Abstract
One of the desired outcomes of dam decommissioning and removal is the recovery of aquatic and riparian ecosystems. To investigate this common objective, we synthesized information from empirical studies and ecological theory into conceptual models that depict key physical and biological links driving ecological responses to removing dams. We define models for three distinct spatial domains: upstream of the former reservoir, within the reservoir, and downstream of the removed dam. Emerging from these models are response trajectories that clarify potential pathways of ecological transitions in each domain. We illustrate that the responses are controlled by multiple causal pathways and feedback loops among physical and biological components of the ecosystem, creating recovery trajectories that are dynamic and nonlinear. In most cases, short-term effects are typically followed by longer-term responses that bring ecosystems to new and frequently predictable ecological condition, which may or may not be similar to what existed prior to impoundment.
Keywords: dam removal, river restoration, disturbance, conceptual models, ecological modeling
Once a river is dammed, is it damned forever? The purposeful removal of dams has accelerated in the last several decades. In the United States alone, over 1400 dams have been deliberately removed since the 1970 s, and the pace of removal will likely continue as many dams approach the end of their engineered life expectancies (Doyle et al. 2008, O’Connor et al. 2015, American Rivers 2018). Although dams are removed for multiple reasons (e.g., safety, costs, loss of function), a common objective is the recovery of ecosystem function, often centered on species of economic and cultural importance (Bednarek 2001). But do ecosystems recover after dam removal? And do they recover to a condition similar to what existed prior to dam emplacement or have factors—both intrinsic and extrinsic—changed such that the newly undammed river enters a new ecological state? These questions are challenging, but understanding and predicting ecological responses to dam removal is crucial for prioritizing which dams to remove and how to remove them (Poff and Hart 2002), as well as for setting realistic expectations about the magnitude and timing of ecological recovery, which may lag far beyond dam removal.
A challenge in understanding and predicting recovery trajectories is that ecological responses vary spatially and temporally. The local and regional context of each dam is distinct, and therefore, the responses to removal are—more often than not—unique (Foley et al. 2017a). In addition, the size and purpose of a dam affects the method and pace of its removal and the magnitude and timing of potential ecological perturbations and recovery. And for rivers with multiple dams, the outcomes of any one dam removal depend on the watershed location (upstream or downstream) and context (e.g., purpose, management practices) of any remaining dams (Skalak et al. 2013, Foley et al. 2017a).
Despite the importance of the physical and ecological context of the specific dam and river, we suggest that ecological responses to dam removal are generally governed by a shared set of physical and biological links and feedback loops. Variation in ecological response is not a function of unique processes operating only at specific locations but, rather, is driven by differences in the strength of shared links and feedback loops common to most dam removals. From this perspective, understanding and predicting ecological responses is enabled by employing a systems approach, which is explicitly focused on these shared causal links and feedbacks among physical and biological components of the ecosystem (Hart et al. 2002, Doyle et al. 2005), while simultaneously accounting for the context-dependent factors, which vary from dam to dam and which control the strength of these linkages (Foley et al. 2017a,b).
The ever-increasing number of empirical dam-removal studies provides the basis for understanding these links and feedback loops (Bellmore et al. 2017a). However, these empirical studies individually have limited inferential power (Hart et al. 2002). Most dam-removal studies are of short duration (1–2 years) and, therefore, provide only narrow windows onto the ecological response at a specific site (Bellmore et al. 2017a). Moreover, in many studies, responses are monitored only for specific species or trophic levels and, therefore, lack the ecological resolution necessary to mechanistically explain observed responses (Bellmore et al. 2017a). Nevertheless, we synthesize these studies by weaving together the threads of empirical information into a tapestry patterned with broader ecologic theory and knowledge. Empirical studies provide information on specific elements of the ecosystem, and conceptual models and theory guide predictions of how the different elements interact—the links and feedback loops that drive system behavior.
Using this approach, we develop conceptual ecological-response models for three distinct spatial domains affected by dam removal: upstream of the former reservoir, within the former reservoir, and downstream of the removed dam (figure 1). We use these conceptual models to explore the ecological responses likely to emerge from the physical and ecological links in each spatial domain, and we illustrate how these models can be used to inform numerical modeling efforts. These models provide a needed systems approach to our conceptual understanding of the ecological responses to dam removal and build on recent syntheses of physical processes (Major et al. 2017), management concerns (Tullos et al. 2016), and the landscape context of biophysical responses to dam removal (Foley et al. 2017a).
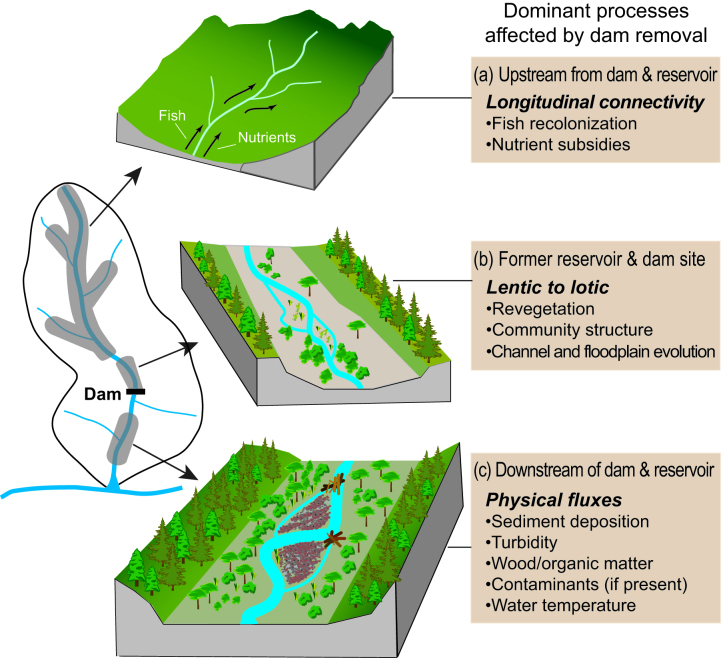
Figure 1.
Spatial domains influenced by dam removal: (a) upstream of the reservoir, (b) within the reservoir or former impoundment, and (c) downstream of the dam. The boxes on the right represent the dominant processes that influence ecological responses in each domain.
Conceptual models of river ecosystem response: Assembling the pieces
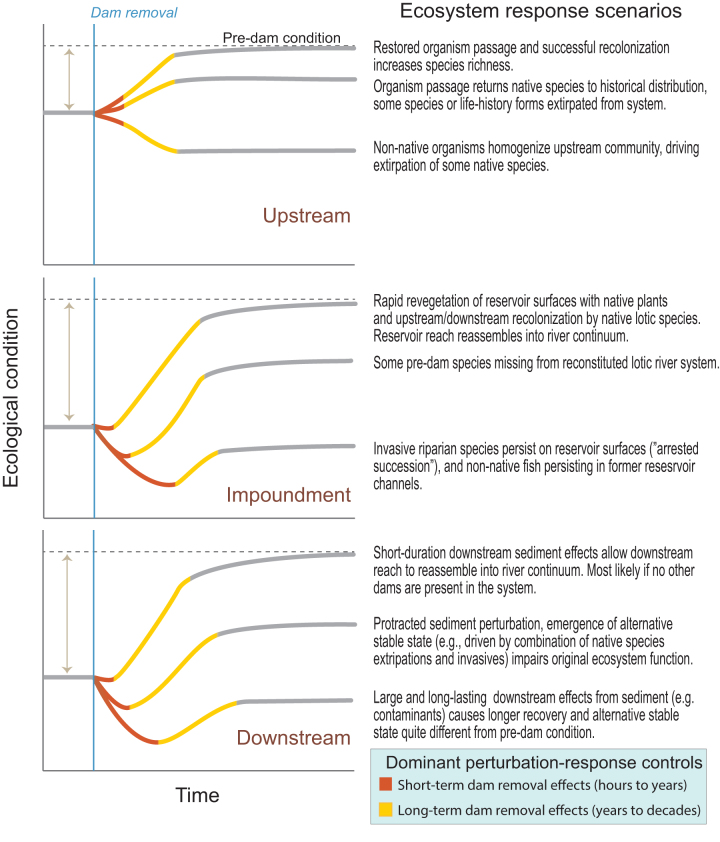
The conceptual models for each spatial domain (figures 2, 3, and 4) are framed as causal-loop diagrams depicting relations among key physical and biological components of the ecosystem. From these links, we postulate longer- and shorter-term ecological responses to dam removal in each domain as a function of overall watershed conditions and history (figure 5). Although some ecological responses to dam removal are conceptually and even quantitatively predictable, responses commonly follow a transient, nonlinear pathway. We refer to this as an ecological response trajectory. Although it is theoretically possible to duplicate a previously observed trajectory, variation in the local and regional context of each dam assures that most dam removals will have different ecological response trajectories, even if they follow similar generalized forms.
