Abstract
The National Cancer Database (NCDB) is a large clinical oncology database developed with data collected from Commission on Cancer (CoC)-accredited facilities. The CoC is managed under the American College of Surgeons, and is a multidisciplinary team that maintains standards in cancer care delivery in health care settings. This database has been used in multiple cancer-focused studies and reports on cancer diagnosis, hospital-level, and patient-related demographics. The focus of this review is to explore and discuss the use of NCDB in colorectal surgery research. Furthermore, our aim for this review is to formulate a guide for researchers who are interested in using the NCDB to complete colorectal research.
Keywords: colorectal cancer, National Cancer Database, Surgery, outcomes
Since the inception of the American College of Surgeons (ACS) in 1913, it has strived to promote standards of surgical care through education and advocacy of its fellows. To that end, it sponsors the National Cancer Database (NCDB) with its cosponsor, the American Cancer Society. The NCDB is a large clinical oncology database collected from Commission on Cancer (CoC)-accredited facilities. The CoC is managed under the ACS and is a multidisciplinary team that maintains standards in cancer care delivery in outpatient and inpatient health care settings. This database has been used in multiple cancer-focused studies, especially in colorectal topics, which will briefly be summarized here. To guide future researchers, the strengths and weaknesses of the NCDB will also be discussed.
Strengths of the National Cancer Database
First, the NCDB is a broad and diverse resource. It is sourced from hospital registry data that have been collected in a standardized fashion since 1989 from over 1,500 CoC-accredited facilities. It is used to follow up patients with malignant neoplastic diseases, their treatments, and their outcomes. The data represent approximately 70% of all newly diagnosed cancer cases, which accounts for 1 million case reports from over 1,500 hospitals. There are currently over 34 million records from cancer centers throughout the United States. The data consist of cancer diagnosis, hospital-level, and patient demographics. Patient-related data include age at diagnosis, sex, race, primary payer, average household income, average education, population density of patient residence, patient comorbidities, postal code, and county of residence. Tumor-related factors include pathologic stage, clinical tumor and nodal stage, histological type, tumor size, grade, and the presence of lymphovascular or perineural invasion. Rare pathologies are also included. Treatment-related factors include the use of neoadjuvant chemoradiation, type of surgery for the primary site, and operative approach. Hospital-related data include facility volume, availability of diagnostic and treatment services, participation in clinical research, and training of resident physicians. The data also consist of patient and hospital identifiers, which assure no duplication of patient information, based on readmissions or new diagnoses, and are subsequently de-identified ( Table 1 ). 1
Table 1. List of variables included in the National Cancer Database.
| Patient-related data | Tumor-related data | Treatment-related data | Hospital-related data |
|---|---|---|---|
| Patient age | Primary site | Diagnostic and staging procedure, days from diagnosis | Facility |
| Sex | Laterality | Diagnostic and staging procedure | Facility type (community, academic, etc.) |
| Spanish origin | Histology | Treatment started, days from diagnosis | Facility location |
| Primary payer | Behavior | First surgical procedure, day from diagnosis | |
| Income | Grade | Definitive surgical procedure, day from diagnosis | |
| Education | Diagnostic confirmation | Surgical procedure of the primary site | |
| Urban/rural | Regional lymph nodes positive | Surgical margins | |
| Circle distance (radius from home to hospital) | Regional lymph nodes examined | Scope of regional LN surgery | |
| Charlson's/Deyo's score | AJCC clinical T | Surgery of other site | |
| Sequence number | AJCC clinical N | Surgical inpatient stay, days from surgery | |
| Class of case | AJCC clinical M | Readmission within 30 d of surgical discharge | |
| Year of diagnosis | AJCC clinical stage group | Reason for no surgery | |
| Palliative care | AJCC pathological T | Radiation, days from diagnosis | |
| 30-day mortality | AJCC pathological N | Radiation therapy | |
| 90-day mortality | AJCC pathological M | Location of radiation therapy | |
| Last contact or death, months from diagnosis | AJCC pathological stage group | Radiation treatment volume | |
| TNM edition number | Regional treatment modality | ||
| Mets at diagnosis | Regional dose | ||
| Mets evaluation | Boost treatment modality | ||
| Size of tumor | Boost dose | ||
| Number of treatments to this volume | |||
| Radiation surgery sequence | |||
| Radiation ended | |||
| Reason for no radiation | |||
| Systemic therapy, day from diagnoses | |||
| Chemotherapy | |||
| Chemotherapy, day from diagnoses | |||
| Hormone therapy | |||
| Hormone therapy, days from diagnosis | |||
| Immunotherapy | |||
| Immunotherapy, days from diagnosis | |||
| Hematologic transplant and endocrine procedures | |||
| Systemic surgery sequence | |||
| Other treatment | |||
| Other treatment, days from diagnosis |
Abbreviations: AJCC, American Joint Committee on Cancers; LN, lymph node.
