Abstract
Background
Palbociclib, a specific inhibitor of CDK4/6, has been shown to provide a survival benefit in hormone receptor-positive advanced breast cancer; however, its resistance and related mechanisms are unclear.
Material/Methods
In this study, we constructed palbociclib-resistant hormone receptor-positive breast cancer cells (MCF-7-P) via culturing with palbociclib for at least 6 months. Quantitative real-time PCR (qRT-PCR) and western blot were used to detect the expression of stemness markers in MCF-7-P and MCF-7 cells. Additionally, cell spheroid formation, Transwell migration, ALDH1 activity, and flow cytometry assays were performed to detect stemness and migration ability of MCF-7-P cells, and the effects of everolimus on MCF-7-P cells stemness and migration ability. Growth inhibition assay was used to examine the effect of everolimus on the sensitivity of palbociclib in MCF-7-P and MCF-7 cells.
Results
MCF-7-P cells had stronger stemness and higher expression of ABCG2 and MDR1. Moreover, PI3K/Akt/mTOR signaling was hyper-activated in MCF-7-P cells. Additionally, everolimus, which is a mTOR inhibitor, attenuated MCF-7-P cells stemness and re-sensitized MCF-7-P cells to palbociclib. Importantly, everolimus enhanced the antitumor effect of palbociclib in palbociclib-sensitive hormone receptor-positive cells (MCF-7 cells).
Conclusions
These findings provide a rationale for future clinical trials of palbociclib and everolimus combination-based therapy in hormone receptor-positive breast cancer.
MeSH Keywords: Breast Neoplasms, Male; Drug Resistance; Stem Cell Research; TOR Serine-Threonine Kinases
Background
Palbociclib was the first inhibitor of cyclin-dependent kinase CDK4/6, developed by Pfizer, and approved to treat hormone receptor-positive (ER+) advanced breast cancer in February 2015 by the Food and Drug Administration [1]. Mechanistically, palbociclib inhibits ER+ breast cancer cell proliferation via impinging upon the G1-S cell cycle checkpoint [2]. However, development of therapeutic resistance is not uncommon in clinical treatment, and the mechanisms contributing to palbociclib resistance are still unclear. Thus, it is important to elucidate the detailed mechanisms, find potential targets, and explore combination therapy, to attenuate or predict palbociclib resistance.
Cancer stem cells are a small subset of cells which exist in tumor tissues and hold the potential of self-renewal and non-directional differentiation, and have been proven to facilitate tumor progression, metastasis, and chemoresistance [3]. PI3K/Akt/mTOR pathway has been shown to be involved in tumor stemness; Mecca et al. indicated that PP242 counteracts glioblastoma cell stemness properties by inhibiting mTORC2/AKT signaling [4]. Chen et al. demonstrated that PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppressed the stemness of colon cancer stem cells [5]. Furthermore, Du et al. showed that microRNA-451 regulated stemness of side population cells via PI3K/Akt/mTOR signaling in multiple myeloma [6]. Thus, targeting the PI3K/Akt/mTOR pathway has become a promising method to eliminate cancer stem cells. Everolimus, a mTOR inhibitor, has been approved to help treat breast cancer [7] and renal carcinoma [8]. However, it is still unclear whether everolimus attenuates ER+ breast cancer stemness.
In this study, we constructed palbociclib-resistant ER+ breast cancer cells (MCF-7-P), and found that MCF-7-P cells had stronger stemness and higher expression of drug resistance-related genes ABCG2 and MDR1 relative to parental MCF-7 cells. Additionally, the PI3K/Akt/mTOR pathway was hyper-activated in MCF-7-P cells. Importantly, we found that everolimus attenuated the stemness of MCF-7-P cells and re-sensitized MCF-7-P cells to palbociclib. Notably, everolimus enhanced the efficiency of palbociclib on parental MCF-7 cells. Altogether, we demonstrated that everolimus, a mTOR inhibitor, could reverse palbociclib-resistance in ER+ human breast cancer cells by attenuating cell stemness through inhibiting the PI3K/Akt/mTOR pathway.
Material and Methods
Cell culture and reagents
Human ER+ breast cancer cell line MCF-7 was purchased from the Chinese Academy of Sciences Cell Bank; DNA fingerprinting analysis was used to confirm its identity. MCF-7 cells were cultured in DMEM medium (Gibco) supplemented with 10% fetal bovine serum at 37°C under humidified air with 5% CO2. Palbociclib (Cat # 1579) and everolimus (Cat # S1120) were purchased from Selleck.