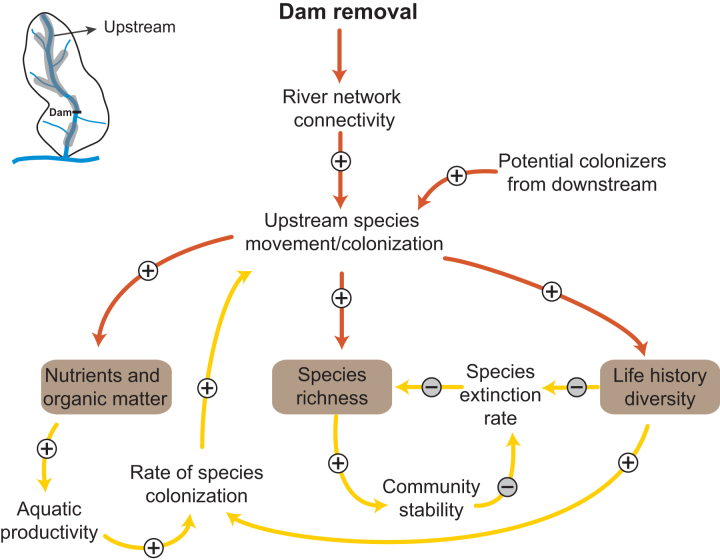
Figure 2.
Causal-loop diagram depicting the cause-and-effect links and associated feedback loops influencing dam removal responses upstream of the former reservoir. Following dam removal, mobile organisms such as fish can recolonize upstream habitats, increasing upstream species richness. Recolonization is self-reinforced by feedback loops that promote productivity and diversity of upstream habitats. The shaded shapes indicate key ecological parameters. The arrows indicate the direction of influence, and the plus and minus signs indicate whether the influence is positive or negative. When they are positive, the variables change in the same direction (when causal variable increases the effected variable also increases or vice versa). When they are negative, the variables change in the opposite direction (when causal variable increases the effected variable decreases or vice versa). Causal links that control responses at short time scales (hours to years) and long time scales (years to decades) are shown in orange and yellow, respectively.
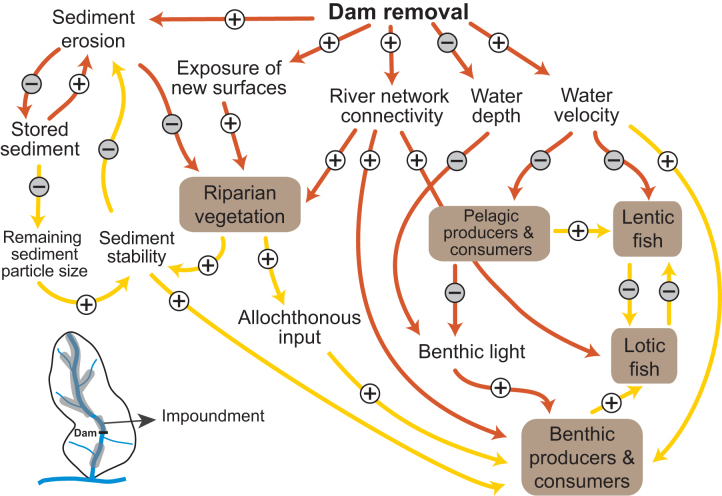
Figure 3.
Causal-loop diagram depicting the cause-and-effect links and associated feedback loops influencing dam removal responses within the former reservoir. Sediment erosion and changes in channel hydraulics alter the environment from one that favors pelagic production and lentic fish assemblages to one that favors benthic production and lotic fish assemblages. The shaded shapes indicate key ecological parameters. The arrows indicate the direction of influence, and the plus and minus signs indicate whether the influence is positive or negative. When they are positive, the variables change in the same direction (when causal variable increases the effected variable also increases or vice versa). When they are negative, the variables change in the opposite direction (when causal variable increases the effected variable decreases or vice versa). Causal links that control responses at short time scales (hours to years) and long time scales (years to decades) are shown in orange and yellow, respectively.
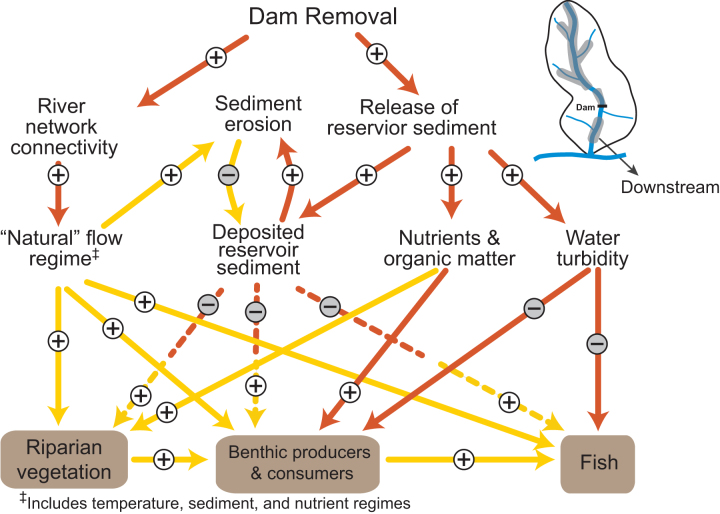
Figure 4.
Causal-loop diagram depicting mechanistic links and feedback loops influencing dam removal responses downstream of a former dam site. Release of sediment, nutrients, and organic matter from the former reservoir effect aquatic organisms and riparian vegetation via numerous causal pathways. Initial deposition of sediments, for example, can bury benthic and riparian organisms, but as this initial sediment pulse is eroded, new habitats for aquatic organisms are created (e.g., spawning gravel for fish). The long-term recovery of species is facilitated by the reestablishment of the natural flow, temperature, sediment, and nutrient regimes to which native organisms are adapted. The shaded shapes indicate key ecological parameters. The arrows indicate the direction of influence, and the plus and minus signs indicate whether the influence is positive or negative. When they are positive, the variables change in the same direction (when causal variable increases the effected variable also increases or vice versa). When they are negative, the variables change in the opposite direction (when causal variable increases the effected variable decreases or vice versa). Causal links that control responses at short time scales (hours to years) and long time scales (years to decades) are shown in orange and yellow, respectively.
Figure 5.
Ecological-response trajectories in upstream, reservoir, and downstream reaches following dam removal. Three hypothetical trajectories are presented for each location, with rationale that explain why these alternative trajectories might emerge. The vertical line on each plot indicates the time of dam removal, and the horizontal line represents the ecological condition that existed prior to dam construction. Recovery to predam conditions are unlikely if natural flow, temperature, sediment, and nutrient regimes remain altered by other dams, if reservoir sediment contains contaminants, and if nonnative species are present. The colored sections of the trajectories indicate the short-term (orange) and long-term (yellow) ecological responses to dam removal.
Short and long term are difficult to define precisely for these conceptual models, because events may occur relatively quickly (e.g., months) for some removals but much slower (e.g., decades) for others. Short-term responses are generally those directly associated with the removal sequence, such as reservoir sediment release and associated habitat and organismal impacts. Long-term responses are those associated with trajectories toward a new dynamic equilibrium, such as the reestablishment of organisms following the initial release of reservoir sediments. The duration of short- and long-term effects is governed by the specific controlling processes, the manner and rate of dam removal, and its overall watershed and ecological context. But in all cases, short-term refers to those physical and ecological responses that occur prior to long-term responses and vice versa.
We focus on the effects of dam removal on taxonomic groups of aquatic and riparian organisms (fishes, aquatic invertebrates, aquatic primary producers, and riparian vegetation). We intentionally omit the identity of specific species and complex food-web interactions in order to make the models broadly generalizable. The models also do not explicitly include local and regional contexts, such as dam size and purpose, the presence of other dams in the river network, and watershed land-use patterns. Although these factors can play large roles in ecological responses to dam removal and can influence the duration of response timescales, conceptual models including all such influences would be intractable and would lack heuristic value. Nevertheless, these generalized models provide a basis for more complex or location-specific conceptual models and, as is described below, a blueprint for quantitative models.
Upstream of the former reservoir: Going against the flow
Conceptual models of river ecosystems frequently emphasize downstream fluxes of nutrients, organic matter, and organisms (Vannote et al. 1980, Newbold et al. 1981, Humphries et al. 2014). However, upstream movement of organisms, such as fish, are also crucial to the function of river ecosystems (Pringle 1997). Dams can reduce biodiversity and productivity by severing these upstream flows (Pess et al. 2008). When dams are removed and longitudinal connectivity is restored, fishes, invertebrates, and commensal microorganisms living on or within these mobile species can recolonize (or initially colonize) upstream habitats. This upstream movement of organisms is a major driver of ecological responses above the former dam and reservoir (figure 2).
As is illustrated in the upstream causal-loop diagram in figure 2, reestablishment of longitudinal connectivity can increase species richness, life history diversity, and the delivery of nutrients and organic matter upstream of the former dam. For example, in the midwestern and eastern United States, low-head dam removals resulted in increased numbers of fish species upstream of the former dam sites (Burdick and Hightower 2006, Catalano et al. 2007, Burroughs et al. 2010, Magilligan et al. 2016). Upstream migration was evident within weeks or months of the dam removals, and up to 95% of all species found downstream of the dams migrated upstream within 1–3 years (Burdick and Hightower 2006, Catalano et al. 2007, Burroughs et al. 2010, Hitt et al. 2012). Colonizers deliver nutrients and organic matter sequestered in downstream habitats (including the ocean in coastal dam removals) that can be incorporated into aquatic and riparian food webs (Gende et al. 2002, Pess et al. 2014). Within a year following the removal of Elwha Dam (one of two large dams removed from the Elwha River, Washington), marine-derived nutrients from adult Pacific salmon were detected upstream of the former dam site in American dippers (Cinclus mexicanus)—an obligate aquatic songbird that feeds on aquatic invertebrates, small fish, and salmon eggs (Tonra et al. 2015).