The quality of the database is ensured with multiple review processes. All hospitals submitting data must be CoC accredited. This accreditation process requires maintenance of a cancer registry with eventual data submission to the CoC, an elected CoC liaison physician and, finally, a CoC survey visit. All data are then collected and submitted, using nationally standardized data items and coding definitions, as described in the CoC Facility Oncology Registry Data Standards (FORD) and nationally standardized data transmission format specifications that guide concise data collection. These data are also derived from the Collaborative Stage Manuals and the American Joint Committee on Cancers (AJCC) Cancer Staging Manual. This manual has undergone multiple revisions, eight of which have occurred over the past 10 years, which is indicative of the quality and quantity of data undergoing constant review.
The data itself are accessible and available to the public under web-based benchmarking applications to promote access to the general public and researchers ( https://www.facs.org/quality%20programs/cancer/ncdb ). The NCDB Public Benchmark Reports include the 14 most commonly diagnosed solid tumors in the United States. Users are provided access to data from six diagnosis years (2003–2013), slightly more than 9 million cases. Users can design queries using data from any one or a combination of three types of hospitals (community, comprehensive community, and academic/teaching facilities), and specify a geographic region or state to narrow the scope of their analysis, making this collection of data one of the most diverse and well-represented databases. As many as three covariates (including patient age, ethnicity, sex, tumor histology, stage, first course therapy, and type of surgical resection) are available for users to define the type of information they wish to review. 1 If greater access is desired, participant user data files (PUFs) are available. These data are Health Insurance Portability and Accountability Act (HIPAA) compliant, and provides information resources researchers can use to review and advance the quality of care delivered to cancer patients through analyses of the available cases. PUFs are available through a biannual application process to investigators associated with CoC-accredited cancer programs.
Weaknesses of the National Cancer Database
While the NCDB is relatively comprehensive for oncologic variables and outcomes, as is the case with any database, there are some restrictions to its use. The data are retrospective in nature, and are also incomplete in multiple areas. Patient-related factors are limited, and family history and extensive comorbidity documentation are lacking. Treatment details are limited, specifically in chemotherapy agents, dose reductions, treatment breaks, or incomplete regimens. Data loss pertaining to these treatments is also likely if patients were being treated at non-CoC facilities prior to surgical treatment. Surgical treatment lacks documentation of tumor location, distance from anal verge, surgeon specialty, operative volume, pathologic response, diagnostic modality, and information on operative planning. As can happen with any database, surgeon preference and experience as well as patient wishes are lost. Additionally, there is limited information on short-term postoperative outcomes, such as surgical site infection, wound complications, other major morbidities, and hospital costs. Furthermore, the database reports all-cause mortality and does not capture cancer-specific survival.
