Abstract
Both variegate and acute intermittent porphyria can manifest with various neurological symptoms. Although acute symptomatic seizures have been previously described, they are typically tonic–clonic and focal impaired awareness seizures. Convulsive status epilepticus and epilepsia partialis continua are rare and have been described on a case report basis. To our knowledge, there are no previously reported cases describing non-convulsive status epilepticus (NCSE) with electroencephalogram (EEG) documentation in the setting of acute porphyria crisis. We report a unique presentation of NCSE, which resolved after administering levetiracetam in a patient with variegate porphyria, without a known seizure disorder.
Keywords: Variegate porphyria, Non-convulsive status epilepticus, Electroencephalogram, Anticonvulsant medication, Acute porphyria crisis
Highlights
-
•
Seizures among acute intermittent porphyria (AIP) patients are not uncommon with prevalence being as high as 10%–20%.
-
•
Non-convulsive status epilepticus (NCSE) in variegate porphyria (VP) patients should be considered in patients with altered mental status and acute porphyric crisis.
-
•
Recognition and avoidance of prophyrogenic anti-seizure medications is important in the treatment of these patients.
-
•
Levetiracetam and hemin are potential treatment combinations for nonconvulsive status epilepticus in acute porphyria crisis patients.
1. Introduction
Variegate porphyria (VP) is an autosomal dominant disorder wherein heme biosynthesis is disrupted due to deficiency of protoporphyrinogen oxidase (PPOX) enzyme. The clinical presentation for VP is similar to acute intermittent porphyria (AIP), which typically presents with altered mentation and unexplained abdominal pain, chest, and back pain.
Neurological complications include peripheral neuropathy, acute encephalopathy, and acute symptomatic seizures. Seizures can affect 10%–20% of patients with acute porphyria; the most commonly reported are tonic–clonic and focal seizures with impaired awareness [1], [2], [3], [4]. Although rare, convulsive status epilepticus and epilepsia partialis continua have been described in case reports [1], [4]. There are no cases previously describing non-convulsive status epilepticus (NCSE) with electroencephalogram (EEG) documentation to our knowledge. We report a unique presentation of NCSE, which resolved after administration of levetiracetam in a patient with nearly 50 years of VP and without a known, prior seizure disorder who presented in an acute porphyria crisis.
2. Methods
We reviewed a case of nonconvulsive status epilepticus secondary to variegate porphyria crisis presenting to a tertiary referral medical center in the Southwest United States. A literature review was performed using PubMed.
3. Results
A 71-year-old woman, with a 50 year history of hereditary variegate porphyria and a history of end-stage renal disease on hemodialysis and coronary artery disease, presented to the emergency department with generalized weakness, lethargy, and confusion.
Upon admission, she was combative, moving all of her extremities, fluent speech, but intermittently confused. Infectious work-up was unrevealing. A chest radiograph, urine toxicology screen, urinalysis, comprehensive blood count, ammonia, and a head computed tomography (CT) were within normal limits. Her comprehensive metabolic profile showed evidence of her known chronic kidney disease. Her cerebrospinal fluid (CSF) analysis was within normal range excluding any infectious causative agent in the central nervous system. Her urine and blood cultures, HBsAg, thyroid stimulating hormone (TSH) and thiamine were unremarkable. Her urine porphobilinogen was elevated, 4.4 μmol/L (reference value is ≤ 1.3 μmol/L), which substantiated her acute porphyria crisis presentation. Interestingly, she had three previous admissions with no prior EEGs for unexplained encephalopathy, which were attributed to her acute porphyria crisis.