Changes in life-history diversity above former dams are not as well documented as changes in species richness and nutrients. However, once-isolated fish populations can reexpress migratory life-history strategies once downstream connection is reestablished (Morita et al. 2000, Pascaul et al. 2001, Quinn et al. 2017). For example, before the Elwha River was dammed, it had a high proportion of stream-type juvenile Chinook salmon that reared in freshwater for 1 year, relative to ocean-type fish that migrated to sea within months of emergence (Pess et al. 2008). The expression of the stream-type life history was generally confined to the colder waters upstream of the former dams. After the Elwha was dammed, Chinook salmon were restricted to warmer reaches lower in the river, promoting an ocean-type life history strategy (Pess et al. 2008). Within the first 3 years after the dam removals, adult Chinook recolonized and began spawning upstream of the former dam sites, and some of these fishes began adopting a stream-type life history.
Following the necessary first step—removing a dam—the recovery process can be reinforced by positive ecological feedback loops (figure 2). These feedbacks likely operate at longer time scales of years to decades and are yet to be observed following dam removal but are nevertheless supported by the broader ecological literature. We note three example ecological feedback loops. First, nutrients and organic matter delivered by organisms colonizing upstream can enhance biological productivity or food availability for consumers in receiving waters. In turn, enhanced productivity may increase the rate of colonization and success of colonizers. This feedback may be particularly important when upstream habitats are recolonized by keystone species that strongly affect aquatic or riparian communities and food webs, such as anadromous salmonids (Gende et al. 2002, Morley et al. 2016) and amphidromous fishes and shrimp in tropical rivers (Pringle et al. 1999). Second, increased life-history diversity promotes species persistence and colonization. Species that exhibit a diversity of life histories are more resilient to environmental change, are less likely to experience local extirpation (Schindler et al. 2010), and are more likely to have migratory variants that can recolonize after disturbances (Waples et al. 2009). Third, having a greater number of species (species richness) may, in some cases, reduce extinction rates by stabilizing community dynamics, as has been postulated through theoretical and mathematical models, as well as observations that more diverse communities are more stable (McCann 2000). For instance, greater species richness is often associated with more complex food webs that have a higher proportion of weak predator–prey interactions that counteract the destabilizing effects of strong interactions (McCann 2000). Moreover, the reestablishment of organism movement across the river network could allow recoupling of previously isolated food webs (by movement among upstream, downstream and tributary habitats); these spatially structured meta food webs may also promote community stability and species persistence (Bellmore et al. 2015). As is indicated in figure 2, these feedback loops likely interact among each other to affect the overall ecological response.
On the basis of these hypothetical causal links and feedbacks, we expect the upstream ecological response trajectory following dam removal to be roughly sigmoidal in shape (figure 5). Responses can occur relatively quickly following dam removal and may be reinforced by positive feedback loops. However, overall ecosystem recovery is limited by the availability of colonizers. Therefore, the recovery process will slow as upstream species and life-history diversity approach the levels found in the downstream river network (Pess et al. 2012). Moreover, if some life-history variants or species have been extirpated while the dam was in place, a full recovery that approaches predam conditions may not be possible without interventions, such as species reintroduction. Altered environmental conditions, particularly flow, sediment, and temperature, may limit the suitability of upstream habitats to possible colonizers (Anderson et al. 2014). In particular, the presence of other upstream dams may limit the spatial scope of recovery and the suitability of upstream habitats. Dam removal may also facilitate the spread of undesirable, nonnative, or highly invasive species upstream of the former dam site (Doyle et al. 2005, Kornis et al. 2015). In such cases, the response trajectory for native species may be negative, creating conflicting conservation outcomes (Fausch et al. 2009, Tullos et al. 2016).
Within the former reservoir: Ponds to rivers
The former reservoir can be the reach most altered physically and ecologically by both dam emplacement and dam removal. When a dam is constructed, the impounded reach is often converted from a flowing river (lotic) to a slower, lake-like (lentic) environment that stores sediment, organic matter, and nutrients and that favors organisms adapted to slower waters (Ward and Stanford 1983). Removing the dam starts a sequence of commonly rapid physical and hydrologic changes, whereby the reservoir reverts back to a flowing river. These profound physical changes trigger large ecological responses within the former reservoir (figure 3).
Conversion from a lentic to a lotic system following dam removal can drive fundamental shifts in community structure (figure 1). As the reservoir is drained, the water depth decreases, and the flow velocity increases. These hydraulic changes, in turn, adversely affect pelagic organisms, such as plankton and lentic-adapted fishes (Foley et al. 2017b). Plankton can be exported downstream, and aquatic vegetation growing in the littoral zone can be stranded on reservoir margins. At the same time, these new hydraulic conditions favor organisms adapted to flowing waters (e.g., Ephemeroptera, Plecoptera, Tricoptera [EPT]; Smokorowski et al. 2011), create better foraging conditions for lotic fish species, and allow more light to penetrate to the streambed, facilitating benthic primary production (Allen and Castillo 2007). The spatial and temporal trajectories of these processes, however, are strongly controlled by the size of the dam and reservoir, the rates and processes of reservoir sediment erosion, and the ensuing channel dynamics within the evolving reservoir reach. For instance, ecological transitions may occur quickly behind shallow run-of-the-river dams, where lotic species predominate before the dam is removed.
Dam removal typically causes erosion of the sediment accumulated in the former reservoir as the base level of the dam is lowered (Major et al. 2017). Dynamic channel processes erode and transport reservoir sediment downstream and can initially create bed conditions too transient to support benthic producers and consumers—particularly during and immediately after removal. However, as sequential hydrologic events winnow these sediments, feedback processes can result in a more stable streambed (Collins et al. 2017). Rapid river incision into stored sediments commonly forms knickpoints (abrupt changes in riverbed slope) that migrate upstream of the former dam site (e.g., Major et al., 2012, Randle et al. 2015, 2017). Downstream of the knickpoint, bank failures and lateral channel migration accelerate overall reservoir erosion (Evans 2007). Reservoirs can lose 50% or more of their impounded sediment volumes within the first few weeks to months after dam removal (Wilcox et al. 2014, Warrick et al. 2015, Major et al. 2017). However, as sequential flows entrain the most mobile sediments, channels tend to stabilize (Collins et al. 2017), facilitating a shift from pelagic to benthic communities. Over years to decades, riparian vegetation can recolonize and further stabilize reservoir terraces and stream banks (Shafroth et al. 2002, Orr and Stanley 2006), although colonization by invasive vegetation species is a management concern (Tullos et al. 2016). Riparian vegetation that establishes after removal also contributes leaf litter and terrestrial invertebrates to the river—important allochthonous inputs providing energy for aquatic food webs (Wallace et al. 1999, Baxter et al. 2005).
Contrasting with upstream habitats, causal links and feedback loops operating in the former reservoir reach commonly produce a shorter-term (days to years) perturbation response to dam removal, largely driven by sediment erosion and dynamic channel processes. Over longer time scales (months to decades), this initial perturbation response will typically transition toward ecological recovery and a new equilibrium condition as reservoir sediment stabilizes and more natural flow, temperature, and sediment regimes are reestablished (figure 5). The strength and duration of the initial perturbation and subsequent geomorphic and ecological responses will vary according to the magnitude of change, which depends on physical aspects such as the dam's size, the reservoir's sediment volume and composition, the watershed area, and the overall sediment supply that accumulated during the dam's presence (Bednarek 2001). Studies of aquatic invertebrate and fish responses to dam removal generally support this trajectory (Bushaw-Newton et al. 2002, Stanley et al. 2002, Dorobek et al. 2015). In a meta-analysis of numerous dam removals, Carlson and colleagues (2018) found that lentic invertebrates first declined in density after dam removal but subsequently recovered within 15–20 months. During the recovery phase, lotic invertebrate taxa, such as EPT, tended to become more prevalent (Bushaw‐Newton et al. 2002), attaining community assemblages similar to upstream free-flowing reference sites (e.g., Stanley et al. 2002). Shifts in the aquatic invertebrate community may not increase species richness or diversity, however, because similar numbers of taxa may be lost and gained in the shift from lentic to lotic conditions. Nevertheless, increases in invertebrate taxa richness, biomass, and density are evident in some former impoundments following dam removal (Thomson et al. 2005, Hansen and Hayes 2012, Carlson et al. 2018). In some settings, fish diversity has increased following dam removal, likely because of restored longitudinal connectivity and the development of more suitable habitats in the former reservoir (Catalano et al. 2007, Foley et al. 2017a, Foley et al. 2017a, 2017b), such as the formation of riffles that are important habitats for many riverine fishes and invertebrates (Cook and Sullivan 2018). Changes in aquatic–terrestrial trophic dynamics may be subtle in former reservoir reaches following dam removal and may be overshadowed by other variables governing riverine ecosystems, such as flow and temperature variability (Sullivan et al. 2018).
Ecological recovery may be rapid—months to years—particularly if the geomorphic response is swift. The long-term ecologic conditions, however, may or may not resemble the predam conditions (figure 5). Similar to the upstream domain, the former reservoir is more likely to trend toward its predam conditions if the species and life-history variants that existed prior to the dam's emplacement are still present and capable of colonizing. Similarly, the presence of nonnative species, incomplete export of sediment from the former reservoir (Tullos et al. 2016), contaminants (Magilligan et al. 2016), and other watershed-scale land-use changes, such as other dams or altered hydrology and water temperature, may prevent former reservoirs from attaining predam ecological conditions (e.g., Hobbs et al. 2009).