While the data have undergone many revisions, release and updates in the registry have to be done frequently, which results in inconsistent data collection. These data include limited reporting for minority groups, due to the populations served by CoC-accredited facilities. 2 As is common with any database, there are errors present in coding, including undercoding and miscoding of data. This is specifically significant in data using procedures as an outcome of measure. In addition, certain procedures have lacked codes since the initiation of the database collection. Finally, current hospitals now require ICD-10 codes, while remaining data use ICD-9 codes.
Examples of Clinical Colorectal Research Using the National Cancer Database
A literature review was completed, identifying articles referenced on the NCDB Web site and PubMed under the search topic “colorectal” and “NCDB” over the past 10 years. Approximately 40 articles were identified, which covered topics ranging from specific colorectal cancer epidemiology to tumor characteristics, patient-related factors, various cancer treatments, and the use of the NCDB to compare national and state guidelines. Illustrative examples from the literature are reviewed later.
Tumor Characteristics
With a large repository of cancer patients, the prevalence and incidence of colorectal tumors is easily identified and studied. Two studies from the same institute reported on cancer treatment and survivorship statistics based on the year of the study. 3 4 They reported colorectal cancer is the second most prevalent disease, with 1.2 million survivors. In 2014, they identified the national treatment standards of CRC, based on disease stage at presentation ( Fig. 1 ).
Fig. 1.
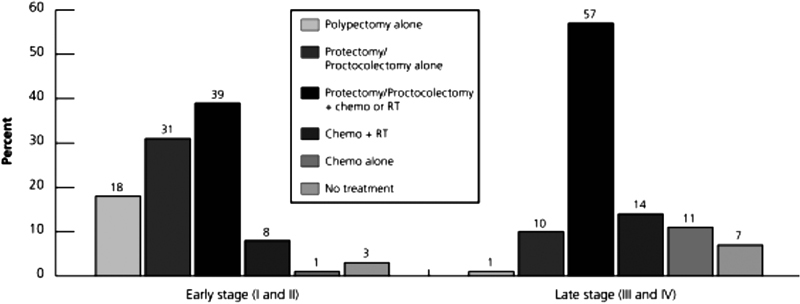
Cancer treatment and survivorship statistics, 2014. Permission to replicate this image was granted by John Wiley and Sons and Copyright Clearance Center (license number: 4010481309374). Source: DeSantis et al; 3 http://onlinelibrary.wiley.com/doi/10.3322/caac.21235/full#caac21235-fig-0006 .
Cancer staging and treatment are influenced by multiple factors. Numerous studies have used the data available in the NCDB to better understand these factors and what prognosis they have on colorectal cancer outcomes. Bilimoria et al assessed the difference in lymph node evaluation by age to report on its influence on overall survival (OS). There was a decrease in the number of nodes examined in elderly individuals (47.7% in <67 years old vs, 41.4% in >78 years old; p < 0.0001), and evaluation of more than 12 nodes was associated with better survival ( p < 0.0001). A second article described the assessment of lymph nodes based on tumor location. On average, more nodes were evaluated in right colon cancers, compared with left colon cancers (median: 12 vs. 8; p < 0.0001). 5 6
In addition to nodal involvement, specific histology of tumors was explored. Mucinous and signet ring colorectal adenocarcinomas were quantified; 10 and 1% existed in the population, respectively. These were most commonly identified in the right colon, and individuals with these diagnoses were more likely to undergo adjuvant chemotherapy. Signet ring adenocarcinoma of the colon had a lower survival rate (hazard ratio: 1.42, 95% confidence interval [CI]: 1.33–1.51), when compared with other cancer pathologies. 7
Neuroendocrine tumors of the hindgut were also studied. Hsu et al quantified appendiceal neuroendocrine tumors. 8 OS in neuroendocrine tumors of the colon and rectum was associated with the depth of invasion, tumor size, lymph node status, and presence of metastatic disease. Additional factors influencing OS in these populations were location of tumor, older age, male gender, lack of insurance, or Medicaid insurance. 9
Patient Characteristics
Patient characteristics and their associated outcomes have been well documented using the NCDB. Age at presentation of colorectal cancer has been examined, and young-onset CRC has been reported as being more prevalent in non-white race, uninsured or Medicaid patients, and in patients who lived in southern and western parts of the United States. 10 Colorectal adenocarcinoma was reported in individuals younger than 18 years; there was no difference in occurrence between gender and race, however, as expected comorbidities increased with age. Individuals younger than 18 years had a higher portion of rectal cancer with signet ring pathology, with worse histologic grade, and a higher rate of radiation therapy. The largest predictors of mortality were cancer stage, Charlson's comorbidity score, poor grade, black race, and male gender. 11