Construction of palbociclib resistant cells
We used a high concentration and short-time culturing method to develop palbociclib-resistant ER+ breast cancer cells (MCF-7-P). Briefly, 3×103 MCF-7 cells were seeded into 96-well plates and treated with 10 μM palbociclib. After 2 weeks, cell clones were collected and expanded to culture with 20 nM palbociclib. The resistance index was confirmed before using in further experiments.
Cell viability assay
Cells were digested and seeded in 96-well plates, and incubated overnight at 37°C in 5% CO2, followed by palbociclib (100 nM) treatment as well as treatment with or without everolimus for 24 hours, 48 hours, and 72 hours. MTT Cell Proliferation and Cytotoxicity Assay Kit (Cat # E606334, Sangon Biotech, Shanghai, China) was used to measure cell viability.
Cell spheroid formation assay
Cell spheroid formation assay was used to evaluate the stemness of cells with different treatment. Briefly, cells were digested and cultured in ultra-low attachment 24-well plates (Corning, Union City, CA, USA) at 1000 cells/well with MammoCult™ Human Medium Kit (Cat #05620, Stemcell Technologies, Vancouver, BC, Canada) for 10 days, followed by measuring spheroid number and size under a microscope fitted with a ruler (more than 50 μM). To evaluate the ability of self-renewal, spheroids were harvested, re-digested and re-cultured with the same condition aforementioned. This was repeated at least 3 times, and the rate of spheroid formation was examined.
Transwell migration assay
Transwell migration assay was used to detect the migration ability of cells. The detailed procedure was described in a previous study [9].
ALDH1 activity assay
ALDEFLUOR™ Kit (Cat #KA3742, Stemcell Technologies) was used to evaluate cell populations with high ALDH1 activity according to the manufacturer’s recommendation.
Flow cytometry assay
To examine the sub-population of stem cells in MCF-7 cells, cells were digested, re-suspended, and stained with the following antibodies: anti-CD44-PE, anti-CD24-APC, IgG1-PE, and IgG1-APC (BD), followed by cell sorting on another flow cytometer (BD C6), and analyzed with BD FACS Diva software.
Quantitative real-time PCR (qRT-PCR)
Quantitative real-time PCR (qRT-PCR) was performed to examine the mRNA level of transcripts. Briefly, cells were washed with phosphate buffer saline, and then FastPure Cell/Tissue Total RNA Isolation Kit (Cat #RC101, Vazyme Biotech Co., Ltd., Nanjing, China) was used to extract RNA from cells with different treatments, followed by synthesizing cDNA with HiScript II Q Select RT SuperMix for qPCR(+gDNA wiper) (Cat #R233-01, Vazyme Biotech Co., Ltd.). Then mRNA expression level was measured with AceQ Universal SYBR qPCR Master Mix (Cat #AQ201, Vazyme Biotech Co., Ltd.) on an ABI Prism 7500 Detection System (Applied Biosystems, Inc.). All samples, including negative control with template, were analyzed at least 3 times. Then mRNA relative expression was normalized to GAPDH expression, and determined using the 2−ΔΔct method.
Western blot
Cells were treated with RIPA Lysis Buffer (Cat #P0013B, Beyotime, Beijing, China), followed by determining protein concentration using Bradford Protein Assay Kit (Cat #P0006, Beyotime). Then 30 μg protein was separated by 10% SDS-PAGE. The protein was transferred onto PVDF membranes (Bio-Rad). Blocking was carried out with 10% non-fat milk for 1.5 hours at 37°C. Then the membranes were washed with TBST for 15 minutes, 3 times, and incubated with the primary antibodies at 4°C overnight. The primary antibodies against ALDH1 (ab129815), Nanog (ab80892), ABCG2 (ab203397), MDR1 (ab3366), p-mTOR (ab84400), cleaved caspase-3 (ab32042), and cleaved PARP (ab32064) were purchased from Abcam. The primary antibodies against β-actin (Cat #60008-1-Ig), p-Akt (Cat #66444-1-Ig), Akt (Cat #10176-2-AP), caspase-3 (Cat #19677-1-AP), PARP (Cat #13371-1-AP), E-cadherin (Cat #20874-1-AP), and vimentin (Cat #10366-1-AP) were purchased from Proteintech. Then membranes were washed with TBST for 15 minutes, 3 times, followed by incubating with HRP-labeled goat anti-rabbit IgG(H+L) (Cat #A0208, Beyotime), or HRP-labeled goat anti-mouse IgG(H+L) (Cat #A0216, Beyotime). ECL Plus (Cat #PE0010, Solarbio) was used to detect chemiluminescent signals in Bio-Rad ChemiDoc™ Touch (CM002554, Bio-Rad).
