Summary
Dynamic processes such as fusion, fission, and trafficking are important in the regulation of cellular organelles, with an abundant literature focused on mitochondria. Mitochondrial dynamics not only help shape its network within cells but also are involved in the modulation of respiration and integrity. Disruptions of mitochondrial dynamics are associated with neurodegenerative disorders. Although proteins that directly bind mitochondria to promote membrane fusion/fission have been studied intensively, machineries that regulate dynamic mitochondrial processes remain to be explored. We have identified an interaction between the mitochondrial fission GTPase Dnm1/DRP1 and the actin-regulatory protein Srv2/CAP at mitochondria. Deletion of Srv2 causes elongated-hyperfused mitochondria and reduces the reserved respiration capacity in yeast cells. Our results further demonstrate that the irregular network morphology in Δsrv2 cells derives from disrupted actin assembly at mitochondria. We suggest that Srv2 functions as a pro-fission factor in shaping mitochondrial dynamics and regulating activity through its actin-regulatory effects.
Subject Areas: Molecular Biology, Cell Biology, Functional Aspects of Cell Biology
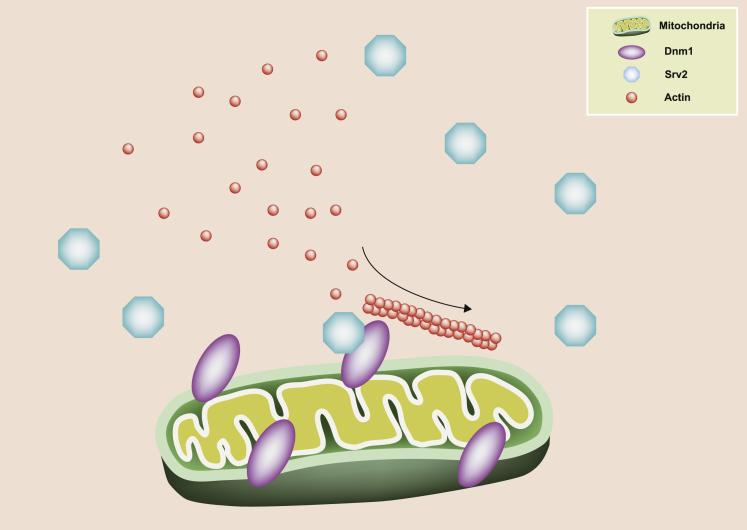
Graphical Abstract
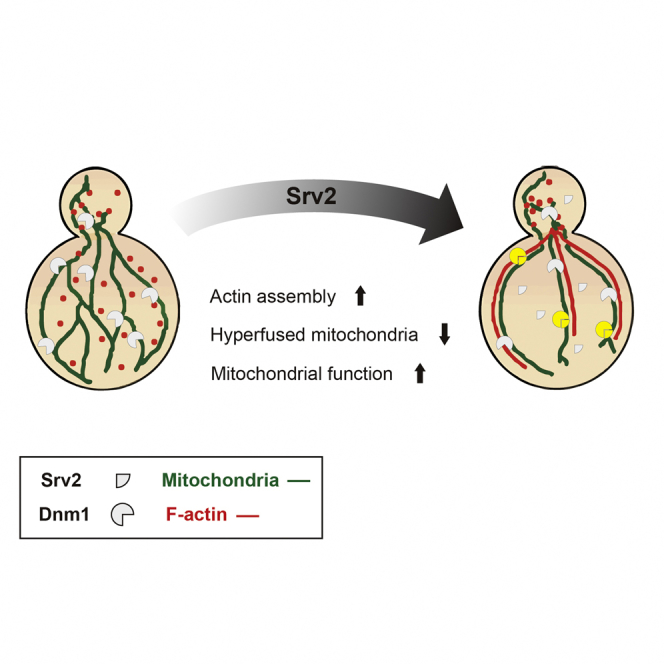
Highlights
-
•
Srv2 interacts with fission protein Dnm1 on mitochondria in yeast cells
-
•
Srv2 deletion causes an irregular, hyperfused-elongated mitochondrial network
-
•
The irregular network derives from loss of Srv2-mediated actin assembly at mitochondria
-
•
Srv2 modulates both mitochondrial dynamics and activity
Molecular Biology; Cell Biology; Functional Aspects of Cell Biology
Introduction
Mitochondria are critical organelles in eukaryotes. Among their known functions, the most significant is to produce energy for cells. Eukaryotic cells preferentially utilize mitochondria to produce ATP through oxidative phosphorylation, with oxygen as the final acceptor of electrons. In addition to its distinctive double membrane and undulating cristae structure, network dynamics also distinguish mitochondria from other organelles. The mitochondrial network is generated through continuous fusion, fission, trafficking, and anchoring. These complementary processes not only shape the network morphology but also aid in maintaining mitochondrial homeostasis and integrity. For instance, the fusion of mitochondria ameliorates functional defects and protects mitochondria from autophagic degradation during starvation (Gomes et al., 2011, Blackstone and Chang, 2011), and fission is critical for mitochondrial positioning and mitophagy (Youle and van der Bliek, 2012, Burman et al., 2017, Böckler et al., 2017).
Disruption of mitochondrial dynamics is correlated with mitochondrial disintegration. Neurons and muscle cells that require ATP for proper function are often prominently affected if mitochondrial function cannot be maintained. Models of Alzheimer, Parkinson, and Huntington diseases exhibit disrupted mitochondrial dynamics and irregular network morphology, prefiguring the importance of well-regulated mitochondrial dynamics (Cho et al., 2009, Shirendeb et al., 2012, Narendra et al., 2009).
Mitochondrial fusion and fission are evolutionarily conserved processes (Westermann, 2010), and large dynamin-related GTPases are required to drive both membrane fusion (MFN1/2 and OPA1) and fission (DRP1) in eukaryotes. In budding yeast, Fzo1 (yeast orthologue of mammalian mitofusins MFN1/2), Ugo1, and Mgm1 (yeast orthologue of OPA1) facilitate mitochondrial outer membrane fusion and inner membrane fusion cooperatively (Wong et al., 2000, Sesaki and Jensen, 2001, Hermann et al., 1998). Cytosolic Dnm1 (yeast orthologue of mammalian DRP1) is recruited to sites of mitochondrial fission by Fis1, Caf4, and Mdv1 to form a distinctive constriction ring (Griffin et al., 2005, Legesse-Miller et al., 2003, Chang and Blackstone, 2007). In addition to these proteins, the endoplasmic reticulum-mitochondria encounter structure (ERMES) is involved in the mitochondrial constriction process; the ER wraps around mitochondria at activated fission sites to facilitate mitochondrial division (Friedman et al., 2011). It appears that not all Dnm1 foci on mitochondria are activated fission sites, and more information is needed to clarify the differences between inactive and active Dnm1 foci.
Mitochondrial transport in Saccharomyces cerevisiae is highly dependent on actin filaments (Hermann et al., 1998, Huckaba et al., 2004, Fehrenbacher et al., 2004). A genetic screen for the essential genes involved in mitochondrial morphogenesis identified multiple actin cytoskeleton-associated proteins (Altmann and Westermann, 2005). In mammalian cells, actin filaments also play a role in ER-mitochondria contact-mediated fission; the dynamic assembly and disassembly of actin is critical for regulating mitochondrial fission (Hatch et al., 2014, Hatch et al., 2016, Ji et al., 2015, Gurel et al., 2015, Li et al., 2015, Moore et al., 2016). However, detailed mechanisms of how actin is involved in mitochondrial dynamics remain unclear.
