Abstract
Metabolic syndrome (MetS) is a constellation of cardiometabolic risk factors, which together predict increased risk of more serious chronic diseases. We propose that one consequence of dietary overnutrition is increased abundance of Gram-negative bacteria in the gut that cause increased inflammation, impaired gut function, and endotoxemia that further dysregulate the already compromised antioxidant vitamin status in MetS. This discussion is timely because “healthy” individuals are no longer the societal norm and specialized dietary requirements are needed for the growing prevalence of MetS. Further, these lines of evidence provide the foundational basis for investigation that poor vitamin C status promotes endotoxemia, leading to metabolic dysfunction that impairs vitamin E trafficking through a mechanism involving the gut-liver axis. This report will establish a critical need for translational research aimed at validating therapeutic approaches to manage endotoxemia—an early, but inflammation-inducing phenomenon, which not only occurs in MetS, but is also prognostic of more advanced metabolic disorders including type 2 diabetes mellitus, as well as the increasing severity of nonalcoholic fatty liver diseases.
Highlights
-
•
Excess dietary energy provokes gut dysbiosis leading to increased inflammation.
-
•
Impairments in gut function contribute to metabolic endotoxemia.
-
•
Endotoxemia depletes vitamin C, which in turn impairs vitamin E trafficking.
-
•
Higher vitamin C intake can restore gut-liver functions and antioxidant status.
Graphical abstract
1. Introduction
Metabolic syndrome (MetS) is at epidemic proportions in the US [1]. The disorder is associated with the obesity epidemic; it is a serious public health problem that leads to the increased prevalence and severity of chronic diseases (e.g. type 2 diabetes, fatty liver disease, heart disease, and stroke) and premature mortality [2], [3]. Consistent with a substantial proportion of Americans having circulating ascorbic acid concentrations indicative of suboptimal or overt deficiency [4], low circulating ascorbic acid concentrations are commonly associated with MetS [5] and the progression to type 2 diabetes [6], [7], [8], [9], [10], [11]. Inadequate vitamin C intakes clearly impair vitamin C status [4]; however, ascorbic acid is also depleted systemically by its reaction with hypochlorous acid (bleach), an anti-microbial agent produced by neutrophils [12]. Although no studies have explicitly tested the hypothesis, the objective of this review is to present evidence supporting that poor vitamin C status in MetS is driven by gut inflammation and barrier dysfunction attributed to excess dietary energy consumption [13]. Importantly, impaired gut barrier function in MetS mediates metabolic endotoxemia by increasing the absorption of bacteria and gut-derived endotoxins (e.g. lipopolysaccharide (LPS)) [13], [14] while also impairing vitamin C absorption [15]. These inter-related events provoke a vicious cycle, both increasing chronic inflammation and oxidative damage. Thus, the following discussion is an overview of an innovative area of investigation to establish the importance of adequate vitamin C status along the gut-liver axis. Improved vitamin C status is hypothesized to alleviate endotoxemia and its consequent pro-inflammatory responses that are suggested to initiate insulin resistance and related metabolic disorders [16], [17]. Further, because LPS triggers intestinal and hepatic inflammation, we propose that improved vitamin C status along the gut-liver axis would restore vitamin E trafficking and bioavailability that is otherwise impaired in MetS persons, likely due to their heightened inflammation in association with poor vitamin C status [18], [19]. Ultimately, research translation of these putative benefits of vitamin C would help establish novel dietary strategies to reduce the growing public health burden of MetS while also providing investigators new tools to evaluate gut-liver functions.
2. Metabolic syndrome: definititon and prevalence
MetS is a cluster of conditions that includes at least 3 of 5 risk factors: hypertension, hyperglycemia, central obesity, hypertriglyceridemia, or low high-density lipoprotein cholesterol (HDL) (Table 1) [20]. Approximately 35% of the American adult population is afflicted with MetS [1]. However, its prevalence occurs in an age-dependent manner such that ≈ 18% of young adults (20–39 y), ≈ 34% of middle-aged adults (40–59 y), and ≈ 47% of older adults > 60 y are afflicted. MetS is a growing epidemic, and its public health impact is emphasized by data indicating that it increases premature mortality by increasing the risk for various disorders characterized by inflammation and oxidative distress (e.g. fatty liver disease, type II diabetes, cardiovascular disease) [2], [3]. Further, many persons with MetS have symptoms that are subclinical, and therefore are left unmanaged from a pharmacological standpoint. Fortunately, MetS development and progression can be averted by improved diet [21], especially increased intake of whole grains, fruits and vegetables, nuts and seeds and decreased intake of refined sugars, white flour and saturated fats [22]. However, these public health recommendations have had little impact [23].
Table 1.
Clinical Criteria of MetS. Individuals classified with MetS must meet a minimum of 3 of the following 5 established clinical criteria [20].
| Risk factor | Cut-off point |
|---|---|
| Waist circumference2 | ≥ 102 cm in men or ≥ 88 cm in women |
| Fasting blood triglycerides | ≥ 150 mg/dL, or the use of pharmacological therapies for hypertriglyceridemia |
| Fasting blood HDL-C | < 40 mg/dL in men or < 50 mg/dL in women, orthe use of pharmacological therapies for reduced HDL-C |
| Blood pressure | Systolic ≥ 130 mmHg and/or diastolic ≥ 85 mmHg, orthe use of medications to manage hypertension |
| Fasting blood glucose | ≥ 100 mg/dL, or pharmacological treatment to decrease hyperglycemia |
3. Implications of inadequate vitamin C in MetS
Data from NHANES indicate that obese adults have 5–12% lower micronutrient intakes along with a higher prevalence of nutrient inadequacy compared with normal weight adults [24]. Vitamin C intakes are poor among the obese with 8% of women and 13% of men having circulating concentrations indicative of vitamin C deficiency [4]. Despite the general recognition that nutritional status in MetS is compromised, nutrition and medical professionals do not advocate for the use of antioxidant supplements because there is a lack of scientific rationale or demonstrated health benefit in humans from the use of these supplements [25]. Thus, a significant need exists to investigate the interactions of antioxidant nutrients in persons who have MetS or are obese, and their impact on intestinal function.
Potentially, inadequate vitamin C status in MetS contributes to small intestinal bacterial overgrowth, transcytosis of enteric bacteria, and an elevation of circulating LPS, which elicits a low-grade inflammatory response. By contrast, oral ingestion of large-dose vitamin C supplements results in limited intestinal vitamin C absorption [26], with excess ascorbic acid remaining in the gut lumen where it can potentially exert beneficial effects both on the intestinal cells and microbiota composition and function.
The gut microbiota composition is an important disease factor [27]. Beneficial changes in gut bacteria composition of persons with MetS who consumed a Mediterranean diet for two years were observed [28]. At baseline, the abundance in MetS of Bacteroides, Eubacterium and Lactobacillus genera was higher than in non-MetS controls patients, whereas the Bacteroides fragilis group, Parabacteroides distasonis, Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii, Fusobacterium nucleatum, Bifidobacterium longum, Bifidobacterium adolescentis, Ruminococcus flavefaciens subgroup and Eubacterium rectale were depleted in MetS (all p-values < 0.05). Following long-term consumption of Mediterranean diet, the populations of P. distasonis, B. thetaiotaomicron, F. prausnitzii, B. adolescentis and B. longum were restored in MetS as compared with the microbiota composition in MetS persons assigned to a low-fat diet (all p-values < 0.05). Moreover, decreased LPS and cardiac event-free survival were associated with long-term consumption of a Mediterranean diet, especially in those who ate fruits and legumes [29], important sources of vitamins C and E, respectively. Thus, diet composition has major effects not only on chronic disease risk, but also on microbiota composition and on endotoxemia.
4. Why would improved vitamin C status decrease endotoxemia in MetS?
4.1. Metabolic endotoxemia
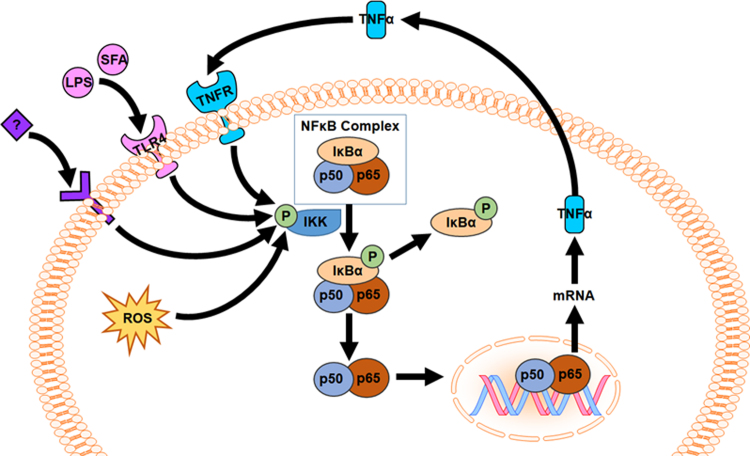
Intestinal bacterial overgrowth is a complication of increased visceral fat that potentiates endotoxemia in MetS [30]. Not only is circulating LPS elevated in MetS, but MetS-associated complications are further increased by inflammation [16]. NF-κB activation is promoted intracellularly by reactive oxygen species (ROS) but also by extracellular receptor-mediated signaling (i.e. LPS activates via Toll-like receptor-4 (TLR4); Fig. 1). Thus, inflammation in MetS is both a cause and consequence of endotoxin-TLR4 signaling, due to its initiating effects on inflammation but also to its downstream potentiation of TNFα-TNFR and ROS generation that exacerbate NFκB activation (Fig. 1).