Downstream of the dam: Here it comes!
Dam-induced changes in natural flow, temperature, sediment, nutrient, and organic matter regimes (Ward and Stanford 1983, Humphries et al. 2014, Wohl et al. 2015) significantly alter the structure and function of downstream ecosystems. In reaches downstream of removed dams, the return of these “natural” regimes—to which many native organisms are adapted—provides an opportunity for ecological recovery (Bednarek 2001). But removing dams also releases decades or more of stored sediment, which affects habitat structure downstream for years or longer (Major et al. 2017). Ecological responses in the downstream domain are determined by the relative effects of initial fluxes of water, sediment, and organic materials from the reservoir reach in conjunction with the longer-term effects of reestablished river network connectivity (figure 4).
Short-term downstream ecological responses (days to years) for most dam removals owe chiefly to reservoir sediment erosion, which increases the downstream transport and deposition of sediment, nutrients, and organic matter and temporarily raises water turbidity (figure 4). The initial deposition of reservoir sediment, in turn, disturbs benthic organisms (algae, invertebrates, and fish eggs) by burial and suffocation (Sethi et al. 2004, Orr et al. 2006) and creates an unstable streambed not suitable for many species (Collier 2002). These effects can temporarily decrease the abundance and richness of downstream periphyton and invertebrate communities after the dam's removal (Chiu et al. 2013, Carlson et al. 2018) and can shift invertebrate assemblages to more disturbance-oriented taxa (Renöfält et al. 2013). For instance, Orr and colleagues (2006) observed significant decreases in benthic chlorophyll a and invertebrate density associated with downstream sediment deposition at two small dam removals in Wisconsin. Increased water turbidity following a dam's removal may also reduce primary production by limiting light penetration (Morley et al. 2008). High turbidity from suspended sediments can also negatively affect fish via reduced foraging efficiency, physical abrasion, clogging of gills, and interference with orientation (Kjelland et al. 2015). However, the greater mobility of fishes than of invertebrates, as well as their adaptation to seasonally high flows and sediment loads, may limit direct mortality. Nonetheless, in some cases, lowered fish abundance after a dam's removal has persisted for as long as 15 years before populations increased (Burroughs et al. 2010). These perturbations are likely to be strongest immediately below the removed dam and to dissipate downstream with sediment diffusion and tributary influences.
Nutrients and organic matter associated with reservoir sediments may buffer aquatic organisms from some of these negative impacts (figure 4). Although this effect has not yet been empirically documented in dam removal studies, increased nutrient loads can result in increased aquatic primary production where the bed is stable and light levels are adequate (Allan and Castillo 2007). Moreover, organic matter from the reservoir may provide food for heterotrophic microbes and invertebrates. This may stabilize higher trophic level production during the initial sediment disturbance by shifting the food web from reliance on green (periphyton, macrophytes) to brown (detritus) sources of energy (Wolkovich et al. 2014). Additional research is needed to quantify the strength of these potentially stabilizing links.
Although sediment deposition may initially perturb aquatic organisms and riparian vegetation, it is also a resource for ecological recovery. Sediment-starved river channels downstream from dams can become incised, armored, and disconnected from their floodplains (Ligon et al. 1995). Deposition and subsequent redistribution of reservoir sediments create new gravel bars, a more heterogeneous streambed, and more suitable spawning habitats for nest-building fishes (Kibler et al. 2011). Entrained reservoir sediments can also aggrade downstream channels and reconnect lateral floodplain habitats (East et al. 2015, Magilligan et al. 2016). Increased channel migration, creation of new gravel bars, and sediment deposition on floodplains provide new surfaces for colonization by pioneer plant species and potentially restore a shifting riparian habitat mosaic (Shafroth et al., 2002, 2016). Moreover, reestablishment of downstream transport of plant seeds from upstream of the former dam may facilitate vegetation recovery on new floodplain surfaces (Cubley and Brown 2016)
Over timescales of years to decades, physical and ecological recovery are strongly controlled by the reestablishment of natural flow, temperature, sediment, nutrient, and organic matter regimes to which native organisms are adapted (Ward and Stanford 1995). For example, the reestablishment of more natural hydrologic and sediment regimes typically creates more dynamic river channels, promoting greater habitat diversity for aquatic and riparian species (Poff et al. 1997, Wohl et al. 2015). This is yet to be documented for many recent dam removals, because many have been relatively small, run-of-the-river dams that did not significantly alter downstream material and energy fluxes. As larger dams are removed, such as the Elwha River dams and the pending removals of those on the Klamath River (California and Oregon), physical and ecological recovery will depend on the extent to which these natural regimes are restored.
Similar to those in the former reservoir, causal links and feedback loops in the downstream domain are likely to produce an initial perturbation response to dam removal—primarily associated with transport and deposition of reservoir sediment—followed by evolution to new geomorphic and ecological conditions associated with reestablishment of unimpeded fluxes of water, energy, and materials from the upper watershed (figure 5). The timing, magnitude, and duration of the initial perturbation response to dam removal depends on the amount and locations of sediment deposited downstream (Orr et al. 2008, Chiu et al. 2013, Tullos et al. 2014, East et al. 2015), which are a function of the amount of sediment stored in the former reservoir and the ability of the river to mobilize this sediment (Major et al. 2017). Recovery follows this initial perturbation as reservoir erosion slows and the downstream sediment pulse disperses, but the overall magnitude of recovery may vary considerably, depending upon local and regional conditions. Evolution of physical and ecological conditions may tend toward a state similar to the dammed condition, the predam condition, or some new condition (figure 5), depending on a broad range of watershed and land-use factors (Foley et al. 2017a). For instance, predam ecological conditions are unlikely if natural flow, temperature, sediment, and nutrient regimes remain altered by other dams, if reservoir sediment contains contaminants, and if nonnative species are present.
Interactions across spatial domains
The river connects all three spatial domains as a corridor for upstream and downstream fluxes of energy, materials, and organisms. Therefore, dam-removal responses in one domain can accelerate or attenuate the rate of change and subsequent recovery in other domains. One obvious interdomain interaction is reservoir sediment erosion and downstream deposition. Prolonged erosion of sediment from the former reservoir could slow downstream ecological recovery. In turn, the rate of downstream ecological recovery could influence the timing, composition, and magnitude of upstream organism colonization. Understanding these links may influence decisions on the rate and style of dam removal. For situations in which voluminous or contaminated reservoir sediments are present, dam removal practitioners may decide to remove or stabilize reservoir sediments as part of the dam-removal process (e.g., Randle and Greimann 2006, Woelfle-Erskine et al. 2012) to protect downstream communities. The condition of the river network upstream of the dam and reservoir may also influence downstream recovery. For example, ecological recovery in the reservoir and downstream reaches depends in part on colonization by organisms from upstream, such as aquatic invertebrates that actively and passively drift downstream (Naman et al. 2016). Downstream recovery may be hampered if the diversity and abundance of these potential colonizers has been compromised by factors such as land use, other dams, and invasive species.
One frequently overlooked interaction is the influence of tributaries and floodplain channels on ecological recovery. Floodplain side channels and tributaries can serve as refuges during the initial downstream sediment disturbance and potentially provide important source populations for river network colonization, assuming they are not buried by sediment (Pess et al. 2008, Peters et al. 2017). For example, adult coho salmon in the Elwha River (Oncorhynchus kisutch) were actively relocated to tributaries upstream of the lower dam to accelerate recolonization early in the dam-removal process when suspended-sediment concentrations and potentially deleterious effects were greatest (Liermann et al. 2017). These transplanted coho salmon immediately spawned, which resulted in levels of smolt out-migrants that were comparable (per stream kilometer) with other established populations in the Pacific Northwest, even during high suspended-sediment levels in the mainstem Elwha River (Liermann et al. 2017).
Quantitative modeling and prediction
Although conceptual models are valuable for generating hypotheses, the many links and feedback loops in these models make it difficult to predict responses at a given location without quantifying the strength and character of these interactions. Our conceptual models provide blueprints for assembling quantitative models, whereby links between system elements are replaced with quantitative statements. The resulting models can be used to numerically model potential ecological responses to dam removal. In some circumstances, models may already exist and could be modified to represent processes in each spatial domain. For instance, population-dynamics models could be used to simulate species recolonization in the upstream domain (e.g., Pess et al. 2012). In former reservoir reaches, hydraulic and sediment-transport models could be linked to habitat-suitability models to explore how dam removal influences the quantity and quality of habitat available for benthic organisms and fishes (e.g., Gillenwater et al. 2006).