Evidence suggests that unidentified factors of mitochondrial dynamics still exist (Koch et al., 2004, Legesse-Miller et al., 2003). For instance, mitochondrial network morphology upon double deletion of the fusion protein Fzo1 and fission protein Dnm1 is similar to that of wild-type yeast, although double deletions still affect mitochondrial inheritance and cell survival rate (Böckler et al., 2017). In mammalian cells, mitochondrial constriction also requires multiple steps mediated by both DRP1 and classical dynamin-2 (Lee et al., 2016). These results indicate that more factors regulating mitochondrial fusion, fission, or both remain to be identified.
In this study, we report that Srv2/CAP interacts with the mitochondrial fission protein Dnm1/DRP1 and functions as a pro-fission factor. Srv2 was initially identified as a factor involved in cAMP/PKA signaling and actin assembly; it binds monomeric actin to sequester available G-actin (Freeman et al., 1995, Vojtek et al., 1991, Gerst et al., 1991). We found that Srv2 deletion causes the mitochondrial network to become hyperfused, likely reflecting its function in regulating actin filament assembly. In addition, the irregularly hyperfused mitochondrial network in Δsrv2 cells is associated with lower reserve respiration capacity. Our finding that Srv2 functions as a pro-fission factor strengthens the argument for involvement of actin in mitochondrial fission and provides insight into the relationship between mitochondrial activity and network morphology.
Results
Srv2/CAP Interacts with Dnm1/DRP1 at Mitochondria in Cells
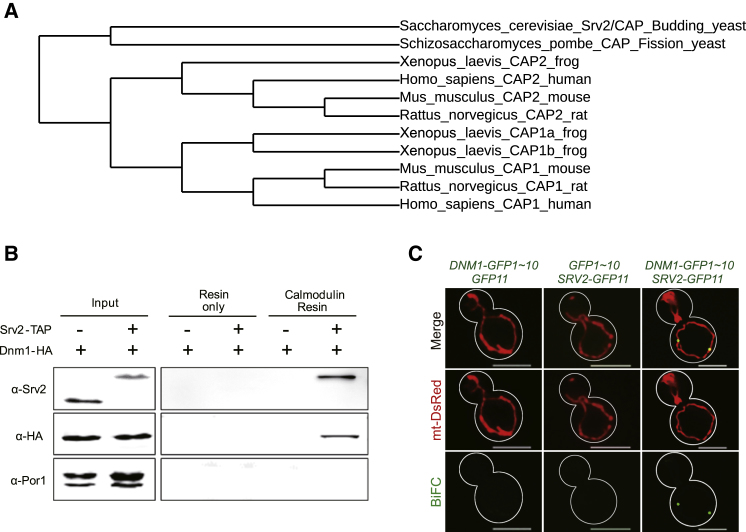
To identify factors involved in mitochondrial dynamics, we performed yeast two-hybrid screening to search for potential factors that interact with the human mitochondrial fission protein DRP1 (splice variant 1, residues 1–736; Figure S1). Other than DRP1 itself, which is known to self-associate (Zhu et al., 2004), the only specific interactor identified was CAP2 (cyclase-associated protein 2). There are two CAP genes, CAP1 and CAP2, in mammalian cells. These two proteins have subtle differences in sequences along with different distributions (Peche et al., 2007). Based on phylogenetic analyses (Dereeper et al., 2010, Chevenet et al., 2006, Edgar, 2004, Guindon and Gascuel, 2003, Castresana, 2000), S. cerevisiae has only a single CAP orthologue, Srv2 (Figure 1A). Srv2 was originally identified as a protein required for RAS-activated adenylate cyclase activity and regulation of cAMP levels in yeast (Fedor-Chaiken et al., 1990). More recent studies have demonstrated that Srv2 binds actin and is required for normal actin turnover and organization (Chaudhry et al., 2010, Chaudhry et al., 2014, Mattila et al., 2004, Balcer et al., 2003). Both cAMP signaling and the actin cytoskeleton have previously been identified to play important roles in mitochondrial dynamics (Li et al., 2015, Chang and Blackstone, 2007). Since a single CAP ortholog in yeast (Srv2) facilitates functional studies, we decided to investigate the role of CAP/Srv2 in mitochondrial dynamics and function in S. cerevisiae first.
Figure 1.
Yeast Srv2 Interacts with Mitochondrial Fission GTPase Dnm1
(A) Phylogenetic tree of Srv2/CAP proteins in different organisms.
(B) Srv2 was fused with the TAP tag, and Dnm1 was tagged with HA epitope. Following calmodulin-affinity chromatography, Srv2-Dnm1 co-precipitation was revealed by immunoblotting for HA. Porin was used as negative control. Note that the Srv2 antibody is less sensitive to Srv2-TAP than Srv2.
(C) Yeasts transformed with DNM1-GFP1∼10 + GFP11, GFP1∼10 + SRV2-GFP11, or DNM1-GFP1∼10 + SRV2-GFP11 were examined using the BiFC assay. mt-DsRed was expressed to label mitochondria. The BiFC signal of SRV2-GFP11 and DNM1-GFP1∼10 revealed the specific interaction of Srv2 with Dnm1 on mitochondria in living cells. Scale bars represent 5 μm.
We performed co-precipitation studies to confirm the interaction between Srv2 and Dnm1 in yeast. C-terminal Srv2 was conjugated with Tandem Affinity Purification tag (TAP-tag) in a Dnm1-HA yeast strain (Ghaemmaghami et al., 2003). Using calmodulin-affinity resin to precipitate Srv2-TAP, we demonstrated that Dnm1 co-precipitates with Srv2 (Figure 1B). The Srv2/Dnm1 co-precipitation was not seen in the Dnm1-HA only strain. In addition, we applied split-GFP-based bimolecular fluorescence complementation (BiFC) (Skarp et al., 2008) to examine the spatial and temporal interaction of Srv2 and Dnm1 in vivo. Dnm1-GFP 1–10 and Srv2-GFP 11 were expressed in yeast cells, and upon complementation we detected that more than 50% of cells contain reconstituted GFP puncta on mitochondria (Figure 1C). When we used either N- or C-terminal fragments instead of full-length Srv2 in BiFC experiments, C-terminal Srv2 demonstrated complementary fluorescence puncta at mitochondria in cells (Figure S2). The co-precipitation and BiFC results indicate that Srv2 and Dnm1 interact with one other on mitochondria in living cells.