Fig. 1.
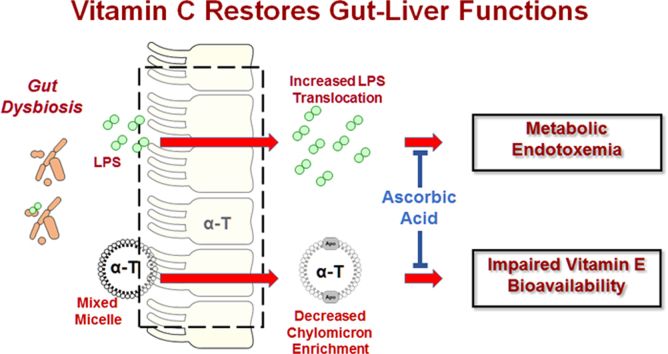
LPS-TLR4-NFκB signaling pathway is an established potentiator of TNFα-dependent inflammation in metabolic disorders. NFκB (e.g. p50-p65) is activated by reactive oxygen species (ROS) and by signaling from Toll-like receptor-4 (TLR4), tumor necrosis factor-α (TNF-α) receptor (TNFR), and other receptors (labeled with “?”). Vitamin C is hypothesized to improve gut barrier function to reduce the absorption of endotoxin (LPS), and its consequent signaling via hepatic TLR4. Thus, decreasing LPS will interrupt the cycle of NFκB activation at the intestine and the liver otherwise driven by inflammatory mediators, e.g. TNF-α.
Normally, LPS is absorbed from the intestine bound to the LPS-binding protein attached to chylomicrons [31]. This chylomicron-dependent mechanism protects against TLR4-induced inflammation by directing LPS to the lymphatics instead of the portal stream, sequestering LPS away from circulating white cells by instead delivering it to the liver for detoxification [32]. However, increased LPS absorption is promoted by a high-fat diet that not only increases chylomicron secretion, but also increases gut barrier dysfunction, thereby allowing LPS passage and increasing circulating LPS, which potentiates endotoxemia-associated inflammation [13], [33], [34], [35].
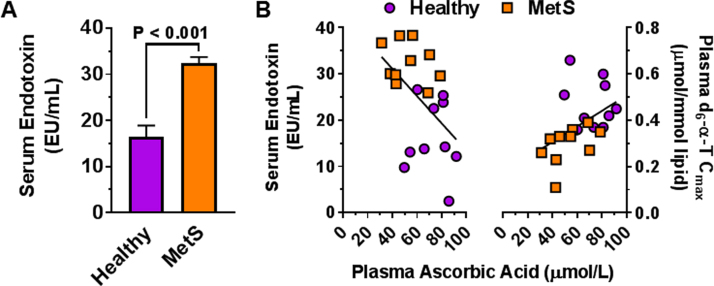
Our assessment of a limited population of relatively healthy adults with MetS shows that, in association with lower vitamin C status, persons with MetS not only have metabolic endotoxemia (unpublished observation; Fig. 2) and increased biomarkers of inflammation but also lower α-tocopherol (α-TOH) bioavailability [18], [19]. These findings suggest a complex interaction between the antioxidant vitamins and endotoxin-induced inflammation, as well as with other dietary antioxidants [36]. Although casuality has yet to be established, hypothetically poor vitamin C status drives metabolic endotoxemia, or conversely heightened inflammation due to endotoxemia depletes systemic vitamin C. In support of the latter possibility, dietary vitamin C intakes (110 mg/d) of MetS persons in our study cohort were above the RDA (defined as 90 mg/d in men and 75 mg/d in women [37]); yet, MetS persons had serum ascorbic acid concentrations that were lower than those of healthy control subjects, who were provided the same rigorously controlled diet.
Fig. 2.
Both endotoxemia and α-T bioavailability are associated with plasma ascorbic acid concentrations. A) Persons with MetS in our study had endotoxin concentrations that were > 2-times higher (p < 0.0001) than those of age- and gender-matched healthy adults without any sex differences (p > 0.05). B) Plasma ascorbic acid concentrations are negatively correlated (r = -0.54, p < 0.015, n = 20) with endotoxin concentrations and are positively correlated (r = 0.61, p < 0.005, n = 20) with the maximum plasma d6-α-tocopherol concentrations (d6-α-T Cmax) during a vitamin E pharmacokinetics trial [18].
Endotoxemia may also further impair the already low ascorbate status in MetS because LPS inhibits absorption of ascorbate [15] by decreasing sodium-dependent vitamin C transporters (SVCT-1 and −2) [15], [38], [39], [40]. Additionally, oxidized ascorbate (i.e. dehydroascorbic acid; DHA) can be taken up by enterocytes via glucose transporters [41], [42]. However, when glucose intakes are high, this mechanism has little benefit on vitamin C status because concentrations of DHA are much lower than that of glucose [43], [44]. Thus, poor vitamin C status in MetS may not only be the result of low dietary vitamin C intakes [4], which are exacerbated by impaired absorption of either ascorbate or DHA.
In support of the concept that antioxidants help to maintain gut barrier function, these nutrients have been reported to be decreased in the gut mucosa of humans with inflammatory bowel disease [45]. Further, endotoxemia in mice initiates obesity and insulin resistance [16], while antioxidants mitigate intestinal inflammation [46]. Importantly, in humans, impaired gut barrier function occurs with scurvy (e.g. [47], [48], [49]).
Mounting evidence indicates that endotoxemia, gut permeability, and NAFLD severity are causally related [50], [51]. Various conditions can impair gut barrier function [52], [53], which then drives inflammation and endotoxemia [54], [55]. LPS is the driver of metabolic endotoxemia and has been proposed as the initiator of increased inflammation in MetS [16], [34]. Indeed, patients with hepatic cirrhosis, a consequence of NAFLD, have increased circulating endotoxin that predicted disease severity [56], [57]. Children with NAFLD also had increased circulating endotoxin [58], which also strongly predicted NAFLD severity scores [58]. A sugar probe test indicated that they experienced increased gut permeability, which correlated with disease severity (i.e. fibrosis, portal inflammation, hepatocyte ballooning) [59]. The findings in both children [58] and adults [56], [57] demonstrate the prognostic value of endotoxin assessments, and support validating approaches to attenuate metabolic endotoxemia.
4.2. Vitamin C and barrier function
Animal studies support the concept that vitamin C can reduce the sequelae of MetS. Although most animals used for basic research are capable of synthesizing vitamin C, notable exceptions are guinea pigs and a mutant strain of rats (osteogenic disorder syndrome; ODS rats). Vitamin C-deficient guinea pigs in response to endotoxemia induction have increased NF-κB responses in association with exaggerated systemic shock and impaired lung phosphatidylcholine biosynthesis [60], [61], [62], while dietary vitamin C supplementation attenuates endotoxemia and intestinal barrier defects [62]. Vitamin C-deficient ODS rats also demonstrate increased endotoxemia associated with increased liver inflammation and gut dysfunction [63], increased gastric mucosal lesions [64], acute phase responses [65], inflammatory chemokine and cytokines [66], and cytokine-induced neutrophil chemoattractant-1 (CINC-1) [66]. They also have increased LDL [67], decreased HDL [68] and impaired synthesis of apo-AI [69]. Thus, the association between vitamin C deficiency and the hallmarks of MetS (i.e. gut barrier dysfunction, increased endotoxemia, inflammation, altered lipoproteins) are supported by studies in vitamin C-deficient animals.
Vitamin C at pharmacological doses has demonstrated benefit to manage sepsis. Intravenous (IV) ascorbate, which temporarily circumvents regulatory mechanisms that limit plasma ascorbate [26], is currently being used to treat sepsis and prevent sepsis-related mortality [70], [71], [72]. Outcomes from IV ascorbate intervention studies provide critical insights into ascorbate function. For example, administration of LPS (IV, 20 IU/kg) to humans in an experimental setting decreased plasma ascorbate concentrations and significantly decreased forearm blood flow reactivity to acetylcholine, while administration of IV ascorbate restored endothelium-dependent vasodilation, showing that ascorbate blocks endotoxemia [73]. In separate studies, patients with septic shock, who were administered IV ascorbate, had significant reductions in pro-inflammatory biomarkers (i.e. C-reactive protein, procalcitonin) [71], thereby demonstrating the close interrelationship of inadequate vitamin C status, endotoxemia, and increased inflammation. In agreement, vitamin C supplementation reverses these effects in vitro [74], in translational models [62], [75], [76], [77], and in humans [71], [72].
4.3. Oxidative distress and intestinal mucosal barrier redox status
Ascorbate reacts as an antioxidant with hypochlorous acid, an anti-microbial agent that is produced by neutrophils via myeloperoxidase (MPO) [12]. Further, intestinal and circulating neutrophils accumulate high ascorbate levels that function to protect themselves from MPO, which is activated during bacterial phagocytosis [37]. Remarkably, MPO also acts as an “ascorbate peroxidase” that directly consumes ascorbate [78]. Thus, the oxidative distress that is evident in MetS consists not only of non-specific lipid peroxidation that consumes α-TOH [79] to potentially increase the risk for NAFLD [80], [81], but is also is a direct consequence of increased inflammatory responses (i.e. MPO) that deplete vitamin C. Further, we have shown that insufficient ascorbate results in the more rapid depletion of α-TOH [82], [83]. Accordingly, fecal concentrations of MPO, as well as calprotectin (an abundant neutrophil protein that is released during inflammation [84]), may be useful measures to evaluate intestinal inflammation and therapeutic benefit of supplemental vitamin C. Similarly, restored α-TOH absorption and gut-liver trafficking that are otherwise dysregulated in MetS [18] would be expected in response improved vitamin C status.
