Abstract
The Rochester Epidemiology Project (REP), a longitudinal population-based database, is the largest epidemiologic database in the world. Originally established at Mayo Clinic in Rochester, Minnesota, the REP has been instrumental in defining the natural history of disease states and the impact of treatment in a well-defined population. In the United States, the REP has made important contributions to the colon and rectal literature, largely because healthcare is fragmented with no unique identifier to longitudinally follow-up with a patient throughout the system over his or her lifespan. Investigation with the REP has provided insight to the economic burden associated with inflammatory bowel disease, the benefit of screening for colorectal cancer, and the natural history of Mekel's and diverticular disease. In addition to practice changing research, the REP can be used as a model for future linkage systems in the United States.
Keywords: Rochester Epidemiology Project, population-based database, inflammatory bowel disease, colon and rectal cancer, diverticulitis
The Rochester Epidemiology Project (REP), officially initiated in 1966, established a comprehensive medical records linkage system for almost all persons residing in Olmsted County, Minnesota. The REP now represents the largest passive epidemiologic database in the world. With continuous funding from the National Institute of Health (NIH) for over 40 years, the REP has been instrumental in defining the natural history of both common and rare disease and impact of treatment in an entire well-defined population. This type of longitudinal population health database is a unique in the United States where a national health system is lacking and strict confidentiality of medical information causes difficulty developing and maintaining such databases. Several obstacles were overcome to link patient-specific health information across diverse health care provider organizations to create the REP. Herein, we present the REP history, the infrastructure, the challenges in maintaining this database in the current era, and how this database has been instrumental in answering questions relevant to colon and rectal surgery.
Linking patient information across a diverse set of medical interfaces such as inpatient hospital stays, outpatient physician visits, and outpatient specialty clinics allows for the study of disease natural history and treatment interventions in an entire community with the goal to improve population wellness. As a linkage system grows in the number of patients and duration of follow-up, it becomes increasingly informative for population-based research if a well-defined population is encompassed. Therefore, an ideal medical linkage system should include four characteristics: (1) a well-defined geographic region; (2) a history of at least 10 years to provide the historical depth needed to answer public health questions; (3) a large number of patients so that rare diseases and medical practices can be adequately studied; and (4) as many variables as possible that can be searched electronically. 1 The REP is an ideal system as all these characteristics are present.
History and Origins of the Rochester Epidemiology Project
Maintained for nearly half a century, the REP has supported an extensive amount of clinical and epidemiologic research. Although officially established in 1966, its origins are traced to events at the Mayo Clinic in the early 1900s. The Mayo Clinic was initially established by William W. Mayo and his two sons, William J. and Charles H. Mayo. Their original patient records were kept in leather-bound ledgers, maintained by the individual physicians. The old ledger system became the basis of continuity of care of individual patients and for describing new surgical techniques and case series. 2 3 These records are part of the REP, and during chart review for the below-mentioned chronic ulcerative colitis study, the authors had opportunity to handle chart from Dr. Charles Mayo and Dr. Balfour. Henry Plummer, a clinical associate of the Mayo Clinic group practice, realized the limitations of the ledger system with its cumbersome entry, difficulty in retrieving, and fragmentation of patient information. In 1907, Plummer introduced a new medical record system where patient information is recorded on unbound paper forms and filed for each patient who was assigned a unique medical record number. This new system allowed for all the information on a single patient to be filed together, under a unique identifying number, and stored in a central location. Importantly, each file was available to any physician for teaching and research purposes. However, as patient volume increased and diagnostic terminology expanded, the Plummer system became strained. Nonetheless, Dr. Plummer's dossier system became the basis for the modern medical record system, pre-EHR, and he is generally credited with the invention of the medical chart.
In the 1930s, Joseph Berkson of the Mayo Clinic established the “Berkson codes.” These were numerical codes to file records by disease and surgical intervention. At that time, punch cards were used to enter the codes, and included on these punch cards was a data element, indicating residency in Rochester, Minnesota, the largest city in Olmsted County. This was the start of having access to epidemiologic data since research could be performed on a well-defined population. Simultaneously, epidemiology developed as a major research methodology in cancer, cardiovascular disease, other chronic conditions, 4 5 and population-based research was being increasingly used to validate previously published data based on small patient series.