SRV2 Deletion Causes a Hyperfused Mitochondrial Network
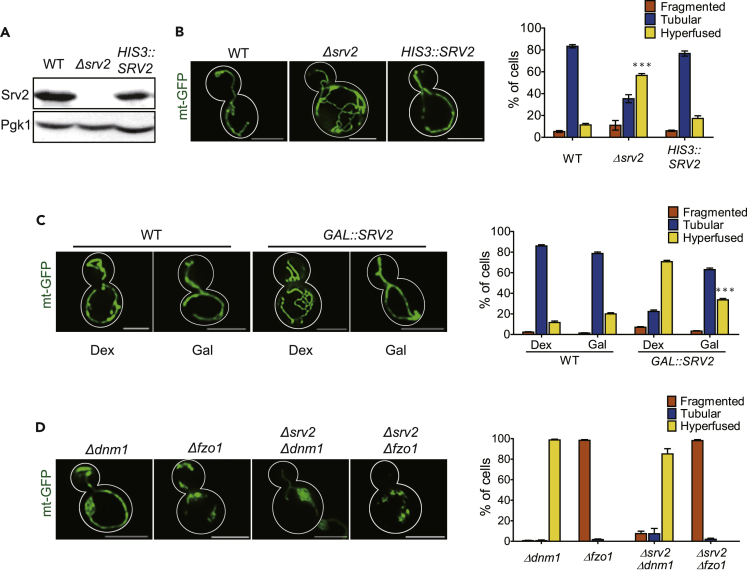
To elucidate the functional role of Srv2 in mitochondrial dynamics, we used direct gene replacement to construct an SRV2 deletion strain using the KanMX cassette (Figure 2A). Mitochondrial network morphology was used as an indicator for the status of mitochondrial dynamics. The mitochondrial network was labeled using mtGFP (Westermann and Neupert, 2000), and its morphology was classified into tubular, fragmented, and elongated-hyperfused categories by blinded observers. We found that 58% of Δsrv2 cells exhibited an elongated-hyperfused mitochondrial network. In addition, these elongated-hyperfused mitochondria in Δsrv2 cells had more branches as compared with the elongated mitochondria in fission-defect Δdnm1 cells. By contrast, <10% of wild-type cells had elongated-hyperfused mitochondria (Figure 2B). To verify that the elongated-hyperfused mitochondrial phenotype was due to SRV2 deletion, we examined mitochondrial morphology in an ectopic Srv2-expressing strain derived from the Δsrv2 strain. Ectopically- expressed Srv2 efficiently complemented the phenotype of the SRV2 deletion, and the ratio of cells with elongated-hyperfused mitochondria in cells ectopically expressing Srv2 approximated that of wild-type cells (Figures 2A and 2B). Next, we constructed a galactose-inducible Srv2 overexpression strain; the ratio of inducible SRV2 cells with elongated-hyperfused mitochondria was close to that of Δsrv2 cells in repressive dextrose medium. When yeasts were switched to galactose medium to induce Srv2 overexpression, the ratio of cells with elongated-hyperfused mitochondria was similar to that of the endogenously expressing cells (Figure 2C). Overexpression of Srv2 did not have any additional effects on mitochondrial structure or distribution. Together, these results demonstrate that the balance of mitochondrial fusion and fission in Δsrv2 cells is shifted toward excess fusion, resulting in elongated-hyperfused mitochondria. Thus, data from SRV2 deletion and galactose-induced overexpression of SRV2 suggest that Srv2 is a pro-fission factor in yeast cells, particularly when combined with the data showing a direct interaction between Srv2 and Dnm1.
Figure 2.
Srv2 Is Required for Maintaining Mitochondrial Dynamics
(A) SRV2 deletion and ectopic expression (HIS3::SRV2) were confirmed by immunoblotting, with Pgk1 as a protein loading control.
(B) Mitochondrial network morphology in wild-type, Δsrv2, and HIS3::SRV2 cells expressing mtGFP was examined by fluorescence microscopy. In the right panel, the mean ratios of cells with hyperfused mitochondria were: wild-type, 11.33%; Δsrv2, 56.67%; and HIS3::SRV2, 17.33%. Statistical data for each strain or condition were obtained from three trials of 100 yeast cells. The mean values ±SE were obtained by averaging the percentages of three counts. ***p < 0.001 vs. wild-type group. Scale bars represent 5 μm.
(C) Effects of ectopic Srv2 induction on mitochondria were examined with GAL::SRV2 cells. The ratio of cells with hyperfused mitochondria in YPDex vs. YPGal medium was: wild-type, 11.67% vs. 20%; GAL::SRV2, 70.67% vs. 33.67%. ***p < 0.001 vs. Dex group.
(D) Mitochondria network classification for Δdnm1, Δfzo1, Δsrv2 Δdnm1, and Δsrv2 Δfzo1 strains. SRV2 depletion had no synergic effects on the mitochondrial morphology in the FZO1 or DNM1 deletion strains.
To clarify further the role of well-known mitochondrial fusion/fission factors in generating the hyperfused mitochondrial phenotype in Δsrv2 cells, we examined mitochondrial network morphology in several double-deletion yeast strains, Δsrv2 Δdnm1 and Δsrv2 Δfzo1. Removal of the SRV2 gene in combination with the mitochondrial fission factor DNM1 or fusion factor FZO1 did not reveal synergic effects (Figure 2D); mitochondrial network morphology in double-deletion strains remained elongated-hyperfused in Δsrv2 Δdnm1 cells and fragmented in Δsrv2 Δfzo1 cells. Collectively, the mitochondrial morphologies in single- and double-deletion strains indicate that these conventional fusion/fission GTPases are decisive factors in generating mitochondrial morphology in Δsrv2 cells.
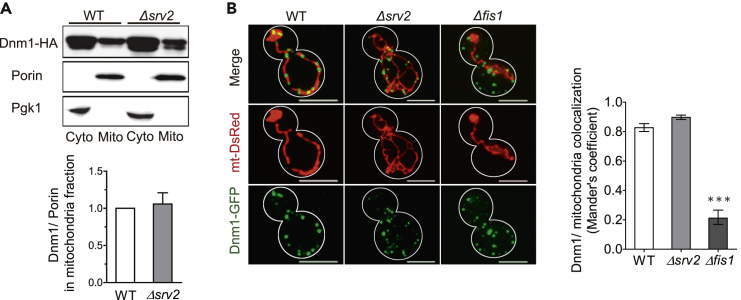
Dnm1 Localization Is Not Affected by SRV2 Deletion
Dnm1 is a cytosolic protein recruited to fission sites on mitochondria to form a fission complex, constricting mitochondria and driving their fission (Kuravi et al., 2006, Griffin et al., 2005, Mozdy et al., 2000). The BiFC assay already demonstrated that Srv2 and Dnm1 interact with one another on mitochondria. To explore the possibility that deletion of SRV2 affects translocation of Dnm1 to mitochondria and reduces fission complex formation, we examined protein levels of Dnm1 in the mitochondrial fraction. Dnm1 protein levels in mitochondrial fractions isolated from both wild-type and Δsrv2 cells were similar, although a slightly lower molecular weight form of Dnm1 was more notable in the Δsrv2 cells (Figure 3A). We labeled mitochondria and Dnm1 with different fluorescence proteins, and the localization of Dnm1 foci at mitochondria was assayed using Mander's colocalization coefficient method (Li et al., 2015, Dunn et al., 2011). We found that SRV2 deletion did not affect the colocalization of Dnm1 and mitochondria. On the other hand, FIS1 deletion dramatically decreased the localization of DNM1 puncta to mitochondria (Figure 3B). Thus, it is unlikely that Srv2 depletion affects Dnm1 recruitment to mitochondria.
Figure 3.
SRV2 Deletion Does Not Affect Dnm1 Distribution
(A) Dnm1-HA levels in mitochondrial and cytosolic fractions of wild-type (WT) and Δsrv2 cells were evaluated by immunoblotting. Pgk1 and porin were used as cytosolic (Cyto) and mitochondrial (Mito) markers, respectively. Mitochondrial Dnm1 levels were normalized to porin levels for comparisons. Mean ± SD from three trials are presented.
(B) Wild-type, Δsrv2, and Δfis1 cells expressing mt-DsRed and Dnm1-GFP were imaged by fluorescence microscopy. Dnm1-GFP foci on mt-DsRed were analyzed using ImageJ software. Mander's coefficient indicates the degree of association of Dnm1-GFP and mt-DsRed. ***p < 0.001 vs. WT group. Means ±SD are presented; 25 yeast cells/strain. Scale bars represent 5 μm.