Another potential benefit of increased vitamin C intake is the improvement in barrier function caused by increasing collagen synthesis in the intestine [77]. This proposed mechanism is consistent with ascorbate's coenzyme function that hydroxylates proline and lysine to cross-link collagen [85]. For example, studies in the T84 human crypt-like epithelial cell line with indomethacin-induced barrier dysfunction show that bacteria cross the epithelium via a transcellular pathway, which is abrogated by treatment with vitamin C [74]. Thus, poor ascorbate status at the gut likely exacerbates barrier dysfunction that increases the translocation of LPS-derived Gram-negative bacteria to potentiate inflammation.
Oxygen may play a critical role in gut health. Oxygen gradients decrease steeply from the upper to lower gastrointestinal tract and also increase from the lumen to the more vascularized epithelial layer. Oxygen regulates microbial colonization with more oxygen-tolerant bacteria in the mucosa (e.g. Helicobacter) whereas obligate anaerobes (e.g. Firmicutes) are luminal [86], [87]. Oxygen gradients also contribute to microbial biogeography by influencing metabolite production and modulating redox effectors (e.g. nitric oxide, hydrogen sulfide, ROS) of either bacteria or host origin [88]. ROS generated by the intestinal epithelium [89], [90] function to exert redox-responsive cell signaling (e.g. thiol redox switches) and maintain gut barrier integrity [91], [92].
Gut dysbiosis in mouse models promotes obesity or MetS [93] and dysregulates insulin secretion [94]. We are not aware of any studies that have directly examined vitamin C alone on microbiota composition or redox function, but when mice were fed an antioxidant supplement they demonstrated decreased gut inflammation [46]. Importantly, in early-weaned piglets with gut dysbiosis and decreased intestinal ROS-detoxifying capacity, a vitamin C-rich antioxidant cocktail improved intestinal redox status in association with increased proportions of commensal bacteria and decreased pathogenic bacteria [95]. A vitamin C-rich functional food also alleviated metabolic endotoxemia and liver steatosis in a rat NAFLD model in association with improved microbiota composition (i.e. greater α-diversity, increased Firmicutes/Bacteroidetes ratio) [96]. Further, studies in vitro with an antioxidant cocktail containing vitamin C facilitated the successful culturing of anaerobic protozoa in association with decreasing the oxidation-reduction potential (i.e. more reduced environment) and increasing acetate, a short chain fatty acid that is an important energy source for these microorganisms [97]. We therefore posit that greater vitamin C intakes can directly attenuate metabolic endotoxemia in MetS by improving gut barrier function, as well as improving microbial diversity and function.
Increased levels of circulating LPS have been observed following strenuous aerobic exercise [98]. After exercise, the plasma ascorbate free radical (Asc•-) increased, which suggests an increase in oxidative distress. However, in this study, oral vitamin C supplementation (1000 mg) decreased LPS concentrations and increased plasma ascorbate from 29 to 121 μM. As might be expected, supplementation also increased the plasma Asc•- level both before and after exercise. [98]
5. Vitamin C as a pro-oxidant: cause for concern?
The Asc•- is generated in vivo by oxidation of ascorbate [99]. The steady-state plasma Asc•- levels are affected by many parameters, including the pH, concentrations of ascorbate and catalytic metals, as well as the flux of oxidants. With appropriate controls, the steady-state level of Asc•- can be used as an indicator of oxidant flux [100]. A healthy level of plasma ascorbate is considered to be in the range of ≈ 40–80 µM. At these levels of ascorbate, the Asc•- concentration in whole blood is typically below the limit of detection using standard electron paramagnetic resonance spectroscopy (EPR) approaches. However, IV ascorbate administration can increase plasma ascorbate concentrations several hundred-fold because it bypasses tight gut-level regulation that otherwise restricts excess absorption of oral vitamin C [26]. For example, following repeated daily IV ascorbate infusions (7,500 mg), peak plasma ascorbate reached ≈2 mM and Asc•- reached a maximum of 200 nM [101]. Despite these high Asc•- concentrations, biomarkers of pro-oxidant injury were not increased [101]. Furthermore, in a phase 1 clinical trial, large doses of IV ascorbate were given, which elevated plasma ascorbate levels to 20–25 mM. Prior to IV ascorbate infusion, Asc•- in whole blood samples was below the limit of detection; however, Asc•- was readily observed immediately after IV ascorbate infusion. Despite this Asc•- increase, the redox state of the GSSG/2GSH couple, an indicator of oxidative distress, was unchanged in erythrocytes and a marker of lipid peroxidation, F2-isoprostanes, actually decreased [102]. These data indicate that high levels of ascorbate and Asc•- do not provoke oxidative distress. Further, a recent systematic review found IV ascorbate, despite achieving very high blood ascorbate concentrations, to be safe [103].
How can the Asc•- increase without inducing adverse effects? Some of the reactions involving vitamin C and its oxidized forms that can occur in vivo are shown in Table 2 [104]. However, unlike many radicals that provoke oxidant damage, Asc•- has limited reactivity. For example, Asc•- does not covalently react with O2 to generate a more oxidizing peroxyl radical, nor does it readily transfer an electron to dioxygen to form superoxide; in fact it reacts readily with superoxide [105]. By the same token, the two-electron oxidation product of ascorbate, DHA also reacts with superoxide [106]. This is because Asc•- is neither a strong oxidant nor a strong reductant. Indeed, one-electron reduction potentials show that Asc•- is both a weak oxidant (Asc•-, H+/AscH-; +282 mV), as well as a weak reductant, (DHA/Asc•-, −174 mV) [99]. The observations above, in combination with the thermodynamics of the DHA/Asc•- /AscH- triad, support that Asc•- is non-toxic from a free radical biology standpoint; but physiologically, it may actually be revealing favorable health benefits. This concept is consistent with observations that administration of IV ascorbate administration sufficient to increase Asc•- has chemoprotective activity [107]. Thus, the increased Asc•- presence indicates that ascorbate is performing its antioxidant and biochemical functions well.
Table 2.
Reactions of ascorbate. a.
| Reactants | Products | |
|---|---|---|
| Asc2-+O2 | → | Asc•- + O2•-b |
| AscH-+ Fe3+ | → | Asc•- + Fe2+ |
| Asc•-+ Fe3+ | → | DHA + Fe2+ |
| 2Asc•-+ H+ | → | AscH- + DHAc |
| AscH-+ DHA | → | 2Asc•- + H+d |
| AscH-+ 2Fe3+ | → | DHA + 2Fe2+ |
Asc2- = ascorbate dianion, AscH- = ascorbate, Asc•- = ascorbate radical, DHA= dehydroascorbic acid, Fe3+ = ferric ion, Fe2+ = ferrous ion, O2 = oxygen, O2•- = superoxide.
This reaction is slow; the reaction of AscH- with O2 is negligible.
Dismutation of Asc•-.
Comproportionation, the reverse of the above reaction.
Asc•- is uniquely different from the vast majority of free radicals. Because it is so unreactive, it can easily be detected by EPR in plasma or serum, provided both ascorbate and O2 are present. Although its formation along with the generation of hydrogen peroxide is catalyzed by redox active metals (e.g. iron) [108], this reaction is limited because of the low levels of redox active iron [109], [110]. Further, normal cells have a large capacity to remove H2O2 [111]. However, in disease, the level of catalytic iron in cells and tissues can increase. A clear example is in iron-overload disorders, ranging from issues arising from blood transfusion to hemochromatosis [112], [113]. Thus, while there may be concern regarding vitamin C-iron interactions leadings to excess H2O2 accumulation in specialized populations, its broad use, including IV ascorbate, has been shown to be quite safe [114].
6. Vitamin C: the critical link to vitamin E adequacy by improving gut-liver function?
The interactions of antioxidants have been recognized by free radical chemists for decades [99], [115]. We previously addressed this question by investigating the turnover kinetics of α-TOH in cigarette smokers; smokers had increased oxidative distress. We found that the disappearance of α-TOH was inversely related to plasma ascorbate levels [83], but could be normalized by vitamin C supplementation [82]. We have now focused on the possibility that α-TOH bioavailability is highly dependent upon adequacy of vitamin C, not only to recycle and maintain α-TOH [99], but also to enhance physiological functions along the gut-liver axis that facilitate trafficking of α-TOH to achieve vitamin E adequacy.