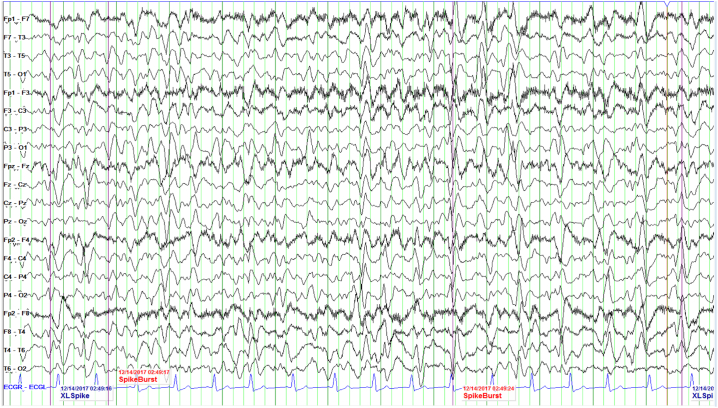
The following day her neurologic exam changed and she became stuporous, responding only to noxious stimuli. A neurology consultation was placed. Her physical examination demonstrated no spontaneous movement of her extremities as well as a lack of deep tendon reflexes. She was nonverbal, and did not respond to verbal cues or follow commands. Her Glasgow Coma Scale was 9 (E3V2M4). An EEG was ordered which showed generalized triphasic waves and independent multifocal sharp waves, concerning for nonconvulsive status epilepticus (Fig. 1).
Fig. 1.
Trend EEG revealed generalized 5–6 Hz theta with admixed 2–3 Hz delta frequency slowing. There was evidence of multifocal, independently occurring, sharp waves in the bilateral cerebral hemispheres occurring most prominently with stimulation. With stimulation, discharges occur with 1–2 Hz periodicity. Additionally, frequent generalized triphasic waves were seen. Triphasic waves were seen in addition to generalized slowing of the background activity. These collective findings in the setting of underlying diffuse cerebral dysfunction suggested the potential for seizures to arise.
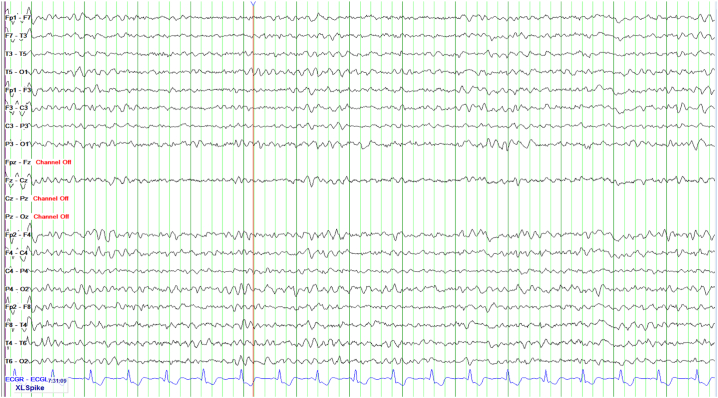
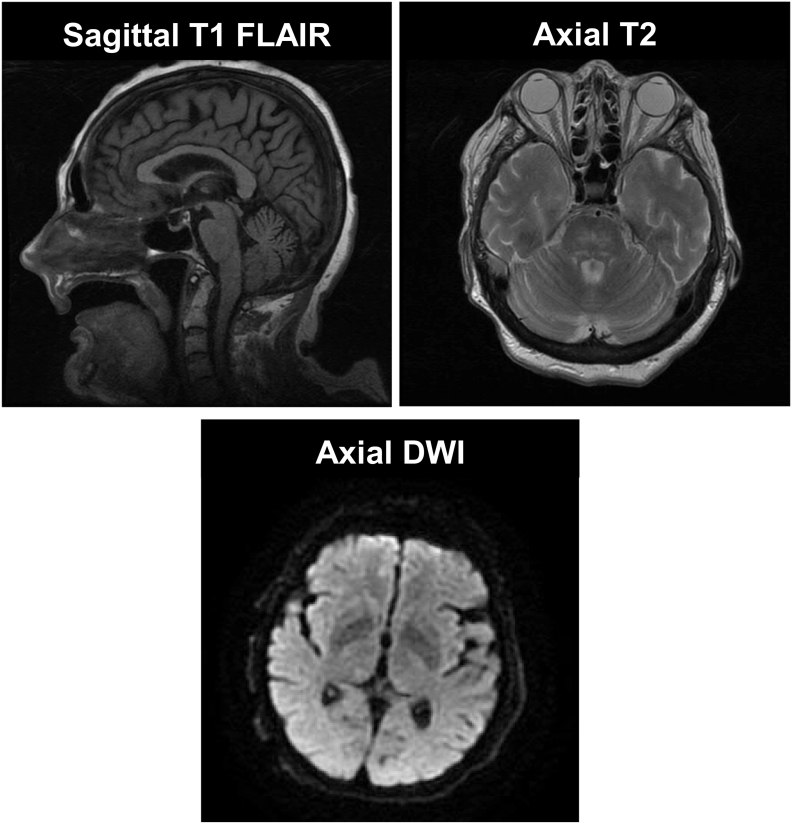
Her EEG pattern improved significantly upon a 2-gram loading dose of intravenous levetiracetam. She continued to receive maintenance levetiracetam doses and her mental status continued to improve (Fig. 2). Brain magnetic resonance imaging (MRI) was unremarkable (Fig. 3). Hemin was administered in the treatment of her acute porphyria. Ultimately, she was discharged home following return to her baseline.
