Abstract
BACKGROUND:
Platelet additive solutions (PAS) are crystalloid nutrient media used in place of plasma for platelet storage. They replace 60%–70% of plasma in platelet components, so the amount of storage plasma can be decreased. Platelets in PAS have lower risk for allergic transfusion reactions with equivalent clinical efficacy for controlling bleeding.
AIM:
The aim of this study is to evaluate the clinical and laboratory efficacy of PAS-platelets.
MATERIALS AND METHODS:
A total of 1674 single donor platelet (SDP) were collected in PAS in the month of June to September 2016 by different apheresis systems. The quality control tests were done on 356 units in 4 months. Total number of SDP were processed with Amicus device (n = 232), Trima Accel (n = 84), and MCS+ (n = 40). The parameters analyzed were antibody titer of anti-A and anti-B, volume, platelet count, pH, bacterial contamination, and reporting of adverse transfusion reaction. Antibody titers were checked by tube technique, and platelet counts were checked by hematology analyzer Sysmex poch 100i. The swirling was checked manually, and pH was checked with pH strips.
RESULTS:
Out of 356, 164 units were O group, 113 units were B group, 68 units were of A group, and the remaining 11 units were of AB Group. Anti-A and anti-B titer was significantly reduced in PAS-SDP and found 1:32 or less for all the units. All the units found negative for bacterial contamination. No transfusion reaction was reported of the units transfused. All other quality parameters for platelets also found satisfactory after implementing the additive solution.
CONCLUSION:
The ABO antibody titers were significantly reduced after addition of PAS. This facilitates the ABO incompatible SDP transfusion and helps in inventory management. The risk of allergic transfusion reaction decreases after reducing the amount of plasma from SDP units. Using PAS-SDP certainly improve the inventory management for platelets with no compromise on clinical and laboratory efficacy.
Keywords: Platelet additive solution, single donor platelet, transfusion reaction
Introduction
Platelet transfusion has an important role in patient care, and single donor platelet (SDP) is widely used component for various thrombocytopenic patients. SDP generally prepared and stored with 200–300 ml of donor plasma and shelf life if 5 days. Due to the large volume of plasma, ABO incompatible SDP transfusion is not practiced routinely.[1]
Platelet additive solutions (PASs) were first developed in the 1980s and continued to be improved over the following years. The use of PAS as replacement for plasma has a number of benefits, both for the quality of the platelet concentrates and for the patients.[1]
PAS are crystalloid nutrient media used in place of plasma for platelet storage. They replace 60%–70% of plasma in platelet components, so the amount of storage plasma can be decreased.[1,2]
PAS provide real and potential benefits. Platelets stored in PAS have been demonstrated to have a lower risk for allergic transfusion reactions. In addition, platelets stored in PAS appear to have equivalent clinical efficacy for controlling bleeding, compared to platelets stored in 100% plasma. The transfusion of platelets stored in PAS versus plasma results in a lower incidence of other plasma-associated transfusion reactions, such as ABO hemolytic reactions and transfusion-related acute lung injury. Further, platelets in PAS are compatible with pathogen reduction technology.[1,2]
PAS-II is the simplest of these additives, containing only sodium chloride, sodium citrate, and sodium acetate. When phosphate was added to PAS-II to act as a buffer, the resulting additive was renamed PAS-III. This addition of phosphate increased the metabolism of glucose, thus resulting in increased lactate production.[2] PAS-III M contains potassium and magnesium to counteract this.[3]
PAS-III M has been shown to be an effective substitute for plasma in both apheresis and buffy coat platelet concentrates stored for up to 12 days. PAS-III M is a third-generation PAS-containing acetate, a substrate for oxidative phosphorylation, phosphate to provide improved buffering capacity as well as potassium, citrate, and magnesium. Storage of platelets in this additive has been shown to reduce the rate of glycolysis, leading to better retention of pH and hypotonic shock response reactivity.[4,5,6]
Aims and objectives
The aim and objectives of this study are to evaluate the clinical and laboratory efficacy of PAS-platelets.
Materials and Methods
The Regional Blood Transfusion Center in Karnataka collects an average of 35,000 blood units per year from nonremunerated voluntary donors only and performs nearly 2500 SDP donations every year. All the whole blood units are collected from voluntary blood donors and tested by nucleic acid test (NAT) along with the serology testing. As a policy blood bank intended to collect all SDPs also from voluntary blood donors and get the NAT testing done. Due to short shelf life and high cost, maintaining inventory of SDPs units was a challenge.
The blood bank started using SDP with PAS III (SSP+, MacoPharma, Moveaux, France) for better inventory management and to facilitate ABO incompatible SDP transfusion. The objective of this prospective cohort study is to perform validation of SDP apheresis using PAS and also to analyze the clinical outcome of using SDP with PAS. A total of 1674 SDP were collected in PAS in the month of June and September 2016 by different apheresis systems. The quality control tests (QCTs) were done on 356 units in 4 months [Figure 1].