The development of a medical records linkage system for Olmsted County came with the arrival of Leonard T. Kurland to Mayo Clinic. Dr. Kurland was trained in epidemiology and understood the unique potential of the Mayo Clinic records system for the generation of accurate natural history data from a geographically defined population. In 1966, he obtained funding from the NIH to establish a patient medical record linkage system between Mayo Clinic and other health care facilities in Rochester and the entirety of Olmsted County ( Fig. 1 ). This was the official start of the REP passive medical records linkage system. 6 7
Fig. 1.
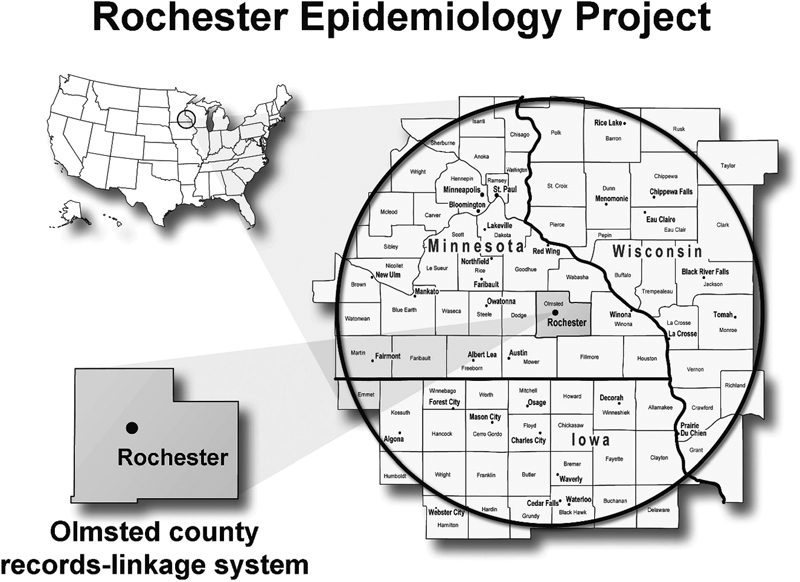
The geography of the Rochester Epidemiology Project. Inset box depicts the 300-mile radius surrounding Olmstead County. Reproduced with permission from Holubar et al, 23 by Wolters Kluwer Health, Inc.
Obstacles for the Rochester Epidemiology Project
For the first time in 1996, the State of Minnesota passed a new law that required general written authorization from each patient before their medical record could be reviewed for research. To comply with this law, Mayo Clinic, Olmsted Medical Center, Rochester Family Medicine Clinic, and other health facilities associated with the REP had to implement a way to obtain written authorization from patients. Patients were contacted twice in writing, with 60 days between each attempt. Lack of response was considered an authorization of medical charts for research. Only 2.1% of patients denied authorization of all REP health care providers, 8 and refusal of research authorization was higher among women, younger patients, and patients with previous diagnoses considered more sensitive (e.g., mental disorders). 9
Then in 2002, the federal Health Insurance Portability and Accountability Act (HIPAA) was implemented. 10 The law allowed for review of existing medical data without informed consent from each patient if investigators obtained a HIPAA waiver from the Mayo Clinic Institutional Review Board (IRB) and Olmsted Medical Center IRB. However, if any contact with the individual was needed for purposes of the study (e.g., surveys and interviews whether by letter, telephone, face to face), then written informed consent and HIPAA authorization was required. This HIPAA waiver allowed investigators using REP to conduct studies with higher participation rates while respecting patients' privacy concerns. 8
Lastly, in the early 2000s, paper charts had become impractical and cumbersome. Under the direction of Dr. Steven Jacobsen, electronic health records gradually replaced the traditional Mayo Clinic paper records with the transition being complete in 2005. Dr. Jacobsen also established an electronic portal to search medical records for any given individual and implemented a permanent linkage system, which was less prone to error than the study-by-study linkage system previously used. 8 In 2006, Walter Rocca and Barbara Yawn became joint directors of REP and under their leadership, additional infrastructure expanded the REP capabilities. They encompassed more health practices (e.g., dental and chiropractors), expanded the geographic coverage to include the eight-county region of southeastern Minnesota ( Fig. 2 ) which more than doubled the population covered, established codes for integration of prescription data, started a program to increase community engagement in REP by introducing the first extramural website to facilitate the use of REP research by external investigators ( http://www.RochesterProject.org ), and formally addressed the demographic and socioeconomic similarities and differences in Olmsted Country compared with the state of Minnesota and the rest of the United States. This last capability was important to establish the generalizability of REP studies to other populations or the entire country. 1
Fig. 2.