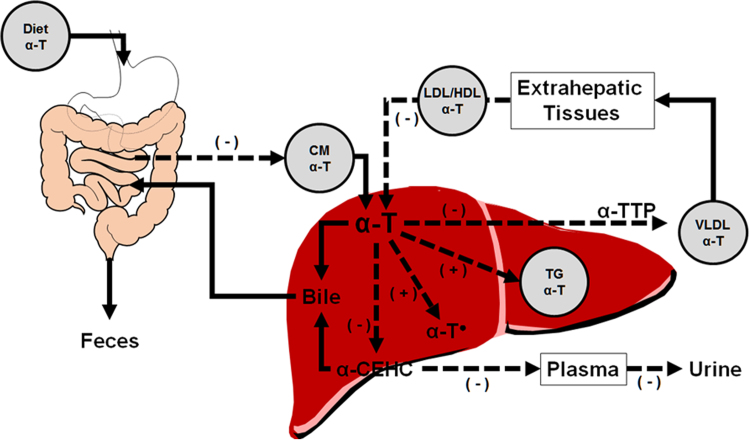
Bioavailability [116] is defined as it relates to α-TOH as the extent and rate of α-TOH incorporation into the circulation, which is dependent upon α-TOH absorption, lipoprotein incorporation, trafficking, and lipoprotein-mediated tissue uptake, as well as hepatic catabolism (Fig. 3) [117]. Increased inflammation and oxidative damage in association with low vitamin C status in persons with MetS potentiated already poor α-TOH status by limiting α-TOH trafficking along the gut-liver axis [18]. These findings likely help to explain why MetS persons are at high-risk for liver dysfunction and liver injury that occurs in NAFLD [118]. Further, it provides a strong premise for the favorable outcomes of clinical interventions involving antioxidants for improved liver health. For example, vitamin E supplementation in persons with nonalcoholic steatohepatitis (NASH) resolved histological evidence of its presence in both adults and children [119], [120], while also improving liver function in adults [119]. Further, low vitamin C status has been postulated to promote the progression of simple steatosis to NASH [81], and an inverse association between vitamin C intake and NAFLD has been observed in a cross sectional study [80]. These findings suggest that antioxidants are a critical factor for normal function of the gut-liver axis.
Fig. 3.
Pathways of α-T trafficking, disposition and catabolism. The “ - " and “+" symbols indicate pathways that are likely altered by MetS. Dietary α-T, in the presence of biliary and pancreatic secretions forms micelles, of which some is absorbed and the remainder excreted in the feces. Enterocytes secrete α-T-containing chylomicrons (CM) into the lymphatics, and following one pass through the circulation, are taken up by the liver. Hepatic α-T has several possible fates: (i) α-TTP can facilitate its transfer to lipoproteins (e.g. VLDL, LDL, HDL) that transport α-T to and from the periphery; (ii) α-T can be excreted in bile; (iii) α-T can be catabolized to α-CEHC, which can be excreted in bile for fecal excretion, or secreted into plasma, transported to the kidney and excreted in urine [117]; (iv) α-T can be oxidized by a peroxyl radical, but this is likely rapidly reduced to α-T by other antioxidants, such as ascorbate [137], or (v) α-T may remain in the liver in lipid droplets. Abbreviations: α-T•, α-tocopheroxyl radical; α-TTP, α-tocopherol transfer protein; CM, chylomicron; VLDL, very low-density lipoprotein; LDL/HDL, low/high-density lipoproteins; TG, triacylglycerides.
6.1. Oxidative distress depletes α-TOH
Increased oxidative distress results in faster disappearance rates of plasma α-TOH in cigarette smokers [83], [121]. Consistent with correlative evidence that low vitamin C status increases the rate of disappearance of α-TOH [83], findings from a randomized placebo-controlled cross-over study demonstrated that vitamin C supplementation (500 mg twice daily; 2-wk) restored the rates of disappearance of α-TOH to levels no different from nonsmoking persons [82]. This observation is consistent with a mechanism by which ascorbate functions as an electron-donor to reduce the α-tocopherol radical (α-TO•) back to α-TOH, the functional form as a donor antioxidant, i.e. it recycles vitamin E [99].
α-TO• + AscH- → α-TOH + Asc•-
Similar to smokers, inadequate vitamin C status in MetS persons could, in part, impair α-TOH status by increasing oxidative α-TOH depletion because biomarkers of oxidative distress and inflammation are higher in MetS persons compared with healthy individuals [18], [19], [122] (Fig. 3). Further, participants with MetS had lower plasma ascorbic acid concentrations despite eating diets with similar amounts of vitamin C as those consumed by healthy subjects for 3 days prior to completing α-TOH pharmacokinetic studies [18]. In those pharmacokinetic studies, α-TOH bioavailability was significantly reduced among MetS persons. Increased oxidative distress and inflammatory biomarkers were also inversely correlated with lower α-TOH enrichment in chylomicrons and VLDL. This outcome suggests that poor α-TOH status in MetS is also due to physiological impairments along the gut-liver axis that prevent adequate α-TOH packaging and delivery to target tissues. Thus, decreased vitamin C status in MetS likely contributes to the further depletion of α-TOH.
6.2. α-Carboxyethyl-hydroxychromanol (α-CEHC): a better measure of α-TOH status
The paradigm in MetS of decreased α-TOH status is best described by the concept of “physiological inadequacy” and is driven in MetS by impaired trafficking of α-TOH along the gut-liver axis [18]. The challenge to interpret the lower α-TOH bioavailability and slower plasma α-TOH turnover observed in MetS [18] is that α-TOH status is difficult to assess in hyperlipidemic individuals. Because circulating lipoproteins transport α-TOH with other lipids (e.g. triglyceride, cholesterol), elevated blood lipids “trap” α-TOH in the circulation. This process yields apparently “normal” plasma α-TOH concentrations that mask the fact that α-TOH concentrations in target tissues are inadequate, thereby permitting oxidative injury [19], [123]. We propose that a urinary vitamin E catabolite, α-CEHC, can be used in conjunction with circulating α-TOH as a more reliable biomarker of α-TOH status, especially among hyperlipidemic persons where even lipid-normalized measures of α-TOH status have limited interpretation [123].
Vitamin E catabolism is not a result of its antioxidant function, but rather from a xenobiotic process that maintains vitamin E homeostasis by preferentially catabolizing non-α-TOH forms of vitamin E [124]. While α-TOH is preferentially secreted into plasma as a function of the hepatic α-TOH transfer protein (α-TTP) [125], cytochrome P450 4F2 initiates xenobiotic metabolism by ω-hydroxylation of the sidechain of various vitamin E forms [124], [126].
During xenobiotic catabolism, the α-TOH-13’-COOH then undergoes several rounds of β-oxidation in the liver [127], which ultimately results in the formation of CEHC with carboxymethylbutyl-hydroxychromanol (CMBHC) as its immediate precursor (Fig. 4) [126]. It has been hypothesized that α-CEHC is synthesized endogenously when hepatic α-TOH concentrations exceed the capacity of α-TTP to facilitate α-TOH secretion from the liver into the circulation [128]. The first carboxy catabolite, α-TOH-13’-COOH, has been proposed as a potent anti-inflammatory agent, which can inhibit 5-lipoxygenase a key enzyme in the biosynthesis of leukotrienes from arachidonic acid [129]. Thus, it is important to maintain sufficiently high vitamin E intakes so that excess α-TOH could be used for this anti-inflammatory function.
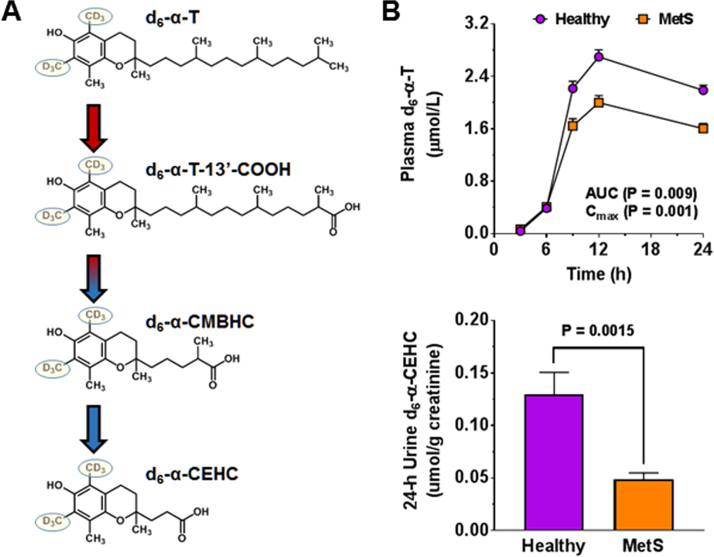
Fig. 4.
Structures of d6-α-T and α-T catabolites (left panel) and markers of vitamin E bioavailability (right panel). Structures of α-T and its metabolites following ω-hydroxylation by cytochrome P450 4F2 [124], conversion to the carboxyl form and β-oxidation to α-CEHC. Also shown are the deuterium labels (D) in the methyl groups that are carried through the catabolic process (A). The plasma d6-α-T concentrations in healthy adults and those with MetS (n = 10/group, upper panel, B) and urinary d6-α-CEHC excretion (lower panel, B) from participants in a pharmacokinetic trial using d6-α-T [18], [19]. Abbreviations: α-CEHC; α-carboxyethyl hydroxychromanol; α-CMBHC, α-carboxymethylbutyl hydroxychroman; d6-α-T, d6-α-tocopherol; d6-α-T-13’COOH, 13’ carboxy-d6-α-T.
Previously, we have reported that decreased α-TOH bioavailability in MetS persons with poor vitamin C status is most likely attributed to increased inflammation that impairs gut-liver functions that promote α-TOH enrichment in intestinal-derived chylomicrons and facilitate its secretion in VLDL from the liver [18]. We also found that this reduced bioavailability of α-TOH, and hence low α-TOH status in MetS, was reflected by less urinary α-CEHC excretion [19]. Thus, measures of α-CEHC in conjunction with α-TOH pharmacokinetic responses can be used to evaluate the extent to which vitamin C supplementation has repaired gut and liver function. This approach provides a sensitive measure of “gut to liver” trafficking of α-TOH and its hepatic catabolism to α-CEHC (Fig. 4). Additionally, lower urinary excretion levels of deuterium-labeled-α-CEHC in MetS were significantly correlated (p < 0.05) with higher plasma concentrations of C-reactive protein, IL-10, IL-6, insulin and lower plasma concentrations of HDL-C, as well as with higher diastolic blood pressure, waist circumference, and BMI. These findings suggest that our observations concerning vitamin E catabolism are related to the heightened inflammation and impaired cardiometabolic health in participants with MetS. Further, insufficient vitamin C status in MetS likely impairs gut-liver functions to drive increased α-TOH requirements. Establishing these interactions of vitamins C and E is important because > 92% of American adults fail to meet dietary recommendations for vitamin E [130]; furthermore, a large proportion of individuals have suboptimal circulating α-TOH concentrations [131].