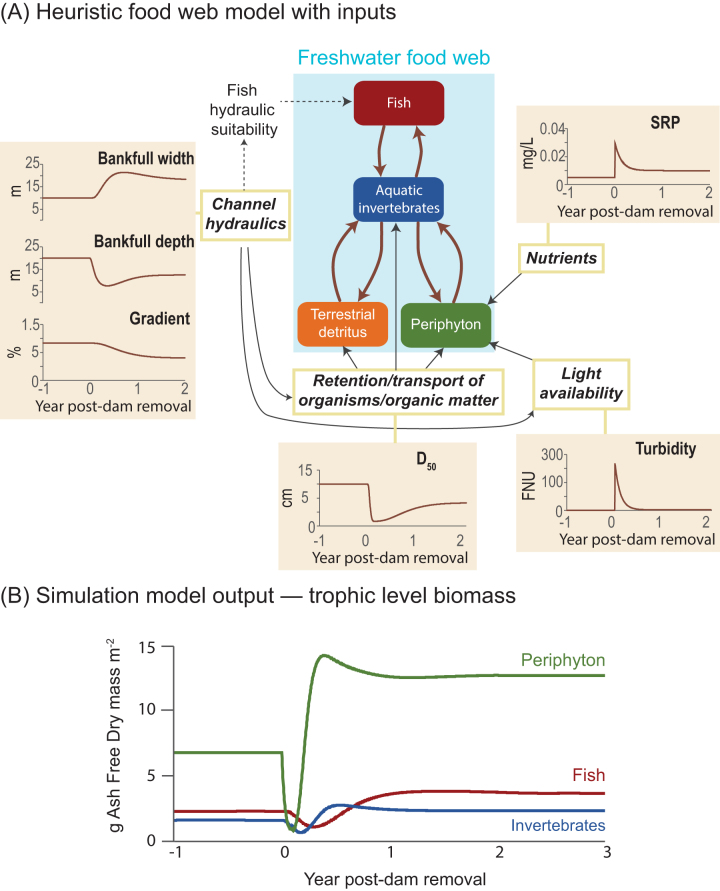
To illustrate how quantitative models can be used to explore ecological responses to dam removal, we simulated response trajectories for aquatic producers (periphyton) and consumers (fish and invertebrates) just downstream of a hypothetical dam removal using the aquatic trophic productivity (ATP) model (Bellmore et al. 2017b), a food-web model that includes many of the response variables of interest in dam removal (figure 6). The ATP model is a dynamic river food-web model (e.g., Power et al. 1995), whereby aquatic organisms—as well as dead organic matter—are compartmentalized into trophic groups that share similar predators and prey (figure 6). The biomass dynamics of this generalized food web and the success of specific trophic groups are linked in the ATP model to the physical, chemical, and hydraulic conditions of the river affected by dam removal (Bellmore et al. 2017b).
Figure 6.
Quantitative models can be used to simulate ecological responses to dam removal. In this case, a river food-web simulation model was used to predict downstream trophic responses to a hypothetical dam removal. The top panel (a) shows how physical and chemical responses to dam removal (shaded graphs) affect the dynamics of the modeled food web (e.g., water turbidity influences the amount of light that reaches the streambed to fuel periphyton production). The bottom panel (b) shows the resultant biomass dynamics of fish, aquatic invertebrates and periphyton. Abbreviations: D50, median particle size of benthic substrate; FNU, formazin nephelometric units; SRP, soluble reactive phosphorus.
We parameterized the model with idealized physical and chemical dam-removal response trajectories. These hypothetical trends indicate possible effects following a rapid dam removal (figure 6). We assumed that water turbidity and nutrient concentrations would peak quickly following dam removal and decay exponentially, that benthic substrate size would first decline with deposition of reservoir sediments but would later coarsen as finer sediments were exported and that bankfull depth would decline, bankfull width would increase, and channel gradient would decrease with deposition of reservoir sediments and reestablishment of natural flow and sediment regimes. In specific applications, these model inputs could be estimated prior to dam removal from physical and hydraulic models (e.g., Cui et al. 2017) or expert opinion. In the model, water turbidity and nutrient concentrations influence the amount of light and nutrients available to fuel periphyton at the base of the food web. Channel morphology (bankfull width, bankfull depth, and gradient) affects channel hydraulics, such as water depth, width, flow velocity, and shear stress acting on the streambed. In turn, water depth and turbidity influence light attenuation, channel width influences the wetted area available for biological production, and water velocity, shear stress, and benthic sediment size influence the mobilization, transport, and retention of benthic organisms and organic matter. For a full description of the ATP model, see Bellmore and colleagues (2017b). Once the APT model was parameterized, we simulated ecological responses across three trophic levels: periphyton, aquatic invertebrates, and fish.
In the model simulation, the three trophic levels followed a similar response trajectory in the downstream domain: declines in biomass during the initial perturbation of the dam removal, followed by recovery to biomass levels that surpassed the preremoval conditions. The initial perturbation response was driven by two primary factors: high turbidity, which reduced available light for periphyton growth, and the deposition of fine-grained reservoir sediment, which created an unstable streambed that was not suitable for periphyton and aquatic invertebrates. But as turbidity declined and the streambed grain size coarsened, the biomass of each trophic level recovered. Final downstream biomasses exceeded preremoval conditions owing to higher assumed background nutrient concentrations (associated with reestablishment of nutrient transport from upstream) and a more biologically retentive channel that was wider and had a lower gradient. The timescale of the modeled response, however, varied among the trophic levels. Periphyton communities on the streambed were highly susceptible to the initial dam-removal disturbance but recovered more quickly because of higher turnover rates than those of invertebrates and fish.
Responses to real dam removals are more complex than the modeled responses presented in the present article (see box 1, figure 7); nonetheless, this simple example illustrates that such models may be able to predict realistic ecological response trajectories. Moreover, such analyses can explore potential responses before dams are removed (figures 6 and 7). For instance, different assumptions and removal strategies (e.g., instantaneous versus phased removal) could be simulated to identify approaches that reduce negative impacts and provide the best chance for long-term ecological recovery. Although simulations themselves are idealized, the process of organizing information into a quantitative framework can promote a greater understanding of the factors that control system dynamics. In the case of dam removal, making informed decisions with the aid of models is crucial, because once a dam is removed, there is no going back.
Box 1. Modeled food-web responses to dam removal on the Elwha River, Washington, United States.
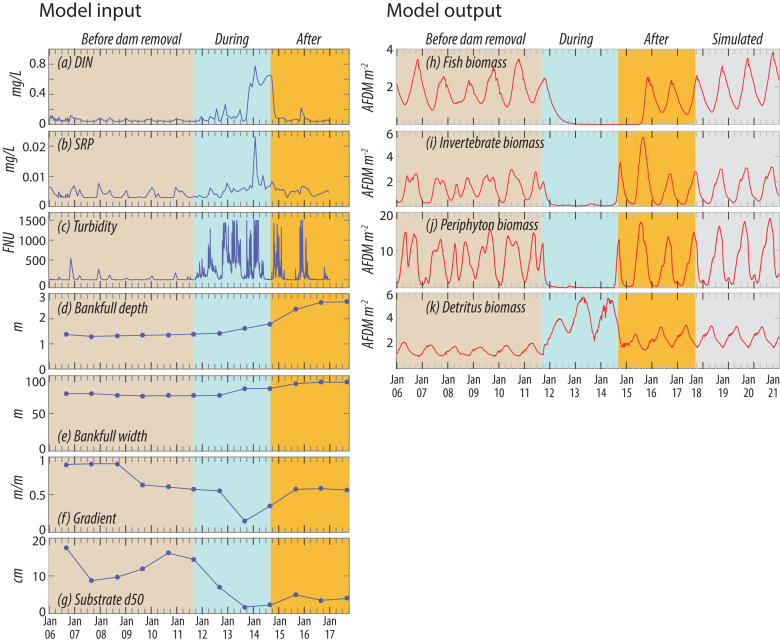
After nearly a century of impoundment, two dams on the Elwha River in northwestern Washington State were removed simultaneously, beginning in September (2011). The 32-m-tall Elwha Dam was built 8 kilometers (km) from the ocean in 1913, and the 64-m Glines Canyon Dam was built 14 km farther upstream in (1927). Both structures lacked fish passage, resulting in precipitous declines in anadromous salmon populations over the nearly100 years of dam emplacement. Dam removal was intended to restore migratory access for seven species of Pacific salmon and steelhead—still present downstream of the lower dam—to pristine spawning and rearing habitats upstream in Olympic National Park. Phasing the removals over 1 and 3 years for Elwha and Glines Canyon Dams, respectively, helped control the release of ∼30 metric tonnes of sediment accumulated in the reservoirs (Randle et al. 2015). This unprecedented release of sediment (65% of total in the first 5 years; Ritchie et al. 2018) resulted in modified channel morphology, fining of the downstream river bed, increased turbidity and a pulse of sediment and nutrients (East et al. 2015, Magirl et al. 2015, Warrick et al. 2015, Ritchie et al. 2018).
We used the ATP model (Bellmore et al. 2017b) to simulate downstream biomass responses for fish, aquatic invertebrates, periphyton and detritus (dead organic matter) following these dam removals. We parameterized the model with measured changes in channel morphology (East et al. 2018), turbidity (Magirl et al. 2015), nutrient concentrations (Washington Department of Ecology, Station 18B070; figure 7), and other location-specific environmental information such as water temperature, discharge, and solar radiation.
Model simulations indicate that dam removal significantly affected trophic productivity (figure 7). In reaches just downstream from Elwha Dam, simulations showed an almost complete loss of fish, invertebrate and periphyton biomass coinciding with dam removal in late (2011). Modeled declines were largely due to the combined effects of high turbidity that limited light availability and periphyton growth and deposition of finer sediments that made benthic habitats unsuitable for periphyton and invertebrates. Biomass values remained low until mid-2014, at which point turbidity decreased to levels that allowed periphyton growth to rebound. Modeled detrital biomass was high during dam removal, reflecting the pulse of detritus from within stored sediments and restored longitudinal connectivity to the upstream river network. The availability of this low-quality detritus, however, was insufficient to offset the loss of higher-quality periphyton as a food source for aquatic invertebrates. Although empirical data directly comparable to model simulations are currently limited, there is evidence to suggest that the downstream ecological community was indeed negatively affected. The density of benthic invertebrates, for instance, declined by almost two orders of magnitude relative to preremoval abundance. Simulations of ecological conditions for 2017–2021 indicated that fish, invertebrate, and periphyton biomass may increase further if turbidity continues to decline and the streambed continues to coarsen (figure 7).