Fig. 2.
Trend EEG shows dramatic improvement after levetiracetam administration.
Fig. 3.
Representative images of patient's brain MRI. There was no evidence of hemorrhage, infarct, or other acute intracranial abnormalities.
Two months later, she followed up in epilepsy clinic with plans to repeat her EEG and continue levetiracetam in the interim. Her mental status and neurological exam were within normal limits.
4. Discussion
Porphyrias are a group of rare metabolic disorders caused by disruption in the heme synthesis pathway. There are eight enzymes involved in this pathway and VP is a type of porphyria caused by mutation in the PPOX gene. The estimated incidence of VP is 1 in 100,000 individuals in the general population of European decent [5].
Neurologic complications include peripheral neuropathy, acute encephalopathy, and acute symptomatic seizures. Although the data on lifetime prevalence of status epilepticus in VP patients is unknown, seven cases of status epilepticus associated with porphyria disorders have been reported [4]. Our patient presented with non-specific symptoms of porphyria crisis upon admission, but later had a distinct deterioration of her mental status, raising the clinical concern for non-convulsive status epilepticus.
Her EEG revealed generalized triphasic and independent multifocal sharp waves which improved dramatically after levetiracetam treatment, suggesting NCSE as the culprit. She underwent initial and repeat serological and neurodiagnostic studies to rule out seizures and further evaluate her acute encephalopathy. Fortunately, her clinical status continued to improve with maintenance levetiracetam as well as administration of hemin in the treatment of her acute porphyria crisis.
Identification and treatment of seizures in patients with porphyrias can be challenging. Treatment of patients with porphyrias can be difficult due to the porphyrogenicity of many anti-seizure medications (Table 1). First line treatment options for seizures and status epilepticus include porphyria precipitating anti-seizure drugs such as phenytoin, valproate, benzodiazepines, and lacosamide [1], [6], [7]. These anti-seizure drugs are metabolized by heme containing cytochrome P450 enzymes in the liver, thereby leading to increased liver heme biosynthesis and deposition of porphyrin complexes [5], [8]. Therefore, if an inappropriate anti-seizure medication is selected, a patient's acute porphyria crisis can be exacerbated. This is an important clinical distinction in treating patients with VP who develop epilepsy and present with drug-resistant seizures while in hospital.
Table 1.
Assessment of antiepileptic drug porphyrogenicity.
| Non-porphyrinogenic antiepileptic drugs | Porphyrinogenic antiepileptic drugs |
|---|---|
| Clobazam | Carbamazepine |
| Clonazepam | Ethosuximide |
| Gabapentin | Felbamate |
| Lacosamide | Oxcarbazepine |
| Levetiracetam | Phenobarbital |
| Phenytoin | |
| Lamotrigine | |
| Clonazepam | |
| Primidone | |
| Tiagabine | |
| Topiramate | |
| Valproate |
Note: Rufinamide, eslicarbazepine, and perampanel are of uncertain porphyrogenicity. These agents are not yet classified and possibly porphyrinogenic [1].
Levetiracetam was effective in the treatment of this patient's NCSE. It has high oral bioavailability and reaches peak plasma concentration in approximately 1 h. More importantly, levetiracetam does not induce hepatic cytochrome P450 enzymes and, therefore, does not increase heme synthesis in VP patients. Approximately 66% of the drug is excreted unchanged in the urine and the remaining metabolites are excreted independent of hepatic enzymes [9].
