while the majority of patients showed significant decrement with tetanic stimulation.[5,9] Decrement–increment pattern was shown in milder stages of OP poisoning. The maximal decrement of the primary CMAP with the second response was seen followed by a gradual increase and accompanying disappearance of repetitive CMAP. The decrement–increment response was thought to occur due to stimulus-induced antidromic backfiring in the nerves, a presynaptic event.[9,18] Another possibility is desensitization of ACh postsynaptic receptors as demonstrated in animal studies.[19] Prolonged receptor desensitization can cause influx of calcium that damages the neuromuscular junction and can cause the subacute neuromuscular syndrome.[20] Similar findings with tetanic stimulation were seen in our patients. The decrement occurred with the second stimulus in some patients and third stimulus in few patients.
CONCLUSION
The presence of repetitive CMAPs, decrement–increment response to tetanic stimulation, and reduced diaphragmatic CMAP amplitude in OP poisoning patients is correlated with neuromuscular paralysis and need for mechanical ventilation. The occurrence of repetitive CMAPs and decrement with tetanic stimulation could also help to evaluate the patients with acute unknown poisoning at casualty.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.De Bleecker JL. Organophosphate and carbamate poisoning. In: Engel AG, editor. Vinken and Bryun's Handbook of Clinical Neurology. 3rd Ser. Vol. 91. Amsterdam: Elsevier; 2008. pp. 401–32. [DOI] [PubMed] [Google Scholar]
- 2.Jeyaratnam J. Acute pesticide poisoning: A major global health problem. World Health Stat Q. 1990;43:139–44. [PubMed] [Google Scholar]
- 3.Singh S, Sharma N. Neurological syndromes following organophosphate poisoning. Neurol India. 2000;48:308–13. [PubMed] [Google Scholar]
- 4.Besser R, Gutmann L, Dillmann U, Weilemann LS, Hopf HC. End-plate dysfunction in acute organophosphate intoxication. Neurology. 1989;39:561–7. doi: 10.1212/wnl.39.4.561. [DOI] [PubMed] [Google Scholar]
- 5.Wadia RS, Chitra S, Amin RB, Kiwalkar RS, Sardesai HV. Electrophysiological studies in acute organophosphate poisoning. J Neurol Neurosurg Psychiatry. 1987;50:1442–8. doi: 10.1136/jnnp.50.11.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayasinghe SS, Pathirana KD, Buckley NA. Effects of acute organophosphorus poisoning on function of peripheral nerves: A cohort study. PLoS One. 2012;7:e49405. doi: 10.1371/journal.pone.0049405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayasinghe SS, Pathirana KD. Slow repetitive nerve stimulation in patients with acute organophosphorous poisoning after clinical recovery. Asia Pac J Med Toxicol. 2013;2:14–8. [Google Scholar]
- 8.Senanayake N, Karalliedde L. Neurotoxic effects of organophosphorus insecticides. An intermediate syndrome. N Engl J Med. 1987;316:761–3. doi: 10.1056/NEJM198703263161301. [DOI] [PubMed] [Google Scholar]
- 9.Singh G, Khurana D. Neurology of acute organophosphate poisoning. Neurol India. 2009;57:119–25. doi: 10.4103/0028-3886.51277. [DOI] [PubMed] [Google Scholar]
- 10.MacLean IC, Mattioni TA. Phrenic nerve conduction studies: A new technique and its application in quadriplegic patients. Arch Phys Med Rehabil. 1981;62:70–3. [PubMed] [Google Scholar]
- 11.Eccles JC, MacFarlane WV. Actions of anti-cholinesterases on endplate potential of frog muscle. J Neurophysiol. 1949;12:59–80. doi: 10.1152/jn.1949.12.1.59. [DOI] [PubMed] [Google Scholar]
- 12.Nagata K, Huang CS, Song JH, Narahashi T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–8. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- 13.van Dijk JG, Lammers GJ, Wintzen AR, Molenaar PC. Repetitive CMAPs: Mechanisms of neural and synaptic genesis. Muscle Nerve. 1996;19:1127–33. doi: 10.1002/(SICI)1097-4598(199609)19:9<1127::AID-MUS7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Singh G, Mahajan R, Whig J. The importance of electrodiagnostic studies in acute organophosphate poisoning. J Neurol Sci. 1998;157:191–200. doi: 10.1016/s0022-510x(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 15.Clark AL, Hobbiger F, Terrar DA. Nature of the anticholinesterase-induced repetitive response of rat and mouse striated muscle to single nerve stimuli. J Physiol. 1984;349:157–66. doi: 10.1113/jphysiol.1984.sp015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager KW, Roberts DV, Wilson A. Neuromuscular function in pesticide workers. Br J Ind Med. 1970;27:273–8. doi: 10.1136/oem.27.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh G, Sidhu UP, Mahajan R, Avasthi G, Whig J. Phrenic nerve conduction studies in acute organophosphate poisoning. Muscle Nerve. 2000;23:627–32. doi: 10.1002/(sici)1097-4598(200004)23:4<627::aid-mus23>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Besser R, Vogt T, Gutmann L, Hopf HC, Wessler I. Impaired neuromuscular transmission during partial inhibition of acetylcholinesterase: The role of stimulus-induced antidromic backfiring in the generation of the decrement-increment phenomenon. Muscle Nerve. 1992;15:1072–80. doi: 10.1002/mus.880151003. [DOI] [PubMed] [Google Scholar]
- 19.Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Good JL, Khurana RK, Mayer RF, Cintra WM, Albuquerque EX. Pathophysiological studies of neuromuscular function in subacute organophosphate poisoning induced by phosmet. J Neurol Neurosurg Psychiatry. 1993;56:290–4. doi: 10.1136/jnnp.56.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]