Figure 7.
Environmental data (a–g) used to parameterize the aquatic trophic productivity model for dam removal on the Elwha River (Washington state), and simulated outputs (h–k) for fish, invertebrate, periphyton and detritus biomass. Abbreviations: AFDM, ash-free dry mass; D50, median particle size of benthic substrate; DIN, dissolved inorganic nitrogen; FNU, formazin nephelometric units; SRP, soluble reactive phosphorus
Conclusions
Empirical dam removal studies and ecological theory support our conceptual models defining the links and feedback loops affecting ecological responses to dam removal. These models define response trajectories that clarify pathways of ecological transitions upstream of the former reservoir, within the former reservoir, and downstream of the former reservoir. Within each spatial domain, these models illustrate that dam-removal responses are controlled by multiple causal pathways and interdomain links, which interact to strengthen or dampen responses. Together, these interconnections create dynamic, nonlinear responses, which are complex but can be predicted if the relative strengths of the dominant links and feedback loops—controlled by local and regional factors—are known.
Our conceptual models can be used in multiple ways to increase understanding of these interconnections. First, dam-removal practitioners can use these models to trace the important causal pathways likely to determine responses at specific locations. These qualitative exercises can improve decision-making and prediction by fostering a holistic understanding of the multiple pathways by which dam removal is likely to influence specific ecological communities. This qualitative understanding can be used to prioritize the physical and ecological variables that should be monitored following removal. Second, these models provide a template for more detailed conceptual models that account for location-specific processes, organisms, watershed context, and management scenarios. For instance, these models are being adapted to evaluate potential ecological responses to dam removals on the Klamath River in Northern California, where several dams are being removed in series. Finally, our models provide a foundation for constructing quantitative models, parameterized with relevant local and regional environmental information. Simulations from these models can provide alternate hypotheses, which can be tested with empirical data following removal. Although modeled results may not match actual outcomes, information gathered during postremoval monitoring can be used to refine model parameter values and model structure, as well as the underlying knowledge and assumptions on which the model is based. For instance, unanticipated results could help identify important feedback loops and local environmental conditions that may be important for predicting outcomes of future dam removals.
We conclude by returning to the original question: When a river is dammed, is it damned forever? Our conceptual models and a growing number of empirical studies suggest that rivers, given the opportunity, can indeed recover substantially from having been dammed. But the structure and function of the ecosystem may not be the same or even similar to what existed prior to dam emplacement. Damming rivers causes changes in ecological communities by extirpation of native species and spread of nonnative and invasive species (Olden 2016). Therefore, the ecological communities that assemble following dam removal may be very different than those that existed before the dam was constructed. Moreover, baseline conditions of the watershed may have changed significantly while the dam was in place. Land use, pollution, the presence of other dams, sediment contamination, climate change, and numerous other factors will constrain the trajectory of both physical and ecological recovery (Foley et al. 2017a). The ability to go back to a predammed state will likely depend on how long the dam existed and the magnitude of its many-faceted effects on the ecosystem. Even if all elements of the ecosystem still exist, it is unlikely they will reassemble in the exact fashion that existed previously (Temperton et al. 2004).
But what is “damned forever”? The perception of ecological recovery following dam removal ultimately depends on societal expectations (Hobbs 2007). Recovery expectations may be high in settings in which vivid recollections of pristine predam conditions still exist. On the Elwha River, for example, a strong written and oral history of large Pacific salmon runs promoted expectations that dam removal would lead to recovery of historical populations. In contrast, dam removal expectations may be substantially different from predam conditions in locations in which these memories have been lost. Managers and practitioners can use models such as those presented in the present article to help stakeholders and community members understand the potential range of ecological responses to dam removal and the most likely trajectories and future conditions, thereby better shaping (and even guiding) more realistic expectations for ecological recovery as well as avoiding undesired outcomes.
Acknowledgments
We gratefully acknowledge funding from the US Geological Survey's John Wesley Powell Center for Analysis and Synthesis, which supported our efforts to synthesize dam removal science. In particular, we thank Jill Baron and Leah Colasuonno at the Powell Center for help and encouragement. Additional funding support was provided by NOAA, USGS, the Bureau of Reclamation, and US Forest Service. We thank all the members of the USGS Powell Center Dam Removal working group for their efforts on this synthesis project. We thank Correigh Greene, Tim Beechie, and three anonymous reviewers for providing insightful comments that substantially improved the content and quality of the manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US government. Data used in this synthesis are publicly available and are listed in the references.
Author Biographical
J. Ryan Bellmore (jbellmore@fs.fed.us) is affiliated with the US Department of Agriculture, Forest Service, Pacific Northwest Research Station, in Juneau, Alaska. George R. Pess is affiliated with the NOAA Fisheries’ Northwest Fisheries Science Center, in Seattle, Washington. Jeffrey J. Duda is affiliated with the US Geological Survey's Western Fisheries Research Center, also in Seattle. Jim E. O’Connor is affiliated with the US Geological Survey's Geology, Minerals, Energy, and Geophysics Science Center, in Portland, Oregon. Amy E. East and Melissa M. Foley are affiliated with the US Geological Survey's Pacific Coastal and Marine Science Center, in Santa Cruz, California. Andrew C. Wilcox is affiliated with the University of Montana's Department of Geosciences, in Missoula. Jon J. Major is affiliated with the US Geological Survey's Cascades Volcano Observatory, in Vancouver, Washington. Patrick B. Shafroth is affiliated with the US Geological Survey's Fort Collins Science Center, in Fort Collins, Colorado. Sarah A. Morley is affiliated with the NOAA Fisheries’ Northwest Fisheries Science Center, in Seattle, Washington. Christopher S. Magirl is the studies chief at the US Geological Survey's Arizona Water Science Center, in Tucson, Arizona. Chauncey W. Anderson is affiliated with the US Geological Survey's Oregon Water Science Center, in Portland, Oregon. James E. Evans is affiliated with the Department of Geology at Bowling Green State University, in Bowling Green, Ohio. Christian E. Torgersen is affiliated with the US Geological Survey's Forest and Rangeland Ecosystem Science Center, Cascadia Field Station, at the University of Washington, in Seattle, Washington. Laura S. Craig is affiliated with American Rivers, in Washington DC.