Figure 1.
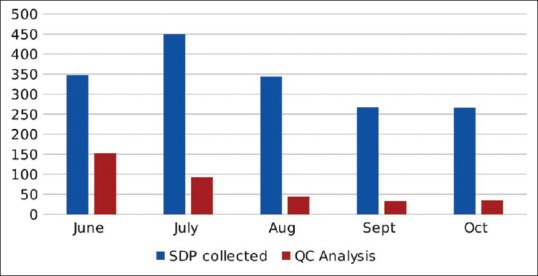
Total number of single donor platelet collected and analyzed for quality control
A total number of SDP were processed with Amicus device (n = 232), Trima Accel (n = 84) and MCS+ (n = 40). Amicus has automated programming for PAS but in Trima and MCS+, SDP was collected as concentrate, and then, PAS was added with sterile connecting device. The PAS to Plasma ratio was 70:30.
In this prospective study, we evaluate the quality of PAS platelet apheresis on the basis of various parameters after approval from organization ethical committee. The parameters analyzed were antibody titer of Anti-A and Anti-B, volume, platelet count, pH, bacterial contamination, and reporting of adverse transfusion reaction. The samples for QCTs are collected on day 4. Antibody titers were checked by tube technique, and platelet counts were checked by hematology analyzer Sysmex Poch 100i. The swirling was checked manually, and pH was checked with pH strips. The specimen for bacterial culture was collected under the aseptic condition and sent to microbiology department. The technique for culture was BD BACTECH automated culture system and reporting was done after 72 h. As a control, we checked antibody titer in the 135 (30%of test samples) donors plasma.
Results
Out of 356, 164 units were O group, 113 units were B group, 68 units were of A group, and the remaining 11 units were of AB group [Figure 2]
Platelet count was <3.0 × 1011 platelets/unit in 14 SDP units, count was between 3.0–4.0 × 1011 platelets/unit in 244 units, and the count was >4.0 × 1011 in 98 units. The mean platelet count was 3.6 × 1011 platelets/unit with range of 2.8–5.8 × 1011 platelets/unit [Figure 3]
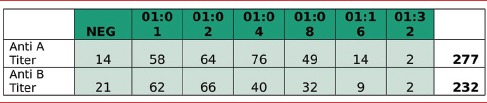
Anti A antibody titer was checked in total 277 units. For antibody Anti-A Titer in SDP with PAS units, 14 samples came negative, 58 samples came 1:1, 64 samples were 1:2, 76 samples came 1:4, 49 samples came 1:8, 14 samples came 1:16 and 02 samples came 1:32 [Table 1]
Anti B antibody was checked in 232 units. For Antibody Anti-B Titer in SDP with PAS units, 21 samples came negative, 62 samples came 1:1, 66 samples came 1:2, 40 samples came 1:4, 32 samples came 1:8, 09 samples came 1:16, and 02 samples came 1:32 [Table 1]
As a control, we checked antibody titer in the 135 (30%) donors plasma. Total 25 were A, 46 were B, 52 were O. We found Anti A and Anti B in the range of 1:512–4048
The mean volume of the SDP collected was 263 ml, and the range was 200–300 ml. Swirling was present in all the SDP Units, and pH of all the SSP units was >6
All the units found negative for bacterial contamination
No transfusion reaction was reported of the units transfused.
Figure 2.
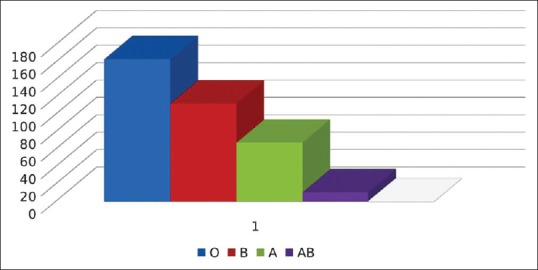
Blood group-wise distribution of single donor platelets
Figure 3.
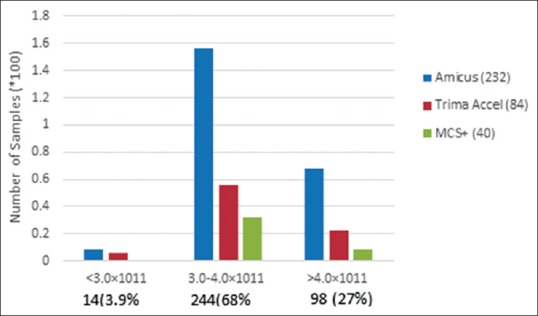
Quality control of single donor platelet: platelet count
Table 1.