Statistical analysis
Data analysis was carried out using GraphPad Prism 5.01 (CA, USA), and presented as mean ± standard deviation. The significance of the differences between the groups were determined using one-way ANOVA with the Tukey-Kramer post-test. Results were considered to be significant when the P value was less than 0.05.
Results
MCF-7-P cells exhibited palbociclib resistance and stronger stemness
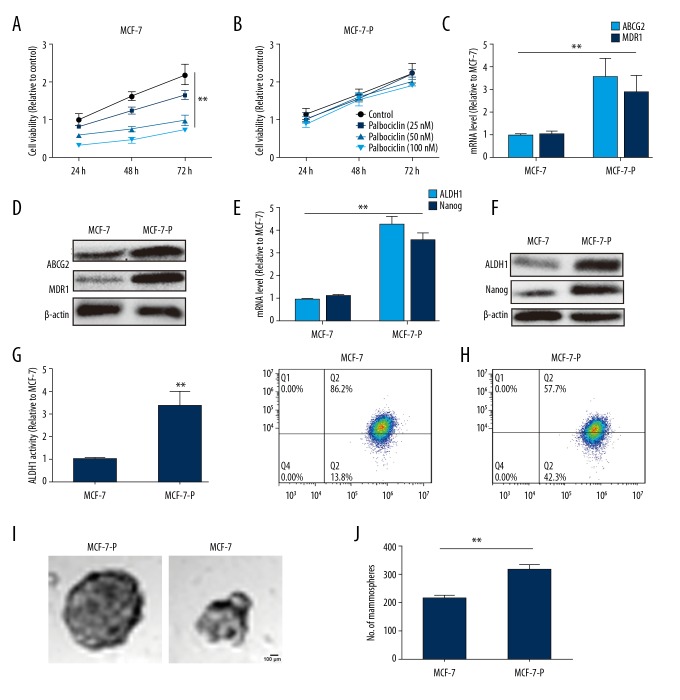
We developed palbociclib-resistant MCF-7 cells (MCF-7-P). First, we confirmed the resistant characteristics of the MCF-7-P cells via cell viability assay. As shown in Figure 1A and 1B, palbociclib at 25 nM, 50 nM, and 100 nM significantly decreased cell viability of MCF-7 cells, but did not affected MCF-7-P cell viability. Consistently, we found the mRNA expression levels of 2 common drug resistance genes MDR1 and ABCG2 involved in resistance to CDK4/6 inhibitors [10], were significantly upregulated in MCF-7-P cells (Figure 1C, 1D). Since cancer stem cells could confer drug resistance [11], we investigated whether MCF-7-P cells had higher stemness. The qRT-PCR and western blot analysis (Figure 1E, 1F) indicated that MCF-7-P cells displayed higher expression of stemness markers ALDH1 and Nanog [12,13]. Notably, MCF-7-P cells displayed higher ALDH1 activity via ALDH1 activity assay (Figure 1G). Additionally, since CD44+/CD24− are well-acknowledged surface markers of breast cancer stem cells [14], we examined the expression in MCF-7-P and MCF-7 cells and found the percentage of CD44+/CD24− cells in MCF-7-P cells was 42.3±0.62%, which was significantly higher than in the parental counterparts of MCF-7 cells which was 13.8% ± 0.65% (Figure 1H). Because previous studies indicated that non-adherent spheroids are highly enriched for cancer stem cells [15,16], we evaluated cell spheroid formation capability, and found that MCF-7-P cells exhibited stronger ability compared with MCF-7 cells, characterized as the increase of spheroid size and number (Figure 1I, 1J). Therefore, we established palbociclib-resistant MCF-7-P cells, and the MCF-7-P cells exhibited higher stemness.
Figure 1.