7. Summary and future research direction
Poor vitamin C status is associated with MetS [5], while better health outcomes concerning metabolic health are associated with higher ascorbate status [132]. Poor ascorbate status in MetS likely is driven by gut inflammation and barrier dysfunction caused by excess fat consumption [13]. This dysfunction increases LPS absorption and causes endotoxemia [13], [14], as well as decreased absorption of ascorbate [15], promoting a cycle of increasing inflammation and oxidative damage that also further decreases both ascorbate and α-TOH status. Inflammation likely also drives systemic depletion of ascorbate, as shown in sepsis patients [133], who despite receiving 50 or 200 mg still become deficient in vitamin C due to heightened immune responses in septic shock that increase the rate of oxidation vitamin C, resulting in its depletion [134]. Taken together, the available data support the premise that endotoxemia promotes poor vitamin C status and exacerbates inflammation along the gut-liver axis that increases the demand for antioxidant defenses, especially vitamins C and E.
Although significant evidence from preclinical studies support the concept of vitamin C-dependent improvements in gut-liver health, the field is currently hampered in its advancement by a lack of clinical studies to evaluate these putative benefits on human health. Not only could translational studies support specialized dietary recommendations for persons with MetS, they may also result in favorable public health outcomes by alleviating the growing prevalence of this metabolic condition. Initial steps towards evidence-based recommendations will require direct hypothesis testing through controlled dietary interventions in MetS persons. Such studies would entail examining dietary vitamin C depletion/repletion on changes in gut barrier dysfunction, metabolic endotoxemia, and trafficking of vitamin E. Such studies are feasible based on the technologies available to assess site-specific gut barrier integrity in humans using sugar probes [135], metagenomics techniques to evaluate microbiota composition and function [136] and isotopically-labeled vitamin E to evaluate gut-liver lipoprotein secretion and trafficking [18]. Outcomes of such studies would have important public health impacts. Specifically, they would promote an understanding of the consequences of poor dietary quality relative to inadequate vitamin C status, which promotes endotoxemia and resulting increased risk of metabolic disease due to inadequate antioxidant vitamin protection.
Acknowledgements
Support provided by DSM Nutrition, the Center for Applied Plant Sciences and Ohio Agricultural Research and Development Center at The Ohio State University, the National Dairy Council, the USDA National Institute of Food and Agriculture, the National Institutes of Health NIDDK (DK081761), NCI (CA169046) and NCATS (UL1TR001070). The sponsors had no influence on the content or interpretation regarding the conclusions of this report.
References
- 1.Aguilar M., Bhuket T., Torres S., Liu B., Wong R.J. Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313:1973–1974. doi: 10.1001/jama.2015.4260. [DOI] [PubMed] [Google Scholar]
- 2.Cornier M.A., Dabelea D., Hernandez T.L., Lindstrom R.C., Steig A.J., Stob N.R., Van Pelt R.E., Wang H., Eckel R.H. The metabolic syndrome. Endocr. Rev. 2008;29:777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzmarzyk P.T., Church T.S., Janssen I., Ross R., Blair S.N. Metabolic syndrome, obesity, and mortality: impact of cardiorespiratory fitness. Diabetes Care. 2005;28:391–397. doi: 10.2337/diacare.28.2.391. [DOI] [PubMed] [Google Scholar]
- 4.Schleicher R.L., Carroll M.D., Ford E.S., Lacher D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES) Am. J. Clin. Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 5.Wei J., Zeng C., Gong Q.Y., Li X.X., Lei G.H., Yang T.B. Associations between dietary antioxidant intake and metabolic syndrome. PLoS One. 2015;10:e0130876. doi: 10.1371/journal.pone.0130876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donin A.S., Dent J.E., Nightingale C.M., Sattar N., Owen C.G., Rudnicka A.R., Perkin M.R., Stephen A.M., Jebb S.A., Cook D.G., Whincup P.H. Fruit. vegetable and vitamin C intakes and plasma vitamin C: cross-sectional associations with insulin resistance and glycaemia in 9-10 year-old children. Diabet. Med. 2016;33:307–315. doi: 10.1111/dme.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamb M.J., Griffin S.J., Sharp S.J., Cooper A.J. Fruit and vegetable intake and cardiovascular risk factors in people with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2017;71:115–121. doi: 10.1038/ejcn.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du H., Li L., Bennett D., Guo Y., Turnbull I., Yang L., Bragg F., Bian Z., Chen Y., Chen J., Millwood I.Y., Sansome S., Ma L., Huang Y., Zhang N., Zheng X., Sun Q., Key T.J., Collins R., Peto R., Chen Z. China Kadoorie Biobank Study. Fresh fruit consumption in relation to incident diabetes and diabetic vascular complications: a 7-y prospective study of 0.5 million Chinese adults. PLoS Med. 2017;14:e1002279. doi: 10.1371/journal.pmed.1002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudjinu H.Y., Sarfo B. Risk factors for type 2 diabetes mellitus among out-patients in Ho, the Volta regional capital of Ghana: a case-control study. BMC Res. Notes. 2017;10:324. doi: 10.1186/s13104-017-2648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson R., Willis J., Gearry R., Skidmore P., Fleming E., Frampton C., Carr A. Inadequate vitamin C status in prediabetes and type 2 diabetes mellitus: associations with glycaemic control, obesity, and smoking. Nutrients. 2017:9. doi: 10.3390/nu9090997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding A.H., Wareham N.J., Bingham S.A., Khaw K., Luben R., Welch A., Forouhi N.G. Plasma vitamin C level, fruit and vegetable consumption, and the risk of new-onset type 2 diabetes mellitus: the European prospective investigation of cancer--Norfolk prospective study. Arch. Intern. Med. 2008;168:1493–1499. doi: 10.1001/archinte.168.14.1493. [DOI] [PubMed] [Google Scholar]
- 12.Pattison D.I., Hawkins C.L., Davies M.J. What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem. Res. Toxicol. 2009;22:807–817. doi: 10.1021/tx800372d. [DOI] [PubMed] [Google Scholar]
- 13.Vors C., Pineau G., Drai J., Meugnier E., Pesenti S., Laville M., Laugerette F., Malpuech-Brugere C., Vidal H., Michalski M.C. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose-effect trial. J. Clin. Endocrinol. Metab. 2015;100:3427–3435. doi: 10.1210/JC.2015-2518. [DOI] [PubMed] [Google Scholar]
- 14.Laugerette F., Alligier M., Bastard J.P., Drai J., Chanseaume E., Lambert-Porcheron S., Laville M., Morio B., Vidal H., Michalski M.C. Overfeeding increases postprandial endotoxemia in men: inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol. Nutr. Food Res. 2014;58:1513–1518. doi: 10.1002/mnfr.201400044. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian V.S., Sabui S., Moradi H., Marchant J.S., Said H.M. Inhibition of intestinal ascorbic acid uptake by lipopolysaccharide is mediated via transcriptional mechanisms. Biochim. Biophys. Acta. 2018;1860:556–565. doi: 10.1016/j.bbamem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., Waget A., Delmee E., Cousin B., Sulpice T., Chamontin B., Ferrieres J., Tanti J.F., Gibson G.R., Casteilla L., Delzenne N.M., Alessi M.C., Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 17.Cani P.D., Delzenne N.M. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr. Opin. Pharmacol. 2009;9:737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Mah E., Sapper T.N., Chitchumroonchokchai C., Failla M.L., Schill K.E., Clinton S.K., Bobe G., Traber M.G., Bruno R.S. alpha-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: a randomized, double-blind, crossover trial. Am. J. Clin. Nutr. 2015;102:1070–1080. doi: 10.3945/ajcn.115.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traber M.G., Mah E., Leonard S.W., Bobe G., Bruno R.S. Metabolic syndrome increases dietary alpha-tocopherol requirements as assessed using urinary and plasma vitamin E catabolites: a double-blind, crossover clinical trial. Am. J. Clin. Nutr. 2017;105:571–579. doi: 10.3945/ajcn.116.138495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Calder P.C., Ahluwalia N., Brouns F., Buetler T., Clement K., Cunningham K., Esposito K., Jonsson L.S., Kolb H., Lansink M., Marcos A., Margioris A., Matusheski N., Nordmann H., O'Brien J., Pugliese G., Rizkalla S., Schalkwijk C., Tuomilehto J., Warnberg J., Watzl B., Winklhofer-Roob B.M. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011;106(Suppl. 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services, US Department of Agriculture 2015–2020 Dietary Guidelines for Americans, 2015.
