Introduction
Cerebral amyloid angiopathy-related inflammation (CAA-ri) is an unusual cause of encephalopathy, seizures and focal neurological deficits.1 2 We report three cases of CAA-ri with minimal symptoms but striking and dynamically evolving brain MRI findings.
Case 1
A 62-year-old man presented with a moderately severe non-radiating frontal headache. Brain MRI 9 months later showed multiple discrete regions of abnormal signal and mild swelling involving white matter and overlying cortex. Susceptibility-weighted imaging (SWI) demonstrated numerous cortical lobar microbleeds throughout both cerebral hemispheres. Repeat MRI another 9 months later showed resolution of many of the parenchymal abnormalities, but with several new regions containing more peripheral microbleeds. Amyloid-PET (using 18F-florbetapir) demonstrated moderate widespread amyloid deposition; CSF analysis showed reduced amyloid-beta 1–42 and high-normal total tau. Formal neuropsychological testing suggested mild compromise in frontal functioning only. The patient was treated with 5 days of intravenous methylprednisolone (1 g daily), followed by an oral taper from prednisolone 60 mg over 8 weeks. Follow-up MRI after 8 months showed almost complete resolution of the parenchymal abnormalities, but with persisting lobar microbleeds. At 24 months following symptom onset, he remains asymptomatic, with stable brain imaging.
Case 2
A 74-year-old man presented with mild subjective memory difficulties only, with no objective neuropsychological deficits. MRI demonstrated a substantial region of abnormal signal in the right temporal and occipital white matter, with no enhancement. Repeat imaging after a few weeks showed partial regression. Over the following 4 years, three further MRIs showed multiple areas of abnormal white matter (sometimes involving cortex as well) within the temporal, parietal and occipital lobes, which largely resolved. SWI demonstrated progressive accumulation of lobar microbleeds, mainly in the affected areas. The patient remains asymptomatic with no change in his subjective cognitive symptoms, without having received immunosuppressive treatment.
Case 3
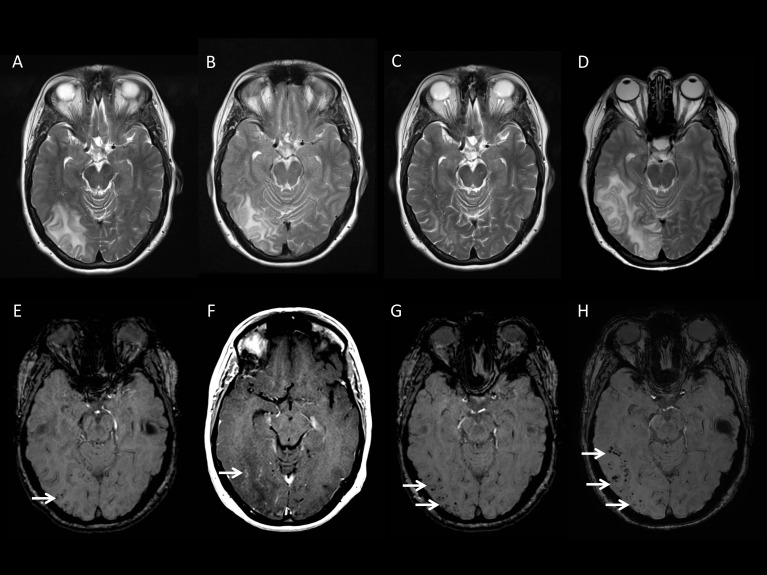
A 54-year-old woman presented with a bright flashing light in her left visual field and a sudden onset headache. After initial CT of the brain demonstrated right-sided occipital hypoattenuation, she was treated for ischaemic stroke and then antiepileptic drugs for presumed seizures. Approximately 6 months later, she developed worsening headache; MRI showed an area of abnormal signal and mild parenchymal swelling in the right temporo-occipital area. A diagnostic brain biopsy showed CAA-ri (Vonsattel grade 3 CAA with associated chronic inflammatory cell infiltration within and around the vessel wall, with angiodestructive and occlusive features). After a further 8 months, she was still experiencing occasional left-sided visual flickering and some subtle memory difficulties. MRI (figure 1) demonstrated progression of the right temporo-occipital abnormality, together with a new separate focus in the anterior right temporal lobe and multiple lobar microbleeds in these regions. Formal neuropsychological testing was normal. Although clinically stable, further MRI 7 weeks later showed extension of the right temporal lobe lesion. She was treated with intravenous methylprednisolone (1 g daily, 5 days, followed by tapering dose prednisolone); 1 month later, the parenchymal signal abnormalities had improved significantly, with no increase in the number of microbleeds.