Race, socioeconomic status, and insurance status seem to play an influential role in the care of colorectal cancer patients. The NCDB identified younger patients, those of black race, Hispanics, and those with limited insurance as more likely to present with advanced disease. Black patients were also less likely to undergo surgical resection of primary cancer (odds ratio [OR]: 1.72, 95% CI: 1.42–2.08; p < 0.001). 12 13 Higher rates of Hispanics, black patients, women, and patients from low socioeconomic status regions were either uninsured or covered with Medicaid or Medicare. 14 15 16 Additionally, no insurance coverage or Medicaid patients had a higher mortality or decreased rate of cancer screening. 14 15 17
Treatment Options
Operative interventions are the primary treatment for colorectal cancer with the addition of adjuvant therapies, and reports on specific interventions have been published using the NCDB. Trends of surgical therapy for stage I rectal cancers have been closely studied. Local excision rates have increased over time, 18 19 and are associated with women, black race, the elderly, and those with private insurance, well- differentiated tumors, and T1 stage cancers. A lower 30-day morbidity rate was associated with local excision; local recurrence, however, was greater in the local excision than in the standard excision population. Patient OS was not influenced, but instead comorbidities played the largest factor ( Fig. 2 ). 19 Stitzenberg and colleagues also examined total mesorectal excision patterns, which were associated with a higher rate of tumor-free margins. Local excision in T2N0, however, had poorer OS than proctectomy and multimodality therapy (OR: 1.39, 95% CI: 1.26–1.53 vs. OR: 1.00, 95% CI: 0.91–1.11). 18
Fig. 2.
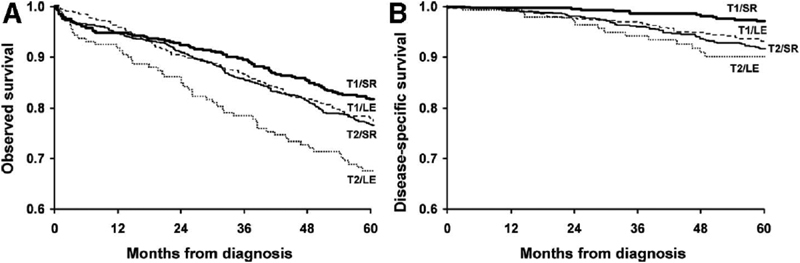
Overall survival (OS; A ) and disease-specific survival (DSS; B ) for T1 and T2 rectal cancer patients treated by local excision (LE) versus standard resection (SR). The number of patients at risk in each group is at time points for every 12 months. Permission to replicate this image was provided by Wolters Kluwer and RightsLink Copyright Clearance Center (license number: 4011051332707). Source: You et al. 19
Furthermore, Nussbaum and colleagues reported on laparoscopic versus open low anterior resections for rectal cancer from years 2010 to 2011, and identified that 65.7% of the procedures were open. Laparoscopic procedures were associated with younger patients, Caucasians, higher income earners, privately insured patients, and in those with lower stage cancer without nodal involvement, but shared similar mortality. Interestingly, they reported open low anterior resection (LAR) was more likely to have positive margins which may indicate some nationwide selection bias to not attempt borderline resectable cases using a laparoscopic approach. 20 The rate of positive circumferential resection margins following rectal cancer resection has been reviewed. In the NCDB, 17.2% of resections had positive circumferential radial margins, and as expected, T4 tumors among the highest. There was no significant difference in mortality between groups and there was no association with receipt of chemoradiation therapy. The type of operation and approach, however, were significantly associated with a positive circumferential radial margin. 21
Finally, disparities were noted in laparoscopic surgical treatment for rectal cancer, with 53.5% of patients undergoing laparoscopic procedures. This intervention was increased in cases with smaller tumor size (<5 cm), neuroendocrine tumor diagnosis, and patients with an income greater than $46,000. Academic and cancer centers reported increased robotic use and these uses were associated with insurance status of the patient. 22 These latter findings represent a concerning disparity.