Anti-A and anti-B titers in platelet additive solutions single donor platelet units
Discussion
Platelet transfusion is required for various different categories for patients including oncology, hematology, and some infections such as Dengue. In India every year, we face challenge of dengue and thousands of people are infected. Many of them have severe thrombocytopenia which requires platelet transfusion. At our institution, majority of platelet transfusion request are for dengue cases, but we do receive requests for cancer patients and other thrombocytopenic conditions. Most of the physician prefers apheresis platelet due to less donor exposure, leukoreduction, and better clinical outcome.
Since SDP is an expensive blood component, therefore, most of the blood banks in India do not keep stock of SDP. As soon as a request of SDP generated, patient relatives are asked to bring the donor, and after processing, same unit is provided. Testing of the units are either done by rapid card method, or few centers use chemiluminescence. Due to the practice of replacement donations and compromised testing, there is a huge risk of transfusion-transmitted infection. Also for critical patients who need urgent platelet transfusion, apheresis platelets are not always available. It is a challenge for clinicians to treat these patients.
In principle, ABO identical platelets are recommended for optimal increments in the counts in patients. However, practically, this is not always feasible due to the scarcity of group-specific platelets, especially SDP's, in times of urgent need. This is compounded by the fact that it has a short shelf life of 5 days, wherein strict adherence to the policy of transfusing ABO identical SDPs can lead to outdate and expiry of platelet.[7]
At our blood center, we decided to break this practice and to get voluntary SDP donors as well as to keep stock of SDP units for critical patients. However, the challenge was cost of keeping all blood group SDPs in shelf was higher and risk of nonutilization of any specific blood group SDP is additional financial burden.
Therefore, we decided to use PAS for apheresis units and to remove most of the plasma and naturally occurring ABO antibodies so that we can provide SDPs without blood group specificity. We observed the significant reduction in ABO titer after adding PAS. The maximum titer was observed in our study was 1:32, and most of the units had titer below 1:8. Similar study Jain et al. have also reported the reduction in Anti A and Anti B titers with PAS and observed maximum titer in PAS SDP 1:64. This decrease in titer may benefit the patients by decreasing the potential for hemolysis if incompatible platelet units have to be issued.[8] Platelets with high titers of naturally occurring isoagglutinins can pose a risk of hemolysis if units are given across ABO barrier and the risk is greater when Group O SDP is released to nongroup O patients.[9] The critical titers depend on the methodology used and may be >1:16 for in vitro hemolysis assay, >1:64–1:100 for direct agglutination, and >1:256–1:400 for indirect antiglobulin test. However, past literature that can clearly define clinically critical titer levels is lacking.[7] Based on work of different researchers, most blood banks follow a cutoff ranging from 128 to 250 for defining high-titer units.[10,11] In our study, we found the ABO titer between 0 and 1:32 which is much less the critical titer value and close to no risk of any hemolysis.
PAS-stored platelets show improved maintenance of pH, combined with a reduction in glucose consumption and lactate production in various studies.[1,12] We also observed that all the PAS SDP units maintained pH >6 till the day 5 of storage. We also found the platelet yield/count in product was in acceptable limits.
PAS platelets are statistically superior and not inferior to plasma-platelets with respect to the transfusion-related adverse reaction rate. Percentages of transfusions with adverse reactions were 1.37% for plasma-platelets, 0.55% for PAS.[13] During our study period, we have not received a single report of adverse reaction from PAS SDP after transfusion of 1674 units. In comparison, we received 16 adverse transfusion reaction reports in the year 2015 from 2432 Plasma SDP transfusions. Another study from de Wildt-Eggen et al. also reported the reduction of adverse transfusion reaction from PAS platelets.[14]
Many studies have suggested that the PAS SDP can be stored for longer period than 5 days. The licensed additive solution would maintain in vitro parameters successfully for up to 9 days. In vivo studies, however, are required to confirm the in vitro results.[15,16]
The published data and studies on the in vitro quality of platelets stored in 70% or even 80% of PAS might encourage transfusion specialists to consider using these PAS in routine blood banking.[4] Although we have not studied the corrective count increment of platelets in patients in our study, studies like Kerkhoffs et al. indicated that there were no significant differences in transfusion responses between PAS III-platelets and plasma-platelets.[17]
Conclusion