Figure 1.
MRI from Case 3, illustrating the incidence of different imaging features of CAA-ri. T2-weighted images obtained 9 months following intitial presentation (A) demonstrate an area of parenchymal signal abnormality in the right temporo-occipital region. SWI from the same time (E) show a few cortical microbleeds. Further imaging obtained 2 months later shows progression of the right temporo-occipital abnormalities on T2-weighted sequences (B) and post-gadolinium T1-weighted images (G) show only subtle enhancement (F). The patient was then treated with corticosteroids, and T2-weighted MRI 5 weeks later shows significant improvement of the abnormalities (C), while SWI demonstrates an increase in the number of cortical microbleeds in the affected area (G). The patient developed new visual symptoms 1 year following her corticosteroid treatment and was reimaged. T2-weighted imaging (D) showed recurrence and extension of the original right temporo-occipital parenchymal abnormalities, with the coincident development of multiple new cortical microbleeds (H). CAA-ri, cerebral amyloid angiopathy-related inflammation; SWI, susceptibility weighted images.
One year after intravenous corticosteroid treatment, while still taking oral steroids, the patient developed headache and new left-sided visual disturbances. MRI showed recurrence and extension of the right-sided temporo-occipital region abnormalities, with local swelling and numerous new cortical microbleeds in the affected area. The patient was once again treated with intravenous corticosteroids (as previously); follow-up MRI 3 months after this showed almost complete regression of the right temporal abnormalities and no change in the appearance or number of peripheral microbleeds.
Discussion
We report three cases of CAA-ri (one definite and two probable, according to proposed criteria for CAA-ri1 2) in which the diagnosis of CAA-ri was made when the patients underwent neuroimaging for mild neurological symptoms. Imaging in all cases showed regions of abnormal gyral signal that waxed and waned over time (months to several years) and involved multiple separate areas, either simultaneously or sequentially, often in the absence of new clinical features. Peripheral lobar microbleeds were observed in all cases and tended to accumulate in areas affected by the abnormal MRI signal and swelling. While headache and positive visual symptoms have been described in CAA-ri, these occurred with more serious neurological symptoms (coma, seizures, altered behaviour, focal neurological deficits3); to the best of our knowledge, CAA-ri presenting with minimal or no symptoms has not previously been described.
These cases highlight several points of interest. The first is the dissociation between the mild clinical features and striking radiological abnormalities; this has been described during the routine follow-up in patients with known CAA-ri.4 Greater MRI availability and an increasing awareness of CAA-ri might thus result in more incidentally diagnosed cases. A recent case series5 described three patients presenting with acute stroke (one ischaemic, two haemorrhagic) with coexistent MRI and cerebrospinal fluid evidence of CAA-ri.
Consistent with previous reports,6–8 our cases closely resemble the amyloid-related imaging abnormalities (ARIA) described in patients with Alzheimer’s disease treated with antiamyloid immunotherapy.9 However, CAA-ri is usually associated with marked neurological disturbances, while ARIA is often asymptomatic or mild.10 Imaging findings consistent with CAA-ri or ARIA have also been described in a small number of patients with Alzheimer’s disease prior to treatment, all of whom were asymptomatic.11 Our cases suggest that spontaneous CAA-ri can also present with minimal symptoms and so might represent a normal physiological mechanism of amyloid clearance.12
Pathological verification remains the gold standard for CAA-ri (only available for one of our patients) and the current clinico-radiological criteria require further validation, particularly for atypical cases, where amyloid- Positron Emission Tomography (PET)13 and cerebrospinal fluid (CSF) findings14 might be helpful. Additionally, minimally symptomatic cases might differ from ‘classical’ CAA-ri; for example, the ApoE ε4 allele is associated with CAA-ri;14 but we did not obtain ApoE information and so cannot investigate this. Although our case reports expand the clinical spectrum of CAA, further longer-term follow-up to better establish the natural history of minimally symptomatic CAA-ri is needed.