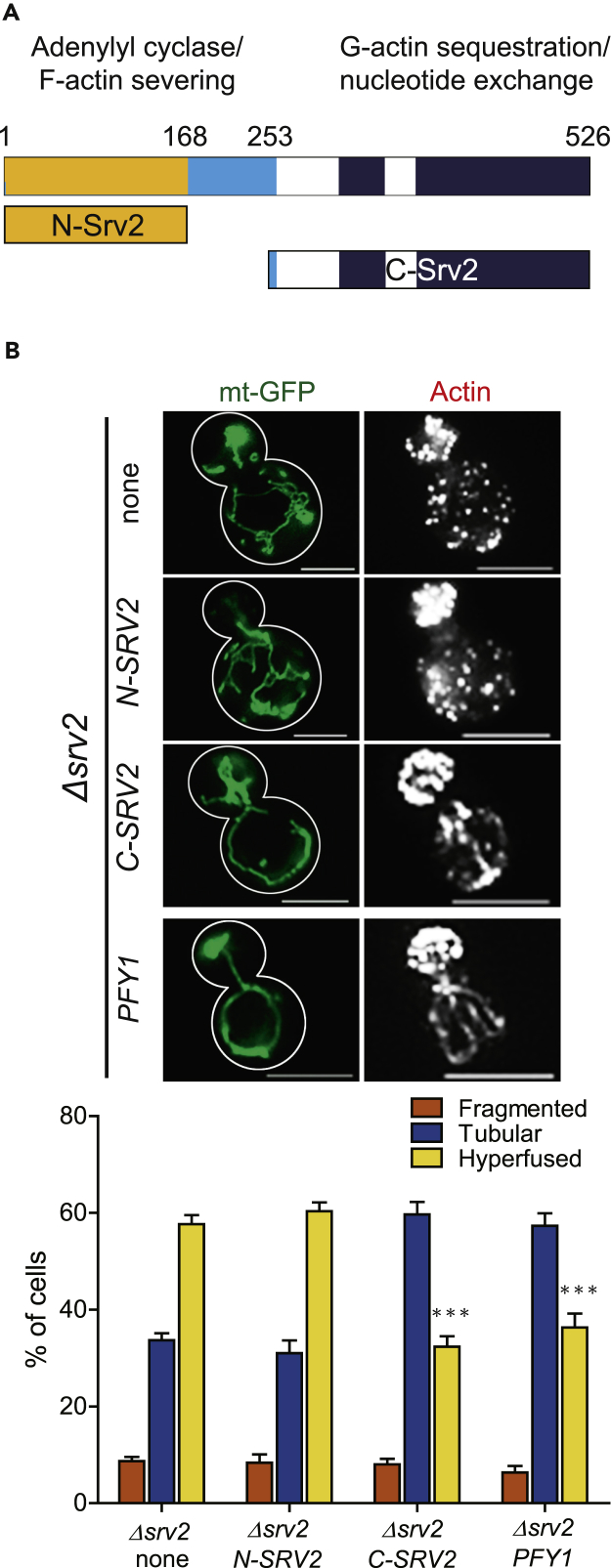
Srv2 Function in Actin Dynamics Is Required for Shaping Mitochondria
Since Dnm1 levels and foci numbers at mitochondria are not affected by SRV2 deletion, it seemed more likely that the elongated-hyperfused mitochondrial phenotype in Δsrv2 cells emanated from loss of a specific Srv2 function. The N-terminal domain of Srv2 has been reported to be involved in cAMP/PKA signaling, and the C-terminal domain is involved in regulating actin dynamics. Overexpression of N-terminal and C-terminal Srv2 fragments can complement cAMP/PKA signaling and rescue defects in actin dynamics in Δsrv2 cells, respectively (Chaudhry et al., 2010, Chaudhry et al., 2014, Mattila et al., 2004, Balcer et al., 2003, Fedor-Chaiken et al., 1990). Overexpression of N- or C-terminal Srv2 fragments in Δsrv2 cells was then used to examine which half of Srv2 could reverse the elongated-hyperfused phenotype and restore mitochondria to a more typical tubular morphology (Figure 4A). Overexpression of C-terminal Srv2 caused 36.67% cells to have elongated-hyperfused mitochondria. However, 57.67% of cells with empty vector and 60.33% of cells with overexpressed N-terminal Srv2 contained elongated-hyperfused mitochondria with the Δsrv2 background. The results demonstrated that overexpression of C-terminal Srv2 has the ability to rescue the mitochondrial phenotype in Δsrv2 cells (Figure 4B). These data also suggest that the function of Srv2 in actin assembly is important for mitochondrial dynamic processes.
Figure 4.
Complementary Experiments Indicated Srv2's Function in Actin Is Critical for Regulating Mitochondria Dynamics
(A) Schematic diagram of Srv2 showing key domains and truncations used in these experiments. Amino acid residue numbers are across the top.
(B) Morphology of mitochondria and F-actin in Δsrv2 cells overexpressing the indicated Srv2 truncations were visualized by fluorescence microscopy. Ratios of cells with hyperfused mitochondria in Δsrv2 yeast transformed with empty vector (none), N-SRV2, C-SRV2, and PFY1 were 57.67%, 60.33%, 36.67%, and 36.33%, respectively. ***p < 0.001 vs. empty vector (none) group. Mitochondrial morphology data in each strain were collected from three trials of 100 yeast cells, with means ±SE plotted. Scale bars represent 5 μm.
To examine further the role of actin assembly in mitochondrial network shape, we overexpressed yeast profilin (Pfy1), an actin monomer-binding protein that is critical for actin organization, in Δsrv2 cells (Yoshida et al., 2013, Sagot et al., 2002, Haarer et al., 1990). Pfy1 overexpression compensates for defects of actin dynamics (Magdolen et al., 1993, Vojtek et al., 1991). When Pfy1 was expressed in Δsrv2 cells, 36.33% of cells contained elongated-hyperfused mitochondria. This number was reduced to a level similar to those cells overexpressing the C-terminal fragment of Srv2 (Figure 4B). Based on the complementation results of yeasts overexpressing the C-terminal domain of Srv2 and full-length Pfy1, Srv2-mediated mitochondrial dynamics appears closely associated with its role in regulating actin dynamics.
Disrupted Srv2-Mediated Actin Dynamics Cause Morphological Changes in the Mitochondrial Network
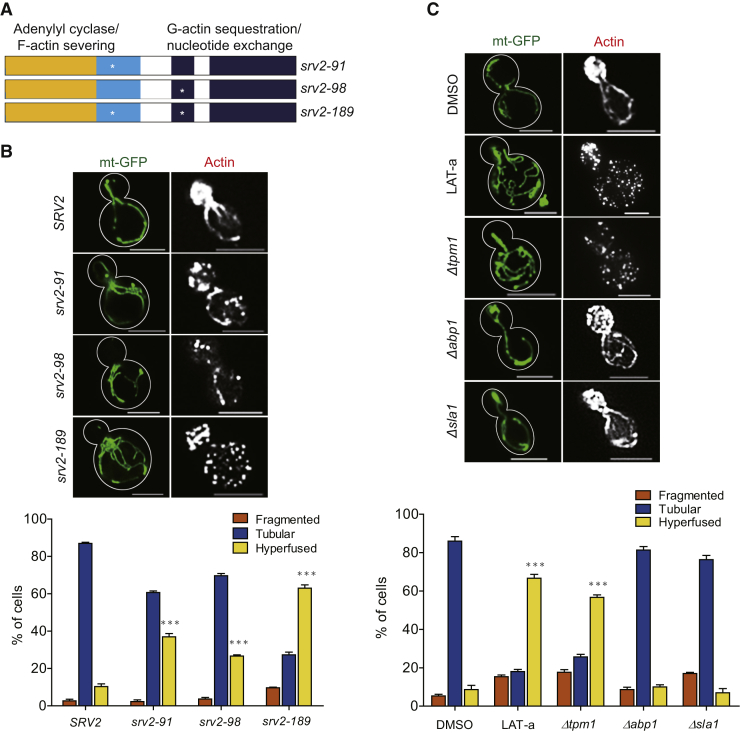
Two SRV2 mutant alleles, srv2-91 and srv2-98, cause actin severing and actin recycling defects, respectively (Chaudhry et al., 2010, Chaudhry et al., 2013). To clarify how Srv2 affects mitochondrial dynamics through its role in regulating actin dynamics, we examined mitochondrial network morphology in yeast strains harboring the srv2-91 and srv2-98 mutant alleles (Figure 5A). The ratio of cells with an elongated-hyperfused mitochondrial network in both strains (37.00% and 26.67%, respectively) was significantly higher than the 11.33% in wild-type yeast cells (Figure 5B). Another mutant allele, srv2-189 (Figure 5A), harbors mutant properties of both srv2-91 and srv2-98, and 63.00% of srv2-189 cells contained elongated-hyperfused mitochondria. This ratio in srv2-189 was very similar to that of the Δsrv2 strain (Figure 5B).