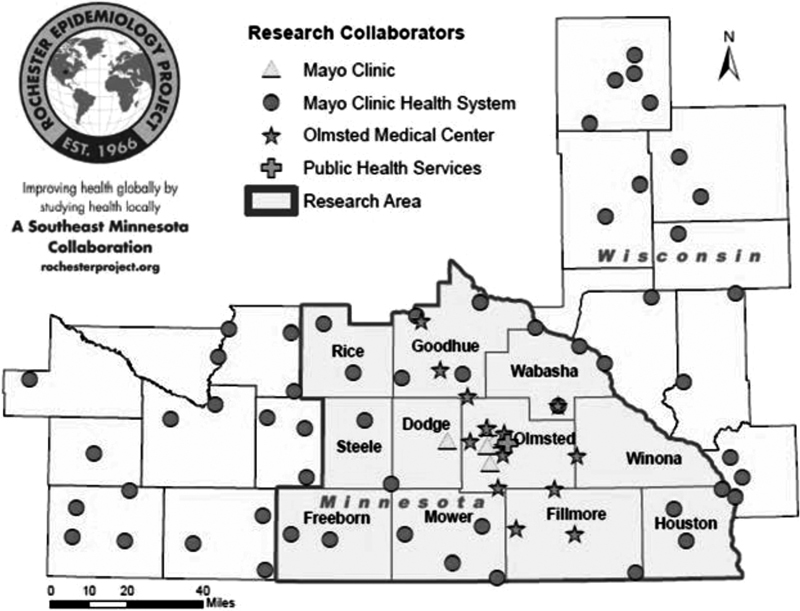
The post-2006 expanded the geographic coverage including the eight-county region of southeastern Minnesota.
Rochester Epidemiology Project in its Current State
Since its inception in 1966 till now, the REP has grown to encompass 502,820 individual patients with 6,239,353 person-years of follow-up. Rather than recording data in a specified format as required in some cohort studies (e.g., Framingham Heart Study), the medical information is recorded in the format dictated by each specific health care provider. Only demographic data, diagnostic codes, surgical procedure codes, and drug prescriptions are currently organized in searchable electronic indexes. 11
A Scientific Steering Committee consists of two coprincipal investigators, an anthropologist, a bioethicist, a biostatistician, an expert in information technology, and a pharmacoepidemiologist leads the REP. A full-time epidemiologist acts as the scientific manager, coordinating the daily organizational activities pertaining to research projects, publications, and interactions with REP users. Two project managers coordinate the financial, personnel, and operational activities, and a third project manager assists with the development of collaborations with new facilities not yet participating in REP. A data management team includes three full-time technology experts, and the statistical team has two part-time statisticians and one part-time data analyst. Four full-time study assistants manage activities of record handling, diagnostic coding, and data verification and correction.
The REP has had continuous NIH funding since 1966. This federal funding is supplemented by research support from the Mayo Clinic. In 2012, the total annual REP budget was $1,370,000. National funding accounted for $770,000 and Mayo Clinic provided an additional $600,000. 11
To date, the REP has been used for over 2,000 publications across nearly every medical discipline. Over the years, several studies have been conducted to investigate the validity of the linkage system, and in one, a random sample of 400 patients found an over-inclusion rate of 2.5% and under-inclusion rate of 1.3%. 8 Thus, its validity has been well established.
A series of steps is required to perform a clinical or epidemiologic study using the REP database. First, multiple medical records from the same individual are linked within and across medical institutions to create a complete medical chart. Second, diagnostic codes, surgical codes, and other coded information is then abstracted and stored in searchable electronic indexes. Each code or piece of information also includes a time frame associated with the intervention or diagnosis allowing investigators to retrieve a list of patients who received a specific diagnosis or procedure within a certain time frame (e.g., all men who underwent a low anterior resection for rectal cancer between 1980 and 2000). Third, a nurse abstractor, physician, or trained investigator reviews all the records to verify diagnoses and apply specific diagnostic criteria. This information can then be abstracted by investigators to perform incidence or prevalence studies, case–control studies, cohort studies, cost or cost-effectiveness studies, and natural history or outcome studies. 11
All epidemiologic studies raise concerns regarding the generalizability from the study sample to the target population and from the target population to other populations or to the entire United States. To address the generalizability of the REP to other parts of the United States, St. Sauver et al 12 compared characteristics of the REP population to the state of Minnesota, the upper Midwest, and the rest of the United States ( Table 1 ). They found that Olmsted County was similar to Minnesota and the rest of the United States in terms of age, sex, and mortality. However, Olmsted County had a higher proportion of white residents (90.3 vs. 75.1%) and a higher socioeconomic status (91.1 vs. 80.4% high-school graduates) than the rest of the United States. Thus, if ethnic or socioeconomic characteristics are important for disease-specific outcomes, this should be considered with generalized findings from the REP. 12
Table 1. Demographic characteristics of Olmsted County, Minnesota, the U.S. white population, and the rest of the U.S. population (1970–2010) a .