MCF-7-P cells exhibit palbociclib resistance and higher stemness. (A) MCF-7 cells were treated with different concentration of palbociclib, and after 24, 48, and 72 hours, the cell viability was analyzed by MTT assay. (B) MCF-7-P cells were treated with different concentration of palbociclib, and after 24, 48, and 72 hours, the cell viability was analyzed by MTT assay. (C) mRNA level of drug resistance-related proteins ABCG2 and MDR1 was detected in MCF-7 and MCF-7-P cells. (D) Protein levels of ABCG2 and MDR1 was examined in MCF-7 and MCF-7-P cells. (E, F) mRNA and protein levels of stemness markers ALDH1 and Nanog were determined in MCF-7 and MCF-7-P cells. (G) ALDH1 activity was measured in MCF-7 and MCF-7-P cells. (H) The CD44+/CD24- cell sub-population was detected in MCF-7 and MCF-7-P cells. (I, J) The cells spheroid formation ability was evaluated in MCF-7 and MCF-7-P cells via measuring the spheroids size and number. Data were presented as mean ± standard deviation; ** P<0.01 versus MCF-7.
PI3K/Akt/mTOR signaling was hyper-activated in MCF-7-P cells and mTOR inhibitor everolimus attenuated MCF-7-P cells stemness
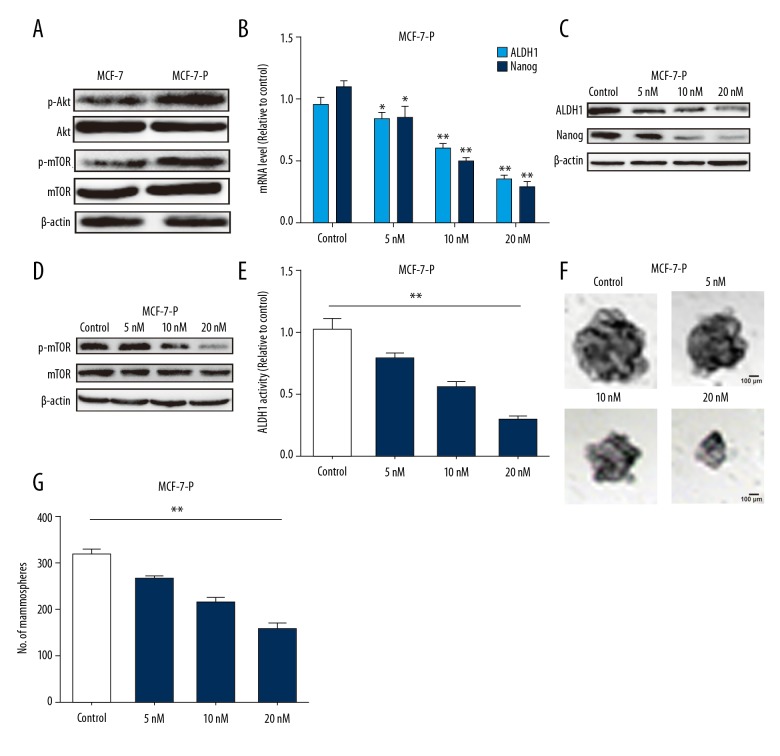
Since PI3K/Akt/mTOR signaling is involved in cancer stem cells formation [17,18], we assumed that this signaling would be hyper-activated in MCF-7-P cells. As expected, the expression level of p-Akt and p-mTOR was significantly increased in MCF-7-P cells (Figure 2A). We examined whether mTOR inhibitor everolimus could attenuate MCF-7-P cells stemness. The qRT-PCR and western blot analysis indicated that everolimus significantly decreased the expression of stemness markers ALDH1 and Nanog in a concentration dependent manner at 5 nM, 10 nM, and 20 nM (Figure 2B, 2C). And p-mTOR expression was suppressed in MCF-7-P cells with everolimus treatment (Figure 2D). Furthermore, ALDH1 activity was attenuated by everolimus treatment in MCF-7-P cells (Figure 2E). Additionally, everolimus decreased the cell spheroid formation ability of MCF-7-P cells in a concentration dependent manner (Figure 2F, 2G). Thus, our results suggested that everolimus could attenuate MCF-7-P cells stemness.
Figure 2.