Figure 5.
Srv2 Regulates Fission by Modulating Actin on Mitochondria
(A) Schematic diagram showing the SRV2 mutant yeast alleles used in these experiments.
(B) Effects of srv2 mutant alleles on mitochondrial network morphology and F-actin assembly were examined using fluorescence microscopy. Ratios of cells with hyperfused mitochondria in SRV2, srv2-91, srv2-98, and srv2-198 strains were 10.33%, 37%, 26.67%, and 63.00%, respectively. ***p < 0.001 vs. SRV2 cells.
(C) Analyses of mitochondrial morphology and F-actin in wild-type (WT) cells treated with latrunculin A along with mutant stains of Δtpm1, Δabp1, and Δsla1 cells were used to verify the effects of F-actin assembly on mitochondrial dynamics. Mitochondrial morphologies for each strain or condition were analyzed from three independent counts of 100 yeast cells. ***p < 0.001 vs. dimethylsulfoxide-treated cells or WT cells. Mean values ±SE were obtained by averaging the percentages of three independent counts. Scale bars represent 5 μm.
Our results thus far suggest that actin-related functions of Srv2 are critical for its modulation of mitochondrial dynamics. To clarify the role of actin in regulating mitochondrial fusion and fission, we treated wild-type yeast with latrunculin A, a compound that sequesters G-actin and inhibits actin polymerization. We found mitochondria network morphology has dose-dependent alteration in response to latrunculin A. Low dosage of latrunculin A treatment caused hyperfused mitochondrial network, whereas high dosage caused fragmented mitochondria. Treatment of cells with 0.5 μM latrunculin A caused three phenotypes—isotropic growth, fewer actin cables, and hyperfused mitochondria—all of which are reminiscent of Δsrv2 phenotypes (Figure 5C). To our surprise, both co-precipitation and BiFC results demonstrated that the Srv2/Dnm1 interaction was not affected, whereas actin polymerization was inhibited under the same latrunculin A treatment condition (Figure S3). Thus, our data demonstrate that actin filament/cable assembly at mitochondria is important for maintaining mitochondrial dynamics in yeast cells.
We next constructed a tropomyosin (TPM1) deletion strain; Tpm1 is a protein that binds to and stabilizes actin cables and filaments (Liu and Bretscher, 1989). In the Δtpm1 strain, we found that 56.67% cells exhibited an elongated-hyperfused mitochondrial network (Figure 5C). However, deletion of two cortical actin-binding proteins, ABP1 (Actin Binding Protein) and SLA1 (Synthetic Lethal with ABP1), did not cause elongated-hyperfused mitochondria (Figure 5C). Both Abp1 and Sla1 are important for actin patch formation. We examined actin cable in these mutant strains by phalloidin staining (Figure 5) and quantified actin cable numbers based on previous studies (Alioto et al., 2016, Higuchi-Sanabria et al., 2016). The Δtpm1 strain has almost no visible actin cables, whereas Δsrv2 and srv2-189 strains have similar actin cable counts that are lower than those of srv2-91 and srv2-98 strains. Both Δabp1 and Δsla1 strains demonstrated minor reduction of actin cable counts compared with wild-type cells (Figure S4). These results from genetic deletion strains, along with data from latrunculin A-treated cells and the Δsrv2 cells, demonstrate that Srv2-mediated actin filament/cable assembly rather than patch formation is more important for maintaining the balance of mitochondrial dynamics.
We also examined the effects of CAP2 deletion on mitochondria in SH-SY5Y cells. Applying CRISPR/Cas9 to delete both alleles of CAP2, we found that a majority of CAP2−/− cells bear a hyperfused mitochondrial network (Figure S5). The CAP2 knockout cells demonstrated that Srv2/CAP2 is important for mitochondrial fission in both mammalian and yeast cells.
Deletion of SRV2 Affects Mitochondrial Activity
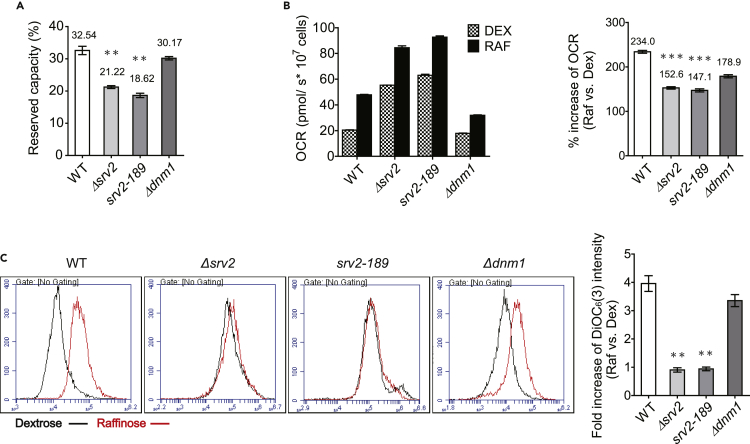
Changes in mitochondrial dynamics are closely associated with adjustments in mitochondrial activity. For instance, Parkin/Pink1 respond to a reduced mitochondrial membrane potential to promote mitochondrial fission (Jin and Youle, 2012, Narendra et al., 2010), and elongation of mitochondria helps to preserve their membrane potential during nutrient starvation (Blackstone and Chang, 2011, Gomes et al., 2011). As SRV2 deletion causes elongation and hyperfusion of the mitochondrial network, it is plausible that mitochondrial activity would not be the same as that of wild-type cells. A high-resolution respirometer (Oroboros Oxygraph 2K) was used to examine the oxygen consumption rate, elucidating the mitochondrial reserve respiration capacity. We treated cells with compounds that inhibit oxidative phosphorylation at different stages of electron transport: triethyltin bromide (TET) to inhibit ATP synthase, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) to increase proton permeability across the mitochondrial inner membrane, and antimycin to inhibit complex III. Oxygen consumption under the stress induced by these pharmacological agents was recorded throughout the procedure. Oxygen consumption rates during each step of the chemical treatments were normalized to flux control factor values to elucidate the reserve respiration capacity (Figure S6). Our results demonstrate that Δsrv2 and srv2-189 cells have a lower reserve respiration capacity as compared with wild-type and Δdnm1 cells (Figure 6A).