| Total population | Aged 18 y and over (%) | Aged 65 y and over (%) | Median age (y) | Male (%) | ||
|---|---|---|---|---|---|---|
| 1970 | Olmsted County | 84,104 | 62.3 | 8.6 | 25.5 | 47.4 |
| Minnesota | 3,804,971 | 63.7 | 10.7 | 26.8 | 49 | |
| U.S. white US population | 177,748,975 | 66.8 | 10.3 | 28.9 | 48.8 | |
| 203,211,926 | 65.7 | 9.9 | 28.1 | 48.7 | ||
| 1980 | Olmsted County | 92,006 | 70.5 | 9.3 | 28.1 | 47.5 |
| Minnesota | 4,075,970 | 71.3 | 11.8 | 29.2 | 49 | |
| U.S. white | 189,035,012 | 73.2 | 11.9 | 31.3 | 48.7 | |
| US population | 226,545,805 | 71.9 | 11.2 | 30 | 48.6 | |
| 1990 | Olmsted County | 106,470 | 72.3 | 10 | 31.6 | 48.5 |
| Minnesota | 4,375,665 | 73.3 | 12.5 | 32.4 | 49 | |
| U.S. white | 199,686,070 | 76.1 | 13.9 | 33.7 | 48.8 | |
| US population | 248,709,873 | 74.4 | 12.6 | 32.8 | 48.7 | |
| 2000 | Olmsted County | 124,277 | 73 | 10.8 | 35 | 49.1 |
| Minnesota | 4,919,479 | 73.8 | 12.1 | 35.4 | 49.5 | |
| U.S. white | 211,460,626 | 76.5 | 14.4 | 37 | 49.1 | |
| US population | 281,421,906 | 74.3 | 12.4 | 35.3 | 49.1 | |
| 2010 | Olmsted County | 144,248 | 74.7 | 12.6 | 36.3 | 48.9 |
| Minnesota | 5,303,925 | 75.8 | 12.9 | 37.4 | 49.6 | |
| U.S. white | 223,553,265 | 78.3 | 15.3 | 40.3 | 49.3 | |
| US population | 308,745,538 | 76 | 13 | 37.2 | 49.2 |
All data obtained from U.S. Census Statistics: 1970, 1980, 1990, 2000, 2010.
The Use of Rochester Epidemiology Project in Colon and Rectal Surgery Research
The REP has made important contributions to the colon and rectal surgery literature. This is largely because within the United States, healthcare remains fragmented with no unique identifier to longitudinally follow-up with a patient throughout the system over his or her lifespan. This, along with privacy laws, discourages the development of population-based disease-specific national databases.
Inflammatory Bowel Disease
There is currently no unified surveillance system for IBD in the United States. Estimates of incidence, prevalence, and death have come largely from administrative data and regional cohorts. This is largely due to the aforementioned challenges of designing and maintaining a population-based disease-specific database. Additional challenges specific to IBD is that IBD is not a “reportable” condition and there are no gold standard laboratory diagnostic testing.