PI3K/Akt/mTOR signaling was hyper-activated in MCF-7-P cells and mTOR inhibitor everolimus attenuated MCF-7-P cells stemness. (A) Expression of p-Akt and p-mTOR was detected in MCF-7 and MCF-7-P cells. (B, C) Expression of stemness markers ALDH1 and Nanog was examined MCF-7-P cells with different concentrations of everolimus treatment. (D) p-mTOR expression was measured in MCF-7-P cells with different concentrations of everolimus treatment. (E) ALDH1 activity was determined in cells depicted in (D). (F, G) The cell spheroid formation ability was evaluated in cells depicted in (C). Data were presented as mean ± standard deviation; * P<0.05, ** P<0.01 versus control.
Everolimus exerted inhibition on MCF-7-P cells migration and epithelial-mesenchymal transition (EMT) process
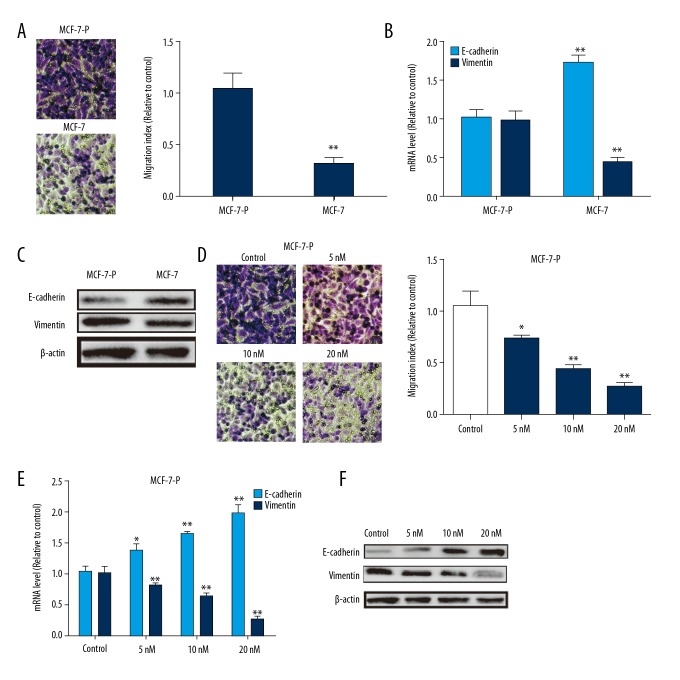
We further investigated whether MCF-7-P cells had higher migration ability and whether everolimus could attenuate it. As shown in Figure 3A, Transwell migration assay showed that MCF-7-P cells had much higher migration ability than parental MCF-7 cells. Consistently, the expression of epithelial marker E-cadherin and mesenchymal marker vimentin was decreased or increased, respectively (Figure 3B, 3C). Remarkably, everolimus significantly decreased cell migration ability of MCF-7-P cells (Figure 3D), and attenuated epithelial-mesenchymal transition (EMT) process, characterized as the increase of E-cadherin expression and decrease of vimentin expression (Figure 3E, 3F).
Figure 3.
Everolimus exerted inhibition on MCF-7-P cells migration and epithelial-mesenchymal transition (EMT) process. (A) Cell migration ability was measured in MCF-7 and MCF-7-P cells. (B, C) Expression of EMT markers (E-cadherin and vimentin) was detected in MCF-7 and MCF-7-P cells. (D) MCF-7-P cells were treated with different concentrations of everolimus, and followed by examining the migration ability via Transwell migration assay. (E, F) EMT markers (E-cadherin and vimentin) expression was determined in cells depicted in (D). Data were presented as mean ± standard deviation; * P<0.05, ** P<0.01 versus control.