Figure 6.
Mitochondrial Activity Is Altered by SRV2 Deletion
(A) High-resolution respirometry was used to assay the oxygen consumption rate (OCR) as described in Methods. OCRs of wild-type, Δsrv2, srv2-189, and Δdnm1 yeast in YPD with mitochondrial respiration complex inhibitors were determined (Figure S6). Reserve capacities (1-Routine respiration/maximal respiration) of wild-type, Δsrv2, srv2-189, and Δdnm1 mitochondria were 32.54%, 21.22%, 18.62%, and 30.17%, respectively. **p < 0.01 vs. WT cells.
(B) OCRs for wild-type, Δsrv2, srv2-189, and Δdnm1 cells were determined in YPD/YPR. Increases of OCR (from YPD to YPR) in wild-type, Δsrv2, srv2-189, and Δdnm1 cells were 2.34-, 1.53-, 1.47-, and 1.79-fold, respectively. ***p < 0.001 vs. WT cells. (C) Mitochondrial membrane potential of wild-type, ∆srv2, srv2-189, and ∆dnm1 yeast in YPD/YPR medium was evaluated by DiOC6 staining followed by flow cytometry. Wild-type (WT) and ∆dnm1 yeast cells showed higher mitochondria membrane potentials in YPR medium, but ∆srv2 and srv2-189 yeast cells did not. Increases of DiOC6 intensity (YPD vs. YPR) in WT, ∆srv2, srv2-189, and ∆dnm1 were 3.94-, 0.90-, 0.93-, and 3.34-fold, respectively. **p < 0.01 vs. WT cells. Mean values ± SE were obtained by averaging the values of three independent experiments.
Owing to catabolite repression in the presence of dextrose, mitochondrial activity would be de-repressed when yeast cells switch to a non-dextrose medium (Gancedo, 1998). To verify further the reserve respiration capacity results, we examined the routine oxygen consumption rate of wild-type, Δdnm1, Δsrv2, and srv2-189 cells in both dextrose and non-dextrose (raffinose) medium (Figure 6B). All four strains had higher oxygen consumption rates in raffinose than dextrose. We then examined the mitochondrial membrane potential in both dextrose and raffinose media, applying DiOC6 to detect the mitochondrial membrane potential using flow cytometry. Cells cultured in raffinose had a higher membrane potential than those cultured in dextrose (Figure 6C). An intriguing finding was that raffinose-related increases in the levels of oxygen consumption and membrane potential in both Δsrv2 and srv2-189 strains were lower than in wild-type and Δdnm1 cells (Figures 6B and 6C). These membrane potential and oxygen consumption rate results are consistent with the reserve respiration capacity results. Together, these data demonstrate that Srv2 defects not only change the mitochondrial network morphology but also impact mitochondrial activity, especially the reserve respiration capacity.
Discussion
SRV2 deletion and mutant alleles cause elongated-hyperfused mitochondria, and this phenotype can be reverted by the overexpression not only of Srv2 but also of Pfy1, indicating that Srv2 modulates mitochondrial dynamics through its function in actin assembly. Deletion of SRV2 not only changed the network morphology of mitochondria but also affected mitochondrial activity; Δsrv2 cells with hyperfused mitochondria have a lower spare respiration capacity when compared with wild-type cells. Furthermore, Δsrv2 cells are unable to increase oxygen consumption and mitochondrial membrane potential as much as wild-type cells when cultured in non-dextrose medium. Based on these findings, we propose that Srv2 serves as a dual-function modulator of both mitochondrial dynamics and activity.
Srv2 was previously known to regulate actin recycling and severing. Both polarized actin cable assembly and patch formation are the major regulatory functions of Srv2/CAP (Toshima et al., 2016). Our results of SRV2 deletion and mutant alleles bring the regulatory mechanisms of mitochondria and actin dynamics together. The yeast two-hybrid, co-precipitation and BiFC results demonstrated the interaction between Dnm1 and Srv2. The interaction was even preserved under the condition when actin cable assembly was inhibited by latrunculin A. However, deletion of SRV2 did not affect Dnm1 localized to mitochondria. This result ruled out the possibility that Srv2 involves in mitochondria dynamics by affecting Dnm1 recruitment to mitochondria.
Over-expressed C-terminal Srv2 and the Pfy1-compensated SRV2 deletion phenotypes demonstrated the involvement of actin in the formation of hyperfused mitochondria in Δsrv2 cells. Mutant alleles that caused minor reduction of actin cable numbers (srv2-91 and srv2-98) led to lower ratio of cells with hyperfused mitochondria than the one caused severe reduction of actin cable numbers (srv2-189). These disrupted actin cable counts in mutant cells along with latrunculin A treatment and TPM1 deletion resulted in hyperfused mitochondria in wild-type cells, suggesting that actin cable formation plays a critical role in mitochondrial dynamics in yeast cells. This notion was further supported by the fact that the hyperfused-elongated mitochondrial network morphology was not seen in Δabp1 or Δsla1 strains that bear actin patch formation defects. However, mitochondrial network morphology in double-deletion strains is elongated-hyperfused in Δsrv2 Δdnm1 cells and fragmented in Δsrv2 Δfzo1 cells. These phenotypes of double-mutant strains suggested that actin cable plays different roles in mitochondrial fusion and fission processes and that Srv2 may be responsible for the differences. The Dnm1/Srv2 interaction may facilitate actin cable assembly on mitochondria. Loss of Srv2 may not only affect actin assembly but also lead to loss of the interaction between mitochondria and actin. Mitochondrial dynamics and activity would be affected either by their interaction with actin cable or when actin assembly at mitochondria is disrupted. We favor a model in which Srv2 mediates mitochondrial dynamics through its function in promoting actin polymerization and stabilizing actin filaments in conjunction with its ability to interact with the fission protein Dnm1 (Figure 7). Potentially, disrupted actin/mitochondria interaction under SRV2 deletion causes either mitochondrial transporting defect or incomplete mitochondrial division. We still have not collected enough results to distinguish between the two possibilities.
Figure 7.
Model of Srv2 Regulating Dynamics and Activity by Modulating Actin Assembly on Mitochondria
The model schematically depicts that the Srv2/Dnm1 interaction facilitates Srv2-mediated modulation of actin polymerization on mitochondria. Actin polymerization is required to regulate mitochondria morphology and activity.
Both SRV2 and DNM1 deletion yeasts possess hyperfused mitochondria with less reserve respiration capacity compared with wild-type cells. The reserve respiration capacity results are in agreement with the mitochondrial membrane potentials measured under conditions without catabolite repression (Figure 6). These data strongly suggest that well-maintained mitochondrial dynamics are required for the proper function of oxidative phosphorylation complexes.
Unexpectedly, the reserve respiration capacities were different in the Δsrv2 and Δdnm1 strains, although both strains contained an elongated-hyperfused mitochondrial network. Even so, we found that the morphology of the mitochondrial network was different between Δsrv2 cells and Δdnm1 cells. The mitochondria in Δsrv2 cells formed an elongated and branched network, whereas in Δdnm1 cells the mesh form of hyperfused mitochondria dominated. The different network morphologies indicate that unidentified factors are involved in the dynamic balance in these strains. A previous study suggested that the machinery of mitochondrial dynamics is correlated with the wild-type and mutant mtDNA expansion rates (Karavaeva et al., 2017, Osman et al., 2015). We speculate that this may underlie the differential reserve respiration capacities of Δsrv2 cells and Δdnm1 cells. However, impacts of different shapes of the network on activity require further study.