Treatment options for stage IV disease have also been captured and studied in the NCDB. Gulack et al evaluated whether resection of the primary tumor without a metastasectomy in stage IV colorectal cancer was associated with improved OS, compared with patients undergoing adjuvant therapies alone. Patients who underwent palliative treatment without curative intent were studied. In patients with stage IV colorectal cancer, 16% had surgery, and the survival rate was 9.2 months in the surgical intervention group, compared with 7.3 months in the adjuvant therapy only group. 23
A few studies assessed the use of adjuvant therapy in the colorectal cancer population. First, Shih et al examined the utilization of immunotherapy among newly diagnosed cancer patients. It was more likely to be administered in patients based on year of diagnosis, and its use was varied by age, race, insurance status, year of diagnosis, and type of treatment facility. 24 Chemotherapy in the T1 node-positive colon cancers was studied by Ganapathi et al. They identified 8.6% of the colorectal cancer patients had lymph node involvement, and slightly greater than two-thirds of the population had chemotherapy. These individuals were frequently younger, with fewer comorbidities, and had private insurance. The chemotherapy group in general had shorter postoperative stays, higher survival rates, and decreased readmission rates. 25
For the use of neoadjuvant chemoradiotherapy for locally advanced rectal cancer, the NCDB has shown that pathologically complete response seems to peak at approximately weeks 10 to 11 after completion of neoadjuvant therapy, with higher volume hospitals utilizing this treatment and, interestingly, applying it to larger tumors. The longer the interval between chemotherapy and surgical intervention was, the higher the tumor down staging (OR: 1.11, 95% CI: 1.02–1.25) and decreased readmission rates occurred (OR: 0.82, 95% CI: 0.70–0.92). 26
Colorectal Cancer Guidelines
Finally, the NCDB has frequently been used as a guide to assess practice patterns. The variation of national treatment patterns for metastatic colorectal cancer was assessed by Krell al. They identified that high-volume hospitals were frequently treating younger patients, those of black race, the privately insured, and patients with multiple comorbidities. There were increased rates of metastasectomy, multiagent chemotherapy, and increased implementation in palliative therapy. 27
Treatment records in the NCDB have also been compared with the National Comprehensive Cancer Network (NCCN) guidelines. Across all colon cancers in 2013, 71% of patients were treated as per the NCCN guidelines. Stage I cancers were more likely to receive standard treatment. Adherence to the NCCN guidelines increased over time and was related to age, comorbidity index, later year of diagnosis, and insurance status. Older patients with pre-existing disease and lower socioeconomic status were less likely to be offered adjuvant therapy. 9 An earlier study in 2007 reported that nonadherence of guidelines was associated with older age, Medicaid, and Medicare or uninsured status. Survival advantage was seen in those who received therapies adherent to the NCCN guidelines. 28
The data within the NCDB have also been used to compare other databases, such as the Veterans Affairs hospital, Ohio Cancer Incidence and Surveillance System, the Mayo Clinic registry, and NSQIP. 29 30 31 32 Overall findings were that compared with these datasets, NCDB performed well with high concordance, but failed to capture all adjuvant therapies and short-term outcomes, suggesting that it is relatively a strong database.