Everolimus inhibited MCF-7-P spheroids viability, migration, EMT, and self-renewal ability
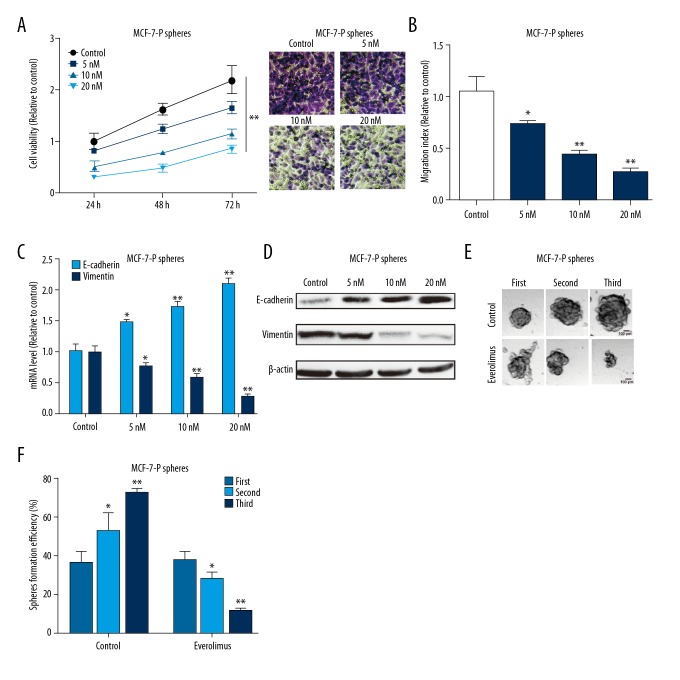
Importantly, we determined whether everolimus could directly inhibit MCF-7-P spheroid progression. MCF-7-P cell spheroids were digested, re-suspended and seeded into 96-well plates; after cells were attached, everolimus was added to the wells. After 24 hours, 48 hours, and 72 hours, cells viability was examined and showed that everolimus significantly decreased cells viability digested by cell spheroids (Figure 4A). Consistent results were obtained in Transwell migration and EMT process assays (Figure 4B–4D). Additionally, the self-renewal ability of MCF-7-P spheroids were evaluated with everolimus (20 nM) treatment, and we found that the self-renewal ability of MCF-7-P spheroids was suppressed by everolimus treatment characterized as the decrease of spheroid-formation efficiency from the first to the third generation (Figure 4E, 4F).
Figure 4.
Everolimus inhibits MCF-7-P spheroids viability, migration, epithelial-mesenchymal transition (EMT), and self-renewal ability. (A) MCF-7-P spheroids were digested and seeded into 96-well plates, followed by different concentrations of everolimus treatment. Then cell viability was determined by MTT assay. (B) Migration ability was detected in MCF-7-P spheroids with different concentrations of everolimus. (C, D) Expression of EMT markers (E-cadherin and vimentin) was measured in MCF-7-P spheroids with different concentrations of everolimus. (E, F) The ability of cells spheroid formation was examined in MCF-7-P spheroids with different concentrations of everolimus. Data were presented as mean ± standard deviation; * P<0.05, ** P<0.01 versus control.
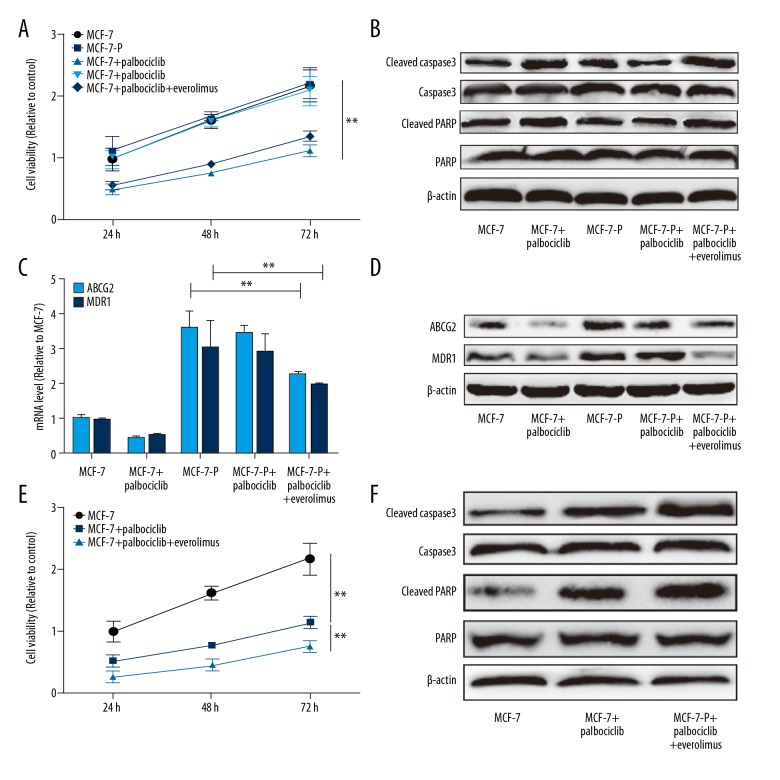
Everolimus attenuated and enhanced palbociclib sensitivity of MCF-7 cells
Finally, we explored whether everolimus could attenuate palbociclib resistance in MCF-7-P cells. Cell viability assay indicated that everolimus rescued palbociclib resistance of MCF-7-P cells characterized as the decrease of cell viability and increase of the expression of apoptosis executors cleaved caspase-3 and cleaved PARP (Figure 5A, 5B). Meanwhile, the expression of drug resistance genes ABCG2 and MDR1 was decreased with combination use of everolimus and palbociclib (Figure 5C, 5D). Additionally, we continued investigating whether everolimus could enhance palbociclib sensitivity in parental MCF-7 cells. As expected, the palbociclib sensitivity of MCF-7 cells was enhanced by everolimus treatment, characterized as the additive inhibition on cell viability (Figure 5E), and additive promotion of the expression of apoptosis executors cleaved caspase-3 and cleaved PARP (Figure 5F). These results indicated that everolimus potentiates the anti-proliferative effects and attenuates resistance of palbociclib in ER+ breast cancer cells.