- 23.Franz M.J., Boucher J.L., Evert A.B. Evidence-based diabetes nutrition therapy recommendations are effective: the key is individualization. Diabetes Metab. Syndr. Obes. 2014;7:65–72. doi: 10.2147/DMSO.S45140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agarwal S., Reider C., Brooks J.R., Fulgoni V.L., 3rd Comparison of prevalence of inadequate nutrient intake based on body weight status of adults in the United States: an analysis of NHANES 2001–2008. J. Am. Coll. Nutr. 2015;34:126–134. doi: 10.1080/07315724.2014.901196. [DOI] [PubMed] [Google Scholar]
- 25.Comerford K.B. Recent developments in multivitamin/mineral research. Adv. Nutr. 2013;4:644–656. doi: 10.3945/an.113.004523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine M., Conry-Cantilena C., Wang Y., Welch R.W., Washko P.W., Dhariwal K.R., Park J.B., Lazarev A., Graumlich J.F., King J., Cantilena L.R. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Round J.L., Mazmanian S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009;9:313–323. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haro C., Garcia-Carpintero S., Alcala-Diaz J.F., Gomez-Delgado F., Delgado-Lista J., Perez-Martinez P., Rangel Zuniga O.A., Quintana-Navarro G.M., Landa B.B., Clemente J.C., Lopez-Miranda J., Camargo A., Perez-Jimenez F. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016;27:27–31. doi: 10.1016/j.jnutbio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Pastori D., Carnevale R., Nocella C., Novo M., Santulli M., Cammisotto V., Menichelli D., Pignatelli P., Violi F. Gut-derived serum lipopolysaccharide is associated with enhanced risk of major adverse cardiovascular events in atrial fibrillation: effect of adherence to Mediterranean diet. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fialho A., Fialho A., Thota P., McCullough A., Shen B. Higher visceral to subcutaneous fat ratio is associated with small intestinal bacterial overgrowth. Nutr. Metab. Cardiovasc. Dis. 2016;26:773–777. doi: 10.1016/j.numecd.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Ghoshal S., Witta J., Zhong J., de Villiers W., Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 2009;50:90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Vreugdenhil A.C., Rousseau C.H., Hartung T., Greve J.W., van 't Veer C., Buurman W.A. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J. Immunol. 2003;170:1399–1405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Moreno J., Garcia-Carpintero S., Gomez-Delgado F., Jimenez-Lucena R., Vals-Delgado C., Alcala-Diaz J.F., Roncero-Ramos I., Rangel-Zuniga O.A., Yubero-Serrano E.M., Malagon M.M., Ordovas J.M., Perez-Martinez P., Lopez-Miranda J., Camargo A. Endotoxemia is modulated by quantity and quality of dietary fat in older adults. Exp. Gerontol. 2018;109:119–125. doi: 10.1016/j.exger.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Li J., Sasaki G.Y., Dey P., Chitchumroonchokchai C., Labyk A.N., McDonald J.D., Kim J.B., Bruno R.S. Green tea extract protects against hepatic NFkappaB activation along the gut-liver axis in diet-induced obese mice with nonalcoholic steatohepatitis by reducing endotoxin and TLR4/MyD88 signaling. J. Nutr. Biochem. 2018;53:58–65. doi: 10.1016/j.jnutbio.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Sapper T.N., Mah E., Moller M.V., Kim J.B., Chitchumroonchokchai C., McDonald J.D., Bruno R.S. Green tea extract treatment reduces NFkappaB activation in mice with diet-induced nonalcoholic steatohepatitis by lowering TNFR1 and TLR4 expression and ligand availability. J. Nutr. Biochem. 2017;41:34–41. doi: 10.1016/j.jnutbio.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Guo Y., Mah E., Bruno R.S. Quercetin bioavailability is associated with inadequate plasma vitamin C status and greater plasma endotoxin in adults. Nutrition. 2014;30:1279–1286. doi: 10.1016/j.nut.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 37.Food and Nutrition Board . National Academy Press; Washington: 2000. Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. [PubMed] [Google Scholar]
- 38.Tsukaguchi H., Tokui T., Mackenzie B., Berger U.V., Chen X.Z., Wang Y., Brubaker R.F., Hediger M.A. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian V.S., Sabui S., Subramenium G.A., Marchant J.S., Said H.M. Tumor necrosis factor alpha reduces intestinal vitamin C uptake: a role for NF-kappaB-mediated signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2018;315:G241–G248. doi: 10.1152/ajpgi.00071.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramanian V.S., Srinivasan P., Wildman A.J., Marchant J.S., Said H.M. Molecular mechanism(s) involved in differential expression of vitamin C transporters along the intestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2017;312:G340–G347. doi: 10.1152/ajpgi.00369.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vera J.C., Rivas C.I., Fischbarg J., Golde D.W. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- 42.Corpe C.P., Eck P., Wang J., Al-Hasani H., Levine M. Intestinal dehydroascorbic acid (DHA) transport mediated by the facilitative sugar transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013;288:9092–9101. doi: 10.1074/jbc.M112.436790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malo C., Wilson J.X. Glucose modulates vitamin C transport in adult human small intestinal brush border membrane vesicles. J. Nutr. 2000;130:63–69. doi: 10.1093/jn/130.1.63. [DOI] [PubMed] [Google Scholar]
- 44.Amir Shaghaghi M., Zhouyao H., Tu H., El-Gabalawy H., Crow G.H., Levine M., Bernstein C.N., Eck P. The SLC2A14 gene, encoding the novel glucose/dehydroascorbate transporter GLUT14, is associated with inflammatory bowel disease. Am. J. Clin. Nutr. 2017;106:1508–1513. doi: 10.3945/ajcn.116.147603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buffinton G.D., Doe W.F. Depleted mucosal antioxidant defences in inflammatory bowel disease. Free Radic. Biol. Med. 1995;19:911–918. doi: 10.1016/0891-5849(95)94362-h. [DOI] [PubMed] [Google Scholar]
- 46.Pierre J.F., Hinterleitner R., Bouziat R., Hubert N.A., Leone V., Miyoshi J., Jabri B., Chang E.B. Dietary antioxidant micronutrients alter mucosal inflammatory risk in a murine model of genetic and microbial susceptibility. J. Nutr. Biochem. 2018;54:95–104. doi: 10.1016/j.jnutbio.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olmedo J.M., Yiannias J.A., Windgassen E.B., Gornet M.K. Scurvy: a disease almost forgotten. Int. J. Dermatol. 2006;45:909–913. doi: 10.1111/j.1365-4632.2006.02844.x. [DOI] [PubMed] [Google Scholar]
- 48.Srikiran P., Bashar A.M., Suresh N., Melchor D., Frida A. Gastrointestinal manifestations of scurvy. Am. J. Gastroenterol. 2000;95 (2623-abstract) [Google Scholar]
- 49.Marik P.E., Hooper M.H. Doctor-your septic patients have scurvy! Crit. Care. 2018;22:23. doi: 10.1186/s13054-018-1950-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanwar P., Nelson J.E., Yates K., Kleiner D.E., Unalp-Arida A., Kowdley K.V. Association between metabolic syndrome and liver histology among NAFLD patients without diabetes. BMJ Open Gastroenterol. 2016;3:e000114. doi: 10.1136/bmjgast-2016-000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirpich I.A., Marsano L.S., McClain C.J. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 2015;48:923–930. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukui H. Increased intestinal permeability and decreased barrier function: does it really influence the risk of inflammation? Inflamm. Intest Dis. 2016;1:135–145. doi: 10.1159/000447252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.France M.M., Turner J.R. The mucosal barrier at a glance. J. Cell Sci. 2017;130:307–314. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimoto M., Uemura M., Nakatani Y., Tsujita S., Hoppo K., Tamagawa T., Kitano H., Kikukawa M., Ann T., Ishii Y., Kojima H., Sakurai S., Tanaka R., Namisaki T., Noguchi R., Higashino T., Kikuchi E., Nishimura K., Takaya A., Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin. Exp. Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- 55.Plovier H., Cani P.D. Microbial impact on host metabolism: opportunities for novel treatments of nutritional disorders? Microbiol. Spectr. 2017:5. doi: 10.1128/microbiolspec.BAD-0002-2016. [DOI] [PubMed] [Google Scholar]
- 56.Lin R.S., Lee F.Y., Lee S.D., Tsai Y.T., Lin H.C., Lu R.H., Hsu W.C., Huang C.C., Wang S.S., Lo K.J. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 57.Chan C.C., Hwang S.J., Lee F.Y., Wang S.S., Chang F.Y., Li C.P., Chu C.J., Lu R.H., Lee S.D. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand. J. Gastroenterol. 1997;32:942–946. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 58.Alisi A., Manco M., Devito R., Piemonte F., Nobili V. Endotoxin and plasminogen activator inhibitor-1 serum levels associated with nonalcoholic steatohepatitis in children. J. Pediatr. Gastroenterol. Nutr. 2010;50:645–649. doi: 10.1097/MPG.0b013e3181c7bdf1. [DOI] [PubMed] [Google Scholar]
- 59.Guercio Nuzio S., Di Stasi M., Pierri L., Troisi J., Poeta M., Bisogno A., Belmonte F., Tripodi M., Di Salvio D., Massa G., Savastano R., Cavallo P., Boffardi M., Ziegenhardt D., Bergheim I., Mandato C., Vajro P. Multiple gut-liver axis abnormalities in children with obesity with and without hepatic involvement. Pediatr. Obes. 2017;12:446–452. doi: 10.1111/ijpo.12164. [DOI] [PubMed] [Google Scholar]