Conclusion
Based on multiple studies, we can safely conclude that the NCDB offers insight into patient- and treatment-related factors associated with cancer care. Little is known regarding cost or specific comorbidities using this database. As a scientific community, the use of large databases, such as the NCDB, should be closely reviewed, as they may impact policy, treatment, and national treatment standards.
References
- 1.American College of Surgeons.National Cancer Database2016; Available at:https://www.facs.org/quality-programs/cancer/ncdb. Accessed August 30, 2016
- 2.Newman L A, Lee C T, Parekh L P et al. Use of the National Cancer Data Base to develop clinical trials accrual targets that are appropriate for minority ethnicity patients: a report from the American College of Surgeons Oncology Group (ACOSOG) Special Population Committee. Cancer. 2006;106(01):188–195. doi: 10.1002/cncr.21592. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis C E, Lin C C, Mariotto A B et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(04):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, DeSantis C, Virgo K et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(04):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Bilimoria K Y, Palis B, Stewart A K et al. Impact of tumor location on nodal evaluation for colon cancer. Dis Colon Rectum. 2008;51(02):154–161. doi: 10.1007/s10350-007-9114-2. [DOI] [PubMed] [Google Scholar]
- 6.Bilimoria K Y, Stewart A K, Palis B E, Bentrem D J, Talamonti M S, Ko C Y. Adequacy and importance of lymph node evaluation for colon cancer in the elderly. J Am Coll Surg. 2008;206(02):247–254. doi: 10.1016/j.jamcollsurg.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Hyngstrom J R, Hu C Y, Xing Y et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19(09):2814–2821. doi: 10.1245/s10434-012-2321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu C, Rashid A, Xing Y et al. Varying malignant potential of appendiceal neuroendocrine tumors: importance of histologic subtype. J Surg Oncol. 2013;107(02):136–143. doi: 10.1002/jso.23205. [DOI] [PubMed] [Google Scholar]
- 9.Chagpar R, Chiang Y J, Xing Y et al. Neuroendocrine tumors of the colon and rectum: prognostic relevance and comparative performance of current staging systems. Ann Surg Oncol. 2013;20(04):1170–1178. doi: 10.1245/s10434-012-2746-z. [DOI] [PubMed] [Google Scholar]
- 10.You Y N, Xing Y, Feig B W, Chang G J, Cormier J N. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med. 2012;172(03):287–289. doi: 10.1001/archinternmed.2011.602. [DOI] [PubMed] [Google Scholar]
- 11.Poles G C, Clark D E, Mayo S W et al. Colorectal carcinoma in pediatric patients: a comparison with adult tumors, treatment and outcomes from the National Cancer Database. J Pediatr Surg. 2016;51(07):1061–1066. doi: 10.1016/j.jpedsurg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Esnaola N F, Stewart A K, Feig B W, Skibber J M, Rodriguez-Bigas M A. Age-, race-, and ethnicity-related differences in the treatment of nonmetastatic rectal cancer: a patterns of care study from the national cancer data base. Ann Surg Oncol. 2008;15(11):3036–3047. doi: 10.1245/s10434-008-0106-9. [DOI] [PubMed] [Google Scholar]
- 13.Halpern M T, Ward E M, Pavluck A L, Schrag N M, Bian J, Chen A Y. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(03):222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 14.Robbins A S, Chen A Y, Stewart A K, Staley C A, Virgo K S, Ward E M. Insurance status and survival disparities among nonelderly rectal cancer patients in the National Cancer Data Base. Cancer. 2010;116(17):4178–4186. doi: 10.1002/cncr.25317. [DOI] [PubMed] [Google Scholar]
- 15.Ward E M, Fedewa S A, Cokkinides V, Virgo K. The association of insurance and stage at diagnosis among patients aged 55 to 74 years in the national cancer database. Cancer J. 2010;16(06):614–621. doi: 10.1097/PPO.0b013e3181ff2aec. [DOI] [PubMed] [Google Scholar]