While administrative data, which are readily available, can assist in population-based studies, these have numerous limitations. Administrative data lack clinical details, concerns about application of standard coding practices between institutions, validation of outcomes, and limitations in deciphering mortality versus lost to follow-up. Thus, regional cohort data such as the REP are useful in studying diseases such as IBD to answer several epidemiologic questions regarding the incidence, cancer incidence, and cost associated with the diagnosis of IBD. In fact, over a 3-year period, more than 90% of county residents are seen at one of the medical sites, capturing nearly all potential IBD cases. 13 Loftus et al used REP to define the incidence, prevalence, and survival of patients with ulcerative colitis (UC) in Olmsted County, Minnesota, and these studies represent likely the best epidemiologic data on IBD in the United States (note in particular Norway and Sweden), which have universal healthcare, also have excellent IBD epidemiologic data. 14
In the late 1990s, there were reports suggesting that Crohn's disease (CD) was increasing and others suggesting an increased mortality among patients with chronic ulcerative colitis (CUC). Between 1940 and 1993, the REP found that CUC had an incidence of 7.6 cases per 100,000 person-years, an adjusted prevalence rate of 229 cases per 100,000, and that increased incidence rates were associated with later calendar years ( p < 0.002), younger age ( p < 0.0001), urban residence ( p < 0.0001), and male sex ( p < 0.003). Overall survival was similar to that expected ( p > 0.2). The REP was unique in its ability to answer these questions due to the ability to capture all patients longitudinally for a population-based epidemiologic study. 14 Later, Loftus et al, again using the REP, showed that incidence rates of both CD and CUC peak in the 20- to 29-year-old age group. 15 Loftus et al's data also suggested that there is not a true bimodal age distribution for IBD as is commonly believed.
Another area of investigation studied by the REP was the relative risk of colon and rectal cancer among patients with IBD. Stonnington et al found a relative risk of 2.4 for colon and rectal cancer among patients with CUC. 13 A later study by Jess et al found no increased risk in CUC patients unless they had extensive colitis, but a slightly increased risk among patients with CD, and a 40-fold risk of small bowel cancer among patients with CD. 16 Additional factors addressed with the REP database included fistula risks, 17 bone fracture risks, 18 19 response to medical therapy, 20 progression of intestinal complications, 21 and requirement for surgery. 22 Again, REP was utilized in determining these important population-based questions since these patients are followed up in a confined medical community allowing for population-based studies, not common to databases seen in referral centers or captured from administrative data.
More recently, Holubar et al from Mayo Clinic looked at costs in healthcare related to CUC in two studies. The first study compared total direct healthcare costs in the 2-year period before surgery and the 2-year period after surgery among patients with UC ( Fig. 3 ). The authors found that costs were significantly reduced in the 2 years following surgery suggesting surgical intervention could be associated with a long-term economic benefit ( Table 2 ). 23 Three years later, the same group performed a follow-up study using REP, to examine the drivers of direct health care costs in the 2 years following surgical versus medical management. In the medical group, disease extent but not severity drove costs, and in the surgical group Brooke ileostomy patients had higher costs than pouch patients; those with pouches had higher costs if they had pouchitis ( Table 3 ). 24 The strength of these two studies is the very precise nature of the cost estimates with statistical bootstrapping, while the weakness was relatively small numbers of patients, but given the population-based nature of the cohorts this represents some of the best real-world data available on the topic.
Fig. 3.
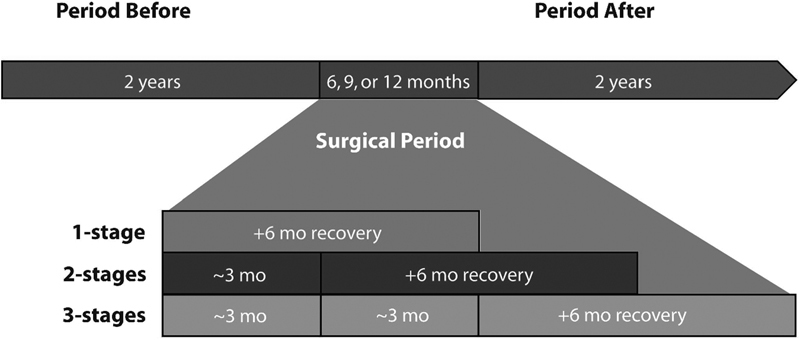
Schematic representation of the periods of observation. Reproduced with permission from Holubar et al, 23 by Wolters Kluwer Health, Inc.
Table 2. Direct healthcare costs of before and after surgery for ulcerative colitis a .
| Subgroup | Period before mean total costs a b | Period after mean total costs a b | Mean cost difference (95% CI) c | p -Value d |
|---|---|---|---|---|
| IPAA ( n = 45) | $15,732 ($10,194) ± $17,943 | $6,436 (1,961) ± 9,820 | $9,296 ($324–$15,628) | <0.001 |
| TPC-BI ( n = 15) | $201,131 ($17,254) ± $10,241 | $7,602 ($5,781) ± $6,784 | $12,529 ($6,467–$18,688) | <0.001 |
Abbreviations: CI, confidence interval; IPAA, ileal pouch–anal anastomosis; TPC-BI, total proctocolectomy with Brooke ileostomy.