- 60.Benito E., Bosch M.A. Impaired phosphatidylcholine biosynthesis and ascorbic acid depletion in lung during lipopolysaccharide-induced endotoxaemia in guinea pigs. Mol. Cell Biochem. 1997;175:117–123. doi: 10.1023/a:1006883628365. [DOI] [PubMed] [Google Scholar]
- 61.Cadenas S., Rojas C., Barja G. Endotoxin increases oxidative injury to proteins in guinea pig liver: protection by dietary vitamin C. Pharmacol. Toxicol. 1998;82:11–18. doi: 10.1111/j.1600-0773.1998.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 62.Abhilash P.A., Harikrishnan R., Indira M. Ascorbic acid suppresses endotoxemia and NF-kappaB signaling cascade in alcoholic liver fibrosis in guinea pigs: a mechanistic approach. Toxicol. Appl. Pharmacol. 2014;274:215–224. doi: 10.1016/j.taap.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Tokuda Y., Miura N., Kobayashi M., Hoshinaga Y., Murai A., Aoyama H., Ito H., Morita T., Horio F. Ascorbic acid deficiency increases endotoxin influx to portal blood and liver inflammatory gene expressions in ODS rats. Nutrition. 2015;31:373–379. doi: 10.1016/j.nut.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Ohta Y., Chiba S., Imai Y., Kamiya Y., Arisawa T., Kitagawa A. Ascorbic acid deficiency aggravates stress-induced gastric mucosal lesions in genetically scorbutic ODS rats. Inflammopharmacology. 2006;14:231–235. doi: 10.1007/s10787-006-1539-z. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda S., Horio F., Kakinuma A. Ascorbic acid deficiency changes hepatic gene expression of acute phase proteins in scurvy-prone ODS rats. J. Nutr. 1998;128:832–838. doi: 10.1093/jn/128.5.832. [DOI] [PubMed] [Google Scholar]
- 66.Horio F., Kiyama K., Kobayashi M., Kawai K., Tsuda T. Ascorbic acid deficiency stimulates hepatic expression of inflammatory chemokine, cytokine-induced neutrophil chemoattractant-1, in scurvy-prone ODS rats. J. Nutr. Sci. Vitaminol. 2006;52:28–32. doi: 10.3177/jnsv.52.28. [DOI] [PubMed] [Google Scholar]
- 67.Horio F., Takahashi N., Makino S., Hayashi Y., Yoshida A. Ascorbic acid deficiency elevates serum level of LDL-cholesterol in a rat mutant unable to synthesize ascorbic acid. J. Nutr. Sci. Vitaminol. 1991;37:63–71. doi: 10.3177/jnsv.37.63. [DOI] [PubMed] [Google Scholar]
- 68.Kono K., Hayakawa M., Asai K., Kuzuya F. Cholesterol metabolism in inherently scorbutic rats (ODS rats) J. Nutr. Sci. Vitaminol. 1988;34:35–45. doi: 10.3177/jnsv.34.35. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda S., Horio F., Yoshida A., Kakinuma A. Ascorbic acid deficiency reduces hepatic apolipoprotein A-I mRNA in scurvy-prone ODS rats. J. Nutr. 1996;126:2505–2511. doi: 10.1093/jn/126.10.2505. [DOI] [PubMed] [Google Scholar]
- 70.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 71.Fowler A.A., 3rd, Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., Gupta S. Medical Respiratory Intensive Care Unit Nursing, Fisher BJ, Natarajan R. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teng J., Pourmand A., Mazer-Amirshahi M. Vitamin C: the next step in sepsis management? J. Crit. Care. 2018;43:230–234. doi: 10.1016/j.jcrc.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 73.Aschauer S., Gouya G., Klickovic U., Storka A., Weisshaar S., Vollbracht C., Krick B., Weiss G., Wolzt M. Effect of systemic high dose vitamin C therapy on forearm blood flow reactivity during endotoxemia in healthy human subjects. Vasc. Pharmacol. 2014;61:25–29. doi: 10.1016/j.vph.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Schoultz I., McKay C.M., Graepel R., Phan V.C., Wang A., Soderholm J., McKay D.M. Indomethacin-induced translocation of bacteria across enteric epithelia is reactive oxygen species-dependent and reduced by vitamin C. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G536–G545. doi: 10.1152/ajpgi.00125.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou G., Kamenos G., Pendem S., Wilson J.X., Wu F. Ascorbate protects against vascular leakage in cecal ligation and puncture-induced septic peritonitis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R409–R416. doi: 10.1152/ajpregu.00153.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kuck J.L., Bastarache J.A., Shaver C.M., Fessel J.P., Dikalov S.I., May J.M., Ware L.B. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem Biophys. Res. Commun. 2018;495:433–437. doi: 10.1016/j.bbrc.2017.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cevikel M.H., Tuncyurek P., Ceylan F., Meteoglu I., Kozaci D., Boylu S. Supplementation with high-dose ascorbic acid improves intestinal anastomotic healing. Eur. Surg. Res. 2008;40:29–33. doi: 10.1159/000108622. [DOI] [PubMed] [Google Scholar]
- 78.Winterbourn C.C., Kettle A.J., Hampton M.B. Reactive oxygen species and neutrophil function. Annu. Rev. Biochem. 2016;85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 79.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei J., Lei G.H., Fu L., Zeng C., Yang T., Peng S.F. Association between dietary vitamin C intake and non-alcoholic fatty liver disease: a cross-sectional study among middle-aged and older adults. PLoS One. 2016;11:e0147985. doi: 10.1371/journal.pone.0147985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ipsen D.H., Tveden-Nyborg P., Lykkesfeldt J. Does vitamin C deficiency promote fatty liver disease development? Nutrients. 2014;6:5473–5499. doi: 10.3390/nu6125473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bruno R.S., Leonard S.W., Atkinson J., Montine T.J., Ramakrishnan R., Bray T.M., Traber M.G. Faster plasma vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic. Biol. Med. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 83.Bruno R.S., Ramakrishnan R., Montine T.J., Bray T.M., Traber M.G. {alpha}-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am. J. Clin. Nutr. 2005;81:95–103. doi: 10.1093/ajcn/81.1.95. [DOI] [PubMed] [Google Scholar]
- 84.Burri E., Beglinger C. The use of fecal calprotectin as a biomarker in gastrointestinal disease. Expert Rev. Gastroenterol. Hepatol. 2014;8:197–210. doi: 10.1586/17474124.2014.869476. [DOI] [PubMed] [Google Scholar]
- 85.Murad S., Grove D., Lindberg K.A., Reynolds G., Sivarajah A., Pinnell S.R. Regulation of collagen synthesis by ascorbic acid. Proc. Natl. Acad. Sci. USA. 1981;78:2879–2882. doi: 10.1073/pnas.78.5.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt T.M., Kao J.Y. A little O2 may go a long way in structuring the GI microbiome. Gastroenterology. 2014;147:956–959. doi: 10.1053/j.gastro.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuda K., Oh K., Ren B., Tickle T.L., Franzosa E.A., Wachtman L.M., Miller A.D., Westmoreland S.V., Mansfield K.G., Vallender E.J., Miller G.M., Rowlett J.K., Gevers D., Huttenhower C., Morgan X.C. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host Microbe. 2015;17:385–391. doi: 10.1016/j.chom.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stacy A., McNally L., Darch S.E., Brown S.P., Whiteley M. The biogeography of polymicrobial infection. Nat. Rev. Microbiol. 2016;14:93–105. doi: 10.1038/nrmicro.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones R.M., Mercante J.W., Neish A.S. Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr. Med. Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jones R.M., Neish A.S. Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 2017;105:41–47. doi: 10.1016/j.freeradbiomed.2016.10.495. [DOI] [PubMed] [Google Scholar]
- 91.Li J., Liu Y., Kim E., March J.C., Bentley W.E., Payne G.F. Electrochemical reverse engineering: a systems-level tool to probe the redox-based molecular communication of biology. Free Radic. Biol. Med. 2017;105:110–131. doi: 10.1016/j.freeradbiomed.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 92.Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 2004;14:679–686. doi: 10.1016/j.sbi.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Ussar S., Griffin N.W., Bezy O., Fujisaka S., Vienberg S., Softic S., Deng L., Bry L., Gordon J.I., Kahn C.R. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kreznar J.H., Keller M.P., Traeger L.L., Rabaglia M.E., Schueler K.L., Stapleton D.S., Zhao W., Vivas E.I., Yandell B.S., Broman A.T., Hagenbuch B., Attie A.D., Rey F.E. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. 2017;18:1739–1750. doi: 10.1016/j.celrep.2017.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., Wang T. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 96.Sanchez-Tapia M., Aguilar-Lopez M., Perez-Cruz C., Pichardo-Ontiveros E., Wang M., Donovan S.M., Tovar A.R., Torres N. Nopal (Opuntia ficus indica) protects from metabolic endotoxemia by modifying gut microbiota in obese rats fed high fat/sucrose diet. Sci. Rep. 2017;7:4716. doi: 10.1038/s41598-017-05096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park T., Yu Z. Aerobic cultivation of anaerobic rumen protozoa, Entodinium caudatum and Epidinium caudatum. J. Microbiol. Methods. 2018;152:186–193. doi: 10.1016/j.mimet.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 98.Ashton T., Young I.S., Davison G.W., Rowlands C.C., McEneny J., Van Blerk C., Jones E., Peters J.R., Jackson S.K. Exercise-induced endotoxemia: the effect of ascorbic acid supplementation. Free Radic. Biol. Med. 2003;35:284–291. doi: 10.1016/s0891-5849(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 99.Buettner G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 100.Buettner G.R., Jurkiewicz B.A. Ascorbate free radical as a marker of oxidative stress: an EPR study. Free Radic. Biol. Med. 1993;14:49–55. doi: 10.1016/0891-5849(93)90508-r. [DOI] [PubMed] [Google Scholar]