Based on phylogenetic analyses, two orthologous CAP proteins in mammalian cells, CAP1 and CAP2, serve similar functions as Srv2 in yeast cells. Although microtubules have been emphasized in mitochondrial dynamics, the role of actin filaments in mitochondrial dynamics in mammalian cells requires further study.
In summary, we have characterized Srv2 as a pro-fission factor that modulates mitochondrial activity. Our work brings the regulatory pathways of actin and mitochondrial dynamics together. As the roles of mitochondrial dynamics in the pathogenesis of neurodegenerative diseases and other diseases are emerging, returning the disrupted equivalence of mitochondrial dynamics to normal will be a potential therapeutic target in the future.
Limitations of the Study
Cytoskeletal systems definitely play a role in mitochondrial dynamics, and mammalian cells have much more sophisticated cytoskeleton systems than yeasts. In addition, mitochondrial transport largely relies on microtubules in mammalian cells. Although we have demonstrated that CAP2-knockout mammalian cells also possess elongated-hyperfused mitochondria, further study is required to clarify how actin assembly on mitochondria is involved in fission and transportation in mammalian cells.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to thank Jin-Jer Lin for his valuable comments on the manuscript, Ann-Shyn Chiang for a microscope objective lens, and David Chan and Janet Shaw for yeast strains. Assistance provided by Jiayee Wu was greatly appreciated. This work was supported by the Ministry of Science and Technology of Taiwan (105-2320-B-007 -005 and 105-2923-B-007 -001 –MY3), Academia Sinica (AS-TP-L107-1), and the Intramural Research Program of the NINDS, NIH.
Author Contributions
Conceptualization, Y-.C.C., C.B., and C-.R.C.; Methodology, T-.H.C., C.B., and C-.R.C.; Investigation, Y-.C.C., W-.L.L., C-.L.C., and C-.R.C.; Writing – Original Draft, Y-.C.C. and C-.R.C.; Writing – Review & Editing, C-.R.C. and C.B.; Funding Acquisition, W-.Y.Y., C.B., and C-.R.C.; Resources, T-.H.C., W-.Y.Y., C.B., and C-.R.C.; Supervision, C.B. and C-.R.C.
Declaration of Interests
The authors declare no competing interests.
Published: January 25, 2019
Footnotes
Supplemental Information includes Transparent Methods, six figures, and two tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.12.021.
Contributor Information
Craig Blackstone, Email: blackstc@ninds.nih.gov.
Chuang-Rung Chang, Email: crchang@life.nthu.edu.tw.
Supplemental Information
References
- Alioto S.L., Garabedian M.V., Bellavance D.R., Goode B.L. Tropomyosin and profilin cooperate to promote formin-mediated actin nucleation and drive yeast actin cable assembly. Curr. Biol. 2016;26:3230–3237. doi: 10.1016/j.cub.2016.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann K., Westermann B. Role of essential genes in mitochondrial morphogenesis in Saccharomyces cerevisiae. Mol. Biol. Cell. 2005;16:5410–5417. doi: 10.1091/mbc.E05-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcer H.I., Goodman A.L., Rodal A.A., Smith E., Kugler J., Heuser J.E., Goode B.L. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 2003;13:2159–2169. doi: 10.1016/j.cub.2003.11.051. [DOI] [PubMed] [Google Scholar]
- Blackstone C., Chang C.-R. Mitochondria unite to survive. Nat. Cell Biol. 2011;13:521–522. doi: 10.1038/ncb0511-521. [DOI] [PubMed] [Google Scholar]
- Böckler S., Chelius X., Hock N., Klecker T., Wolter M., Weiss M., Braun R.J., Westermann B. Fusion, fission, and transport control asymmetric inheritance of mitochondria and protein aggregates. J. Cell Biol. 2017;216:2481–2498. doi: 10.1083/jcb.201611197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman J.L., Pickles S., Wang C., Sekine S., Vargas J.N.S., Zhang Z., Youle A.M., Nezich C.L., Wu X., Hammer J.A., Youle R.J. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 2017;216:3231–3247. doi: 10.1083/jcb.201612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Chang C.-R., Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- Chaudhry F., Breitsprecher D., Little K., Sharov G., Sokolova O., Goode B.L. Srv2/cyclase-associated protein forms hexameric shurikens that directly catalyze actin filament severing by cofilin. Mol. Biol. Cell. 2013;24:31–41. doi: 10.1091/mbc.E12-08-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F., Jansen S., Little K., Suarez C., Boujemaa-Paterski R., Blanchoin L., Goode B.L. Autonomous and in trans functions for the two halves of Srv2/CAP in promoting actin turnover. Cytoskeleton (Hoboken) 2014;71:351–360. doi: 10.1002/cm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry F., Little K., Talarico L., Quintero-Monzon O., Goode B.L. A central role for the WH2 domain of Srv2/CAP in recharging actin monomers to drive actin turnover in vitro and in vivo. Cytoskeleton (Hoboken) 2010;67:120–133. doi: 10.1002/cm.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevenet F., Brun C., Banuls A.-L., Jacq B., Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.-H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Audic S., Claverie J.-M., Blanc G. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 2010;10:8. doi: 10.1186/1471-2148-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K.W., Kamocka M.M., McDonald J.H. A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 2011;300:C723–C742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor-Chaiken M., Deschenes R.J., Broach J.R. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell. 1990;61:329–340. doi: 10.1016/0092-8674(90)90813-t. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher K.L., Yang H.-C., Gay A.C., Huckaba T.M., Pon L.A. Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr. Biol. 2004;14:1996–2004. doi: 10.1016/j.cub.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Freeman N.L., Chen Z., Horenstein J., Weber A., Field J. An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiae cyclase-associated protein. J. Biol. Chem. 1995;270:5680–5685. doi: 10.1074/jbc.270.10.5680. [DOI] [PubMed] [Google Scholar]
- Friedman J.R., Lackner L.L., West M., DiBenedetto J.R., Nunnari J., Voeltz G.K. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998;62:334–361. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst J.E., Ferguson K., Vojtek A., Wigler M., Field J. CAP is a bifunctional component of the Saccharomyces cerevisiae adenylyl cyclase complex. Mol. Cell. Biol. 1991;11:1248–1257. doi: 10.1128/mcb.11.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S., Huh W.-K., Bower K., Howson R.W., Belle A., Dephoure N., O'Shea E.K., Weissman J.S. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Gomes L.C., Di Benedetto G., Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin E.E., Graumann J., Chan D.C. The WD40 protein Caf4p is a component of the mitochondrial fission machinery and recruits Dnm1p to mitochondria. J. Cell Biol. 2005;170:237–248. doi: 10.1083/jcb.200503148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon S., Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Gurel P.S., Mu A., Guo B., Shu R., Mierke D.F., Higgs H.N. Assembly and turnover of short actin filaments by the formin INF2 and profilin. J. Biol. Chem. 2015;290:22494–22506. doi: 10.1074/jbc.M115.670166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer B.K., Lillie S.H., Adams A.E.M., Magdolen V., Bandlow W., Brown S.S. Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J. Cell Biol. 1990;110:105–114. doi: 10.1083/jcb.