Source: Reproduced with permission from Holubar et al, 23 by Wolters Kluwer Health, Inc.
Estimated costs per patient are reported in 2007 constant dollars.
Values are presented as mean (median) % standard deviation.
Bootstrap 95% CI using the percentile method.
Paired t -test.
Table 3. Two-year direct health care costs after surgical and medical therapy for CUC a .
| Time 1 surgical cohort ( n = 60) |
Time 2 surgical cohort ( n = 60) |
p -Value | Time 1 medical cohort ( n = 60) |
Time 2 medical cohort ( n = 60) |
p | |
|---|---|---|---|---|---|---|
| No. of CUC drugs, median | 0 (0–1) a | 0 (0–2) a | 0.5 | 1 (0–3) a | 1 (0–2) a | 0.01 |
| 5-ASA compounds, n (%) | – | – | – | 37 (61.7) | 33 (55) | <0.0002 |
| Antibiotics, n (%) | 3 (5) | 5 (8.3) | 0.02 | – | – | – |
| Steroids, n (%) | 3 (5) b | 3 (5) c | 0.86 | 14 (23.3) | 5 (8.3) | 0.02 |
| Immunomodulators, n (%) | – | 2 (3.3%) d | – | 5 (8.3) | 7 (11.7) | <0.0002 |
| Anti-TNF-α-Ab, n (%) | – | – | – | 1 (1.7) | – | – |
Abbreviations: CUC, chronic ulcerative colitis; TNF, tumor necrosis factor.
Source: Reproduced with permission from Holubar et al, 23 by Wolters Kluwer Health, Inc.
Estimated costs per patient are reported in 2009 constant dollars.
Values are presented as mean (median) ± standard deviation.
Bootstrap 95% CI by using the percentile method.
Paired t -test.
Thus, while a national linkage system within the United States to capture all patients with the diagnosis of IBD would be useful, the REP is one of the closest representatives. The research regarding IBD has been a landmark population-based research and has shed light to treating providers regarding several unanswered questions.
Colon and Rectal Cancer
The REP is particularly useful in the study of colon and rectal cancer for similar reasons to that of IBD. Due to reports claiming an increasing incidence of colorectal cancer in the United States, Beard et al 25 used the REP to look at the incidence of colorectal cancer among men and women by decade from 1940 to 1990. The group did not find an increased incidence of colorectal cancer among men or women in a community where medical records were complete. While the overall incidence was not increasing, Beart et al used the REP to demonstrate an increasing ratio of proximal to distal colorectal carcinomas in a population-based study. 26
Interestingly, a later study by Gupta et al found an overall proportion of screen-detected colorectal cancer rose from 8 to 17% between 1980 and 2000 and the annual adjusted polypectomy rates increased from 86 to 320 per 100,000 ( p < 0.001) while the overall incidence of CRC decreased. These findings underscored the fact that screening is improving clinical outcomes. Interestingly, the same study reported an increase in right-sided colon cancers and a concurrent decrease in left-sided colon cancers. 27
Meckel's Diverticulum
Two REP studies have examined diverticular disease. The first, a study by Cullen et al, 28 Annals of Surgery 1994, examined the epidemiology and management of Meckel's diverticulum. The authors sought to determine the optimal management of incidentally discovered (asymptomatic) Meckel's diverticulum during surgery for another indication: resection versus leaving in situ (expectant management).
Epidemiologically, the authors found that more than 50% of the complicated cases occurred in those below 30 years of age, and men had a higher age-adjusted incidence of developing complicated Meckel's relative to women, but that all genders and all age groups were at risk of developing complications from a Meckel's diverticulum.
They found that the lifetime risk of developing complicated Meckel's disease was 6.4%, with a 12% short-term morbidity, and 2% mortality, and a 7% long-term surgical complication rate. On the other hand after incidental management, complications occurred in only 1 to 2%. They also found that if performed incidentally, the majority (92%) of patients were managed by diverticulectomy, with small bowel resection in only 6%, while in complicated cases, these rates were 65 and 31%. Therefore, the authors recommended routinely removing the incidentally found Meckel's in most patients and in most age groups.
Sigmoid Diverticulitis
In 2015, Bharucha et al 29 examined the natural history of diverticulitis using the REP. Given concerns over the fact that most studies of diverticulitis exclude outpatient, they felt the population-based nature of the REP would be ideal to address this weakness in the literature.