Transfusion of only ABO-compatible platelets is not always feasible due to limited availability and maintaining the daily SDP inventory is a challenge for most of the blood banks. Since the ABO antibody titers were significantly reduced after addition of PAS, therefore, it facilitates the ABO incompatible SDP transfusion and helps in inventory management. Immediate availability of SDP units also helps in managing critical thrombocytopenic patients with very low platelet count. All the basic quality parameters are well maintained in PAS platelets such as platelet counts, pH, and sterility. The risk of allergic transfusion reaction decreases after reducing the amount of plasma from SDP units. Using PAS-SDP certainly improve the inventory management for platelets with no compromise on clinical and laboratory efficacy, in fact, better clinical outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Johnson L, Winter KM, Hartkopf-Theis T, Reid S, Kwok M, Marks DC, et al. Evaluation of the automated collection and extended storage of apheresis platelets in additive solution. Transfusion. 2012;52:503–9. doi: 10.1111/j.1537-2995.2011.03314.x. [DOI] [PubMed] [Google Scholar]
- 2.Gulliksson H, Larsson S, Kumlien G, Shanwell A. Storage of platelets in additive solutions: Effects of phosphate. Vox Sang. 2000;78:176–84. doi: 10.1159/000031177. [DOI] [PubMed] [Google Scholar]
- 3.de Wildt-Eggen J, Schrijver JG, Bins M, Gulliksson H. Storage of platelets in additive solutions: Effects of magnesium and/or potassium. Transfusion. 2002;42:76–80. doi: 10.1046/j.1537-2995.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 4.Ringwald J, Zimmermann R, Eckstein R. The new generation of platelet additive solution for storage at 22 degrees C: Development and current experience. Transfus Med Rev. 2006;20:158–64. doi: 10.1016/j.tmrv.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Ringwald J, Walz S, Zimmermann R, Zingsem J, Strasser E, Weisbach V, et al. Hyperconcentrated platelets stored in additive solution: Aspects on productivity and in vitro quality. Vox Sang. 2005;89:11–8. doi: 10.1111/j.1423-0410.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 6.Gulliksson H, AuBuchon JP, Vesterinen M, Sandgren P, Larsson S, Pickard CA, et al. Storage of platelets in additive solutions: A pilot in vitro study of the effects of potassium and magnesium. Vox Sang. 2002;82:131–6. doi: 10.1046/j.1423-0410.2002.drfgv158.x. [DOI] [PubMed] [Google Scholar]
- 7.Romphruk AV, Cheunta S, Pakoate L, Kumpeera P, Sripara P, Paupairoj C, et al. Preparation of single donor platelet with low antibody titers for all patients. Transfus Apher Sci. 2012;46:125–8. doi: 10.1016/j.transci.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Jain P, Tendulkar A, Gupta A. First Indian initiative for preparation of low-titer group “O” single-donor platelets with platelet additive solution. Asian J Transfus Sci. 2018;12:10–6. doi: 10.4103/ajts.AJTS_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saunders C, Rowe G, Wilkins K, Holme S, Collins P. In vitro storage characteristics of platelet concentrates suspended in 70% SSP+(TM) additive solution versus plasma over a 14-day storage period. Vox Sang. 2011;101:112–21. doi: 10.1111/j.1423-0410.2011.01468.x. [DOI] [PubMed] [Google Scholar]
- 10.Tynngård N, Trinks M, Berlin G. In vitro properties of platelets stored in three different additive solutions. Transfusion. 2012;52:1003–9. doi: 10.1111/j.1537-2995.2011.03417.x. [DOI] [PubMed] [Google Scholar]
- 11.Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high-titer” anti-A/A, B: Implications for transfusion policy. Transfusion. 2004;44:805–8. doi: 10.1111/j.1537-2995.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 12.Oikawa S, Sasaki D, Kikuchi M, Sawamura Y, Itoh T. Comparative in vitro evaluation of apheresis platelets stored with 100% plasma versus bicarbonated ringer's solution with less than 5% plasma. Transfusion. 2013;53:655–60. doi: 10.1111/j.1537-2995.2012.03773.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohn CS, Stubbs J, Schwartz J, Francis R, Goss C, Cushing M, et al. A comparison of adverse reaction rates for PAS C versus plasma platelet units. Transfusion. 2014;54:1927–34. doi: 10.1111/trf.12597. [DOI] [PubMed] [Google Scholar]
- 14.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC, et al. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: A prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Meer PF, Gulliksson H, Aubuchon JP, Prowse C, Richter E, de Wildt-Eggen J, et al. Interruption of agitation of platelet concentrates: Effects on in vitro parameters. Vox Sang. 2005;88:227–34. doi: 10.1111/j.1423-0410.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 16.Vassallo RR, Adamson JW, Gottschall JL, Snyder EL, Lee W, Houghton J, et al. In vitro and in vivo evaluation of apheresis platelets stored for 5 days in 65% platelet additive solution/35% plasma. Transfusion. 2010;50:2376–85. doi: 10.1111/j.1537-2995.2010.02693.x. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhoffs JL, van Putten WL, Novotny VM, Te Boekhorst PA, Schipperus MR, Zwaginga JJ, et al. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol. 2010;150:209–17. doi: 10.1111/j.1365-2141.2010.08227.x. [DOI] [PubMed] [Google Scholar]