Figure 5.
Everolimus attenuated and enhanced palbociclib sensitivity of MCF-7 cells. (A) MCF-7-P cells were treated with palbociclib with or without everolimus, and followed by cell viability assay. (B) Expression of the apoptosis executors, cleaved caspase-3 and cleaved PARP, was detected in cells depicted in (A). (C, D) Expression of drug resistance genes ABCG2 and MDR1 was examined in cells depicted in (A). (E) MCF-7 cells were treated with palbociclib with or without everolimus and followed by detecting cell viability after 24, 48, and 72 hours. (F) Expression of the executors cleaved caspase-3 and cleaved PARP was detected in cells depicted in (E). Data were presented as mean ± standard deviation; ** P<0.01 versus control.
Discussion
Great successes with the clinical application of CDK4/6 inhibitor palbociclib have been reported for ER+ breast cancer, and clinical experiments in other tumors are in progress. However, development of drug resistance is common in the clinical setting, and thus it is necessary to elucidate the underlying mechanisms contributing to resistance, and explore combination treatment methods for patients with palbociclib resistance.
In the present study, palbociclib-resistant ER+ breast cancer cells were established, and we found that the PI3K/Akt/mTOR signaling was hyper-activated in MCF-7-P cells, indicating that palbociclib resistance was associated with PI3K/Akt/mTOR signaling, these results were consistent with a previous study [19]. However, resistance to CDK4/6 inhibitors could be contributed to other pathways, such as disruption of the RB pathway, as mediated by loss of the retinoblastoma tumor suppressor (RB), cyclin D1 and CDK6 amplification, and cyclin E-CDK2 activation [20]. Importantly, we demonstrated that the mTOR inhibitor everolimus could enhance palbociclib sensitivity and attenuated palbociclib resistance; these results indicated that everolimus might be used in combination therapy for ER+ breast cancer with or without palbociclib resistance.
Since cancer stem cells contributed to drug resistance and we identified that MCF-7-P cells had stronger stemness, this phenomenon was considered consistent with previous studies that indicated that transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion [21], and that miR-302 inhibits the tumorigenicity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways [22]. Furthermore, we isolated MCF-7-P cells with stemness via cell spheroid formation assay, and the effects of everolimus on their progression and self-renewal ability were evaluated. Encouragingly, we showed that everolimus could inhibit MCF-7-P sphere cells viability, migration, and spheroid formation ability, indicating that everolimus could potentially kill breast cancer stem cells. To the best of our knowledge, this is the first study indicating that everolimus could kill breast cancer stem cells. However, it is unclear whether everolimus holds similar effects in other tumors, which should be explored in future works.