There first finding was that the incidence of diverticulitis is on the increase in recent decades; in fact, it has more than doubled from 115/100,000 person-years in the 1980s, to 245/100,000 person-years as of 2007. The second finding was that, although the incidence of diverticulitis increasing steadily with advancing age, the greatest increase was in those below 50 years of age. Of note, during these same decades, the use of abdominal computed tomography to assist in the diagnosis increased by 287%.
Regarding recurrent diverticulitis, after their first attack of uncomplicated diverticulitis, the cumulative incidence of recurrence was 8% at 1 year, 17% at 5 years, and 22% at 10 years, with younger age and female gender being independent risk factor for recurrence. After a second recurrence, these numbers increased to 19% at 1 year, 44% at 5 years, and 55% at 10 years.
Complications of diverticulitis were relatively uncommon, occurring in only 12% of the cohort (3,222), with age over 60 years being the main predictor. However, 74% of those who developed complications did so during their first episode. Finally, of the 454 patients who had surgery, 250 were for complications and 125 were for emergency cases, all of which had complicated diverticulitis. Mortality was increased in complicated disease (hazard ratio: 1.36, 95% confidence interval: 1.14–1.62). Overall, these data substantially add to, and validate, what is known about the natural history of diverticular disease.
Conclusions
Successful examples of linkage systems have been implemented in the United Kingdom, 4 6 7 30 Australia, 8 and Canada. 31 However, similar systems have been challenged in the United States due to a lack of a national health system. 2 Some health plans such as Kaiser Permanente have been able to develop a medical linkage system within a given plan, but does not include patients outside that plan. In recent years, several attempts have been made at publically accessible databases for research. However, this has been limited by increased concerns regarding confidentially surrounding patients' health records. And, even if a national database was initiated, the historical depth is lacking—at least for now. Thus, the REP is uniquely positioned in its breadth, depth, and resourcefulness. Its population of nearly 500,000 patients followed up for nearly half a century, the REP provides the infrastructure to perform practice changing population-based research. The ability to generate long-term incidence, prevalence, and mortality rates has become one of the hallmarks of the REP. Simultaneously, it serves as a model for the development of future linkage systems in the United States.
References
- 1.St Sauver J L, Grossardt B R, Yawn B P, Melton L J, III, Rocca W A. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(09):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conway P H, VanLare J M. Improving access to health care data: the Open Government strategy. JAMA. 2010;304(09):1007–1008. doi: 10.1001/jama.2010.1249. [DOI] [PubMed] [Google Scholar]
- 3.Melton L J., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(03):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 4.Acheson E D. The Oxford Record Linkage Study: a review of the method with some preliminary results. Proc R Soc Med. 1964;57:269–274. [PMC free article] [PubMed] [Google Scholar]
- 5.Bureau U C. Washington, DC: US GPO; 1982. 1980 Census of Population. Volume I: Characteristics of the Population. [Google Scholar]
- 6.Gill L, Goldacre M, Simmons H, Bettley G, Griffith M. Computerised linking of medical records: methodological guidelines. J Epidemiol Community Health. 1993;47(04):316–319. doi: 10.1136/jech.47.4.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kendrick S W, Douglas M M, Gardner D, Hucker D. Best-link matching of Scottish health data sets. Methods Inf Med. 1998;37(01):64–68. [PubMed] [Google Scholar]
- 8.Holman C D, Bass A J, Rouse I L, Hobbs M S. Population-based linkage of health records in Western Australia: development of a health services research linked database. Aust N Z J Public Health. 1999;23(05):453–459. doi: 10.1111/j.1467-842x.1999.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 9.Dean J M, Vernon D D, Cook L, Nechodom P, Reading J, Suruda A. Probabilistic linkage of computerized ambulance and inpatient hospital discharge records: a potential tool for evaluation of emergency medical services. Ann Emerg Med. 2001;37(06):616–626. doi: 10.1067/mem.2001.115214. [DOI] [PubMed] [Google Scholar]