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch A.L., Gurel P.S., Higgs H.N. Novel roles for actin in mitochondrial fission. J. Cell Sci. 2014;127:4549–4560. doi: 10.1242/jcs.153791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch A.L., Ji W.-K., Merrill R.A., Strack S., Higgs H.N. Actin filaments as dynamic reservoirs for Drp1 recruitment. Mol. Biol. Cell. 2016;27:3109–3121. doi: 10.1091/mbc.E16-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann G.J., Thatcher J.W., Mills J.P., Hales K.G., Fuller M.T., Nunnari J., Shaw J.M. Mitochondrial fusion in yeast requires the transmembrane GTPase Fzo1p. J. Cell Biol. 1998;143:359–373. doi: 10.1083/jcb.143.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Sanabria R., Vevea J.D., Charalel J.K., Sapar M.L., Pon L.A. The transcriptional repressor Sum1p counteracts Sir2p in regulation of the actin cytoskeleton, mitochondrial quality control and replicative lifespan in Saccharomyces cerevisiae. Microb. Cell. 2016;3:79–88. doi: 10.15698/mic2016.02.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckaba T.M., Gay A.C., Pantalena L.F., Yang H.-C., Pon L.A. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J. Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W.-k., Hatch A.L., Merrill R.A., Strack S., Higgs H.N. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S.M., Youle R.J. PINK1- and Parkin-mediated mitophagy at a glance. J. Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavaeva I.E., Golyshev S.A., Smirnova E.A., Sokolov S.S., Severin F.F., Knorre D.A. Mitochondrial depolarization in yeast zygotes inhibits clonal expansion of selfish mtDNA. J. Cell Sci. 2017;130:1274–1284. doi: 10.1242/jcs.197269. [DOI] [PubMed] [Google Scholar]
- Koch A., Schneider G., Lüers G.H., Schrader M. Peroxisome elongation and constriction but not fission can occur independently of dynamin-like protein 1. J. Cell Sci. 2004;117:3995–4006. doi: 10.1242/jcs.01268. [DOI] [PubMed] [Google Scholar]
- Kuravi K., Nagotu S., Krikken A.M., Sjollema K., Deckers M., Erdmann R., Veenhuis M., van der Klei I.J. Dynamin-related proteins Vps1p and Dnm1p control peroxisome abundance in Saccharomyces cerevisiae. J. Cell Sci. 2006;119:3994–4001. doi: 10.1242/jcs.03166. [DOI] [PubMed] [Google Scholar]
- Lee J.E., Westrate L.M., Wu H., Page C., Voeltz G.K. Multiple dynamin family members collaborate to drive mitochondrial division. Nature. 2016;540:139–143. doi: 10.1038/nature20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legesse-Miller A., Massol R.H., Kirchhausen T. Constriction and Dnm1p recruitment are distinct processes in mitochondrial fission. Mol. Biol. Cell. 2003;14:1953–1963. doi: 10.1091/mbc.E02-10-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Xu S., Roelofs B.A., Boyman L., Lederer W.J., Sesaki H., Karbowski M. Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J. Cell Biol. 2015;208:109–123. doi: 10.1083/jcb.201404050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989;57:233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- Magdolen V., Drubin D.G., Mages G., Bandlow W. High levels of profilin suppress the lethality caused by overproduction of actin in yeast cells. FEBS Lett. 1993;316:41–47. doi: 10.1016/0014-5793(93)81733-g. [DOI] [PubMed] [Google Scholar]
- Mattila P.K., Quintero-Monzon O., Kugler J., Moseley J.B., Almo S.C., Lappalainen P., Goode B.L. A high-affinity interaction with ADP-actin monomers underlies the mechanism and in vivo function of Srv2/cyclase-associated protein. Mol. Biol. Cell. 2004;15:5158–5171. doi: 10.1091/mbc.E04-06-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A.S., Wong Y.C., Simpson C.L., Holzbaur E.L.F. Dynamic actin cycling through mitochondrial subpopulations locally regulates the fission-fusion balance within mitochondrial networks. Nat. Commun. 2016;7:12886. doi: 10.1038/ncomms12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdy A.D., McCaffery J.M., Shaw J.M. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J. Cell Biol. 2000;151:367–380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D., Tanaka A., Suen D.-F., Youle R.J. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy. 2009;5:706–708. doi: 10.4161/auto.5.5.8505. [DOI] [PubMed] [Google Scholar]
- Narendra D.P., Jin S.M., Tanaka A., Suen D.-F., Gautier C.A., Shen J., Cookson M.R., Youle R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman C., Noriega T.R., Okreglak V., Fung J.C., Walter P. Integrity of the yeast mitochondrial genome, but not its distribution and inheritance, relies on mitochondrial fission and fusion. Proc. Natl. Acad. Sci. U S A. 2015;112:E947–E956. doi: 10.1073/pnas.1501737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peche V., Shekar S., Leichter M., Korte H., Schröder R., Schleicher M., Holak T.A., Clemen C.S., Ramanath-Y B., Pfitzer G. CAP2, cyclase-associated protein 2, is a dual compartment protein. Cell. Mol. Life Sci. 2007;64:2702–2715. doi: 10.1007/s00018-007-7316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot I., Rodal A.A., Moseley J., Goode B.L., Pellman D. An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 2002;4:626–631. doi: 10.1038/ncb834. [DOI] [PubMed] [Google Scholar]
- Sesaki H., Jensen R.E. UGO1 encodes an outer membrane protein required for mitochondrial fusion. J. Cell Biol. 2001;152:1123–1134. doi: 10.1083/jcb.152.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirendeb U.P., Calkins M.J., Manczak M., Anekonda V., Dufour B., McBride J.L., Mao P., Reddy P.H. Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease. Hum. Mol. Genet. 2012;21:406–420. doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarp K.-P., Zhao X., Weber M., Jäntti J. Use of bimolecular fluorescence complementation in yeast Saccharomyces cerevisiae. Methods Mol. Biol. 2008;457:165–175. doi: 10.1007/978-1-59745-261-8_12. [DOI] [PubMed] [Google Scholar]
- Toshima J.Y., Horikomi C., Okada A., Hatori M.N., Nagano M., Masuda A., Yamamoto W., Siekhaus D.E., Toshima J. Srv2/CAP is required for polarized actin cable assembly and patch internalization during clathrin-mediated endocytosis. J. Cell Sci. 2016;129:367–379. doi: 10.1242/jcs.176651. [DOI] [PubMed] [Google Scholar]
- Vojtek A., Haarer B., Field J., Gerst J., Pollard T.D., Brown S., Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991;66:497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial dynamics in model organisms: what yeasts, worms and flies have taught us about fusion and fission of mitochondria. Semin. Cell Dev. Biol. 2010;21:542–549. doi: 10.1016/j.semcdb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Westermann B., Neupert W. Mitochondria-targeted green fluorescent proteins: convenient tools for the study of organelle biogenesis in Saccharomyces cerevisiae. Yeast. 2000;16:1421–1427. doi: 10.1002/1097-0061(200011)16:15<1421::AID-YEA624>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Wong E.D., Wagner J.A., Gorsich S.W., McCaffery J.M., Shaw J.M., Nunnari J. The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria. J. Cell Biol. 2000;151:341–352. doi: 10.1083/jcb.151.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Ohnuki S., Yashiroda Y., Ohya Y. Profilin is required for Ca2+ homeostasis and Ca2+-modulated bud formation in yeast. Mol. Genet. Genomics. 2013;288:317–328. doi: 10.1007/s00438-013-0752-x. [DOI] [PubMed] [Google Scholar]
- Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P.-P., Patterson A., Stadler J., Seeburg D.P., Sheng M., Blackstone C. Intra- and intermolecular domain interactions of the C-terminal GTPase effector domain of the multimeric dynamin-like GTPase Drp1. J. Biol. Chem. 2004;279:35967–35974. doi: 10.1074/jbc.M404105200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.