Footnotes
Contributors: GB reviewed the literature and the cases and generated the manuscript and figure. DA and JB cared for the patients and revised the manuscript. MEA reviewed all neuroimaging, contributed to the figure and revised the manuscript. DJW cared for the patients, contributed to the study conception and design, interpretation of cases and manuscript revision.
Funding: GB receives funding from the Rosetrees Trust. DJW receives research support from the Stroke Association, the British Heart Foundation and the Rosetrees Trust. This work was undertaken at UCLH/UCL which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Chung KK, Anderson NE, Hutchinson D, et al. . Cerebral amyloid angiopathy related inflammation: three case reports and a review. J Neurol Neurosurg Psychiatry 2011;82:20–6. 10.1136/jnnp.2009.204180 [DOI] [PubMed] [Google Scholar]
- 2. Auriel E, Charidimou A, Gurol ME, et al. . Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol 2016;73:197–202. 10.1001/jamaneurol.2015.4078 [DOI] [PubMed] [Google Scholar]
- 3. Castro Caldas A, Silva C, Albuquerque L, et al. . Cerebral amyloid angiopathy associated with inflammation: report of 3 cases and systematic review. J Stroke Cerebrovasc Dis 2015;24:2039–48. 10.1016/j.jstrokecerebrovasdis.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 4. DiFrancesco JC, Touat M, Caulo M, et al. . Recurrence of cerebral amyloid angiopathy-related inflammation: a report of two cases from the iCAβ international network. J Alzheimers Dis 2015;46:1071–7. 10.3233/JAD-150070 [DOI] [PubMed] [Google Scholar]
- 5. Renard D, Wacongne A, Thouvenot E. Radiologically isolated cerebral amyloid angiopathy-related inflammation. J Stroke Cerebrovasc Dis 2017;26:e218–20. 10.1016/j.jstrokecerebrovasdis.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 6. Werring DJ, Sperling R. Inflammatory cerebral amyloid angiopathy and amyloid-modifying therapies: variations on the same ARIA? Ann Neurol 2013;73:439–41. 10.1002/ana.23891 [DOI] [PubMed] [Google Scholar]
- 7. Piazza F, Winblad B. Amyloid-Related Imaging Abnormalities (ARIA) in immunotherapy trials for alzheimer’s disease: need for prognostic biomarkers? J Alzheimers Dis 2016;52:417–20. 10.3233/JAD-160122 [DOI] [PubMed] [Google Scholar]
- 8. DiFrancesco JC, Longoni M, Piazza F. Anti-Aβ Autoantibodies in Amyloid Related Imaging Abnormalities (ARIA): candidate biomarker for immunotherapy in alzheimer’s disease and cerebral amyloid angiopathy. Front Neurol 2015;6:207 10.3389/fneur.2015.00207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sperling R, Salloway S, Brooks DJ, et al. . Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 2012;11:241–9. 10.1016/S1474-4422(12)70015-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sevigny J, Chiao P, Bussière T, et al. . The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 2016;537:50–6. 10.1038/nature19323 [DOI] [PubMed] [Google Scholar]
- 11. Carlson C, Estergard W, Oh J, et al. . Prevalence of asymptomatic vasogenic edema in pretreatment Alzheimer’s disease study cohorts from phase 3 trials of semagacestat and solanezumab. Alzheimers Dement 2011;7:396–401. 10.1016/j.jalz.2011.05.2353 [DOI] [PubMed] [Google Scholar]
- 12. Weller RO, Hawkes CA, Kalaria RN, et al. . White matter changes in dementia: role of impaired drainage of interstitial fluid. Brain Pathol 2015;25:63–78. 10.1111/bpa.12218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carmona-Iragui M, Fernández-Arcos A, Alcolea D, et al. . Cerebrospinal fluid anti-amyloid-β autoantibodies and amyloid PET in cerebral amyloid angiopathy-related inflammation. J Alzheimers Dis 2016;50:1–7. 10.3233/JAD-150614 [DOI] [PubMed] [Google Scholar]
- 14. Piazza F, Greenberg SM, Savoiardo M, et al. . Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol 2013;73:449–58. 10.1002/ana.23857 [DOI] [PubMed] [Google Scholar]