References cited
- Allen JD, Castillo MM.. 2007. Stream Ecology: Structure and Function of Running Waters, Second Edition Springer. [Google Scholar]
- American Rivers 2018. American Rivers Dam Removal Database. Figureshare. Fileset. 10.6084/m9.figureshare.5234068.v4. [DOI] [Google Scholar]
- Anderson JH, Pess GR, Carmichael RW, Ford MJ, Cooney TD, Baldwin CM, McClure MM. 2014. Planning Pacific salmon and steelhead reintroductions aimed at long-term viability and recovery. North American Journal of Fisheries Management 34: 72–93. [Google Scholar]
- Baxter CV, Fausch KD, Saunders WC. 2005. Tangled webs: Reciprocal flows of invertebrate prey link streams and riparian zones. Freshwater Biology 50: 201–220. [Google Scholar]
- Bednarek AT. 2001. Undamming rivers: A review of the ecological impacts of dam removal. Environmental Management 27: 803–814. [DOI] [PubMed] [Google Scholar]
- Bellmore JR, Baxter CV, Connolly PJ. 2015. Spatial complexity reduces interaction strengths in the meta‐food web of a river floodplain mosaic. Ecology 96: 274–283. [DOI] [PubMed] [Google Scholar]
- Bellmore JR, Duda JJ, Craig LS, Greene SL, Torgersen CE, Collins MJ, Vittum K. 2017a. Status and trends of dam removal research in the United States. Wiley Interdisciplinary Reviews: Water 4: e1164.10.1002/wat2.1164. [Google Scholar]
- Bellmore JR, Benjamin JR, Newsom M, Bountry JA, Dombroski D. 2017b. Incorporating food web dynamics into ecological restoration: A modeling approach for river ecosystems. Ecological Applications 27: 814–832. [DOI] [PubMed] [Google Scholar]
- Burdick SM, Hightower JE.. 2006. Distribution of spawning activity by anadromous fishes in an Atlantic slope drainage after removal of a low-head dam. Transactions of the American Fisheries Society 135: 1290–1300. [Google Scholar]
- Burroughs BA, Hayes DB, Klomp KD, Hansen JF, Mistak J. 2010. The effects of the Stronach Dam removal on fish in the Pine River, Manistee County, Michigan. Transactions of the American Fisheries Society 139: 1595–1613. [Google Scholar]
- Bushaw-Newton KL, et al. 2002. An integrative approach towards understanding ecological responses to dam removal: The Manatawny Creek study. Journal of the American Water Resources Association 38: 1581–1599. [Google Scholar]
- Carlson PE, Donadi S, Sandin L. 2018. Responses of macroinvertebrate communities to small dam removals: Implications for bioassessment and restoration. Journal of Applied Ecology. doi:10.1111/1365-2664.13102. [Google Scholar]
- Catalano MJ, Bozek MA, Pellett TD. 2007. Effects of dam removal on fish assemblage structure and spatial distributions in the Baraboo River, Wisconsin. North American Journal of Fisheries Management 27: 519–530. [Google Scholar]
- Chiu M-C, Yeh C-H, Sun Y-H, Kuo M-H. 2013. Short-term effects of dam removal on macroinvertebrates in a Taiwan stream. Aquatic Ecology 47: 245–252. [Google Scholar]
- Collier KJ. 2002. Effects of flow regulation and sediment flushing on instream habitat and benthic invertebrates in a New Zealand river influenced by a volcanic eruption. River Research and Applications 18: 213–226. [Google Scholar]
- Collins MJ, et al. 2017. Channel response to sediment release: Insights from a paired analysis of dam removal. Earth Surface Processes and Landforms 42: 1636–1651. [Google Scholar]
- Cook DR, Sullivan SM.. 2018. Associations between riffle development and aquatic biota following lowhead dam removal. Environmental Monitoring and Assessment 190: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubley E, Brown RL.. 2016. Restoration of hydrochory following dam removal on the Elwha River, Washington. River Research and Applications 32: 1566–1575. [Google Scholar]
- Cui Y, Booth DB, Monschke J, Gentzler S, Roadifer J, Greimann B, Cluer B. 2017. Analyses of the erosion of fine sediment deposit for a large dam-removal project: An empirical approach. International Journal of River Basin Management 15: 103–114. [Google Scholar]
- Dorobek A, Sullivan SM, Kautza A. 2015. Short-term consequences of lowhead dam removal for fish assemblages in an urban river system. River Systems 21: 125–139. [Google Scholar]
- Doyle MW, Stanley EH, Orr CH, Selle AR, Sethi SA, Harbor JM. 2005. Stream ecosystem response to small dam removal: Lessons from the Heartland. Geomorphology 71: 227–244. [Google Scholar]
- Doyle MW, Stanley EH, Havlick DG, Kaiser MJ, Steinbach G, Graf WL, Galloway GE, Riggsbee JA. 2008. Aging infrastructure and ecosystem restoration. Science 319: 286–287. [DOI] [PubMed] [Google Scholar]
- East AE, et al. 2015. Large-scale dam removal on the Elwha River, Washington, USA: River channel and floodplain geomorphic change. Geomorphology 228: 765–786. [Google Scholar]
- East AE, Logan JB, Mastin MC. 2018. River-channel topography and sediment grain size on the Elwha River, Washington 2006 to 2017. US Geological Survey. 10.5066/F76972SC. [DOI] [Google Scholar]
- Evans JE. 2007. Sediment impacts of the 1994 failure of IVEX dam (Chagrin River, NE Ohio): A test of channel evolution models. Journal of Great Lakes Research 33: 90–102. [Google Scholar]
- Fausch KD, Rieman BE, Dunham JB, Young MK, Peterson DP. 2009. Invasion versus isolation: Trade‐offs in managing native salmonids with barriers to upstream movement. Conservation Biology 23: 859–870. [DOI] [PubMed] [Google Scholar]
- Foley MM, et al. 2017a. Landscape context and the biophysical response of rivers to dam removal in the United States. PLOS ONE 12(art. e0180107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley MM, et al. 2017b. Dam removal: Listening in. Water Resources Research 53: 5229–5246. [Google Scholar]
- Gende SM, Edwards RT, Willson MF, Wipfli MS. 2002. Pacific salmon in aquatic and terrestrial ecosystems. BioScience 52: 917–928. [Google Scholar]
- Gillenwater D, Granata T, Zika U. 2006. GIS-based modeling of spawning habitat suitability for walleye in the Sandusky River, Ohio, and implications for dam removal and river restoration. Ecological Engineering 28: 311–323. [Google Scholar]
- Hansen J, Hayes D.. 2012. Long-term implications of dam removal for macroinvertebrate communities in Michigan and Wisconsin Rivers, United States. River Research and Applications 28: 1540–1550. [Google Scholar]
- Hart DD, Johnson TE, Bushaw-Newton KL, Horwitz RJ, Bednarek AT, Charles DF, Kreeger DA, Velinsky DJ. 2002. Dam removal: Challenges and opportunities for ecological research and river restoration. BioScience 52: 669–682. [Google Scholar]
- Hitt NP, Eyler S, Wofford JE. 2012. Dam removal increases American eel abundance in distant headwater streams. Transactions of the American Fisheries Society 141: 1171–1179. [Google Scholar]
- Hobbs RJ. 2007. Setting effective and realistic restoration goals: Key directions for research. Restoration Ecology 15: 354–357. [Google Scholar]
- Hobbs RJ, Higgs E, Harris JA. 2009. Novel ecosystems: Implications for conservation and restoration. Trends in Ecology and Evolution 24: 599–605. [DOI] [PubMed] [Google Scholar]
- Humphries P, Keckeis H, Finlayson B. 2014. The river wave concept: Integrating river ecosystem models. BioScience 64: 870–882. [Google Scholar]
- Kibler K, Tullos D, Kondolf M. 2011. Evolving expectations of dam removal outcomes: Downstream geomorphic effects following removal of a small, gravel‐filled dam. Journal of the American Water Resources Association 47: 408–423. [Google Scholar]
- Kjelland ME, Woodley CM, Swannack TM, Smith DL. 2015. A review of the potential effects of suspended sediment on fishes: Potential dredging-related physiological, behavioral, and transgenerational implications. Environment Systems and Decisions 35: 334–350. [Google Scholar]
- Kornis MS, Weidel BC, Powers SM, Diebel MW, Cline TJ, Fox JM, Kitchell JF. 2015. Fish community dynamics following dam removal in a fragmented agricultural stream. Aquatic Sciences 77: 465–480. [Google Scholar]
- Liermann M, Pess G, McHenry M, McMillan J, Elofson M, Bennett T, Moses R. 2017. Relocation and recolonization of coho salmon in two tributaries to the Elwha River: Implications for management and monitoring. Transactions of the American Fisheries Society 146: 955–966. [Google Scholar]
- Ligon FK, Dietrich WE, Trush WJ. 1995. Downstream ecological effects of dams. BioScience 45: 183–192. [Google Scholar]
- Magilligan FJ, Nislow KH, Kynard BE, Hackman AM. 2016. Immediate changes in stream channel geomorphology, aquatic habitat, and fish assemblages following dam removal in a small upland catchment. Geomorphology 252: 158–170. [Google Scholar]
- Magirl CS, Hilldale RC, Curran CA, Duda JJ, Straub TD, Domanski M, Foreman JR. 2015. Large-scale dam removal on the Elwha River, Washington, USA: Fluvial sediment load. Geomorphology 246: 669–686. [Google Scholar]
- Major JJ, East AE, O’Connor JE, Grant GE, Wilcox AC, Magirl CS, Collins MJ, Tullos DD. 2017. Geomorphic responses to dam removal in the United States: A two-decade perspective. 355–283 in Tsutsumi D, Laronne JB, eds. Gravel-Bed Rivers: Processes and Disasters. Wiley. [Google Scholar]
- Major JJ, et al. 2012. Geomorphic Response of the Sandy River, Oregon, to Removal of Marmot Dam. US Geological Survey Professional Paper no. 1792.