Conclusions
These findings demonstrated that acquired CDK4/6 resistance was associated with PI3K/Akt/mTOR signaling and increasing of cells stemness and that these effects were attenuated by everolimus treatment. Our results provide the basis for the next steps in pre-clinical investigation on combination or sequential therapeutic strategies with everolimus and palbociclib in the clinic.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.McCain J. First-in-class CDK4/6 inhibitor palbociclib could usher in a new wave of combination therapies for HR+, HER2− breast cancer. P T. 2015;40(8):511–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Rocca A, Farolfi A, Bravaccini S, et al. Palbociclib. Targeting the cell cycle machinery in breast cancer. Expert Opin Pharmacother. 2014;15(3):407–20. doi: 10.1517/14656566.2014.870555. [DOI] [PubMed] [Google Scholar]
- 3.Knutson TP, Truong TH, Ma S, et al. Post-translationally modified progesterone receptors direct ligand-specific expression of breast cancer stem cell-associated gene programs. Journal of Hematology & Oncology. 2017;10(1):89. doi: 10.1186/s13045-017-0462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mecca C, Giambanco I, Bruscoli S, et al. Counteracts glioblastoma cell proliferation, migration, invasiveness and stemness properties by inhibiting mTORC2/AKT. Front Cell Neurosci. 2018;12:99. doi: 10.3389/fncel.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Shao R, Li F, et al. PI3K/Akt/mTOR pathway dual inhibitor BEZ235 suppresses the stemness of colon cancer stem cells. Clin Exp Pharmacol Physiol. 2015;42(12):1317–26. doi: 10.1111/1440-1681.12493. [DOI] [PubMed] [Google Scholar]
- 6.Du J, Liu S, He J, et al. MicroRNA-451 regulates stemness of side population cells via PI3K/Akt/mTOR signaling pathway in multiple myeloma. Oncotarget. 2015;6(17):14993–5007. doi: 10.18632/oncotarget.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhakal A, Matthews CM, Levine EG, et al. Efficacy of palbociclib combinations in hormone receptor-positive metastatic breast cancer patients after prior everolimus treatment. Clinical Breast Cancer. 2018 doi: 10.1016/j.clbc.2018.04.015. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchler T, Bortlicek Z, Poprach A, et al. Efficacy of everolimus in second- and third-line therapy for metastatic renal cell carcinoma: A registry-based analysis. Urol Oncol. 2014;32(5):569–75. doi: 10.1016/j.urolonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Hu J, Li X, Guo X, et al. The CCR2 3′UTR functions as a competing endogenous RNA to inhibit breast cancer metastasis. J Cell Sci. 2017;130(19):3399–413. doi: 10.1242/jcs.202127. [DOI] [PubMed] [Google Scholar]
- 10.de Gooijer MC, Zhang P, Thota N, et al. P-glycoprotein and breast cancer resistance protein restrict the brain penetration of the CDK4/6 inhibitor palbociclib. Invest New Drugs. 2015;33(5):1012–19. doi: 10.1007/s10637-015-0266-y. [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Docherty F, Brown HK, et al. Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J. 2015;29(8):3141–50. doi: 10.1096/fj.14-266379. [DOI] [PubMed] [Google Scholar]
- 12.Yang M, Liu P, Huang P. Cancer stem cells, metabolism, and therapeutic significance. Tumour Biol. 2016;37(5):5735–42. doi: 10.1007/s13277-016-4945-x. [DOI] [PubMed] [Google Scholar]
- 13.Valent P, Bonnet D, De Maria R, et al. Cancer stem cell definitions and terminology: The devil is in the details. Nat Rev Cancer. 2012;12(11):767–75. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez HR, Gadre SM, Tan M, et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 2018;25(7):1239–58. doi: 10.1038/s41418-018-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17(10):1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu R, Shi P, Nie Z, et al. Mifepristone suppresses basal triple-negative breast cancer stem cells by down-regulating KLF5 expression. Theranostics. 2016;6(4):533–44. doi: 10.7150/thno.14315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wang C, Bu G. Curcumin inhibits the growth of liver cancer stem cells through the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin signaling pathway. Exp Ther Med. 2018;15(4):3650–58. doi: 10.3892/etm.2018.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Zhou N, Wang J, et al. Quercetin suppresses breast cancer stem cells (CD44(+)/CD24(−)) by inhibiting the PI3K/Akt/mTOR-signaling pathway. Life Sci. 2018;196:56–62. doi: 10.1016/j.lfs.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301–13. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido-Castro AC, Goel S. CDK4/6 inhibition in breast cancer: Mechanisms of response and treatment failure. Curr Breast Cancer Rep. 2017;9(1):26–33. doi: 10.1007/s12609-017-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He S, Roberts PJ, Sorrentino JA, et al. Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci Transl Med. 2017;9(387) doi: 10.1126/scitranslmed.aal3986. pii: eaal3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin SL, Chang DC, Ying SY, et al. MicroRNA miR-302 inhibits the tumorigenecity of human pluripotent stem cells by coordinate suppression of the CDK2 and CDK4/6 cell cycle pathways. Cancer Res. 2010;70(22):9473–82. doi: 10.1158/0008-5472.CAN-10-2746. [DOI] [PubMed] [Google Scholar]