- 101.Muhlhofer A., Mrosek S., Schlegel B., Trommer W., Rozario F., Bohles H., Schremmer D., Zoller W.G., Biesalski H.K. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur. J. Clin. Nutr. 2004;58:1151–1158. doi: 10.1038/sj.ejcn.1601943. [DOI] [PubMed] [Google Scholar]
- 102.Welsh J.L., Wagner B.A., van't Erve T.J., Zehr P.S., Berg D.J., Halfdanarson T.R., Yee N.S., Bodeker K.L., Du J., Roberts L.J., 2nd, Drisko J., Levine M., Buettner G.R., Cullen J.J. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013;71:765–775. doi: 10.1007/s00280-013-2070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nauman G., Gray J.C., Parkinson R., Levine M., Paller C.J. Systematic review of intravenous ascorbate in cancer clinical trials. Antioxidants. 2018;7 doi: 10.3390/antiox7070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cabelli D.E., Bielski B.H.J. Kinetics and mechanism for the oxidation of ascorbic acid/ascorbate by HO2/O2- (hydroperoxyl/superoxide) radicals. A pulse radiolysis and stopped-flow photolysis study. J. Phys. Chem. 1983;87:1809–1812. [Google Scholar]
- 106.Sun Y., Pham A.N., Waite T.D. The effect of vitamin C and iron on dopamine-mediated free radical generation: implications to Parkinson's disease. Dalton Trans. 2018;47:4059–4069. doi: 10.1039/c7dt04373b. [DOI] [PubMed] [Google Scholar]
- 107.Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E., Choyke P.L., Pooput C., Kirk K.L., Buettner G.R., Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Buettner G.R., Jurkiewicz B.A. Catalytic metals, ascorbate and free radicals: combinations to avoid. Radiat. Res. 1996;145:532–541. [PubMed] [Google Scholar]
- 109.Schoenfeld J.D., Sibenaller Z.A., Mapuskar K.A., Wagner B.A., Cramer-Morales K.L., Furqan M., Sandhu S., Carlisle T.L., Smith M.C., Abu Hejleh T., Berg D.J., Zhang J., Keech J., Parekh K.R., Bhatia S., Monga V., Bodeker K.L., Ahmann L., Vollstedt S., Brown H., Shanahan Kauffman E.P., Schall M.E., Hohl R.J., Clamon G.H., Greenlee J.D., Howard M.A., Schultz M.K., Smith B.J., Riley D.P., Domann F.E., Cullen J.J., Buettner G.R., Buatti J.M., Spitz D.R., Allen B.G. O2(-) and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;31(487–500):e488. doi: 10.1016/j.ccell.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood J.C. Estimating tissue iron burden: current status and future prospects. Br. J. Haematol. 2015;170:15–28. doi: 10.1111/bjh.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doskey C.M., Buranasudja V., Wagner B.A., Wilkes J.G., Du J., Cullen J.J., Buettner G.R. Tumor cells have decreased ability to metabolize H2O2: implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016;10:274–284. doi: 10.1016/j.redox.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mainous A.G., 3rd, Wright R.U., Hulihan M.M., Twal W.O., McLaren C.E., Diaz V.A., McLaren G.D., Argraves W.S., Grant A.M. Elevated transferrin saturation, health-related quality of life and telomere length. Biometals. 2014;27:135–141. doi: 10.1007/s10534-013-9693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Puliyel M., Mainous A.G., 3rd, Berdoukas V., Coates T.D. Iron toxicity and its possible association with treatment of cancer: lessons from hemoglobinopathies and rare, transfusion-dependent anemias. Free Radic. Biol. Med. 2015;79:343–351. doi: 10.1016/j.freeradbiomed.2014.10.861. [DOI] [PubMed] [Google Scholar]
- 114.Padayatty S.J., Sun A.Y., Chen Q., Espey M.G., Drisko J., Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Golumbic C., Mattill H.A. Antioxidants and the autoxidation of fats. XIII. The antioxygenic action of ascorbic acid in association with tocopherols, hydroquinones and related compounds. J. Am. Chem. Soc. 1941;63:1279–1280. [Google Scholar]
- 116.J. Le Drug bioavailability. Merck Manual Professional Version. Merck Sharp & Dohme Corp. Updated: November, 2017. 〈https://www.merckmanuals.com/professional/clinical-pharmacology/pharmacokinetics/drug-bioavailability〉. (Accessed 19 December 2018).
- 117.Traber M.G. Mechanisms for the prevention of vitamin E excess. J. Lipid Res. 2013;54:2295–2306. doi: 10.1194/jlr.R032946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Demir M., Lang S., Steffen H.M. Nonalcoholic fatty liver disease - current status and future directions. J. Dig. Dis. 2015;16:541–557. doi: 10.1111/1751-2980.12291. [DOI] [PubMed] [Google Scholar]
- 119.Chalasani N.P., Sanyal A.J., Kowdley K.V., Robuck P.R., Hoofnagle J., Kleiner D.E., Unalp A., Tonascia J., Group N.C.R. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemp. Clin. Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lavine J.E., Schwimmer J.B., Van Natta M.L., Molleston J.P., Murray K.F., Rosenthal P., Abrams S.H., Scheimann A.O., Sanyal A.J., Chalasani N., Tonascia J., Unalp A., Clark J.M., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R. Nonalcoholic Steatohepatitis Clinical Research Network. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor A.W., Bruno R.S., Traber M.G. Women and smokers have elevated urinary F(2)-isoprostane metabolites: a novel extraction and LC-MS methodology. Lipids. 2008;43:925–936. doi: 10.1007/s11745-008-3222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bobe G., Cobb T.J., Leonard S.W., Aponso S., Bahro C.B., Koley D., Mah E., Bruno R.S., Traber M.G. Increased static and decreased capacity oxidation-reduction potentials in plasma are predictive of metabolic syndrome. Redox Biol. 2017;12:121–128. doi: 10.1016/j.redox.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sokol R.J., Heubi J.E., Iannaccone S.T., Bove K.E., Balistreri W.F. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N. Engl. J. Med. 1984;310:1209–1212. doi: 10.1056/NEJM198405103101901. [DOI] [PubMed] [Google Scholar]
- 124.Sontag T.J., Parker R.S. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 2002;277:25290–25296. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 125.Traber M.G., Sokol R.J., Burton G.W., Ingold K.U., Papas A.M., Huffaker J.E., Kayden H.J. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J. Clin. Investig. 1990;85:397–407. doi: 10.1172/JCI114452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sontag T.J., Parker R.S. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J. Lipid Res. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 127.Mustacich D.J., Leonard S.W., Patel N.K., Traber M.G. Alpha-tocopherol beta-oxidation localized to rat liver mitochondria. Free Radic. Biol. Med. 2010;48:73–81. doi: 10.1016/j.freeradbiomed.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Traber M.G. Vitamin E inadequacy in humans: causes and consequences. Adv. Nutr. 2014;5:503–514. doi: 10.3945/an.114.006254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pein H., Ville A., Pace S., Temml V., Garscha U., Raasch M., Alsabil K., Viault G., Dinh C.P., Guilet D., Troisi F., Neukirch K., Konig S., Bilancia R., Waltenberger B., Stuppner H., Wallert M., Lorkowski S., Weinigel C., Rummler S., Birringer M., Roviezzo F., Sautebin L., Helesbeux J.J., Seraphin D., Mosig A.S., Schuster D., Rossi A., Richomme P., Werz O., Koeberle A. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat. Commun. 2018;9:3834. doi: 10.1038/s41467-018-06158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maras J.E., Bermudez O.I., Qiao N., Bakun P.J., Boody-Alter E.L., Tucker K.L. Intake of alpha-tocopherol is limited among US adults. J. Am. Diet. Assoc. 2004;104:567–575. doi: 10.1016/j.jada.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 131.McBurney M.I., Yu E.A., Ciappio E.D., Bird J.K., Eggersdorfer M., Mehta S. Suboptimal serum alpha-tocopherol concentrations observed among younger adults and those depending exclusively upon food sources, NHANES 2003–2006. PLoS One. 2015;10:e0135510. doi: 10.1371/journal.pone.0135510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pearson J.F., Pullar J.M., Wilson R., Spittlehouse J.K., Vissers M.C.M., Skidmore P.M.L., Willis J., Cameron V.A., Carr A.C. Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: findings of the CHALICE cohort study. Nutrients. 2017:9. doi: 10.3390/nu9080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Carr A.C., Pullar J.M., Bozonet S.M., Vissers M.C. Marginal ascorbate status (hypovitaminosis C) results in an attenuated response to vitamin C supplementation. Nutrients. 2016:8. doi: 10.3390/nu8060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M., Hypovitaminosis C. and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mitchell C.M., Davy B.M., Halliday T.M., Hulver M.W., Neilson A.P., Ponder M.A., Davy K.P. The effect of prebiotic supplementation with inulin on cardiometabolic health: rationale, design, and methods of a controlled feeding efficacy trial in adults at risk of type 2 diabetes. Contemp. Clin. Trials. 2015;45:328–337. doi: 10.1016/j.cct.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Del Valle-Pinero A.Y., Van Deventer H.E., Fourie N.H., Martino A.C., Patel N.S., Remaley A.T., Henderson W.A. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin. Chim. Acta. 2013;418:97–101. doi: 10.1016/j.cca.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cadenas E., Packer L., Traber M.G. Antioxidants, oxidants, and redox impacts on cell function - a tribute to Helmut Sies. Arch. Biochem. Biophys. 2016;595:94–99. doi: 10.1016/j.abb.2015.11.012. [DOI] [PubMed] [Google Scholar]