- 10.Victor T W, Mera R M. Record linkage of health care insurance claims. J Am Med Inform Assoc. 2001;8(03):281–288. doi: 10.1136/jamia.2001.0080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rocca W A, Yawn B P, St Sauver J L, Grossardt B R, Melton L J., III History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.St Sauver J L, Grossardt B R, Leibson C L, Yawn B P, Melton L J, III, Rocca W A. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(02):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stonnington C M, Phillips S F, Zinsmeister A R, Melton L J., III Prognosis of chronic ulcerative colitis in a community. Gut. 1987;28(10):1261–1266. doi: 10.1136/gut.28.10.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftus E V, Jr, Silverstein M D, Sandborn W J, Tremaine W J, Harmsen W S, Zinsmeister A R. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut. 2000;46(03):336–343. doi: 10.1136/gut.46.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loftus C G, Loftus E V, Jr, Harmsen W S et al. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis. 2007;13(03):254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- 16.Jess T, Simonsen J, Nielsen N M et al. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60(03):318–324. doi: 10.1136/gut.2010.223396. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz D A, Loftus E V, Jr, Tremaine W J et al. The natural history of fistulizing Crohn's disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(04):875–880. doi: 10.1053/gast.2002.32362. [DOI] [PubMed] [Google Scholar]
- 18.Loftus E V, Jr, Achenbach S J, Sandborn W J, Tremaine W J, Oberg A L, Melton L J., III Risk of fracture in ulcerative colitis: a population-based study from Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2003;1(06):465–473. doi: 10.1016/s1542-3565(03)00185-x. [DOI] [PubMed] [Google Scholar]
- 19.Loftus E V, Jr, Crowson C S, Sandborn W J, Tremaine W J, O'Fallon W M, Melton L J., III Long-term fracture risk in patients with Crohn's disease: a population-based study in Olmsted County, Minnesota. Gastroenterology. 2002;123(02):468–475. doi: 10.1053/gast.2002.34779. [DOI] [PubMed] [Google Scholar]
- 20.Faubion W A, Jr, Loftus E V, Jr, Harmsen W S, Zinsmeister A R, Sandborn W J. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(02):255–260. doi: 10.1053/gast.2001.26279. [DOI] [PubMed] [Google Scholar]
- 21.Thia K T, Sandborn W J, Harmsen W S, Zinsmeister A R, Loftus E V., Jr Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139(04):1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peyrin-Biroulet L, Harmsen W S, Tremaine W J, Zinsmeister A R, Sandborn W J, Loftus E V., Jr Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004) Am J Gastroenterol. 2012;107(11):1693–1701. doi: 10.1038/ajg.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holubar S D, Long K H, Loftus E V, Jr, Wolff B G, Pemberton J H, Cima R R. Long-term direct costs before and after proctocolectomy for ulcerative colitis: a population-based study in Olmsted County, Minnesota. Dis Colon Rectum. 2009;52(11):1815–1823. doi: 10.1007/DCR.0b013e3181b327a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holubar S D, Pendlimari R, Loftus E V, Jr et al. Drivers of cost after surgical and medical therapy for chronic ulcerative colitis: a nested case-cohort study in Olmsted County, Minnesota. Dis Colon Rectum. 2012;55(12):1258–1265. doi: 10.1097/DCR.0b013e31826e4f49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beard C M, Spencer R J, Weiland L H, O'Fallon W M, Melton L J., III Trends in colorectal cancer over a half century in Rochester, Minnesota, 1940 to 1989. Ann Epidemiol. 1995;5(03):210–214. doi: 10.1016/1047-2797(94)00107-5. [DOI] [PubMed] [Google Scholar]
- 26.Beart R W, Melton L J, III, Maruta M, Dockerty M B, Frydenberg H B, O'Fallon W M. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26(06):393–398. doi: 10.1007/BF02553382. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A K, Melton L J, III, Petersen G M et al. Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol. 2005;3(02):150–158. doi: 10.1016/s1542-3565(04)00664-0. [DOI] [PubMed] [Google Scholar]
- 28.Cullen J J, Kelly K A, Moir C R, Hodge D O, Zinsmeister A R, Melton L J., IIISurgical management of Meckel's diverticulum. An epidemiologic, population-based study Ann Surg 199422004564–568., discussion 568–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharucha A E, Parthasarathy G, Ditah I et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. Am J Gastroenterol. 2015;110(11):1589–1596. doi: 10.1038/ajg.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walley T, Mantgani A.The UK general practice research database Lancet 1997350(9084):1097–1099. [DOI] [PubMed] [Google Scholar]
- 31.Roos L L, Menec V, Currie R J. Policy analysis in an information-rich environment. Soc Sci Med. 2004;58(11):2231–2241. doi: 10.1016/j.socscimed.2003.08.008. [DOI] [PubMed] [Google Scholar]