- McCann KS. 2000. The diversity stability debate. Nature 405: 228–233. [DOI] [PubMed] [Google Scholar]
- Morita K, Yamamoto S, Hoshino N. 2000. Extreme life history change of white-spotted char (Salvelinus leucomaenis) after damming. Canadian Journal of Fisheries and Aquatic Sciences 57: 1300–1306. [Google Scholar]
- Morley SA, Duda JJ, Coe HJ, Kloehn KK, McHenry ML. 2008. Benthic invertebrates and periphyton in the Elwha River basin: Current conditions and predicted response to dam removal. Northwest Science 82: 179–196. [Google Scholar]
- Morley SA, Coe HF, Duda JJ, Dunphy LS, McHenry ML, Beckman BR, Elofson M, Sampson EM, Ward L. 2016. Seasonal variation exceeds effects of salmon carcass additions on benthic food webs in the Elwha River. Ecosphere 7: e01422 10.1002/ecs2.1422. [Google Scholar]
- Naman SM, Rosenfeld JS, Richardson JS. 2016. Causes and consequences of invertebrate drift in running waters: From individuals to populations and trophic fluxes. Canadian Journal of Fisheries and Aquatic Sciences 73: 1292–1305. [Google Scholar]
- Newbold JD, Elwood JW, O’Neill RV, Winkle WV. 1981. Measuring nutrient spiralling in streams. Canadian Journal of Fisheries and Aquatic Sciences 38: 860–863. [Google Scholar]
- O’Connor JE, Duda JJ, Grant GE. 2015. 1000 dams down and counting. Science 348: 496–497. [DOI] [PubMed] [Google Scholar]
- Olden JD. 2016. Challenges and opportunities for fish conservation in dam-impacted waters. 107–142Closs GP, Krkosek M, Olden JD, eds. Conservation of Freshwater Fishes. Cambridge University Press. [Google Scholar]
- Orr CH, Kroiss SJ, Rogers KL, Stanley EH. 2008. Downstream benthic responses to small dam removal in a coldwater stream. River Research and Applications 24: 804–822. [Google Scholar]
- Orr CH, Rogers KL, Stanley EH. 2006. Channel morphology and P uptake following removal of a small dam. Journal of the North American Benthological Society 25: 556–568. [Google Scholar]
- Orr CH, Stanley EH.. 2006. Vegetation development and restoration potential of drained reservoirs following dam removal in Wisconsin. River Research and Applications 22: 281–295. [Google Scholar]
- Pascual M, Bentzen P, Riva Rossi C, Mackey G, Kinnison MT, Walker R. 2001. First documented case of anadromy in a population of introduced rainbow trout in Patagonia, Argentina. Transactions of the American Fisheries Society 130: 53–67. [Google Scholar]
- Pess GR, McHenry ML, Beechie TJ, Davies J. 2008. Biological impacts of the Elwha River dams and potential salmonid responses to dam removal. Northwest Science 82: 72–90. [Google Scholar]
- Pess GR, Hilborn R, Kloehn K, Quinn TP. 2012. The influence of population dynamics and environmental conditions on pink salmon (Oncorhynchus gorbuscha) recolonization after barrier removal in the Fraser River, British Columbia, Canada. Canadian Journal of Fisheries and Aquatic Sciences 69: 970–982. [Google Scholar]
- Pess GR, Quinn TP, Gephard SR, Saunders R. 2014. Re-colonization of Atlantic and Pacific rivers by anadromous fishes: Links between life history and the benefits of barrier removal. Reviews in Fish Biology and Fisheries 24: 881–900. [Google Scholar]
- Peters RJ, Liermann M, McHenry ML, Bakke P, Pess GR. 2017. Changes in streambed composition in salmonid spawning habitat of the Elwha River during dam removal. Journal of the American Water Resources Association 53: 871–885. [Google Scholar]
- Poff LN, Hart DD.. 2002. How dams vary and why it matters for the emerging science of dam removal. BioScience 52: 659–668. [Google Scholar]
- Poff NL, Allan JD, Bain MB, Karr JR, Prestegaard KL, Richter BD, Sparks RE, Stromberg JC. 1997. The natural flow regime. BioScience 47: 769–784. [Google Scholar]
- Power ME, Sun A, Parker G, Dietrich WE, Wootton JT. 1995. Hydraulic food-chain models. BioScience 45: 159–167. [Google Scholar]
- Pringle CM, Hemphill N, McDowell WH, Bednarek A, March JG. 1999. Linking species and ecosystems: Different biotic assemblages cause interstream differences in organic matter. Ecology 80: 1860–1872. [Google Scholar]
- Pringle CM. 1997. Exploring how disturbance is transmitted upstream: Going against the flow. Journal of the North American Benthological Society 16: 425–438. [Google Scholar]
- Quinn TP, Bond MH, Brenkman SJ, Paradis R, Peters RJ. 2017. Re-awakening dormant life history variation: Stable isotopes indicate anadromy in bull trout following dam removal on the Elwha River, Washington. Environmental Biology of Fishes 100: 1659–1671. [Google Scholar]
- Randle TJ, Bountry JA, Ricthie A, Wille K. 2015. Large-scale dam removal on the Elwha River, Washington, USA: Erosion of reservoir sediment. Geomorphology 246: 709–728. [Google Scholar]
- Randle TJ, Greimann B.. 2006. Dam decommissioning and sediment management. 8–1–8–34 in Sedimentation and River Hydraulics Group , eds. Erosion and Sedimentation Manual. US Bureau of Reclamation. [Google Scholar]
- Renöfält BM, Lejon AGC, Jonsson M, Nilsson C. 2013. Long-term taxon-specific responses of macroinvertebrates to dam removal in a mid-sized Swedish stream. River Research and Applications 29: 1082–1089. [Google Scholar]
- Ritchie AC, Curran CA, Magirl CS, Bountry JA, Hilldale RC, Randle TJ, Duda JJ. 2018. Data in support of 5-year sediment budget and morphodynamic analysis of Elwha River following dam removals. US Geological Survey. 10.5066/F7PG1QWC. [DOI] [Google Scholar]
- Schindler DE, Hilborn R, Chasco B, Boatright CP, Quinn TP, Rogers LA, Webster MS. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465: 609–612. [DOI] [PubMed] [Google Scholar]
- Sethi SA, Selle AR, Doyle MW, Stanley EH, Kitchel HE. 2004. Response of unionid mussels to dam removal in Koshkonong Creek, Wisconsin (USA). Hydrobiologia 525: 157–165. [Google Scholar]
- Shafroth PB, Friedman JM, Auble GT, Scott ML, Braatne JH. 2002. Potential responses of riparian vegetation to dam removal. BioScience 52: 703–712. [Google Scholar]
- Shafroth PB, Perry LG, Rose CA, Braatne JH. 2016. Effects of dams and geomorphic context on riparian forests of the Elwha River, Washington. Ecosphere 7: e01621. [Google Scholar]
- Skalak KJ, Benthem AJ, Schenk ER, Hupp CR, Galloway JM, Nustad RA, Wiche GJ. 2013. Large dams and alluvial rivers in the Anthropocene: The impacts of the Garrison and Oahe Dams on the Upper Missouri River. Anthropocene 2: 51–64. [Google Scholar]
- Smokorowski KE, Metcalfe RA, Finucan SD, Jones N, Marty J, Power M, Pyrce RS, Steele R. 2011. Ecosystem level assessment of environmentally based flow restrictions for maintaining ecosystem integrity: A comparison of a modified peaking versus unaltered river. Ecohydrology 4: 791–806. [Google Scholar]
- Stanley E, Luebke M, Doyle MW, Marshall DW. 2002. Short–term changes in channel form and macroinvertebrate communities following low-head dam removal. Journal of the North American Benthological Society 21: 172–187. [Google Scholar]
- Sullivan SMP, Manning DWP, Davis RP. 2018. Do the ecological impacts of dam removal extend across the aquatic-terrestrial boundary. Ecosphere 9: e02180 10.1002/ecs.2.2180. [Google Scholar]
- Temperton VM, Hobbs RJ, Nuttle T, Halle S. 2004. Assembly Rules and Restoration Ecology: Bridging the Gap between Theory and Practice. Island Press. [Google Scholar]
- Thomson JR, Hart DD, Charles DF, Nightengale TL, Winter DM. 2005. Effects of removal of a small dam on downstream macroinvertebrate and algal assemblages in a Pennsylvania stream. Journal of the North American Benthological Society 24: 192–207. [Google Scholar]
- Tonra CM, Sager-Fradkin K, Morley SA, Duda JJ, Marra PP. 2015. The rapid return of marine-derived nutrients to a freshwater food web following dam removal. Biological Conservation 192: 130–134. [Google Scholar]
- Tullos DD, Collins MJ, Bellmore JR, Bountry JA, Connolly PJ, Shafroth PB, Wilcox AC. 2016. Synthesis of common management concerns associated with dam removal. Journal of the American Water Resources Association 52: 1179–1206. [Google Scholar]
- Tullos DD, Finn DS, Walter C. 2014. Geomorphic and ecological disturbance and recovery from two small dams and their removal. PLOS ONE 9 (art. e108091). 10.1371/journal.pone.0108091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137. [Google Scholar]
- Waples R, Beechie T, Pess G. 2009. Evolutionary history, habitat disturbance regimes, and anthropogenic changes: What do these mean for resilience of Pacific salmon populations? Ecology and Society 14: 3. [Google Scholar]
- Wallace JB, Eggert SL, Meyer JL, Webster JR. 1999. Effects of resource limitation on a detrital-based ecosystem. Ecological Monographs 69: 409–442. [Google Scholar]
- Ward JV, Stanford JA.. 1995. Ecological connectivity in alluvial river ecosystems and its disruption by flow regulation. River Research and Applications 11: 105–119. [Google Scholar]
- Ward JV, Stanford JA.. 1983. The serial discontinuity concept of lotic ecosystems. 29–42 in Fontaine TD, Bartell SM, eds. Dynamics of Lotic Ecosystems. Ann Arbor Science. [Google Scholar]
- Warrick JA, Bountry JA, East AE, Magirl CS, Randle TJ, Gelfenbaum G, Ritchie AC, Pess GR, Leung V, Duda JJ. 2015. Large-scale removal on the Elwha River, Washington, USA: Source-to-sink sediment budget and synthesis. Geomorphology 246: 729–750. [Google Scholar]
- Wilcox AC, O’Connor JE, Major JJ. 2014. Rapid reservoir erosion, hyperconcentrated flow, and downstream deposition triggered by breaching of 38 m tall Condit Dam, White Salmon River, Washington. Journal of Geophysical Research—Earth Surface 119: 1376–1394. [Google Scholar]
- Woelfle-Erskine C, Wilcox AC, Moore JN. 2012. Combining historical and process perspectives to infer ranges of geomorphic variability and inform river restoration in a wandering gravel‐bed river. Earth Surface Processes and Landforms 37: 1302–1312. [Google Scholar]
- Wohl E, Lane SN, Wilcox AC. 2015. The natural sediment regime in rivers: Broadening the foundation for ecosystem management. BioScience 65: 358–371. [Google Scholar]
- Wolkovich EM, Allesina S, Cottingham KL, Moore JC, Sandin SA, de Mazancourt C. 2014. Linking the green and brown worlds: The prevalence and effects of multichannel feeding in food webs. Ecology 95: 3376–3386. [Google Scholar]