- 16.Halpern M T, Pavluck A L, Ko C Y, Ward E M. Factors associated with colon cancer stage at diagnosis. Dig Dis Sci. 2009;54(12):2680–2693. doi: 10.1007/s10620-008-0669-0. [DOI] [PubMed] [Google Scholar]
- 17.Robbins A S, Pavluck A L, Fedewa S A, Chen A Y, Ward E M. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27(22):3627–3633. doi: 10.1200/JCO.2008.20.8025. [DOI] [PubMed] [Google Scholar]
- 18.Stitzenberg K B, Sanoff H K, Penn D C, Meyers M O, Tepper J E. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol. 2013;31(34):4276–4282. doi: 10.1200/JCO.2013.49.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You Y N, Baxter N N, Stewart A, Nelson H. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg. 2007;245(05):726–733. doi: 10.1097/01.sla.0000252590.95116.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nussbaum D P, Speicher P J, Ganapathi A Met al. Laparoscopic versus open low anterior resection for rectal cancer: results from the national cancer data base J Gastrointest Surg 20151901124–131., discussion 131–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rickles A S, Dietz D W, Chang G J et al. High rate of positive circumferential resection margins following rectal cancer surgery: a call to action. Ann Surg. 2015;262(06):891–898. doi: 10.1097/SLA.0000000000001391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabriel E, Thirunavukarasu P, Al-Sukhni E, Attwood K, Nurkin S J. National disparities in minimally invasive surgery for rectal cancer. Surg Endosc. 2016;30(03):1060–1067. doi: 10.1007/s00464-015-4296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulack B C, Nussbaum D P, Keenan J E et al. Surgical resection of the primary tumor in stage IV colorectal cancer without metastasectomy is associated with improved overall survival compared with chemotherapy/radiation therapy alone. Dis Colon Rectum. 2016;59(04):299–305. doi: 10.1097/DCR.0000000000000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih Y C, Elting L S, Halpern M T. Factors associated with immunotherapy use among newly diagnosed cancer patients. Med Care. 2009;47(09):948–958. doi: 10.1097/MLR.0b013e31819a5b2b. [DOI] [PubMed] [Google Scholar]
- 25.Ganapathi A M, Speicher P J, Englum B R et al. Adjuvant chemotherapy for t1 node-positive colon cancers provides significant survival benefit. Dis Colon Rectum. 2014;57(12):1341–1348. doi: 10.1097/DCR.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Probst C P, Becerra A Z, Aquina C T et al. Extended intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(02):430–440. doi: 10.1016/j.jamcollsurg.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krell R W, Regenbogen S E, Wong S L. Variation in hospital treatment patterns for metastatic colorectal cancer. Cancer. 2015;121(11):1755–1761. doi: 10.1002/cncr.29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boland G M, Chang G J, Haynes A B et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(08):1593–1601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Roessel P, Rouse R V, Wren S M. Care within a Veterans hospital: earlier detection of colon cancer. Surg Endosc. 2007;21(08):1434–1440. doi: 10.1007/s00464-006-9184-6. [DOI] [PubMed] [Google Scholar]
- 30.Mallin K, Palis B E, Watroba N et al. Completeness of American Cancer Registry Treatment Data: implications for quality of care research. J Am Coll Surg. 2013;216(03):428–437. doi: 10.1016/j.jamcollsurg.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Shi Q, You Y N, Nelson H et al. Cancer registries: a novel alternative to long-term clinical trial follow-up based on results of a comparative study. Clin Trials. 2010;7(06):686–695. doi: 10.1177/1740774510380953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkow R P, Kmiecik T E, Bentrem D J et al. Effect of including cancer-specific variables on models examining short-term outcomes. Cancer. 2013;119(07):1412–1419. doi: 10.1002/cncr.27891. [DOI] [PubMed] [Google Scholar]