There have been various mechanisms described; however, the underlying pathophysiology of seizures in patients with porphyria has not been fully elucidated. In one study, Tracy and colleagues report that patients with porphyria can also present with syndrome of inappropriate antidiuretic hormone (SIADH) which can be a possible contributing factor in seizures [11]. Solinas and colleagues report the trigger of these seizures is probably related to metabolic imbalance and to the intrinsic epileptogenic role of some porphyrins [10]. Additional studies have reported that neural damage can follow a porphyric attack which may predispose to brain lesions that may be epileptogenic [10], [11]. Furthermore, porphyria attacks are also exacerbated by environmental factors such as alcohol, drugs, hormonal, and dietary changes. These factors amplify the deficiency of PPOX enzyme which leads to deposition of porphyrin complexes [5]. We speculate that there could also be a role for protoporphyrinogen IX to have an epileptogenic role given its dysfunction in the role of VP.
5. Conclusions
This case highlights the importance of consideration of nonconvulsive status epilepticus in patients with known variegate porphyria and acute encephalopathy. Additionally, 10%–20% of patients with acute intermittent porphyria in relapse have been noted to manifest acute symptomatic seizures which warrant added caution while treating patients with anti-seizure medication in porphyria crisis [1]. Recognition of seizures is critical so that appropriate anti-seizure medications are selected so as to avoid exacerbation of porphyria. Levetiracetam could be a preferred choice of treatment for NCSE in patients with variegate porphyria. Hemin treatment should be started urgently to treat porphyria crisis and help reduce porphyrin complex deposition.
Acknowledgments
We thank Barbara J. Weisser for her assistance with manuscript formatting, and EEG technicians for preparation of the electroencephalogram image.
Footnotes
Conflict of Interest: None of the authors have any conflict of interest to declare.
References
- 1.Balestrini S., Hart Y., Thunell S., Sisodiya S.M. Safe use of perampanel in a carrier of variegate porphyria. Pract Neurol. 2016;16(3):217–219. doi: 10.1136/practneurol-2015-001305. [DOI] [PubMed] [Google Scholar]
- 2.Bissell D.M., Wang B. Acute hepatic porphyria. J Clin Transl Hepatol. 2015;3(1):17–26. doi: 10.14218/JCTH.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bylesjo I., Forsgren L., Lithner F., Boman K. Epidemiology and clinical characteristics of seizures in patients with acute intermittent porphyria. Epilepsia. 1996;37(3):230–235. doi: 10.1111/j.1528-1157.1996.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 4.Tran T.P., Leduc K., Savard M., Dupre N., Rivest D., Nguyen D.K. Acute porphyria presenting as epilepsia partialis continua. Case Rep Neurol. 2013;5(2):116–124. doi: 10.1159/000353279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Porphyria Foundation Variegate porphyria. 2017. http://wwwporphyriafoundationcom/about-porphyria/types-of-porphyria/VP
- 6.Bonkowsky H.L., Sinclair P.R., Emery S., Sinclair J.F. Seizure management in acute hepatic porphyria: risks of valproate and clonazepam. Neurology. 1980;30(6):588–592. doi: 10.1212/wnl.30.6.588. [DOI] [PubMed] [Google Scholar]
- 7.Gaspard N., Jirsch J., Hirsch L.J. 2018. Nonconvulsive status epilepticus. [UpToDate] [Google Scholar]
- 8.Paul F., Meencke H.J. Levetiracetam in focal epilepsy and hepatic porphyria: a case report. Epilepsia. 2004;45(5):559–560. doi: 10.1111/j.0013-9580.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Patsalos P.N. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther. 2000;85(2):77–85. doi: 10.1016/s0163-7258(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 10.Solinas C., Vajda F.J. Epilepsy and porphyria: new perspectives. J Clin Neurosci. 2004;11(4):356–361. doi: 10.1016/j.jocn.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Tracy J.A., Dyck P.J. Porphyria and its neurologic manifestations. Handb Clin Neurol. 2014;120:839–849. doi: 10.1016/B978-0-7020-4087-0.00056-5. [DOI] [PubMed] [Google Scholar]